Abstract
The existence of constitutive activity for G protein-coupled receptors (GPCRs) was first described in 1980s. In 1991, the first naturally occurring constitutively active mutations in GPCRs that cause diseases were reported in rhodopsin. Since then, numerous constitutively active mutations that cause human diseases were reported in several additional receptors. More recently, loss of constitutive activity was postulated to also cause diseases. Animal models expressing some of these mutants confirmed the roles of these mutations in the pathogenesis of the diseases. Detailed functional studies of these naturally occurring mutations, combined with homology modeling using rhodopsin crystal structure as the template, lead to important insights into the mechanism of activation in the absence of crystal structure of GPCRs in active state. Search for inverse agonists on these receptors will be critical for correcting the diseases cause by activating mutations in GPCRs. Theoretically, these inverse agonists are better therapeutics than neutral antagonists in treating genetic diseases caused by constitutively activating mutations in GPCRs.
Keywords: G protein-coupled receptor, disease, constitutively active mutation, inverse agonist, mechanism of activation, transgenic model
1. Introduction
G protein-coupled receptors (GPCRs) are versatile signaling molecules at the cell surface. Our sensing of the outside world, including vision, smell, and taste, rely on these receptors. The inter-cellular communications also depend on them (Bockaert & Pin, 1999). They transduce a large variety of extracellular signals, including light, odorants, ions, lipids, catecholamines, neuropeptides and large glycoprotein hormones (Bockaert & Pin, 1999). Analysis of the human genome revealed at least 799 unique GPCRs (Gloriam et al., 2007). One widely adopted scheme classify the GPCRs into five families, with the most important and extensively studied being Family A (Class 1), rhodopsin-like, Family B (Class 2), secretin-like, and Family C (Class 3) metabotropic glutamate receptor-like (Bockaert & Pin, 1999; Kolakowski, 1994). Another classification scheme, with the acronym GRAFS, divide GPCRs into five families: Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2 and Secretin (Fredriksson et al., 2003).
GPCRs consist of seven transmembrane α-helices (TMs) connected by alternating extracellular and intracellular loops (ELs and ILs), with the N terminus extracellular and the C terminus intracellular. The seven TMs are arranged in a counter-clock fashion as viewed from the extracellular surface. The crystal structures of rhodopsin, opsin, β1-, and β2-adrenergic receptor (AR) at high resolution confirmed this topology (Cherezov et al., 2007; Palczewski et al., 2000; Park.et al, 2008; Rasmussen et al., 2007; Rosenbaum et al., 2007; Warne et al., 2008).
Since almost all known physiological processes are regulated by GPCRs, it is easy to appreciate that dysfunctions in these signaling pathways will lead to various pathological states. Since the discovery of the first naturally occurring mutation in GPCRs causing human disease (Dryja et al., 1990), the list for diseases caused by GPCR mutations keeps expanding (for an exhaustive list see (Schoneberg et al., 2004)). A number of excellent general reviews on diseased GPCRs have been published (Arvanitakis et al., 1998; Parnot et al., 2002; Rao & Oprian, 1996; Schoneberg et al., 2004; Schoneberg et al., 2002; Shenker, 1995; Spiegel, 1996; Spiegel & Weinstein, 2004; Spiegel et al., 1993; Tao, 2006) together with many reviews on mutations in individual GPCRs.
In addition to the diseases caused by loss-of-function mutations in GPCRs, dysfunction in basal activity can also cause diseases. In this review, I will briefly introduce constitutive activation of GPCRs. I will then summarize several diseased states caused by either constitutive activation or loss of constitutive activity. Selected examples of animal models expressing constitutively active GPCRs will then be reviewed. Insights gained from detailed studies of these naturally occurring mutations in these as well as related GPCRs, by site-directed mutagenesis and homology modeling, are highlighted. Finally, the potential of inverse agonists in treating the diseases caused by activating mutations are reviewed.
2. Constitutive activation of GPCRs
In 1984, Cerione et al. first described that β2-AR was active in the absence of hormone in a reconstituted system consisting of purified receptor and the stimulatory heterotrimeric G protein, Gs (Cerione et al., 1984). Subsequently, Costa and Herz showed that in membranes prepared from NG108-15 cells, a neuroblastoma-glioma cell line expressing high levels of endogenous δ opioid receptor, some competitive antagonists exhibit negative intrinsic activity; they decrease the GTPase activity in the absence of agonist (Costa & Herz, 1989), suggesting the existence of basal activity in the endogenous δ opioid receptor. These negative antagonists are called inverse agonists (for a recent historical review, see (Costa & Cotecchia, 2005)).
With the freshly cloned subtypes of AR cDNAs at hand, Lefkowitz’s lab soon showed that mutations in α1B-, α2-, and β2-ARs result in dramatic increases in constitutive activity (Cotecchia et al., 1990; Ren et al., 1993; Samama et al., 1993). These studies led them to propose the extended ternary complex model for GPCR activation (Samama et al., 1993). In this model, the wild type (WT) receptor exists in equilibrium between inactive and active conformations. Agonists stabilize the receptor in active conformation, whereas inverse agonists stabilize the receptor in inactive conformation. Neutral antagonists have equal affinities for inactive and active conformations. This model was subsequently modified to more complex models such as cubic ternary complex model (Weiss et al., 1996) to accommodate the existence of multiple active conformations. However, the underlying principals of Lefkowitz’s model remain valid.
Not only mutations can cause constitutive activation, many WT receptors also have considerable constitutive activities. In addition to δ opioid receptor described above, other prominent examples of WT receptors with high constitutive activity include CB1 cannabinoid receptor, growth hormone secretagogue (GHS) receptor (GHSR) (see below), and melancortin-1 receptor (MC1R) (see (Seifert & Wenzel-Seifert, 2002) for an exhaustive list). Members of the same subfamily of related receptors frequently have different constitutive activity. A classical example is that of the dopamine receptors, with the D1B having high constitutive activity (Tiberi & Caron, 1994). Similarly, β2-AR has significantly higher constitutive activity than β1-AR (Chakir et al., 2003). The constitutive activity has physiological significance as shown by mutations that selectively eliminates the constitutive activity result in human diseases (see Section 4).
As detailed below, constitutively active mutation in rhodopsin was soon discovered in humans. Cone and colleagues were the first to report a naturally occurring constitutively active mutation in GPCRs that bind diffusible ligands. They showed that the somber allele in mice is due to a constitutively active mutation in the MC1R (Robbins et al., 1993). The mutation, E92K (2.62) (see next paragraph regarding the numbering system), in the extracellular half of TM2, increased the basal signaling to about 60% of the maximal response achieved by the WT receptor. This pioneering report was quickly followed with a series of constitutively active mutations in other GPCRs that cause diseases in humans. This is the focus of this review.
Ballesteros and Weinstein proposed a numbering system that facilitates the comparison of data obtained from different GPCRs (Ballesteros & Weinstein, 1995). In this system, two numbers denote the location of each residue. The first number denotes the helix. The second number denotes the relative position of the residue to the most highly conserved residue in that TM, decreasing towards the N terminus, and increasing towards the C terminus. The most highly conserved residue is given a number of 50. For example, there is a signature motif towards the end of TM3, DRY. Arginine is the most highly conserved residue in TM3, therefore numbered as 3.50. Hence Asp is designated as 3.49, whereas Tyr is designated as 3.51. The mouse MC1R mutation described above, E92K, is designated E92K (2.62). This numbering system is used here to highlight the fact that indeed many mutations affect the same loci, which is not easy to appreciate from the amino acid numbering of the different receptors.
3. Diseases caused by constitutively active mutations in GPCRs
Constitutively active mutations have also been frequently referred to as “gain-of-function mutations”. However, there are in fact three types of gain-of-function mutations (Refetoff et al., 2001). The first one is constitutive activation. The second one is increased sensitivity to the agonist (decreased EC50, concentration of agonist needed to cause 50% maximal response). The third one is lowering of the specificity. Both of the latter types of mutations have been identified. A classical example of the lowering of EC50 is the calcium-sensing receptor, where increased sensitivity to the plasma calcium concentration cause autosomal dominant or sporadic hypocalcemia (Baron et al., 1996; Pollak et al., 1994) (recently reviewed in (Hu & Spiegel, 2007)). Only a single mutation has been found to cause constitutive activation in the calcium-sensing receptor (Zhao et al., 1999). Good examples of the lowering of specificity can be found with the glycoprotein hormone receptors. The three receptors, thyroid stimulating hormone (TSH) receptor (TSHR), luteinizing hormone (LH)/chorionic gonadotropin (CG) receptor (LHCGR), and follicle-stimulating hormone (FSH) receptor, usually only recognize their cognate ligands. However, mutations can lower this specificity. A mutation in TSHR was identified that respond to high levels of human CG (hCG) present during early pregnancy, causing familial gestational hyperthyroidism (Rodien et al., 1998). Another example is mutations in FSHR that result in inappropriate response to hCG and in some cases to TSH, causing familial spontaneous ovarian hyperstimulation syndrome (OHSS) and hyperthyroidism during pregnancy (see below).Since the current manuscript is on diseases associated with the constitutive activation of the GPCRs, the latter two types of mutations will not be discussed in detail herein.
3.1. Rhodopsin mutations and retinitis pigmentosa or congenital stationary night blindness
Rhodopsin, the receptor for vision, is a unique GPCR in that the inverse agonist 11-cis-retinal is covalently bound to the protein moiety, opsin, through a protonated Schiff base linkage to the ε-amino group of K296 in TM7 (7.43). The inverse agonist keeps the basal activity of rhodopsin to almost nonexistent. With absorption of a single photon, the inverse agonist 11-cis-retinal isomerizes to all-trans-retinal, and rhodopsin, after undergoing conformational changes, is converted to metarhodopsin II, the active form of rhodopsin. Consequent activation of transducin, the G protein in rod cells, initiates the photo-transduction cascade.
Keen et al. reported the first constitutively active mutation in rhodopsin, K296E, in autosomal dominant retinitis pigmentosa (ADRP) (Keen et al., 1991). These patients from a British family, compared to the patients caused by loss-of-function mutations in rhodopsin, have a more severe phenotype and earlier onset. They developed cataracts in their thirties and forties. Oprian and colleagues showed that this mutant is constitutively active (Robinson et al., 1992). Because the site of attachment for 11-cis-retinal was mutated, the constitutive activity of the mutant is not suppressed by 11-cis-retinal, the inverse agonist for rhodopsin, potentially explaining the phenotypes of patients harboring this mutation (Robinson et al., 1992). Constitutive activity interferes with rod sensitivity, which is usually kept quiescent by the covalently bound inverse agonist. This so called “dark-light” (perception of light in darkness) will result in impaired sensing to dim but not bright light (Sieving et al., 1995). Subsequently, another mutation at the same site, K296M, was identified in an American family (Sullivan et al., 1993). This mutation was also shown to cause constitutive activation (Rim & Oprian, 1995).
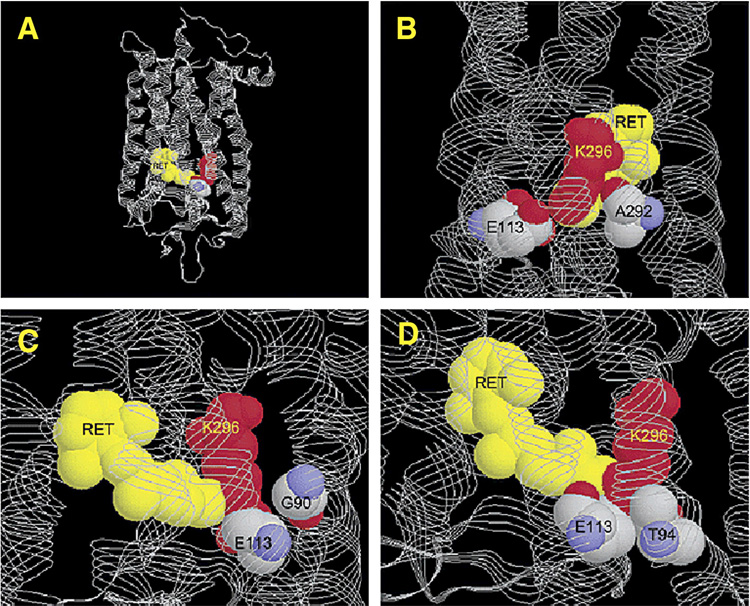
Constitutive activation of rhodopsin can also cause congenital night blindness (CNB). Dryja and colleagues reported the first rhodopsin mutation in CNB (Dryja et al., 1993). This mutation, A292E (7.39), is located about one helical turn away from K296. The mutant behaves similarly as the WT rhodopsin in the presence of chromophore. However, the mutant apoprotein opsin, in the absence of chromophore, could constitutively activate transducin (Dryja et al., 1993). It was suggested that the newly introduced Glu competes with E113 for ionic interactions with the positively charged nitrogen on K296, therefore break the salt-bridge between K296 and E113 resulting in activation of the protein (Rao & Oprian, 1996; Gross et al., 2003) (Fig. 1)
Fig. 1.
Structure of bovine rhodopsin, with coordinates from PDB entry 1L9H. Shown in A is the overall structure of rhodopsin. Shown in B–D are close-up views of the active site highlighting the locations of the three night blindness mutations: (B) A292, (C) G90, and (D) T94. Reprinted with permission from A. K. Gross et al. 2003. Biochemistry 42:2009–2015. Copyright (2003). American Chemical Society.
Another rhodopsin mutation, G90D (2.57), was reported to also cause CNB (Sieving et al., 1995). The patients have extensive night blindness with early-onset. However, unlike patients with retinitis pigmentosa, they do not exhibit any apparent retinal damage until much later in life. Functional studies showed that indeed G90D constitutively activates rhodopsin (Rao et al., 1994). Detailed biochemical and Fourier-transform infrared spectroscopy experiments showed that G90D has important features of metarhodopsin in the dark, such as deprotonation of E113 (Zvyaga et al., 1994). In the crystal structure of rhodopsin, G90 are in close proximity to K296 (Gross et al., 2003) (Fig. 1). The G90D mutation also causes constitutive activation by disrupting the salt bridge between K296 and E113 through competition of the newly introduced Asp with E113 for the positively charged K296 (Rao & Oprian, 1996). Data from the double mutant G90D/E113Q showed that indeed the newly introduce Asp can compensate for the loss of the Schiff base counterion at E113 (Rao et al., 1994).
Recently, another mutation, T94I (2.61), was found to cause CNB (al-Jandal et al., 1999). Functional studies showed that T94I rhodopsin could bind 11-cis-retinal. The mutant rhodopsin, when bound with 11-cis-retinal, is not active in the dark. However, the opsin is constitutively active (Gross et al., 2003; Ramon et al., 2003). Structural analysis showed that T94 is in close proximity to E113 counterion, therefore involved in maintaining the inactive conformation of the receptor. Fig. 1 showed that indeed all three mutations causing CNB are located near the salt bridge between E113 and K296 (Gross et al., 2003). All five constitutively active mutants disrupt the salt bridge critical for maintaining the ground state of rhodopsin (Kim et al., 2004; Rao & Oprian, 1996).
Why some mutations in rhodopsin cause CNB whereas others cause degenerative ADRP? CNB is a mild disease in which rod cells showed little or no degeneration whereas ADRP is a severe disease that progress from decreased rod cell sensitivity to loss of the photoreceptor cells and total blindness. It was hypothesized that the different phenotypes caused by constitutively active rhodopsin mutations might be explained by their differential abilities to bind 11-cis-retinal (Rao et al., 1994). The mutant rhodopsins associated with ADRP (K296E and K296M) cannot bind 11-cis-retinal (because K296, the site of chromophore attachment, is mutated), so all the mutant apoprotein are constitutively active, resulting in retinal degeneration. The mutant rhodopsins associated with CNB (G90D, T94I and A292E) can bind to 11-cis-retinal; therefore the majority of the mutant rhodopsins are kept inactive by the bound 11-cis-retinal. Only a small portion of the mutant proteins is in the active opsin form (not bound to 11-cis-retinal). This signaling, while raising the dark-adapted threshold (Sieving et al., 1995), does not result in retinal degeneration (Rao et al., 1994). For K296E and K296M, the constitutive signaling, without intermittent termination by 11-cis-retinal binding (as the mutants that cause CNB), ultimately results in apoptosis of the photoreceptor cells. Mutant mice that cannot produce 11-cis-retinal (therefore unliganded opsin is constitutively active) also have retinal degeneration (Woodruff et al., 2003). In summary, mutations that cause continuous activation of the photo-transduction pathway will result in ADRP, whereas mutations that can terminate the constitutive signaling led to the mild CNB (Lem & Fain, 2004).
3.2. TSHR mutations and hyperthyroidism
TSH is a large glycoprotein hormone produced by thyrotropes of the anterior pituitary. It is composed of two subunits, an α-subunit identical to that of LH and FSH and a TSH-specific β-subunit. TSH is essential for thyroid hormone synthesis and secretion as well as for cell proliferation and differentiation in the thyroid gland (Rapoport et al., 1998). TSH exerts its effects by interacting with cell surface TSHR. The primary pathway activated by TSHR is Gs and cyclic adenosine monophosphate (cAMP). At high TSH concentrations and high level of TSHR expression, TSHR activation also increases inositol phosphate (IP) and calcium by coupling to Gq/11 and subsequent activation of phospholipase C (PLC). Indeed, it has been shown that human TSHR can couple to members of all four families of G proteins (Laugwitz et al., 1996). A recent study provided in vivo evidence that the IP/Ca2+ pathway is important for hormone synthesis (Grasberger et al., 2007).
Because of the prominent roles of TSH in thyroid gland growth and function, TSHR abnormalities are involved in the pathogenesis of a variety of thyroid diseases. Activating mutations of the TSHR cause toxic adenoma as well as familial and sporadic nonautoimmune hyperthyroidism, whereas inactivating mutations of the TSHR cause hypothyroidism and resistance to TSH (reviewed in (Davies et al., 2005; Kopp, 2001)).
Parma et al. were the first to report that constitutively active mutations in the TSHR gene cause functioning thyroid adenoma with hyperthyroidism (Parma et al., 1993). They identified two somatic mutations, D619G (6.30) and A623I (6.34) in TM6 in three out of eleven hyperfunctioning thyroid adenomas. Since this pioneering report, 46 additional constitutively active mutations have been identified in toxic adenoma as well as in familial and sporadic nonautoimmune hyperthyroidism (Table 1). As shown clearly in Table 1, the mutations cluster around IL3 and TM6, consistent with studies in both TSHR and other GPCRs demonstrating the critical importance of this region in maintaining the WT receptor in inactive conformation.
Table 1.
Naturally occurring constitutively active mutations in TSHR
These activating mutations are heterozygous. In the hot nodules, the mutations are somatic. Genomic DNAs isolated from nearby normal tissue or leukocyte revealed WT genotype. The reported frequency of TSHR mutation in hot nodules ranges from 8% in Japanese patients (Takeshita et al., 1995) to 82% in European patients (Parma et al., 1997) (reviewed in (Arturi et al., 2003)). In familial cases, the transmission mode is autosomal dominant. Germline activating TSHR mutations cause hereditary or sporadic (de novo) toxic thyroid hyperplasia in the absence of autoimmunity. Different expressivity was observed, with age of onset of hyperthyroidism ranging from early infancy to adulthood (Van Sande et al., 1995). Some patients with activating TSHR mutations presented with sub-clinical hyperthyroidism. Aggressive treatment such as surgical or radioactive removal of the whole thyroid gland is usually required to prevent relapse (Van Sande et al., 1995). Additional radioiodine therapy is frequently required.
Functional studies showed that all mutants examined constitutively activate the cAMP pathway (see the references cited in Table 1; most of the case reports studied the functional properties of the mutants identified, at least in terms of basal cAMP production). Wild-type TSHR already has a significant level of constitutive activity when expressed heterologously. Expression of these mutant TSHRs further increased basal cAMP levels compared with cells expressing WT TSHR. Most of the mutants do not have increased basal levels of IPs. Only a few mutants, such as I486F, I486M, and I568T (Parma et al., 1995), and Δ613–621 (Wonerow et al., 2000), cause constitutive activation of the PLC-IP pathway, suggesting that activation of cAMP pathway is the cause of these thyroid diseases.
It has been proposed that the TSHR is an oncogene. Several studies investigated the oncogenic potential of the TSHR mutants in vitro. When the constitutively active mutant (CAM) TSHRs were expressed in FRTL-5, a permanent but untransformed rat thyroid cell line, TSH-independent proliferation (Fournes et al., 1998; Ludgate et al., 1999; Porcellini et al., 1997) and neoplastic transformation (anchorage-independence) (Fournes et al., 1998) were observed. (FRTL-5 cells need TSH stimulation to proliferate. In the absence of TSH, these cells become quiescent but viable for several weeks. When these cells are introduced into nude mice, tumorigenesis is observed.) Constitutively active Gsα mutant expressed under the same promoter (bovine thyroglobulin gene promoter), although resulting in TSH-independent cell growth and raised intracellular cAMP level, was not tumorigenic (Fournes et al., 1998). Expression of A263I TSHR by retroviral transduction in human primary thyrocytes also induced formation of colonies, suggesting that signaling of the mutant TSHR is sufficient to initiate thyroid tumorigenesis (Ludgate et al., 1999). Although it is believed that the cAMP-protein kinase A (PKA) signal transduction pathway is the major driver for cell proliferation, other effectors, such as IP, phosphatidyl inositol-3 kinase, Gβγ-mediated signaling, small GTPases (such as Ras, RhoA, and Rap1), and cAMP-dependent but PKA-independent pathway, might also be involved (Fournes et al., 1998; Medina & Santisteban, 2000; Rivas & Santisteban, 2003).
Fuhrer and coworkers compared the constitutive activity measured in COS-7 (monkey embryonic kidney cells as used in most of the previous studies) versus rat FRTL-5 and human thyrocytes (Fuhrer et al., 2003a). They showed that the constitutive activities of the seven TSHR mutants tested in thyroid cells do not correlate with their constitutive activities in COS-7 cells, highlighting the contribution of cellular context to the measured activities. In FRTL-5 cells and human thyrocytes, these mutant TSHRs show a similar order of potency. Furthermore, mutants with the highest constitutive activity (in terms of basal cAMP levels) may fail to induce proliferation, perhaps due to complex counter-regulatory mechanisms at the level of phosphodiesterases and cAMP regulatory element modulator isoforms, as well as receptor desensitization (Fuhrer et al., 2003a; Persani et al., 2000).
Interestingly, activating mutations of TSHR may also be the etiology of feline hyperthyroidism, a common feline endocrinopathy similar to human toxic nodular goiter.Watson et al. screened 134 nodules and found nine somatic mutations (not present in the leukocyte genomic DNA) in the feline TSHR gene (Watson et al., 2005). These mutations include M452T, S504R, V508R, R530Q, V557L, T631A, T631F, D632Y, and D632H. Five of these mutations were identified in human toxic nodular goiter and shown to cause constitutive activation, suggesting that they might be involved in the pathogenesis of feline hyperthyroidism. Functional studies of these mutations in cat TSHR, especially basal cAMP levels, are needed to reach definitive conclusions regarding their roles in the pathogenesis of feline hyperthyroidism.
3.3. LHCGR mutations and male-limited precocious puberty/Leydig cell adenoma
In males, androgen (primarily testosterone) is produced by interstitial Leydig cells in the testis under the stimulation of LH. At puberty, the hypothalamus-pituitary-gonad axis becomes active. Increased pulsatile secretion of gonadotropin-releasing hormone results in increased serum LH level. LH stimulates the testis to produce testosterone, which in turn initiates the development of male secondary sexual characteristics, including growth of testis and penis, growth of pubic hair and beard, and deepening of voice. The receptor mediating LH action, the LHCGR, therefore plays a critical role in pubertal development (Ascoli et al., 2002). Defects in LHCGR signaling cause hypergonadotropic hypogonadism (Latronico & Segaloff, 1999; Themmen & Huhtaniemi, 2000). Because of the critical roles of testosterone in the development of secondary sexual characteristics at puberty and its effect on bone growth, abnormal production of testosterone in pre-pubertal boys will stimulate rapid growth and virilization. However, due to premature epiphyseal closure, the adult heights of these boys are decreased (Shenker, 2002).
Familial male-limited precocious puberty (FMPP) or testotoxicosis is an autosomal-dominant gonadotropin-independent disorder. Robert King Stone described the first case in 1852. He described a boy of four years old with a height of a ten year old and “If the child’s face is concealed, the examiner would declare his figure to be that of miniature man, perfectly developed and at least 21 years of age” (quoted from (Shenker, 1998)). The patient’s father also had precocious puberty: “…I may observe that the father presented extreme precocity, having experienced his first sexual indulgence at the age of 8 years” (also quoted from (Shenker, 1998)). It was more than 140 years later that Shenker and colleagues showed that mutations in the LHCGR gene resulting in constitutive activation could cause premature increase in testosterone production, leading to FMPP (Shenker et al., 1993). The first such mutation identified was D578G (6.44) in TM6 (Shenker et al., 1993). The mutation co-segregates with the disease in multiple families (Kawate et al., 1995; Kremer et al., 1993; Shenker et al., 1993). Functional studies showed that indeed the mutant is constitutively active. When expressed in COS-7 cells, basal cAMP production is dramatically increased (3 – 4.5 fold) with the D578G LHCGR (Shenker et al., 1993; Yano et al., 1994). Since the initial identification of D578G mutation, 14 additional activating mutations have been identified in LHCGR gene causing FMPP (Table 2).
Table 2.
Naturally occurring constitutively active mutations in LHCGR
| Mutation | Location (Ballesteros numbering when applicable) | References |
|---|---|---|
| L368P | TM1 (1.41) | (Latronico et al., 2000b) |
| A373V | TM1 (1.46) | (Gromoll et al., 1998) |
| M398T | TM2 (2.42) | (Evans et al., 1996; Kraaij et al., 1995; Yano et al., 1996) |
| L457R | TM3 (3.43) | (Latronico et al., 1998) |
| I542L | TM5 (5.54) | (Laue et al., 1995) |
| D564G | TM6 (6.30) | (Laue et al., 1995) |
| A568V | TM6 (6.34) | (Latronico et al., 1995a) |
| M571I | TM6 (6.37) | (Kosugi et al., 1995 Kremer et al., 1993) |
| A572V | TM6 (6.38) | (Yano et al., 1995) |
| I575L | TM6 (6.41) | (Laue et al., 1996) |
| T577I | TM6 (6.43) | (Kosugi et al., 1995) |
| D578E | TM6 (6.44) | (Wu et al., 1999) |
| D578G | TM6 (6.44) | (Shenker et al., 1993) |
| D578Y | TM6 (6.44) | (Laue et al., 1995) |
| C581R | TM6 (6.45) | (Laue et al., 1995) |
These subjects have normal reproductive function in adulthood so the constitutive activity does not disrupt the normal reproductive axis function. The reason for the lack of abnormality is not well understood. One reason might be that the constitutively active receptor is down regulated due to the inherent instability of constitutively active receptor (Smit et al., 1996). Another reason is that the constitutively active mutant receptor induces phosphodiesterase that might result in decreased signaling in vivo (Shinozakiet al., 2003). There is only one report of an adult patient harboring a LHCGR mutation with testicular seminoma and Leydig cell hyperplasia (Martin et al., 1998). Careful follow-up of the pediatric patients is needed to see whether there are other pathological conditions associated with the constitutively active mutations. Currently, the main goals for treating these pediatric patients are to delay epiphyseal closure so they can reach their predicted final heights as well as to limit other symptoms associated with high testosterone levels, such as acne, spontaneous erections, psychological and social stress, and aggressive behavior (Jeha et al., 2006). Female carriers of these activating mutations do not have precocious puberty, likely due to the fact that both LH and FSH are needed for ovarian steroidogenesis. For example, the appearance of LHCGR depends on FSH stimulation in ovaries but not in testes. Whether excess androgen production in adult women will cause polycystic ovary syndrome remains to be investigated. So far, the evaluations of the reproductive endocrine status revealed no evidence of sub-clinical abnormalities in older females with constitutively active LHCGR mutations (Latronico et al., 2000a; Rosenthal et al., 1996).
Functional studies of these mutants have shown that they are all constitutively active on the major signaling pathway, Gs. Cells expressing these mutants have increased cAMP production in the absence of agonist. There are some evidences of correlation of the basal activity with age of presentation and severity. D578Y, which has twice the basal activity of D578G, was found in boys with very early-onset and severe testotoxicosis (Kosugi et al., 1996; Laue et al., 1995). Most of the mutants do not cause constitutive activation of the PLC-IP pathway. There are only a few exceptions such as D578Y (Kosugi et al., 1996) and D578H (Liu et al., 1999).
The original mutation, D578G, responds to hCG stimulation with similar EC50 and maximal response (Rmax) as the WT LHCGR. Most of the mutants described above also respond to hCG stimulation. The exceptions are L457R, I542L, I575L, and C581R. These four mutants have diminished or absent responses to hCG stimulation, although they bind to hCG normally (Kremer et al., 1999; Latronico et al., 1998; Laue et al., 1995).
Since LH can also stimulate Leydig cell proliferation, it was hypothesized that activating mutations of LHCGR might cause Leydig-cell tumors. Shenker and colleagues first identified D578H mutation from Leydig-cell adenomas in three boys (Liu et al., 1999). This same mutation has since been identified in two other studies from Leydig-cell tumors from three additional boys of different ethnic backgrounds (Canto et al., 2002; Richter-Unruh et al., 2002). In vitro experiments showed that this mutant is extremely active. The basal cAMP level is 28 times that of WT basal cAMP level; that is about 80% of the Rmax in the WT LHCGR. It also increases basal IP production significantly (7-fold, equal to 70% of Rmax) (Liu et al., 1999). It was hypothesized that the dual activation of the cAMP and IP pathways might be a contributor to the neoplastic transformation of Leydig cells by D578H mutation. Ascoli and colleagues performed detailed comparisons of three CAMs: L457R and D578Y (associated with precocious puberty and Leydig cell hyperplasia) and D578H (associated with Leydig cell adenomas) (Hirakawa & Ascoli, 2003; Hirakawa et al., 2002; Min & Ascoli, 2000; Min et al., 2002). These experiments showed that these CAMs constitutively activate the same G proteins (including Gs, Gi/o, and Gq/11), resulting in signaling by cAMP, IP and ERK1/2 pathways. They concluded that the signaling pathways activated by these three CAMs are the same, as well as the same as ligand-activated WT receptor (reviewed in (Ascoli, 2007)). Therefore the cause for D578H-induced Leydig-cell adenoma remains to be determined.
After the first laboratory-generated CAMs were reported, it was quickly found that these CAMs are constitutively phosphorylated and desensitized (Pei et al., 1994). What about the naturally occurring mutants? Although there is constitutive desensitization, it is obvious that it cannot compensate the constitutive activation, since the constitutive activity does result in pathological states.
Of the three LHCGR CAMs studied (L457R, D578H, and D578Y), two (L457R and D578H) had increased basal phosphorylation (Min & Ascoli, 2000). After hCG stimulation, they showed little or no response with increased cAMP production. They also failed to increase phosphorylation after hCG stimulation (Min & Ascoli, 2000). However, all three mutants have increased rates for internalizing hCG which were not decreased by over-expression of arrestin, suggesting that internalization in these mutants bypass many of the steps (such as activation, phosphorylation, and arrestin binding) usually followed by the WT receptor after agonist-binding (Min & Ascoli, 2000). Further studies revealed different postendocytotic fates of receptor-hormone complex between L457R and WT (Galet & Ascoli, 2006). Unlike the WT receptor, most of the L457R is recycled to the cell surface, rather than being routed to the lysosome for degradation, which could contribute to prolonged signaling augmenting the apparent constitutive activity (Galet & Ascoli, 2006).
The LHCGR is known to form dimers or oligomers (Roess et al., 2000; Tao et al., 2004; Urizar et al., 2005) and hCG treatment increases dimer formation (Tao et al., 2004). Using fluorescence resonance energy transfer, it was shown that D578H and D578Y constitutively dimerize. In contrast, in the WT LHCGR, the proportion of the receptor in the dimer form is minimal in the absence of hormone stimulation (Lei et al., 2007). Whether this constitutive dimerization is a common property of other CAMs as well is not known at present.
3.4. FSHR mutations and spontaneous ovarian hyperstimulation syndrome
The FSHR is critically involved in regulating reproductive function (Simoni et al., 1997). In the males, FSH is important for spermatogenesis, whereas in females FSH is absolutely required for follicle growth. Because of the critical importance of FSH in regulating cell proliferation as well as the fact that activating mutations in the TSHR and LHCGR cause thyroid tumors and Leydig cell adenoma (see above), several groups hypothesized that constitutively active mutations in the FSHR might constantly stimulate granulosa cell proliferation, therefore resulting in tumor formation. Unfortunately, no germline or somatic mutations were found in these studies (Fuller et al., 1998; Giacaglia et al., 2000; Latronico & Segaloff, 1999; Ligtenberg et al., 1999). One inactivating (not activating) mutation identified was later found to be due to contamination (Kotlar et al., 1998; Kotlar et al., 1997).
The first constitutively active mutation in FSHR was identified in an unusual case of a hypophysectomized (due to pituitary tumor) but fertile man (Gromoll et al., 1996). This patient, although lacking gonadotropin, had normal testis volume and spermatogenesis with androgen replacement therapy alone. He fathered three children. Since in primates, unlike in rodents, spermatogenesis cannot be achieved with testosterone alone (Schaison et al., 1993), the authors hypothesized that there might be an activating mutation in the FSHR gene in this patient to achieve residual FSH activity. Sequencing showed that indeed this patient harbors a heterozygous D567G (6.30) mutation (corresponding to D564G in LHCGR). Functional studies showed that the mutant receptor resulted in a small but consistent 1.5-fold increase in basal cAMP level compared with WT FSHR (Gromoll et al., 1996). When the mutant was expressed in a mouse Sertoli cell line, a 3-fold increase in basal cAMP level was observed (Simoni et al., 1997). Studies with transgenic mice showed that this small increase does have functional implications in vivo (see below).
Normally, there is strict specificity in the interaction of gonadotropin with their cognate receptors. Therefore, FSHR only interacts with FSH, but not TSH, LH or hCG. However, mutations in the receptors might result in relaxing of this stringency. An earlier example is the TSHR mutation that results in increased response to hCG by the mutant TSHR, resulting in gestational thyrotoxicosis (Rodien et al., 1998) (see above). With the FSHR, recent studies showed that mutations that decrease the specificity of the mutant FSHR were the cause of OHSS (De Leener et al., 2008; De Leener et al., 2006; Montanelli et al., 2004a; Smits et al., 2003; Vasseur et al., 2003). In OHSS, there are two important components: growth of multiple follicles and the luteinization of these follicles (Delbaere et al., 2005). Most cases of OHSS are iatrogenic due to in vitro fertilization treatments. Only about 5% of the cases are spontaneous.
The mutations recently identified in FSHR gene from OHSS patients include T449A (3.32), T449I, I545T (5.45), D567G (6.30), and D567N. All mutations are heterozygous. The mutant receptors showed a clear dose-dependent response to hCG stimulation. Although the sensitivity is much lower than the cognate LHCGR, the enormous increase in serum hCG during the first trimester of pregnancy can cause significant stimulation of FSHR resulting in over-stimulation of the ovaries. Since the large extracellular domain is responsible for binding to the glycoprotein hormones, these mutations, located in the transmembrane domains, are likely not directly involved in interacting with the ligands. Rather, global conformational changes induced by these mutations might affect the extracellular loops or the amino terminus, resulting in relaxed specificity (Vasseur et al., 2003). In fact, ligand binding studies cannot detect specific binding of the mutant receptors to hCG reflecting the very low affinity for hCG (De Leener et al., 2008; Smits et al., 2003; Vasseur et al., 2003). Some sporadic cases without FSHR mutation might be explained by the promiscuous activation of WT FSHR by the extremely high hCG levels during the first trimester of pregnancy (De Leener et al., 2008).
Interestingly, some of the mutants, including T449A, I545T, D567G, and D567N, are constitutively active. It is likely that constitutive activation is not required for the development of OHSS, since patients with trophoblastic disease (with higher hCG levels than normal pregnancy) also have spontaneous ovarian hyperstimulation (Ludwig et al., 1998; Montanelli et al., 2004a). In addition, T449I and a mutation in the extracellular domain of the FSHR (S128Y) identified in patients with OHSS do not cause constitutive activation (De Leener et al., 2008). Therefore, the loss of specificity is most likely the etiology of spontaneous OHSS in the patients carrying FSHR mutations.
3.5. PTHR1 mutations and Jansen’s metaphyseal chondrodysplasia
Parathyroid hormone (PTH)/PTH-related peptide (PTHrP) type 1 receptor (PTHR1) is expressed in bone, kidney, and growth plate chondrocytes, maintaining the circulating concentrations of calcium and phosphorus within narrow limits. Therefore it is a central regulator of both bone development and ion homeostasis. Activating mutations of PTHR1 result in a special bone disorder called Jansen’s metaphyseal chondrodysplasia (JMC). This disease is characterized by short limb dwarfism (short stature with extremely short limbs) due to severe abnormalities of growth plate, micrognathia (small lower jaw), hypercalcemia, and hypophosphatemia. The patients have increased bone remodeling. Hence markers for both osteoblastic (such as serum alkaline phosphatase and osteocalcin) and osteoclastic (such as urinary hydroxyproline excretion) activities are increased. These biochemical changes are reminiscent of primary hyperparathyroidism. However, unlike primary hyperparathyroidism, serum PTH and PTHrP levels are normal or undetectable. Urinary excretion of cAMP is increased, suggesting PTH/PTHrP-independent generation of cAMP. The pattern of inheritance is autosomal dominant, although many cases are sporadic (Juppner, 1996).
Since JMC patients have either normal or undetectable serum levels of PTH, yet they have hypercalcemia, constitutively active mutation in PTHR1 was hypothesized to be a potential cause. The first PTHR1 mutation was identified from a JMC patient that changes codon 223 in TM2 from histidine to arginine (Schipani et al., 1995) (PTHR1 is a member of Family B GPCRs. The Ballesteros and Weinstein numbering system are not used for these receptors since they do not have a clear-cut highly conserved residue in each TM as Family A GPCRs). Since that initial report, three additional mutations were identified from JMC patients: T410P (Schipani et al., 1996) and T410R (Bastepe et al., 2004) in TM6, and I458R in TM7 (Schipani et al., 1999).
PTHR1 is primarily coupled to Gs. In vitro expression of these mutants showed that the basal cAMP levels are increased, and these increases are dependent on the amount of plasmids transfected (hence the number of the receptors on the cell surface) (Bastepe et al., 2004; Schipani et al., 1999; Schipani et al., 1996). PTHR1 is also coupled to Gq/11; therefore receptor activation also increases IP production. However, of the mutants studied (H223R, T410P, and I458R), none causes constitutive activation of this pathway (Bastepe et al., 2004; Schipani et al., 1999; Schipani et al., 1996). H223R also failed to increase IP production after PTH or PTHrP stimulation (Schipani et al., 1996). There seems to have some genotype-phenotype correlation. For example, the constitutive activity of T410R is lower than that of T410P. The phenotype is also less severe in the patients harboring T410R (Bastepe et al., 2004).
Expression of H223R or T410P PTHR1 with arrestin3-green fluorescent protein in HEK-293T cells resulted in agonist-independent recruitment of arrestin3 to the cell surface, whereas arrestin3 was distributed uniformly in cytoplasm in cells expressing WT PTHR1 (Ferrari & Bisello, 2001). In the absence of ligand, H223R and WT were predominantly localized on the cell surface, whereas T410P was partly localized intracellularly, indicating constitutive intracellular trafficking. T410P PTHR1 also internalizes antagonists, whereas WT or H223R do not. Therefore, cellular localization and internalization characteristics are different between the two naturally occurring CAMs, H223R and T410P (Ferrari & Bisello, 2001).
In summary, constitutively active mutations in GPCRs were clearly shown to cause several diseases. Most of the diseases are associated with the endocrine system with the exception of rhodopsin. It should be noted that several receptors were hypothesized to be potential causes of some diseases if constitutively activated, according to their physiological roles. However, extensive search for these constitutively active mutations have failed. The case for FSHR was mentioned before. There are a few other examples. No activating mutations were found in angiotensin II AT1A receptor in hyperfunctioning adrenal Conn adenoma (Davies et al., 1997), despite the critical importance of angiotensin II in regulating aldosterone secretion in adrenal gland. Similarly, in the melanocortin-2 receptor, no constitutively active mutations were identified in a variety of adrenal tumors (Latronico et al., 1995b; Light et al., 1995), although melanocortin-2 receptor mediates adrenocorticotropin’s function in stimulating cell proliferation. In the gonadotropin-releasing hormone receptor, no activating mutations were identified from pituitary gonadotroph adenomas (Kaye et al., 1997).
4. Diseases caused by loss of constitutive activity in GPCRs
4.1. MC4R mutations and obesity
Mutations in the melanocrtin-4 receptor (MC4R) gene are the most common monogenic form of obesity. Amazingly, in a small protein with 332 amino acids, more than 130 mutations, mostly missense mutations, have been identified from various patient populations (see (Tao, 2005, 2006) for earlier reviews). Roughly one third of the codons were found to be mutated. The MC4R, coupled to Gs, has some constitutive activity. In most of the earlier studies, the basal cAMP levels of the mutants were not studied (Donohoue et al., 2003; Gu et al., 1999; G. Ho & MacKenzie, 1999; Lubrano-Berthelier et al., 2003; Nijenhuis et al., 2003; Tao & Segaloff, 2003; Vaisse et al., 2000; VanLeeuwen et al., 2003; Yeo et al., 2003).
In 2004, Vaisse and coworkers suggested that loss of constitutive activity in the mutant MC4Rs is one cause of obesity (Srinivasan et al., 2004). They showed that several mutations in the extracellular N terminus resulted in no changes in other functions except for the loss of basal activity when compared with the WT MC4R. They suggested that the constitutive activity of the MC4R transmits a tonic satiety signal. Defects in this tonic signaling could cause obesity (Srinivasan et al., 2004).
Subsequently, we and others studied the basal activities in other MC4R mutants. In our studies of ten MC4R mutants identified from obese patients with binge eating disorder as well as other obese or non-obese subjects, we showed that five mutants, I102S (2.62) and I102T in TM2, A154D in IL2, F202L (5.48) in TM5, and N240S (6.30) in TM6 have decreased basal activities, whereas the other four mutants, including T11A in the extracellular domain, F51L (1.39) in TM1, T112M in EL1, and S295P (7.46) in TM7, have normal basal activities (Tao & Segaloff, 2005). Of the three mutants identified from obese Chinese subjects, including Y35C and C40R in the extracellular domain and M218T in IL3, all have normal basal activities (Rong et al., 2006).
Another evidence supporting the potential importance of the constitutive activity of the MC4R in regulating energy balance is the unique existence of endogenous inverse agonists in the melanocortin system. For the MC4R, Agouti-related protein (AgRP) is an inverse agonist (Haskell-Luevano & Monck, 2001; Nijenhuis et al., 2001) (reviewed in (Adan, 2006; Adan & Kas, 2003)). The balance between the actions of the agonists and the inverse agonist determines the activity of the MC4R. For a mutant receptor, even if the agonist action were not altered, increased affinity for AgRP would be predicted to result in defective receptor activity. Therefore several studies have also investigated the affinity of the mutant receptors for AgRP. O’Rahilly and colleagues first showed that a mutant in the carboxyl terminus (I316S) had decreased affinity for the agonist but unchanged affinity for the antagonist, therefore with an altered balance for them (Yeo et al., 2003). Of the three MC4R variants identified in Chinese subjects, all had normal binding affinities towards AgRP (Rong et al., 2006). Haskell-Luevano and colleagues systematically studied the binding of 40 naturally occurring MC4R mutants towards multiple agonists and the antagonist AgRP. They identified mutants with both increased and decreased AgRP potency as measured by AgRP ability to antagonizing agonist signaling (Xiang et al., 2006).
Paradoxically, some of the mutant MC4Rs identified from obese patients have increased constitutive activity compared with WT MC4R. The first CAM MC4R identified, L250Q (6.40) in TM6, has very high constitutive activity (Vaisse et al., 2000). Even when the expression of the mutant receptor is only half of the WT receptor, it still exerts high constitutive activity (Xiang et al., 2006). Subsequently, other mutations with high constitutive activity were identified from either obese or overweight subjects (Hinney et al., 2006; Hinney et al., 2003; Valli-Jaakola et al., 2004). Theses mutants, including S127L (3.30) in TM3, H158R in IL2, and P230L in IL3, all significantly increased basal cAMP levels compared with WT MC4R. As explained above, loss of constitutive activity is consistent with obesity pathogenesis. Increased constitutive activity is expected to result in lean phenotype or perhaps even associated with anorexia nervosa. Indeed, studies have shown that some variants such as I251L and V103I are associated with protection from obesity (Stutzmann et al., 2007; Young et al., 2007). Therefore whether these constitutively active mutations are indeed the cause of obesity remains unknown. For L250Q, slightly decreased expression of the mutant receptor was reported (Vaisse et al., 2000). For S127L, impaired signaling to the agonist was reported (Valli-Jaakola et al., 2004). These defects might balance the constitutive activity in vivo. One caveat is that the functional studies were performed in heterologous cells and may not faithfully mimic the response in neuronal cells. Furthermore, constitutive desensitization or down-regulation might decrease the constitutive signaling in vivo. Another reason might be the induction of phosphodiesterases in vivo that degrades the cAMP produced therefore attenuates the signal generated by these neurons (Persani et al., 2000; Shinozaki et al., 2003).
Interestingly, two naturally occurring variants were also identified from different strains of pigs. We showed that one mutation, D298N (7.49), which mutates a highly conserved Asp in TM7 (of the N/DPXXY motif), cause a small decrease in basal activity, although it does not change the affinity for AgRP (Fan et al., 2008). The other mutant, R236H in IL3, has the same basal activity and binding affinity for AgRP as WT porcine MC4R (Fan et al., 2008). The relevance of these two variants in modulating pig growth traits is doubtful, consistent with the genetic studies (reviewed in (Fan et al., 2008)).
It should be pointed out that for the related melanocortin-3 receptor (MC3R), the WT receptor has no constitutive activity (Tao, 2007). Of the mutant MC3Rs identified so far, none changed the basal activity (Tao, 2007; Tao & Segaloff, 2004).
4.2. GHSR mutations and familial short stature/obesity
The field of GHS and ghrelin is a fascinating story. In the 1970s, Bowers and colleagues developed a series of small synthetic peptides that stimulate growth hormone (GH) secretion (Bowers, 1998). Subsequently, Merck developed peptidomimetics such as MK-0677 that could be administered orally (Patchett et al., 1995). This group then cloned the GHSR from pituitary and hypothalamus in 1996 as an orphan receptor with the endogenous ligand unknown (Howard et al., 1996). Subsequently, ghrelin was shown to be the endogenous cognate ligand for GHSR (Kojima et al., 1999). Ghrelin is unique in having a special post-translational modification at the third residue, Ser3, octanoylation, that is essential for binding to the receptor and its biological activities.
Extensive studies showed that ghrelin/GHSR system is important in regulating both energy homeostasis and growth hormone secretion (reviewed in (Davenport et al., 2005)). As one way of brain-gut communication, ghrelin regulates both short-term energy balance, through its stimulatory effect on food intake and inhibitory effect on fatty acid oxidation, as well as long-term energy homeostasis. In the arcuate nucleus, ghrelin activates the neuropeptide Y (NPY)/AgRP neuron (Nakazato et al., 2001). Ghrelin is the first circulating hormone that can stimulate food intake with peripheral administration. The other orexigenic hormones, such as NPY, AgRP, orexins, and melanin-concentrating hormone, all failed to stimulate food intake following systemic administration (Hosoda et al., 2002). In addition, ghrelin has many other functions, such as stimulation of gastric acid secretion and motility, anti-proliferative actions on cancer cells, and vasodilatory effect likely by acting on the smooth muscle (Davenport et al., 2005).
Because of the critical importance of ghrelin on regulating GH secretion and energy homeostasis, dysfunctional GHSR was expected to cause short stature due to deficient GH secretion and/or obesity. The first screening effort for mutations in GHSR identified two missense variants, A204E in EL2 from an obese patient and F279L (6.51) in TM6 from a child with short stature (Wang et al., 2004). Subsequently, A204E mutation was identified in two unrelated families with familial short stature (Pantel et al., 2006). The mode of inheritance is dominant. Heterozygous individuals already exhibit the phenotype. However, there is incomplete penetrance: not all individuals harboring the heterozygous mutation have short stature (Pantel et al., 2006). The causative role of GHSR in obesity is less conclusive. Although there is genetic linkage and association of GHSR with obesity in humans (Baessler et al., 2005), the first screening effort obtained inconclusive evidence regarding GHSR mutation and obesity (Wang et al., 2004). In the patients described in the Pantel et al. study, some with A204E mutations were not obese (Pantel et al., 2006). The mechanism of any loss-of-function mutations that are associated with obesity is not clear. Ghrelin is a potent hunger signal postulated to be a meal initiation factor (Cummings et al., 2001). Loss-of-function would be predicted to protect from obesity leading to a lean phenotype, such as in ghrelin and/or GHSR knockout mice (Pfluger et al., 2008; Wortley et al., 2005; Zigman et al., 2005).
GHSR is coupled to Gq/11, therefore receptor activation leads to increased levels of IP and Ca++. Schwartz and colleagues showed that human GHSR has very high constitutive activity, at about 50% of ghrelin-stimulated signaling (Holst et al., 2003). This constitutive activity is likely to be relevant in vivo. For example, GHSR knockout leads to more severe defect in energy balance than ghrelin knockout (Pfluger et al., 2008). Functional studies showed that A204E GHSR can signal normally to GHSR but have diminished constitutive activity (Pantel et al., 2006). Although binding capacity was decreased due to low cell surface expression, the binding affinity for ghrelin was not affected. A previous study showed that F279L had decreased binding for MK-0677 (Feighner et al., 1998). Schwartz and colleagues showed that F279 is part of a cluster of aromatic residues located at the cytoplasmic ends of TM6 and TM7 that is important for maintaining the active conformation of the WT receptor, mutations of these residues result in loss of the constitutive activity (Holst et al., 2004). Kopin and colleagues recently performed extensive functional characterization of the two mutants and two variants reported in the single nucleotide polymorphism database, I134T (3.43) in TM3 and V160M (4.42) in TM4 (G. Liu et al., 2007). They showed that V160M and F279L have significantly reduced constitutive activity whereas A204E is devoid of constitutive activity (basal signaling is similar to mock transfected cells) (Liu et al., 2007).
5. Lessons learned from animal models expressing constitutively active GPCRs
Expression of the mutant GPCRs in mice not only confirms the involvement of the mutations in the pathogenesis of the diseases, but also it can reveal novel physiological and pathological roles of the receptors.
The mutation associated with ADRP, K296E, was shown to be constitutively active in vitro (see above). However, the mutant receptor is not constitutively active in transgenic animals (Li et al., 1995). It was shown that the mutant rhodopsin is phosphorylated (likely by rhodopsin kinase) and bound with arrestin forming inactive complexes (Li et al., 1995). In vitro studies produced conflicting results. One study suggested that constitutively active rhodopsins are not constitutively phosphorylated (Robinson et al., 1994) whereas another study showed that these mutants are constitutively phosphorylated by rhodopsin kinase (Rim & Oprian, 1995).
Haywood et al. devised a clever strategy to investigate the functional relevance of the first naturally occurring constitutively active mutation in FSHR, D567G (Haywood et al., 2002). Transgenic mice expressing D567G-FSHR were produced in gonadotropin-deficient background, the hpg mice. These mice cannot produce gonadotropin-releasing hormone; therefore there is no LH or FSH production. Transgenic hpg mice expressing D567G-FSHR had increased testis weight, containing mature Sertoli cells and postmeiotic germ cells absent in hpg controls. Sertoli cells isolated from transgenic mice had increased (2-fold) basal cAMP level demonstrating that indeed the mutant FSHR is constitutively active. Comparison of transgenic mice expressing FSH or D567G-FSHR with LHCGR knockout mice showed that in mice, full Sertoli cell proliferation could be accomplished by FSH activity alone (Allan et al., 2004). To investigate its action in maintaining rather than initiating spermatogenesis, D567G-FSHR was expressed in normal mice. Analysis of the testes from these mice showed that total Sertoli and spermatogonia cell numbers were increased, indicating constitutive FSH-like activity (Allan et al., 2006).
A transgenic mouse line in which expression of a constitutively active PTHR1, H223R, targeted to the growth plate by the rat α1 (II) collagen promoter, was established (Schipani et al., 1997b). These mice recapitulate the phenotypes of JMC patients with short and deformed limbs. They showed delayed mineralization, decelerated chondrocyte maturation in skeletal segments that are formed by the endochondral process, and prolonged presence of hypertrophic chondrocytes with delay of vascular invasion (Schipani et al., 1997b). The transgene also corrects most of the bone abnormalities in PTHR1 knockout mice, although the PTHR1 null mice with the transgene still die perinatally (Soegiarto et al., 2001). Expression of the same CAM in osteoblasts by placing its expression under the control of the type I collagen promoter showed that both the osteoblast and osteoclast numbers were increased, suggesting that PTH activation of PTHR1 in osteoblasts increase both bone-forming and bone-resorbing activities (Calvi et al., 2001). In another transgenic mouse line with the CAM expression targeted to the osteoblasts under the control of Col1a1 promoter, mutant mice have decreased disuse-induced bone loss due to decreased osteoclastic activity (Ono et al., 2007). Analysis of the bone marrow system of these mice revealed that PTHR1 regulates the establishment of the hematopoietic stroma compartment and of the skeletal stem cells (Kuznetsov et al. 2004).
6. Insights into mechanism of activation of GPCRs
Detailed characterizations of the naturally occurring constitutively active mutations of GPCRs that cause diseases described above not only provide novel insights into the physiological and pathophysiological roles of the underlying system, but also shed lights on the structure-function relationships of the GPCRs, especially the mechanism of activation. In addition, using the clues provided by the experiments of nature, scientists have generated multiple mutations at the diseased loci to examine which amino acids can cause constitutive activation. In addition, homologous mutations in other related GPCRs or in the same GPCRs from other species were also made to gain a better understanding of the mechanism of activation of related receptors. In many of these studies, experimental data were combined with homology modeling using the crystal structure of inactive rhodopsin as the template. With the recent reports of the β1- and β2-AR crystal structures, some of the future studies will likely also use these structures as templates. As mentioned earlier, rhodopsin is a unique receptor with the ligand covalently bound to the opsin moiety. All other receptors, including β2-AR, bind diffusible ligands. Although the crystal structures of rhodopsin and β2-AR are very similar, with the root mean squared deviation for the alpha carbon backbone of the TMs at 1.56 Å(Rasmussen et al., 2007), there are some significant differences, such as a more open conformation for the β2-AR, weaker interactions between the cytoplasmic ends of TM3 and TM6, the lack of “ionic lock” between D/E (3.49) and R (3.50) as well as E (6.30). Modeling based on information from multiple structures might provide a better representation of other GPCRs that still wait for direct structural studies.
One question that remains to be addressed unequivocally is how accurate a representation the constitutively active mutation is of the ligand-induced active conformation. The consensus is that CAM is an intermediate conformation (R’) between the inactive (R) and fully activated (R*) conformations. In fact, it is widely accepted that there is an ensemble of active conformations and different agonists may induce and/or stabilize different active conformations (Maudsley et al., 2005; Whistler et al., 2002). The conformational changes associated with ligand binding involve both disruption of interactions that constrain the WT receptor in inactive state and the establishment of new interactions maintaining the receptor in active conformation. Some CAMs mimic the breakage of interactions that constrain the WT receptor (for example, mutations of A293 in IL3 of the α1B-AR into any other residue will cause constitutive activation (Kjelsberg et al., 1992)), whereas some mutants mimic the establishment of new interactions (for example, only the introduction of Arg in L460 in human FSHR cause constitutive activation, other mutations at this locus do not cause constitutive activation (Tao et al., 2000). In the related LHCGR, mutations that induce a positive charge (Lys, Arg and His) cause constitutive activation (Shinozaki et al., 2001).
With these caveats in mind, I summarize below some of the studies that exploited the naturally occurring mutations in more detail and lessons learned.
6.1. Multiple mutations of the diseased locus
To gain a better understanding of the mechanism of activation in the naturally occurring mutants, investigators have generated multiple mutations at the diseased loci to see which amino acids are tolerated and which ones are not. From these results, the potential interactions involved could be predicted.
Since LHCGR activating mutations cluster around the IL3 and TM6 (Table 2), several investigators have studied in detail the importane of this region in LHCGR activation. Kosugi et al. mutated D578 to multiple residues (Kosugi et al., 1996). They showed that replacement of D578 with Gly, Ser, Leu, Tyr, or Phe cause constitutive activation, whereas replacement to Asn do not cause constitutive activation, suggesting that the ability of D578 acting as a hydrogen bond acceptor, rather than its negative charge, is critical for constraining the receptor in inactive conformation (Kosugi et al., 1996). Computer modeling and further mutagenesis experiments suggested that D578 forms hydrogen bonds with both N615 (7.45) and N619 (7.49) (Angelova et al., 2002; Lin et al., 1997). Mutations that disrupt these hydrogen bonds cause constitutive activation (Angelova et al., 2000).
Fanelli performed extensive molecular dynamic simulations of the WT and CAMs of the LHCGR (Angelova et al., 2002; Fanelli, 2000; Fanelli et al., 2004; Zhang et al., 2005; Zhang et al., 2007). These computational experiments suggested that the activation of the LHCGR is accompanied by the breakage of a salt bridge between the cytoplasmic ends of TM3 and TM6 (R3.50-D6.30). The result is that several residues at the cytoplasmic ends of TM3 and TM6 are more exposed, leading to increased solvent accessible surface area (SAS value). The residues used for calculating SAS values were revised several times. The most recently used residues include R464 (3.50), T467 (3.53), I468 (3.54), and K563 (6.29). For example, for the L457R, molecular dynamic simulations suggested that the introduced Arg forms a salt bridge with D578 (6.44), the locus of the first constitutively active mutation reported. Segaloff and colleagues generated disruptive and reciprocal mutants to test this hypothesis. The results of these experiments showed that a mutant designed to disrupt this salt bridge (L457R/D578N) resulted in dramatic decrease in constitutive activity, whereas a reciprocal mutant L457D/D578R (the salt bridge can still form in this mutant except that the positions of the two residues were switched) has similar high constitutive activity as L457R, suggesting that indeed there might be a salt bridge in L457R mutant that contributes to the constitutive activation of the mutant receptor (Zhang et al., 2005) (reviewed in (Latronico & Segaloff, 2007)).
Multiple mutations of some of the FSHR loci identified that result in constitutive activation have been performed. Combination of multiple mutagenesis and molecular modeling suggested that weakening of interhelical locks between TM6 and TM3 or TM6 and TM7 was responsible for the constitutive activity of D567 (6.30) and T449 (3.32) mutants (Montanelli et al., 2004b). A common feature of several members of Family A GPCRs is an ionic lock between D (6.30) and R (3.50) (Angelova et al., 2002; Ballesteros et al., 2001; Gether, 2000; Greasley et al., 2002). Any mutation that weakens or disrupts this interaction results in constitutive activation. The recently reported opsin structure showed that indeed this ionic lock is broken in opsin (Park et al., 2008). For FSHR, a negatively charged residue is needed at D (6.30) to maintain the receptor in inactive conformation. Especially, mutations introducing a basic residue such as Arg or Lys cause in repulsion of the newly introduced residue and R (3.50), resulting in high constitutive activity (Montanelli et al., 2004b). Similarly, T449 (3.32), together with S (3.36), forms hydrogen bonds with H (7.42). Mutations that disrupt these interactions result in constitutive activation (Montanelli et al., 2004b). I545 (5.54) was mutated to Ala, Phe, Leu, Asn, and Val in addition to the naturally occurring mutation Thr (De Leener et al., 2008). After correcting for differences in cell surface expression, it was shown that some mutants such as I545L, I545N, I545F, and I545T cause constitutive activation, whereas others such as I545V and I545A do not cause constitutive activation. Molecular modeling suggested that I545, at the intracellular end of TM3, is part of a tight packing involving residues from TM3 (L460 and T464) and TM6 (I579). Mutations that disrupt the packing either by establishing new interactions (I545T or I545N) or by disrupting the local structure (I545F and I545L) cause constitutive activation, where those that do not are not constitutively active (De Leener et al., 2008). Our previous data showing that L460R mutation can cause constitutive activation is consistent with this hypothesis (Tao et al., 2000).
Saturation mutagenesis (mutation of a residue to all other 19 natural amino acids) was also performed at the H223 and T410 loci in PTHR1 (Schipani et al., 1997a). Mutation of H223 to a basic residue (Arg or Lys) causes constitutive activation, whereas all the other mutants were not constitutively active. Substitution of T410 with any other residue causes a certain degree of constitutive activation, suggesting that mutation of T410 results in disruption of an interaction that constrains the WT receptor in inactive conformation (Schipani et al., 1997a).
6.2. Homologous mutations in other GPCRs
Members of the superfamily of GPCRs are divided into families and subfamilies. Members of subfamilies usually share high homology. For example, of the five melanocortin receptors, all can bind adrenocorticotropin and are about 50% homologous. However, they do exhibit important differences in signaling. For example, the WT MC4R has some constitutive activity, whereas the WT MC3R has no basal activity. Closely related receptors also have different susceptibilities to mutation-induced constitutive activation, as shown by the examples described here.
Another fruitful approach employed by numerous investigators is to generate homologous mutations in related receptors corresponding to the naturally occurring mutations that we know cause constitutive activation. Indeed, naturally occurring activating mutations have been found in the same locations in related receptors. For example, L3.43 were mutated to Arg in both LHCGR (Latronico et al., 1998) and TSHR (Kosugi et al., 2000; Trulzsch et al., 2001). D6.30 were mutated in LHCGR, FSHR, and TSHR (see above). Similarly, I/V5.54 was mutated in LHCGR (to Leu) (Laue et al., 1995) or TSHR (to Phe or Leu) (Esapa et al., 1999). Some of the positions mutated in LHCGR were also sites for constitutively active mutations in other Family A GPCRs (reviewed in (Shenker, 2002)).
Inspection of the naturally occurring activating mutations in the glycoprotein hormone receptors revealed that the TSHR has the most mutations, followed by the LHCGR, and finally the FSHR (see above). This is the same rank order of the basal activity of the WT glycoprotein hormone receptors, with the TSHR the noisiest and the FSHR the most quiescent (Feng et al., 2008; Van Sande et al., 1995). This may not be a coincidence. WT TSHR is the least constrained, therefore a minor disturbance in the interactions constraining the WT receptor can further destabilize the receptor, resulting in constitutive activation, even against the noisy WT state (Van Sande et al., 1995). For the FSHR, the WT receptor is highly constrained. Small disturbance in the interactions constraining the WT receptor may not be able to destabilize the receptor enough to generate measurable constitutive activity. Laboratory-designed mutants are consistent with this suggestion.
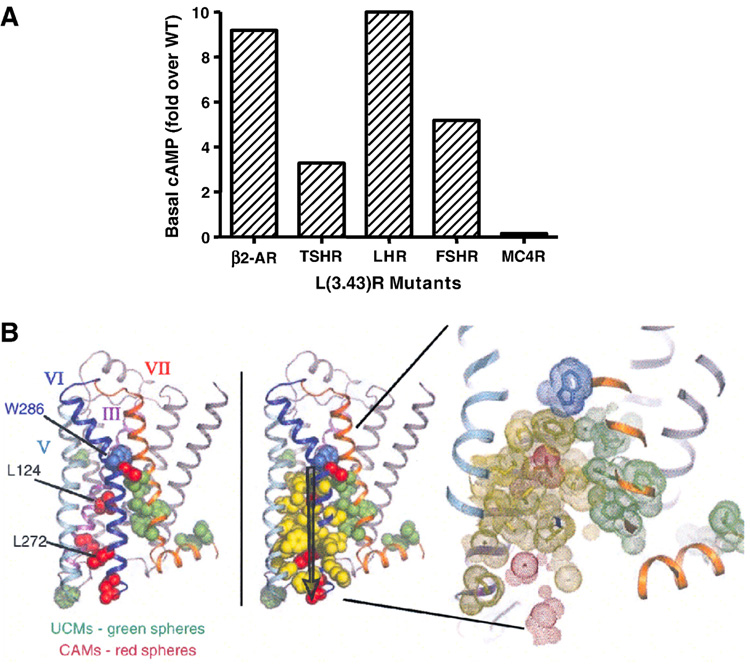
As described earlier, several activating mutations were identified in LHCGR that cause male-imited precocious puberty. Hsueh’s group generated four mutants in the FSHR corresponding to those activating mutations in LHCGR. Surprisingly, they showed that all four mutations do not cause constitutive activation in FSHR (Kudo et al., 1996). After the identification of the LHCGR L457R (3.43) activating mutation (Latronico et al., 1998), we generated the corresponding mutation in the FSHR (Tao et al., 2000). We showed that the corresponding mutation L460R causes a strong constitutive activation, increasing basal cAMP level five-fold (Fig. 2). When L460 was mutated to Lys, Ala, or Asp, none of the mutants are constitutively active. L (3.43) is highly conserved in Family A GPCRs, present in 74% family members (Mirzadegan et al., 2003). Therefore we generated additional mutants in the β2-AR. We showed that mutations of the homologous residue (L124) to Arg, Lys, or Ala all resulted in constitutive activation (Tao et al., 2000). These results suggest that L3.43 is important in stabilizing the inactive conformation of GPCRs. This conclusion is further supported by the following studies. In the TSHR, the corresponding mutation (L512R) has been identified from patients with hyperthyroidism (Kosugi et al., 2000; Trulzsch et al., 2001). L512R is constitutively active. In addition, alanine-scanning mutagenesis from Hulme and colleagues also showed that Leu3.43 is important for maintaining the inactive conformation in the rat M1 muscarinic receptor (Lu & Hulme, 1999). Mutation in C5a receptor at this locus also causes constitutive activation (Baranski et al., 1999). Recent crystal structure of β2-AR showed that this Leu, as well as L272 (6.34), are linked through packing interactions with those residues that when mutated cause defective agonist-induced activation (indicating these residues are important for forming intramolecular interactions that stabilize active conformation) (Fig. 2). It was suggested that signaling generated by ligand binding might be propagated through this packing interaction, mediated by rotameric toggle switch at W286 (6.48), to the cytoplasmic half of the receptor, resulting in G protein coupling/activation. However, as always, there are exceptions. I generated the corresponding mutation in MC4R. The mutant receptor (L140R) was not constitutively active. In fact, the mutant receptor has lower basal activity compared with WT MC4R (Tao YX, unpublished observations) (Fig. 2). This result suggests that there are important differences in the role of L3.43 in maintaining the inactive conformation in the MC4R versus the other GPCRs cited above.
Fig. 2.
A. Basal activities of L(3.43)R mutants in several GPCRs. Data were obtained from references listed in the article with the exception of MC4R that is my own unpublished data. B. Packing interactions of CAMs, including L124 (3.43), with uncoupling mutations in the β2-AR. Panel B is reprinted with permission from D. M. Rosenbaum et al. 2007. Science 318:1266–1273. Copyright (2007). AAAS.
The roles of L3.43 in mediating agonist-induced activation of the receptors seem to be different among the different receptors. For example, in both LHCGR and FSHR, L(3.43)R mutation leads to severely impaired hormone-induced signaling (Latronico et al., 1998; Tao et al., 2000; Shinozaki et al., 2001). However, in β2-AR, the mutation does not affect agonist-induced signaling (Tao et al., 2000).
For the TSHR, it was hypothesized that the large extracellular domain acts as an inverse agonist constraining the unliganded receptor in inactive conformation. Extensive studies have shown that a serine in the extracellular domain (S281 in the TSHR or S277 in the LHCGR) is critical for constraining the WT receptors in inactive conformation (Ho et al., 2001; Nakabayashi et al., 2000; Parma et al., 1997; Vlaeminck-Guillem et al., 2002). It was shown that in the LHCGR, constitutive activation induced by S277N mutation requires all the leucine rich repeats of the ectodomain (Sangkuhl et al., 2002). A corresponding mutation (S273I) in the FSHR has been shown to also cause constitutive activation (Montanelli et al., 2004b).
It was assumed that the human FSHR (hFSHR) is more resistant to mutation-induced constitutive activation than the TSHR or LHCGR. To study this further, we recently performed a detailed comparison of the susceptibility to mutation-induced constitutive activation in the LHCGR versus the FSHR (Zhang et al., 2007). Based on data from LHCGR, nine homologous mutations were made in FSHR. These experiments showed that only three FSHR mutants have significant constitutive activity. The levels of constitutive activities in the FSHR CAMs are also lower than those in the LHCGR. However, modeling suggested that the mechanisms through which homologous mutations cause constitutive activation are similar between the FSHR and LHCGR (Zhang et al., 2007).
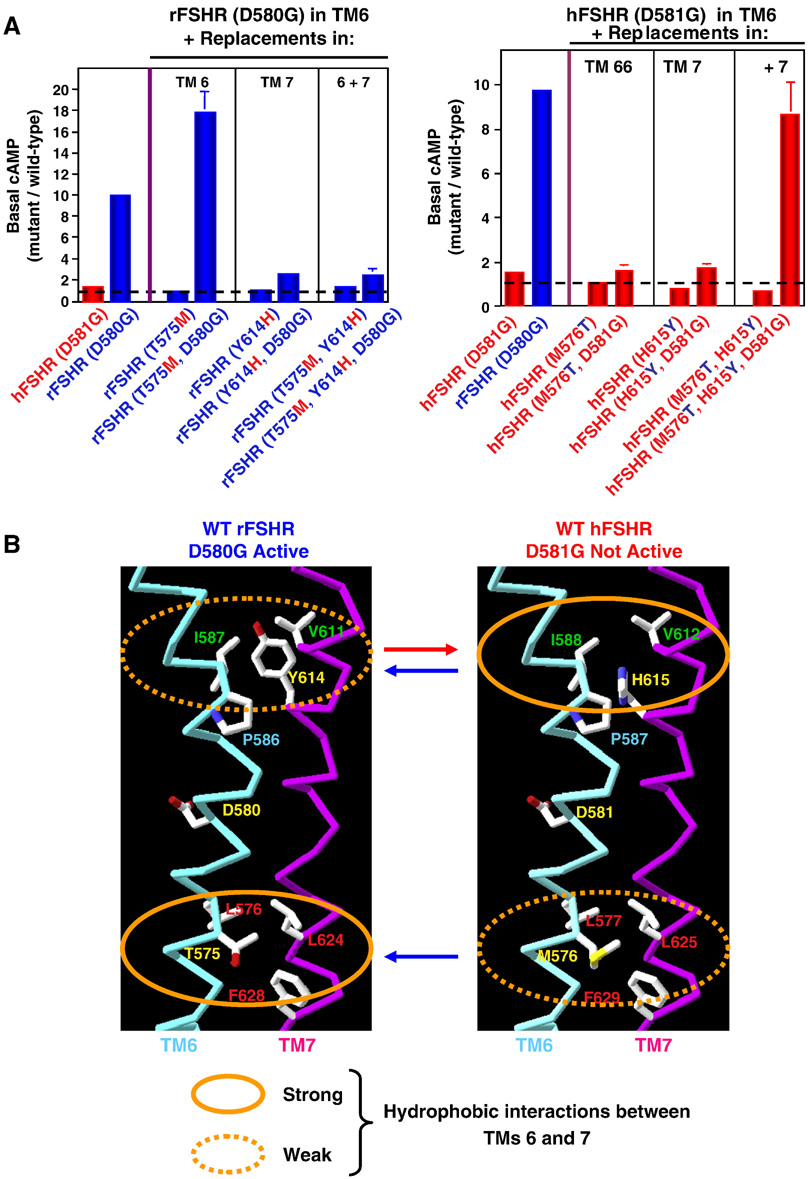
Although we confirmed Hsueh’s data on the hFSHR D581G (6.44) (not constitutively active), we found unexpectedly the corresponding mutation in rat FSHR (rFSHR) (D580G) caused very robust constitutive activation (Tao et al., 2002). The basal cAMP of D580G rFSHR is increased ~10-fold compared with the WT rFSHR. Since the rat and human FSHRs are highly homologous, at 89% over the full-length receptors, and 95% in the TMs, we sought to identify the molecular determinants of this dramatic difference in the two receptors in susceptibility to D(6.44)G mutation-induced constitutive activation. Using chimera, we showed that switching the extracellular domains do not affect the mutation-induced constitutive activation. Hence, the chimera with the extracellular domain of hFSHR and TMs of rFSHR were constitutively active when the D(6.44)G was introduced. The chimera with the extracellular domain of rFSHR and the TMs of hFSHR was not constitutively active when the D(6.44)G mutation was introduced. These results suggest that the molecular determinants dictating the two receptors to D(6.44)G-induced constitutive activation lie in the serpentine region of the receptors. We therefore used reciprocal site-directed mutagenesis to identify these molecular determinants. We showed that two residues, one each in TM6 and TM7, could account for this dramatic difference. The double mutant M576T/H615Y hFSHR, although not constitutively active, becomes constitutively active when the D(6.44)G mutation was introduced. Similarly, when Y614 in rFSHR was mutated to the corresponding residue in hFSHR, His, the D(6.44)G mutant receptor is not constitutively active any more (Fig. 3). Homology modeling suggested that differences in hydrophobic interactions between TM6 and TM7 of the two FSHRs might account for the different susceptibility to D(6.44)G mutation-induced constitutive activation (Tao et al., 2002) (Fig. 3). In hFSHR, hydrophobic interaction between I588 in TM6 and V612 in TM7 is prevented in the rFSHR by the steric and polar interference from H615. Stronger hydrophobic interactions in the cytoplasmic ends of TM6 and TM7 are necessary for D(6.44)G mutation-induced constitutive activation. In rFSHR, four residues, including T575 and L576 in TM6 and L624 and F628 in TM7, have hydrophobic interactions. However, in hFSHR, the side chain of M576 is predicted to project to the lipid bilayer therefore cannot participate in the hydrophobic interaction. Hence in hFSHR, only three residues form hydrophobic interactions (Tao et al., 2002). Hsueh’s group showed that different interactions between TM5 and TM6 are responsible for the different susceptibility to D(6.30)G mutation-induced constitutive activation in hFSHR (where the mutant is not constitutively active) and LHCGR (where the mutant is constitutively active) (Kudo et al., 1996).
Fig. 3.
Different susceptibility in rFSHR and hFSHR to D(6.44)G-mutation induced constitutive activation. Panel A showed that two mutations, one in TM6 and one in TM7, can reverse the susceptibility to D(6.44)G-mutation induced constitutive activation. Panel B is a model of the two receptors suggesting that different hydrophobic interactions in TM6 and TM7 might be responsible for the different susceptibility to D(6.44)G-mutation induced constitutive activation. These data and models were modified and reprinted with permission from Tao et. al. 2002. Mol. Endocrinol. 16:1881–1892. Copyright (2002). The Endocrine Society.
Homologous mutations of H223R and T410P mutations were also made in other PTHR1 as well as related Family B GPCRs (Schipani et al., 1997a). Interestingly, these mutations result in constitutive activation in the opossum receptor but not in rat receptor. The molecular determinants of this difference in susceptibility to mutation-induced activation have not been elucidated. When these mutations were introduced into PTHR2, calcitonin receptor, secretin receptor, growth hormone releasing hormone receptor, glucagon-like peptide 1 receptor, and corticotropin-releasing hormone receptor, no constitutive activation was observed (Schipani et al., 1997a). However, when the homologous mutations were introduced into rat glucagon receptor, H178R (corresponding to H223R in PTHR1) was constitutively active, whereas T352P (corresponding to T410P in PTHR1) was not (T352A is constitutively active) (Hjorth et al., 1998).
7. Therapeutic implications for inverse agonists
When the inverse agonism was first described, there was reservation on whether it was of therapeutic relevance or just an interesting laboratory observation (Milligan et al., 1995). Studies performed during the past decade or so have provided unequivocal evidences on the physiological relevance of constitutive activity, suggesting the potential therapeutic values of modulating the constitutive activity. Kenakin reviewed the activity of 380 antagonists for 73 receptors that show constitutive activity and found 85% of these antagonists were inverse agonists (Kenakin, 2004). Some of the most highly prescribed drugs are inverse agonists. Prominent examples include the beta-blockers propranolol, alprenolol, pindolol, and timolol used for treating hypertension, angina pectoris, and arrhythmia that act on the β2-AR, metoprolol and bisoprolol used for treating hypertension, coronary heart disease, and arrhythmias by acting on β1-AR, cetirizine and loratadine for treating allergies and hay fever by acting on histamine H1 receptor, cimetidine and ranitidine acting on the histamine H2 receptor for treating heartburn and peptic ulcers, prazosin and phentolamine for treating hypertension by acting on α1-adrenergic receptors, atropine used as premedication for anesthesia, and pirenzepine used for treatment of peptic ulcer, respectively, that act on the muscarinic receptors, and naloxone used for treating heroin overdose by acting on μ-opioid receptor (Kenakin, 2004). As recently suggested by Kenakin, translational research of inverse agonism will show whether new therapeutic options will emerge (Kenakin, 2006), especially for silencing the constitutively active mutant receptors that cause the diseases described herein.
Govardhan and Oprian (Govardhan & Oprian, 1994) did the first proof-of-principal experiment on the therapeutic potential of inverse agonist in treating diseases due to constitutively active mutations in GPCRs. In that study, they set out to find the inhibitor that can fit three criteria: it should inactivate the mutant rhodopsins in the dark and in light, it should not affect the WT rhodopsin, and it should be able to cross the blood-retina barrier. They showed that amine derivatives of 11-cis-retinal could accomplish this on K296G, a laboratory-generated CAM. However, they are not active on the naturally occurring mutants K296E and K296M, likely due to the greater steric bulk of Glu and Met side chains in comparison to Gly (Govardhan & Oprian, 1994). Subsequently, they synthesized a retinylamine analog that is one carbon shorter than the parent 11-cis-retinal and showed that this compound is indeed an effective inhibitor of both the K296E and K296M mutants. The analog inhibited constitutive activation of transducin as well as their constitutive phosphorylation by rhodopsin kinase (Yang et al., 1997).
Currently, the treatments for diseases caused by constitutively active mutations in the TSHR gene include removal of the thyroid gland with additional radiotherapy. For diseases caused by activating LHCGR gene mutations, current treatments include antiandrogens such as cyproterone acetate and bicalutamide, aromatase inhibitors such as testolactone and anastrozole, and cytochrome P450 inhibitor such as ketoconazole (Almeida et al., 2008). Again, the major goal is to decrease the skeleton maturation to increase the adult height of the affected boys. Variable success was observed. For OHSS patients with constitutively active FSHR gene mutations, the treatment was careful observation or termination of pregnancy to remove the source of hCG. No inverse agonists have been tested in these systems. Recently, small molecule agonists and antagonists have been identified for LHCGR and FSHR (reviewed in (Blakeney et al., 2007; Heitman & IJzerman, 2008)) although it is not known whether any of the antagonists are inverse agonists and their potential utility in treating these genetic diseases.
In the MC4R, activation of the receptor results in decreased food intake and increased energy expenditure and inhibition of the receptor results in increased food intake and decreased energy expenditure. Several groups have tried to use MC4R antagonists for treating cancer cachexia. Marks and coworkers showed that intracerebroventricular injection of the natural inverse agonist AgRP prevented anorexia and loss of lean mass in mice bearing a syngenic sarcoma or caused by uremia (Cheung et al., 2005; Marks et al., 2001). Wisse and coworkers showed that intracerebroventricular injection of the MC4R antagonist SHU9119 (a peptide analogue of α-MSH) completely reversed cancer anorexia in mice with prostate cancer (Wisse et al., 2001). Both AgRP and SHU9119 are non-specific antagonists for the neural MCRs, blocking the action of both the MC3R and MC4R. Further studies showed that the effects of these antagonists are mediated by the MC4R not the MC3R (Marks et al., 2003). These studies established the principle that MC4R antagonism results in increased food intake and decreased energy expenditure, resulting in less lean mass loss in chronic disease state. However, peptides are not practical therapeutic approach. Subsequently, a small molecule antagonist acting as inverse agonist for the MC4R developed by Vos and colleagues, ML00253764, was also shown to prevent tumor-induced anorexia (Nicholson et al., 2006; Vos et al., 2004). Another small molecule compound developed by Neurocrine Biosciences, NBI-12i, was also shown to ameliorate tumor-induced anorexia and loss of lean mass (Markison et al., 2005). However, this compound was shown to be a competitive MC4R antagonist. Recently, it was shown that oral administration of a novel MC4R antagonist 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-6-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)propionyl]piperazine increased food intake in tumor-bearing mice (Chen et al., 2007). Several excellent reviews were published recently summarizing the studies on MC4R antagonism as potential therapeutic approach for cachexia, acute inflammation, and renal failure (Deboer & Marks, 2006; DeBoer & Marks, 2006; Foster & Chen, 2007). Further studies are needed to see whether there is any advantage of using inverse agonist versus neutral competitive antagonist in treating cachexia caused by cancer or other chronic diseases. It would also be of great interest to see whether small molecule mimetics of AgRP can be used for enhancing appetite in diseased states.
Because of the critical importance of ghrelin/GHSR in regulating GH secretion and energy balance, low molecular weight agonists for GHSR could be used to treat GH deficiency and cachexia due to chronic diseases. It was proposed that these agonists might be used to restore a youthful GH profile in senior citizens. GHSR antagonists could be used for treating obesity. Holst and Schwartz hypothesized that GHSR antagonists could be used before meals to antagonize the action of high levels of endogenous ghrelin and inverse agonists could be used to decrease the constitutive activity of GHSR between meals so there would be no craving for snack (Holst & Schwartz, 2004). Both approaches reduced hunger signal from GHSR (Holst & Schwartz, 2004). They first reported that [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-substance P is a full inverse agonist decreasing the basal activity of the WT GHSR to the level in untransfected cells (Holst et al., 2003). Another peptide, D-Lys3-GHRP-6, was shown to be an inverse agonist in seabream GHSR (Chan et al., 2004). Several recent studies reported a number of small molecule GHSR antagonists (Demange et al., 2007; Esler et al., 2007; Liu et al., 2004; Liu et al., 2006; Moulin et al., 2007; Moulin et al., 2008a; Moulin et al., 2008b; Rudolph et al., 2007; Serby et al., 2006; Xin et al., 2006; Xin et al., 2005; Zhao et al., 2004; Zhao et al., 2005). Some of these compounds were found to decrease food intake and body weight. In addition, since ghrelin were shown to also play a role in controlling glucose-induced insulin secretion (Dezaki et al., 2008), some of these compounds were reported to have glucose-lowering effects mediated by enhanced glucose-dependent insulin secretion (Esler et al., 2007; Rudolph et al., 2007). However, none of them were reported to be inverse agonists. Many of these studies did not investigate whether the compounds reported are inverse agonists or not. Functional studies measured intracellular calcium level rather than IP production that fail to detect the constitutive activity of GHSR (Holst et al., 2003) therefore masking any potential inverse agonistic activity. Inverse agonism can only be measured in a system with constitutive activity. Clinical trial to test Holst and Schwartz's hypothesis of combining neutral antagonists and inverse agonists to combat obesity has to wait for the development of safe inverse agonists.
One concern for using inverse agonists as therapeutics is the up-regulation of receptor numbers resulting in tolerance (Milligan & Bond, 1997). This was first observed with the H2 histamine receptor (Smit et al., 1996). The inverse agonists cimetidine and ranitidine but not the neutral antagonist burimamide induced up-regulation of the exogenously expressed H2 receptor, providing a plausible explanation for the tolerance to the inverse agonists after prolonged clinical use (Smit et al., 1996). Milligan and colleagues showed that inverse agonists also up-regulate β2-and α1B-ARs (Lee et al., 1997; MacEwan & Milligan, 1996). Therefore achieving a high degree of suppressing the constitutive activity without the undesired effect of up-regulating receptor expression (with its corresponding increase in basal signaling) is the challenge for any drug discovery efforts trying to exploit inverse agonists as treatment modalities for the diseases caused by constitutively active mutations in GPCRs.
8. Conclusions
Since the pioneering reports of constitutively active mutations associated with either special phenotype (dominant black color in mice with MC1R mutations) or diseases (retinitis pigmentosa, hyperthyroidism or male-limited precocious puberty) in early 1990s, tremendous progresses have been made during the past 15 years or so. Constitutively active mutations in several additional receptors were found to cause other diseases. In addition, the loss of constitutive activity in the mutant receptors can also cause disease, suggesting the critical importance of the basal activity in the WT receptor in normal physiology. The detailed functional studies of these naturally occurring mutations, in combination with homology modeling using rhodopsin as the template, provided novel insights into the mechanism of activation of these GPCRs. Transgenic animals expressing some of these mutants were generated. The studies of these humanized animal models yielded novel insights into the physiological functions of these receptors. Clinical studies, combined with in vitro functional expression studies, as well as studies with genetically modified animals, significantly advanced our understanding of these genetic diseases and provided models for identifying new therapeutic options. For the diseases caused by constitutively active mutations, inverse agonists are expected to have better therapeutic values compared with neutral antagonists. Further medicinal chemistry studies are expected to yield better drugs for treating these genetic diseases.
Acknowledgements
I am very grateful to Dr. Deborah L. Segaloff at The University of Iowa Carver College of Medicine for her superb mentoring during my stay in her laboratory. She introduced me to the field of diseased GPCRs. I studied constitutive activation of gonadotropin receptor during my post-doctoral training with her. I also thank her for giving me the freedom to pursue my independent line of investigation on the neural melanocortin receptors in her lab. Due to space constraints, I cannot cite all the important studies relevant to the topics covered in this review. I apologize to those investigators. My studies on the neural melanocortin receptors and human obesity were supported by American Heart Association (0265236Z), National Institutes of Health R15DK077213, and intramural support at Auburn University (Competitive Research Grant, Biogrant, and Animal Health and Disease Research).
Abbreviations
- ADRP
autosomal dominant retinitis pigmentosa
- AgRP
Agouti-related protein
- AR
adrenergic receptor
- CAM
constitutively active mutant
- CNB
congenital night blindness
- EC50
the concentration that results in 50% maximal response
- EL
extracellular loop
- FMPP
familial male-limited precocious puberty
- FSH
follicle stimulating hormone
- FSHR
FSH receptor
- GH
growth hormone
- GHS
growth hormone secretagogue
- GHSR
growth hormone secretagogue receptor
- GPCR
G protein-coupled receptor
- hCG
human chorionic gonadotropin
- hFSHR
human FSHR
- IL
intracellular loop
- IP
inositol phosphate
- JMC
Jansen’s metaphyseal chondrodysplasia
- LH
luteinizing hormone
- LHCGR
LH/CG receptor
- MCR
melanocortin receptor
- MC1R
melanocortin-1 receptor
- MC3R
melanocortin-3 receptor
- MC4R
melanocortin-4 receptor
- OHSS
ovarian hyperstimulation syndrome
- PLC
phospholipase C
- POMC
pro-opiomelanocortin
- PTH
parathyroid hormone
- PTHrP
PTH related peptide
- PTHR1
PTH/PTHrP receptor type 1
- rFSHR
rat FSHR
- SAS
solvent accessible surface
- TM
transmembrane α-helix
- TSH
thyroid stimulating hormone
- TSHR
TSH receptor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan RA. Constitutive receptor activity series: endogenous inverse agonists and constitutive receptor activity in the melanocortin system. Trends Pharmacol Sci. 2006;27:183–186. doi: 10.1016/j.tips.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Adan RA, Kas MJ. Inverse agonism gains weight. Trends Pharmacol Sci. 2003;24:315–321. doi: 10.1016/S0165-6147(03)00130-5. [DOI] [PubMed] [Google Scholar]
- Akcurin S, Turkkahraman D, Tysoe C, Ellard S, De Leener A, Vassart G, Costagliola S. A family with a novel TSH receptor activating germline mutation (p.Ala485Val) Eur J Pediatr. 2008 doi: 10.1007/s00431-007-0659-9. in press. [DOI] [PubMed] [Google Scholar]
- al-Jandal N, Farrar GJ, Kiang AS, Humphries MM, Bannon N, Findlay JB, Humphries P, Kenna PF. A novel mutation within the rhodopsin gene (Thr-94-Ile) causing autosomal dominant congenital stationary night blindness. Hum Mutat. 1999;13:75–81. doi: 10.1002/(SICI)1098-1004(1999)13:1<75::AID-HUMU9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Alberti L, Proverbio MC, Costagliola S, Weber G, Beck-Peccoz P, Chiumello G, Persani L. A novel germline mutation in the TSH receptor gene causes non-autoimmune autosomal dominant hyperthyroidism. Eur J Endocrinol. 2001;145:249–254. doi: 10.1530/eje.0.1450249. [DOI] [PubMed] [Google Scholar]
- Allan CM, Garcia A, Spaliviero J, Jimenez M, Handelsman DJ. Maintenance of spermatogenesis by the activated human (Asp567Gly) FSH receptor during testicular regression due to hormonal withdrawal. Biol Reprod. 2006;74:938–944. doi: 10.1095/biolreprod.105.048413. [DOI] [PubMed] [Google Scholar]
- Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, Handelsman DJ. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology. 2004;145:1587–1593. doi: 10.1210/en.2003-1164. [DOI] [PubMed] [Google Scholar]
- Almeida MQ, Brito VN, Lins TS, Guerra-Junior G, de Castro M, Antonini SR, Arnhold IJ, Mendonca BB, Latronico AC. Long-term treatment of familial male-limited precocious puberty (testotoxicosis) with cyproterone acetate or ketoconazole. Clin Endocrinol. 2008;69:93–98. doi: 10.1111/j.1365-2265.2007.03160.x. [DOI] [PubMed] [Google Scholar]
- Angelova K, Fanelli F, Puett D. A model for constitutive lutropin receptor activation based on molecular simulation and engineered mutations in transmembrane helices 6 and 7. J Biol Chem. 2002;277:32202–32213. doi: 10.1074/jbc.M203272200. [DOI] [PubMed] [Google Scholar]
- Angelova K, Narayan P, Simon JP, Puett D. Functional role of transmembrane helix 7 in the activation of the heptahelical lutropin receptor. Mol Endocrinol. 2000;14:459–471. doi: 10.1210/mend.14.4.0439. [DOI] [PubMed] [Google Scholar]
- Arseven OK, Wilkes WP, Jameson JL, Kopp P. Substitutions of tyrosine 601 in the human thyrotropin receptor result in increase or loss of basal activation of the cyclic adenosine monophosphate pathway and disrupt coupling to Gq/11. Thyroid. 2000;10:3–10. doi: 10.1089/thy.2000.10.3. [DOI] [PubMed] [Google Scholar]
- Arturi F, Scarpelli D, Coco A, Sacco R, Bruno R, Filetti S, Russo D. Thyrotropin receptor mutations and thyroid hyperfunctioning adenomas ten years after their first discovery: unresolved questions. Thyroid. 2003;13:341–343. doi: 10.1089/105072503321669811. [DOI] [PubMed] [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Gershengorn MC. Constitutively signaling G-protein-coupled receptors and human disease. Trends Endocrinol Metab. 1998;9:27–31. doi: 10.1016/s1043-2760(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Ascoli M. Potential Leydig cell mitogenic signals generated by the wild-type and constitutively active mutants of the lutropin/choriogonadotropin receptor (LHR) Mol Cell Endocrinol. 2007:260–262. 244–248. doi: 10.1016/j.mce.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Baessler A, Hasinoff MJ, Fischer M, Reinhard W, Sonnenberg GE, Olivier M, Erdmann J, Schunkert H, Doering A, Jacob HJ, Comuzzie AG, Kissebah AH, Kwitek AE. Genetic linkage and association of the growth hormone secretagogue receptor (ghrelin receptor) gene in human obesity. Diabetes. 2005;54:259–267. doi: 10.2337/diabetes.54.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, Javitch JA. Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995:366–428. [Google Scholar]
- Baranski TJ, Herzmark P, Lichtarge O, Gerber BO, Trueheart J, Meng EC, Iiri T, Sheikh SP, Bourne HR. C5a receptor activation: genetic identification of critical residues in four transmembrane helices. J Biol Chem. 1999;274:15757–15765. doi: 10.1074/jbc.274.22.15757. [DOI] [PubMed] [Google Scholar]
- Baron J, Winer KK, Yanovski JA, Cunningham AW, Laue L, Zimmerman D, Cutler GB., Jr Mutations in the Ca2+-sensing receptor gene cause autosomal dominant and sporadic hypoparathyroidism. Hum Mol Genet. 1996;5:601–606. doi: 10.1093/hmg/5.5.601. [DOI] [PubMed] [Google Scholar]
- Bastepe M, Raas-Rothschild A, Silver J, Weissman I, Wientroub S, Juppner H, Gillis D. A form of Jansen's metaphyseal chondrodysplasia with limited metabolic and skeletal abnormalities is caused by a novel activating parathyroid hormone (PTH)/PTH-related peptide receptor mutation. J Clin Endocrinol Metab. 2004;89:3595–3600. doi: 10.1210/jc.2004-0036. [DOI] [PubMed] [Google Scholar]
- Biebermann H, Schoneberg T, Hess C, Germak J, Gudermann T, Gruters A. The first activating TSH receptor mutation in transmembrane domain 1 identified in a family with nonautoimmune hyperthyroidism. J Clin Endocrinol Metab. 2001;86:4429–4433. doi: 10.1210/jcem.86.9.7888. [DOI] [PubMed] [Google Scholar]
- Biebermann H, Schoneberg T, Krude H, Gudermann T, Gruters A. Constitutively activating TSH-receptor mutations as a molecular cause of non-autoimmune hyperthyroidism in childhood. Langenbecks Arch Surg. 2000;385:390–392. doi: 10.1007/s004230000145. [DOI] [PubMed] [Google Scholar]
- Blakeney JS, Reid RC, Le GT, Fairlie DP. Nonpeptidic ligands for peptide-activated G protein-coupled receptors. Chem Rev. 2007;107:2960–3041. doi: 10.1021/cr050984g. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers CY. Growth hormone-releasing peptide (GHRP) Cell Mol Life Sci. 1998;54:1316–1329. doi: 10.1007/s000180050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P, Gordon D, Chiefari E, Yong S, DeJong S, Pitale S, Russo D, Filetti S. A Phe 486 thyrotropin receptor mutation in an autonomously functioning follicular carcinoma that was causing hyperthyroidism. Thyroid. 2000;10:1009–1012. doi: 10.1089/thy.2000.10.1009. [DOI] [PubMed] [Google Scholar]
- Canto P, Soderlund D, Ramon G, Nishimura E, Mendez JP. Mutational analysis of the luteinizing hormone receptor gene in two individuals with Leydig cell tumors. Am J Med Genet. 2002;108:148–152. doi: 10.1002/ajmg.10218. [DOI] [PubMed] [Google Scholar]
- Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. The mammalian β2-adrendergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984;23:4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Chakir K, Xiang Y, Yang D, Zhang SJ, Cheng H, Kobilka BK, Xiao RP. The third intracellular loop and the carboxyl terminus of β2-adrenergic receptor confer spontaneous activity of the receptor. Mol Pharmacol. 2003;64:1048–1058. doi: 10.1124/mol.64.5.1048. [DOI] [PubMed] [Google Scholar]
- Chan CB, Leung PK, Wise H, Cheng CH. Signal transduction mechanism of the seabream growth hormone secretagogue receptor. FEBS Lett. 2004;577:147–153. doi: 10.1016/j.febslet.2004.08.088. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang W, Tucci F, Tran JA, Fleck BA, Hoare SR, Joppa M, Markison S, Wen J, Sai Y, Johns M, Madan A, Chen T, Chen CW, Marinkovic D, Arellano M, Saunders J, Foster AC. Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4receptor for the potential treatment of cachexia. J Med Chem. 2007;50:5249–5252. doi: 10.1021/jm070806a. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115:1659–1665. doi: 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Cotecchia S. Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol Sci. 2005;26:618–624. doi: 10.1016/j.tips.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at δ opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S, Exum S, Caron MG, Lefkowitz RJ. Regions of the α1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci U S A. 1990;87:2896–2900. doi: 10.1073/pnas.87.8.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. International Union of Pharmacology. LVI.Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57:541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- Davies E, Bonnardeaux A, Plouin PF, Corvol P, Clauser E. Somatic mutations of the angiotensin II (AT1) receptor gene are not present in aldosterone-producing adenoma. J Clin Endocrinol Metab. 1997;82:611–615. doi: 10.1210/jcem.82.2.3764. [DOI] [PubMed] [Google Scholar]
- Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115:1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leener A, Caltabiano G, Erkan S, Idil M, Vassart G, Pardo L, Costagliola S. Identification of the first germline mutation in the extracellular domain of the follitropin receptor responsible for spontaneous ovarian hyperstimulation syndrome. Hum Mutat. 2008;29:91–98. doi: 10.1002/humu.20604. [DOI] [PubMed] [Google Scholar]
- De Leener A, Montanelli L, Van Durme J, Chae H, Smits G, Vassart G, Costagliola S. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab. 2006;91:555–562. doi: 10.1210/jc.2005-1580. [DOI] [PubMed] [Google Scholar]
- de Roux N, Polak M, Couet J, Leger J, Czernichow P, Milgrom E, Misrahi M. A neomutation of the thyroid-stimulating hormone receptor in a severe neonatal hyperthyroidism. J Clin Endocrinol Metab. 1996;81:2023–2026. doi: 10.1210/jcem.81.6.8964822. [DOI] [PubMed] [Google Scholar]
- Deboer MD, Marks DL. Cachexia: lessons from melanocortin antagonism. Trends Endocrinol Metab. 2006;17:199–204. doi: 10.1016/j.tem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Marks DL. Therapy insight: Use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- Delbaere A, Smits G, De Leener A, Costagliola S, Vassart G. Understanding ovarian hyperstimulation syndrome. Endocrine. 2005;26:285–290. doi: 10.1385/ENDO:26:3:285. [DOI] [PubMed] [Google Scholar]
- Demange L, Boeglin D, Moulin A, Mousseaux D, Ryan J, Berge G, Gagne D, Heitz A, Perrissoud D, Locatelli V, Torsello A, Galleyrand JC, Fehrentz JA, Martinez J. Synthesis and pharmacological in vitro and in vivo evaluations of novel triazole derivatives as ligands of the ghrelin receptor. 1. J Med Chem. 2007;50:1939–1957. doi: 10.1021/jm070024h. [DOI] [PubMed] [Google Scholar]
- Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118:239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Donohoue PA, Tao YX, Collins M, Yeo GSH, O'Rahilly S, Segaloff DL. Deletion of codons 88–92 of the melanocortin-4 receptor gene: a novel deleterious mutation in an obese female. J Clin Endocrinol Metab. 2003;88:5841–5845. doi: 10.1210/jc.2003-030903. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Berson EL, Rao VR, Oprian DD. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet. 1993;4:280–283. doi: 10.1038/ng0793-280. [DOI] [PubMed] [Google Scholar]
- Dryja V, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Duprez L, Hermans J, Van Sande J, Dumont JE, Vassart G, Parma J. Two autonomous nodules of a patient with multinodular goiter harbor different activating mutations of the thyrotropin receptor gene. J Clin Endocrinol Metab. 1997a;82:306–308. doi: 10.1210/jcem.82.1.3691. [DOI] [PubMed] [Google Scholar]
- Duprez L, Parma J, Costagliola S, Hermans J, Van Sande J, Dumont JE, Vassart G. Constitutive activation of the TSH receptor by spontaneous mutations affecting the N-terminal extracellular domain. FEBS Lett. 1997b;409:469–474. doi: 10.1016/s0014-5793(97)00532-2. [DOI] [PubMed] [Google Scholar]
- Duprez L, Parma J, Van Sande J, Allgeier A, Leclere J, Schvartz C, Delisle MJ, Decoulx M, Orgiazzi J, Dumont J, et al. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet. 1994;7:396–401. doi: 10.1038/ng0794-396. [DOI] [PubMed] [Google Scholar]
- Esapa CT, Duprez L, Ludgate M, Mustafa MS, Kendall-Taylor P, Vassart G, Harris PE. A novel thyrotropin receptor mutation in an infant with severe thyrotoxicosis. Thyroid. 1999;9:1005–1010. doi: 10.1089/thy.1999.9.1005. [DOI] [PubMed] [Google Scholar]
- Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, Bullock W, Daly M, Decarr L, Li Y, Milardo L, Molstad D, Zhu J, Gardell SJ, Livingston JN, Sweet LJ. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148:5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- Evans BAJ, Bowen DJ, Smith PJ, Clayton PE, Gregory JW. A new point mutation in the luteinising hormone receptor gene in familial and sporadic male limited precocious puberty: genotype does not always correlate with phenotype. J Med Genet. 1996;33:143–147. doi: 10.1136/jmg.33.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZC, Sartin JL, Tao YX. Pharmacological analyses of two naturally occurring porcine melanocortin-4 receptor mutations in domestic pigs. Domest Anim Endocrinol. 2008;34:383–390. doi: 10.1016/j.domaniend.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Fanelli F. Theoretical study on mutation-induced activation of the luteinizing hormone receptor. J Mol Biol. 2000;296:1333–1351. doi: 10.1006/jmbi.2000.3516. [DOI] [PubMed] [Google Scholar]
- Fanelli F, Verhoef-Post M, Timmerman M, Zeilemaker A, Martens JW, Themmen AP. Insight into mutation-induced activation of the luteinizing hormone receptor:molecular simulations predict the functional behavior of engineered mutants at M398. Mol Endocrinol. 2004;18:1499–1508. doi: 10.1210/me.2003-0050. [DOI] [PubMed] [Google Scholar]
- Feighner SD, Howard AD, Prendergast K, Palyha OC, Hreniuk DL, Nargund R, Underwood D, Tata JR, Dean DC, Tan CP, McKee KK, Woods JW, Patchett AA, Smith RG, Van der Ploeg LH. Structural requirements for the activation of the human growth hormone secretagogue receptor by peptide and nonpeptide secretagogues. Mol Endocrinol. 1998;12:137–145. doi: 10.1210/mend.12.1.0051. [DOI] [PubMed] [Google Scholar]
- Feng X, Muller T, Mizrachi D, Fanelli F, Segaloff DL. An intracellular loop (IL2) residue confers different basal constitutive activities to the human lutropin receptor and human thyrotropin receptor through structural communication between IL2 and helix 6, via helix 3. Endocrinology. 2008;149:1705–1717. doi: 10.1210/en.2007-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Bisello A. Cellular distribution of constitutively active mutant parathyroid hormone (PTH)/PTH-related protein receptors and regulation of cyclic adenosine 3',5'-monophosphate signaling by beta-arrestin2. Mol Endocrinol. 2001;15:149–163. doi: 10.1210/mend.15.1.0587. [DOI] [PubMed] [Google Scholar]
- Foster AC, Chen C. Melanocortin-4 receptor antagonists as potential therapeutics in the treatment of cachexia. Curr Top Med Chem. 2007;7:1131–1136. doi: 10.2174/156802607780906663. [DOI] [PubMed] [Google Scholar]
- Fournes B, Monier R, Michiels F, Milgrom E, Misrahi M, Feunteun J. Oncogenic potential of a mutant human thyrotropin receptor expressed in FRTL-5 cells. Oncogene. 1998;16:985–890. doi: 10.1038/sj.onc.1201626. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Holzapfel HP, Wonerow P, Scherbaum WA, Paschke R. Somatic mutations in the thyrotropin receptor gene and not in the Gsα protein gene in 31 toxic thryoid nodules. J Clin Endocrinol Metab. 1997;82:3885–3891. doi: 10.1210/jcem.82.11.4382. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Lewis MD, Alkhafaji F, Starkey K, Paschke R, Wynford-Thomas D, Eggo M, Ludgate M. Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology. 2003a;144:4018–4030. doi: 10.1210/en.2003-0438. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Tannapfel A, Sabri O, Lamesch P, Paschke R. Two somatic TSH receptor mutations in a patient with toxic metastasising follicular thyroid carcinoma and non-functional lung metastases. Endocr Relat Cancer. 2003b;10:591–600. doi: 10.1677/erc.0.0100591. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Warner J, Sequeira M, Paschke R, Gregory J, Ludgate M. Novel TSHR germline mutation (Met463Val) masquerading as Graves' disease in a large Welsh kindred with hyperthyroidism. Thyroid. 2000;10:1035–1041. doi: 10.1089/thy.2000.10.1035. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Verity K, Shen Y, Mamers P, Jobling T, Burger HG. No evidence of a role for mutations or polymorphisms of the follicle-stimulating hormone receptor in ovarian granulosa cell tumors. J Clin Endocrinol Metab. 1998;83:274–279. doi: 10.1210/jcem.83.1.4509. [DOI] [PubMed] [Google Scholar]
- Galet C, Ascoli M. A constitutively active mutant of the human lutropin receptor (hLHR-L457R) escapes lysosomal targeting and degradation. Mol Endocrinol. 2006;20:2931–2945. doi: 10.1210/me.2006-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Giacaglia LR, Kohek MBdF, Carvalho FM, Fragoso MC, Mendonca B, Latronico AC. No evidence of somatic activating mutations on gonadotropin receptor genes in sex cord stromal tumors. Fertil Steril. 2000;74:992–995. doi: 10.1016/s0015-0282(00)01565-x. [DOI] [PubMed] [Google Scholar]
- Gloriam DE, Fredriksson R, Schioth HB. The G protein-coupled receptor subset of the rat genome. BMC Genomics. 2007;8:338. doi: 10.1186/1471-2164-8-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardhan CP, Oprian DD. Active site-directed inactivation of constitutively active mutants of rhodopsin. J Biol Chem. 1994;269:6524–6527. [PubMed] [Google Scholar]
- Grasberger H, Van Sande J, Hag-Dahood Mahameed A, Tenenbaum-Rakover Y, Refetoff S. A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J Clin Endocrinol Metab. 2007;92:2816–2820. doi: 10.1210/jc.2007-0366. [DOI] [PubMed] [Google Scholar]
- Greasley PJ, Fanelli F, Rossier O, Abuin L, Cotecchia S. Mutagenesis and modelling of the a1b-adrenergic receptor highlight the role of the helix 3/helix 6 interface in receptor activation. Mol Pharmacol. 2002;61:1025–1032. doi: 10.1124/mol.61.5.1025. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Partsch C-J, Simoni M, Nordhoff V, Sippell WG, Nieschlag E, Saxena BB. A mutation in the first transmembrane domain of the lutropin receptor causes male precocious puberty. J Clin Endocrinol Metab. 1998;83:476–480. doi: 10.1210/jcem.83.2.4579. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Simoni M, Nieschlag E. An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hyophysectomized man. J Clin Endocrinol Metab. 1996;81:1367–1370. doi: 10.1210/jcem.81.4.8636335. [DOI] [PubMed] [Google Scholar]
- Gross AK, Rao VR, Oprian DD. Characterization of rhodopsin congenital night blindness mutant T94I. Biochemistry. 2003;42:2009–2015. doi: 10.1021/bi020613j. [DOI] [PubMed] [Google Scholar]
- Gu W, Tu Z, Kleyn PW, Kissebah A, Duprat L, Lee J, Chin W, Maruti S, Deng N, Fisher SL, Franco LS, Burn P, Yagaloff KA, Nathan J, Heymsfield S, Albu J, Pi-Sunyer FX, Allison DB. Identification and functional analysis of novel human melanocortin-4 receptor variants. Diabetes. 1999;48:635–639. doi: 10.2337/diabetes.48.3.635. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- Haywood M, Tymchenko N, Spaliviero J, Koch AE, Jimenez M, Gromoll J, Simoni M, Nordhoff V, Handelsman DJ, Allan CM. An activated human follicle-stimulating hormone (FSH) receptor stimulates FSH-like activity in gonadotropin-deficient transgenic mice. Mol Endocrinol. 2002;16:2582–2591. doi: 10.1210/me.2002-0032. [DOI] [PubMed] [Google Scholar]
- Heitman LH, IJzerman AP. G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: A case for GnRH, LH, FSH, and GPR54 receptor ligands. Med Res Rev. 2008 doi: 10.1002/med.20129. in press. [DOI] [PubMed] [Google Scholar]
- Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, Scherag A, Nguyen TT, Schlumberger P, Rief W, Vollmert C, Illig T, Wichmann HE, Schafer H, Platzer M, Biebermann H, Meitinger T, Hebebrand J. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab. 2006;91:1761–1769. doi: 10.1210/jc.2005-2056. [DOI] [PubMed] [Google Scholar]
- Hinney A, Hohmann S, Geller F, Vogel C, Hess C, Wermter AK, Brokamp B, Goldschmidt H, Siegfried W, Remschmidt H, Schafer H, Gudermann T, Hebebrand J. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J Clin Endocrinol Metab. 2003;88:4258–4267. doi: 10.1210/jc.2003-030233. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Ascoli M. A constitutively active somatic mutation of the human lutropin receptor found in Leydig cell tumors activates the same families of G proteins as germ line mutations associated with Leydig cell hyperplasia. Endocrinology. 2003;144:3872–3878. doi: 10.1210/en.2003-0365. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Galet C, Ascoli M. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR): a novel experimental paradigm to study the functional properties of the hLHR. Endocrinology. 2002;143:1026–1035. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- Hjorth SA, Orskov C, Schwartz TW. Constitutive activity of glucagon receptor mutants. Mol Endocrinol. 1998;12:78–86. doi: 10.1210/mend.12.1.0045. [DOI] [PubMed] [Google Scholar]
- Ho G, MacKenzie RG. Functional characterization of mutations in melanocortin-4 receptor associated with human obesity. J Biol Chem. 1999;274:35816–35822. doi: 10.1074/jbc.274.50.35816. [DOI] [PubMed] [Google Scholar]
- Ho SC, Van Sande J, Lefort A, Vassart G, Costagliola S. Effects of mutations involving the highly conserved S281HCC motif in the extracellular domain of the thyrotropin (TSH) receptor on TSH binding and constitutive activity. Endocrinology. 2001;142:2760–2767. doi: 10.1210/endo.142.7.8246. [DOI] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279:53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25:113–117. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Holzapfel HP, Wonerow P, von Petrykowski W, Henschen M, Scherbaum WA, Paschke R. Sporadic congenital hyperthyroidism due to a spontaneous germline mutation in the thyrotropin receptor gene. J Clin Endocrinol Metab. 1997;82:3879–3884. doi: 10.1210/jcem.82.11.4378. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum C, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med. 2007;11:908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeha GS, Lowenthal ED, Chan WY, Wu SM, Karaviti LP. Variable presentation of precocious puberty associated with the D564G mutation of the LHCGR gene in children with testotoxicosis. J Pediatr. 2006;149:271–274. doi: 10.1016/j.jpeds.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Juppner H. Jansen's metaphyseal chondrodysplasia. A disorder due to a PTH/PTHrP receptor gene mutation. Trends Endocrinol Metab. 1996;7:157–162. doi: 10.1016/1043-2760(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Kawate N, Kletter GB, Wilson BE, Netzloff ML, Menon KMJ. Identification of constitutively activating mutation of the luteinising hormone receptor in a family with male limited gonadotrophin independent precocious puberty (testotoxicosis) J Med Genet. 1995;32:553–554. doi: 10.1136/jmg.32.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye PV, Hapgood J, Millar RP. Absence of mutations in exon 3 of the GnRH receptor in human gonadotroph adenomas. Clin Endocrinol. 1997;47:549–554. doi: 10.1046/j.1365-2265.1997.3131127.x. [DOI] [PubMed] [Google Scholar]
- Keen TJ, Inglehearn CF, Lester DH, Bashir R, Jay M, Bird AC, Jay B, Bhattacharya SS. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991;11:199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- Kenakin T. The physiological significance of constitutive receptor activity. Trends Pharmacol Sci. 2006;26:603–605. [Google Scholar]
- Khoo DH, Parma J, Rajasoorya C, Ho SC, Vassart G. A germline mutation of the thyrotropin receptor gene associated with thyrotoxicosis and mitral valve prolapse in a Chinese family. J Clin Endocrinol Metab. 1999;84:1459–1462. doi: 10.1210/jcem.84.4.5620. [DOI] [PubMed] [Google Scholar]
- Kim JM, Altenbach C, Kono M, Oprian DD, Hubbell WL, Khorana HG. Structural origins of constitutive activation in rhodopsin: Role of the K296/E113 salt bridge. Proc Natl Acad Sci U S A. 2004;101:12508–12513. doi: 10.1073/pnas.0404519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MC, Lefkowitz RJ. Constitutive activation of the a1B-adrenergic receptor by all amino acid substitutions at a single site. J Biol Chem. 1992;267:1430–1433. [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kolakowski LF., Jr GCRDb: a G-protein-coupled receptor database. Receptors Channels. 1994;2:1–7. [PubMed] [Google Scholar]
- Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci. 2001;58:1301–1322. doi: 10.1007/PL00000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp P, Jameson JL, Roe TF. Congenital nonautoimmune hyperthyroidism in a nonidentical twin caused by a sporadic germline mutation in the thyrotropin receptor gene. Thyroid. 1997a;7:765–770. doi: 10.1089/thy.1997.7.765. [DOI] [PubMed] [Google Scholar]
- Kopp P, Muirhead S, Jourdain N, Gu WX, Jameson JL, Rodd C. Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281⤍isoleucine) in the extracellular domain of the thyrotropin receptor. J Clin Invest. 1997b;100:1634–1639. doi: 10.1172/JCI119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp P, van Sande J, Parma J, Duprez L, Gerber H, Joss E, Jameson JL, Dumont JE, Vassart G. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med. 1995;332:150–154. doi: 10.1056/NEJM199501193320304. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Hai N, Okamoto H, Sugawa H, Mori T. A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur J Endocrinol. 2000;143:471–477. doi: 10.1530/eje.0.1430471. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Mori T, Shenker A. The role of Asp578 in maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. J Biol Chem. 1996;271:31813–31817. doi: 10.1074/jbc.271.50.31813. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Van Dop C, Geffner ME, Rabl W, Carel JC, Chaussain JL, Mori T, Merendino JJ, Jr, Shenker A. Characterization of heterogeneous mutations causing constitutive activation of the luteinizing hormone receptor in familial male precocious puberty. Hum Mol Genet. 1995;4:183–188. doi: 10.1093/hmg/4.2.183. [DOI] [PubMed] [Google Scholar]
- Kotlar T, Young RH, Albanese C, Crowley WF, Jr, Scully RE, Jameson JL. Absence of mutations in the FSH receptor in ovarian granulosa cell tumors. J Clin Endocrinol Metab. 1998;83:3001. doi: 10.1210/jcem.83.8.5033-1. [DOI] [PubMed] [Google Scholar]
- Kotlar TJ, Young RH, Albanese C, Crowley WF, Jr, Scully RE, Jameson JL. A mutation in the follicle-stimulating hormone receptor occurs frequently in human ovarian sex cord tumors. J Clin Endocrinol Metab. 1997;82:1020–1026. doi: 10.1210/jcem.82.4.3870. [DOI] [PubMed] [Google Scholar]
- Kraaij R, Post M, Kremer H, Milgrom E, Epping W, Brunner HG, Grootegoed AJ, Themmen APN. A missense mutation in the second transmembrane segment of the luteinizing hormone receptor causes familiar male precocious puberty. J Clin Endocrinol Metab. 1995;80:3168–3172. doi: 10.1210/jcem.80.11.7593421. [DOI] [PubMed] [Google Scholar]
- Kremer H, Mariman E, Otten BJ, Moll GW, Jr, Stoelinja GB, Wit JM, Jansen M, Drop SL, Faas B, Ropers H-H, Brunner HG. Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum. Mol. Genet. 1993;2:1779–1783. doi: 10.1093/hmg/2.11.1779. [DOI] [PubMed] [Google Scholar]
- Kremer H, Martens JW, van Reen M, Verhoef-Post M, Wit JM, Otten BJ, Drop SL, Delemarre-van de Waal HA, Pombo-Arias M, De Luca F, Potau N, Buckler JM, Jansen M, Parks JS, Latif HA, Moll GW, Epping W, Saggese G, Mariman EC, Themmen AP, Brunner HG. A limited repertoire of mutations of the luteinizing hormone (LH) receptor gene in familial and sporadic patients with male LH-independent precocious puberty. J Clin Endocrinol Metab. 1999;84:1136–1140. doi: 10.1210/jcem.84.3.5515. [DOI] [PubMed] [Google Scholar]
- Kudo M, Osuga Y, Kobilka BK, Hsueh AJW. Transmembrane regions V and VI of the human luteinizing hormone receptor are required for constitutive activation by a mutation in the third intracellular loop. J Biol Chem. 1996;271:22470–22478. doi: 10.1074/jbc.271.37.22470. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi L, Kronenberg HM, Schipani E, Robey PG, Bianco P. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167:1113–1122. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico A, Segaloff D. Naturally occurring mutations of the luteinizing hormone receptor: Lessons learned about reproductive physiology and G protein-coupled receptors. Am J Hum Genet. 1999;65:949–958. doi: 10.1086/302602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico AC, Abell AN, Arnhold IJP, Liu X, Lins TSS, Brito VN, Billerbeck AE, Segaloff DL, Mendonca BB. A unique constitutively activating mutation in the third transmembrane helix f the luteinizing hormone receptor causes sporadic male gonadotropin independent precocious puberty. J Clin Endocrinol Metab. 1998;83:2435–2440. doi: 10.1210/jcem.83.7.4968. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Anasti J, Arnhold IJP, Mendonca BB, Domenice S, Albano MC, Zachman K, Wajchenberg BL, Tsigos C. A novel mutation of the luteinizing hormone receptor gene causing male gonadotropin-independent precocious puberty. J. Clin. Endocrin. Metab. 1995a;80:2490–2494. doi: 10.1210/jcem.80.8.7629248. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Lins TS, Brito VN, Arnhold IJ, Mendonca BB. The effect of distinct activating mutations of the luteinizing hormone receptor gene on the pituitary-gonadal axis in both sexes. Clin Endocrinol. 2000a;53:609–613. doi: 10.1046/j.1365-2265.2000.01135.x. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Reincke M, Mendonca BB, Arai K, Mora P, Allolio B, Wajchenberg BL, Chrousos GP, Tsigos C. No evidence for oncogenic mutations in the adrenocorticotropin receptor gene in human adrenocortical neoplasms. J Clin Endocrinol Metab. 1995b;80:875–877. doi: 10.1210/jcem.80.3.7883845. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Segaloff DL. Insights learned from L457(3.43)R, an activating mutant of the human lutropin receptor. Mol Cell Endocrinol. 2007;260–262:287–293. doi: 10.1016/j.mce.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico AC, Shinozaki H, Guerra G, Jr, Pereira MA, Lemos Marini SH, Baptista MT, Arnhold IJ, Fanelli F, Mendonca BB, Segaloff DL. Gonadotropin-independent precocious puberty due to luteinizing hormone receptor mutations in Brazilian boys: A novel constitutively activating mutation in the first transmembrane helix. J Clin Endocrinol Metab. 2000b;85:4799–4805. doi: 10.1210/jcem.85.12.7071. [DOI] [PubMed] [Google Scholar]
- Laue L, Chan W-C, Hsueh A, Kudo M, Hsu SY, Wu S, Blomberg L, Cutler GB. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci U S A. 1995;92:1906–1910. doi: 10.1073/pnas.92.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue L, Wu SM, Kudo M, Hsueh AJW, Cutler GB, Jelly DH, Diamond FB, Chan WY. Heterogeneity of activating mutations of the human luteinizing hormone receptor in male-limited precocious puberty. Biochem Mol Med. 1996;58:192–198. doi: 10.1006/bmme.1996.0048. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A. 1996;93:116–120. doi: 10.1073/pnas.93.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Cotecchia S, Milligan G. Up-regulation of the levels of expression and function of a constitutively active mutant of the hamster α1B-adrenoceptor by ligands that act as inverse agonists. Biochem J. 1997;325:733–739. doi: 10.1042/bj3250733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Hagen GM, Smith SM, Liu J, Barisas G, Roess DA. Constitutively-active human LH receptors are self-associated and located in rafts. Mol Cell Endocrinol. 2007;260–262:65–72. doi: 10.1016/j.mce.2005.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Fain GL. Constitutive opsin signaling: night blindness or retinal degeneration? Trends Mol Med. 2004;10:150–157. doi: 10.1016/j.molmed.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Li T, Franson WK, Gordon JW, Berson EL, Dryja TP. Constitutive activation of phototransduction by K296E opsin is not a cause of photoreceptor degeneration. Proc Natl Acad Sci U S A. 1995;92:3551–3555. doi: 10.1073/pnas.92.8.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light K, Jenkins PJ, Weber A, Perrett C, Grossman A, Pistorello M, Asa SL, Clayton RN, Clark AJ. Are activating mutations of the adrenocorticotropin receptor involved in adrenal cortical neoplasia? Life Sci. 1995;56:1523–1527. doi: 10.1016/0024-3205(95)00114-l. [DOI] [PubMed] [Google Scholar]
- Ligtenberg MJ, Siers M, Themmen AP, Hanselaar TG, Willemsen W, Brunner HG. Analysis of mutations in genes of the follicle-stimulating hormone receptor signaling pathway in ovarian granulosa cell tumors. J Clin Endocrinol Metab. 1999;84:2233–2234. doi: 10.1210/jcem.84.6.5739. [DOI] [PubMed] [Google Scholar]
- Lin Z, Shenker A, Pearlstein R. A model of the lutropin/choriogonadotropin receptor: insights into the structural and functional effects of constitutively activating mutations. Protein Eng. 1997;10:501–510. doi: 10.1093/protein/10.5.501. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu G, Xin Z, Serby MD, Zhao H, Schaefer VG, Falls HD, Kaszubska W, Collins CA, Sham HL. Novel isoxazole carboxamides as growth hormone secretagogue receptor (GHS-R) antagonists. Bioorg Med Chem Lett. 2004;14:5223–5226. doi: 10.1016/j.bmcl.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu M, Xin Z, Zhao H, Serby MD, Kosogof C, Nelson LT, Szczepankiewicz BG, Kaszubska W, Schaefer VG, Falls HD, Lin CW, Collins CA, Sham HL, Liu G. Optimization of 2,4-diaminopyrimidines as GHS-R antagonists:side chain exploration. Bioorg Med Chem Lett. 2006;16:1864–1868. doi: 10.1016/j.bmcl.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Liu G, Duranteau L, Carel J-C, Monroe J, Doyle DA, Shenker A. Leydig-cell tumors caused by an activating mutation of the gene encoding the luteinizing hormone receptor. N Engl J Med. 1999;341:1731–1736. doi: 10.1056/NEJM199912023412304. [DOI] [PubMed] [Google Scholar]
- Liu G, Fortin JP, Beinborn M, Kopin AS. Four missense mutations in the ghrelin receptor result in distinct pharmacological abnormalities. J Pharmacol Exp Ther. 2007;322:1036–1043. doi: 10.1124/jpet.107.123141. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun Y, Dong Q, He M, Cheng CH, Fan F. A novel TSHR gene mutation (Ile691Phe) in a Chinese family causing autosomal dominant non-autoimmune hyperthyroidism. J Hum Genet. 2008;53:475–478. doi: 10.1007/s10038-008-0257-3. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Hulme EC. The functional topography of transmembrane domain 3 of the m1 muscarinic acetylcholine receptor, revealed by scanning mutagenesis. J Biol Chem. 1999;274:7309–7315. doi: 10.1074/jbc.274.11.7309. [DOI] [PubMed] [Google Scholar]
- Lubrano-Berthelier C, Durand E, Dubern B, Shapiro A, Dazin P, Weill J, Ferron C, Froguel P, Vaisse C. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12:145–153. doi: 10.1093/hmg/ddg016. [DOI] [PubMed] [Google Scholar]
- Ludgate M, Gire V, Crisp M, Ajjan R, Weetman A, Ivan M, Wynford-Thomas D. Contrasting effects of activating mutations of GαS and the thyrotropin receptor on proliferation and differentiation of thyroid follicular cells. Oncogene. 1999;18:4798–4807. doi: 10.1038/sj.onc.1202864. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Gembruch U, Bauer O, Diedrich K. Ovarian hyperstimulation syndrome (OHSS) in a spontaneous pregnancy with fetal and placental triploidy:information about the general pathophysiology of OHSS. Hum Reprod. 1998;13:2082–2087. doi: 10.1093/humrep/13.8.2082. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ, Milligan G. Inverse agonist-induced up-regulation of the human β2-adrenoceptor in transfected neuroblastoma X glioma hybrid cells. Mol Pharmacol. 1996;50:1479–1786. [PubMed] [Google Scholar]
- Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, Fleck BA, Brown BT, Marks DL. The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology. 2005;146:2766–2773. doi: 10.1210/en.2005-0142. [DOI] [PubMed] [Google Scholar]
- Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144:1513–1523. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- Martin MM, Wu SM, Martin AL, Rennert OM, Chan WY. Testicular seminoma in a patient with a constitutively activating mutation of the luteinizing hormone/chorionic gonadotropin receptor. Eur J Endocrinol. 1998;139:101–106. doi: 10.1530/eje.0.1390101. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Martin B, Luttrell LM. The origins of diversity and specificity in G protein-coupled receptor signaling. J Pharmacol Exp Ther. 2005;314:485–494. doi: 10.1124/jpet.105.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Santisteban P. Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur J Endocrinol. 2000;143:161–178. doi: 10.1530/eje.0.1430161. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bond RA. Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci. 1997;18:468–474. doi: 10.1016/s0165-6147(97)01139-5. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bond RA, Lee M. Inverse agonism: pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol Sci. 1995;16:10–13. doi: 10.1016/s0165-6147(00)88963-4. [DOI] [PubMed] [Google Scholar]
- Min L, Ascoli M. Effect of activating and inactivating mutations on the phosphorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol Endocrinol. 2000;14:1797–1810. doi: 10.1210/mend.14.11.0555. [DOI] [PubMed] [Google Scholar]
- Min L, Galet C, Ascoli M. The association of arrestin-3 with the human lutropin/choriogonadotropin receptor depends mostly on receptor activation rather than on receptor phosphorylation. J Biol Chem. 2002;277:702–710. doi: 10.1074/jbc.M106082200. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanelli L, Delbaere A, Di Carlo C, Nappi C, Smits G, Vassart G, Costagliola S. A mutation in the follicle-stimulating hormone receptor as a cause of familial spontaneous ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 2004a;89:1255–1258. [PubMed] [Google Scholar]
- Montanelli L, Van Durme JJ, Smits G, Bonomi M, Rodien P, Devor EJ, Moffat-Wilson K, Pardo L, Vassart G, Costagliola S. Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol. 2004b;18:2061–2073. doi: 10.1210/me.2004-0036. [DOI] [PubMed] [Google Scholar]
- Moulin A, Demange L, Berge G, Gagne D, Ryan J, Mousseaux D, Heitz A, Perrissoud D, Locatelli V, Torsello A, Galleyrand JC, Fehrentz JA, Martinez J. Toward potent ghrelin receptor ligands based on trisubstituted 1,2,4-triazole structure. 2.Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem. 2007;50:5790–5806. doi: 10.1021/jm0704550. [DOI] [PubMed] [Google Scholar]
- Moulin A, Demange L, Ryan J, M'Kadmi C, Galleyrand JC, Martinez J, Fehrentz JA. Trisubstituted 1,2,4-triazoles as ligands for the ghrelin receptor: on the significance of the orientation and substitution at position 3. Bioorg Med Chem Lett. 2008a;18:164–168. doi: 10.1016/j.bmcl.2007.10.113. [DOI] [PubMed] [Google Scholar]
- Moulin A, Demange L, Ryan J, Mousseaux D, Sanchez P, Berge G, Gagne D, Perrissoud D, Locatelli V, Torsello A, Galleyrand JC, Fehrentz JA, Martinez J. New trisubstituted 1,2,4-triazole derivatives as potent ghrelin receptor antagonists. 3. Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem. 2008b;51:689–693. doi: 10.1021/jm701292s. [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Kudo M, Kobilka B, Hsueh AJ. Activation of the luteinizing hormone receptor following substitution of Ser-277 with selective hydrophobic residues in the ectodomain hinge region. J Biol Chem. 2000;275:30264–30271. doi: 10.1074/jbc.M005568200. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317:771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Garner KM, VanRozen RJ, Adan RA. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem. 2003;278:22939–22945. doi: 10.1074/jbc.M211326200. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Fukata S, Hishinuma A, Kudo T, Ohye H, Ito M, Kubota S, Amino N, Kuma K, Miyauchi A. Sporadic congenital hyperthyroidism due to a germline mutation in the thyrotropin receptor gene (Leu 512 Gln) in a Japanese patient. Endocr J. 2006;53:735–740. doi: 10.1507/endocrj.k06-090. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Nagayama Y, Amino N, Hishinuma A, Takano T, Yoshida H, Kubota S, Fukata S, Kuma K, Miyauchi A. A novel thyrotropin receptor germline mutation (Asp617Tyr) causing hereditary hyperthyroidism. Endocr J. 2007;54:927–934. doi: 10.1507/endocrj.k07-088. [DOI] [PubMed] [Google Scholar]
- Nogueira CR, Kopp P, Arseven OK, Santos CL, Jameson JL, Medeiros-Neto G. Thyrotropin receptor mutations in hyperfunctioning thyroid adenomas from Brazil. Thyroid. 1999;9:1063–1068. doi: 10.1089/thy.1999.9.1063. [DOI] [PubMed] [Google Scholar]
- Ono N, Nakashima K, Schipani E, Hayata T, Ezura Y, Soma K, Kronenberg HM, Noda M. Constitutively active parathyroid hormone receptor signaling in cells in osteoblastic lineage suppresses mechanical unloading-induced bone resorption. J Biol Chem. 2007;282:25509–25516. doi: 10.1074/jbc.M610782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton MP, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest. 2006;116:760–768. doi: 10.1172/JCI25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Cochauz P, Gervy C, Mockel J, Dumont J, Vassart G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Hermans J, Rocmans P, Van Vliet G, Costagliola S, Rodien P, Dumont JE, Vassart G. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thyroid adenomas. J Clin Endocrinol Metab. 1997;82:2695–2701. doi: 10.1210/jcem.82.8.4144. [DOI] [PubMed] [Google Scholar]
- Parma J, Van Sande J, Swillen S, Tonacchera M, Dumont J, Vassart G. Somatic mutations causing constitutive activity of the thyrotropin receptor are the major cause of hyperfunctioning thyroid adenomas: identification of additional mutations activating both the cyclic adenosine 3',5'-monophosphate and inositol phosphate-Ca2+ cascades. Mol Endocrinol. 1995;9:725–733. doi: 10.1210/mend.9.6.8592518. [DOI] [PubMed] [Google Scholar]
- Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- Paschke R, Tonacchera M, Van Sande J, Parma J, Vassart G. Identification and functional characterization of two new somatic mutations causing constitutive activation of the thyrotropin receptor in hyperfunctioning autonomous adenomas of the thyroid. J Clin Endocrinol Metab. 1994;79:1785–1789. doi: 10.1210/jcem.79.6.7989485. [DOI] [PubMed] [Google Scholar]
- Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng V, Chan WW, Butler B, Hickey G, Jacks T, Schleim K, Pong SS, Chaung LYP, Chen HY, Frazier E, Leung KH, Chiu SHL, Smith RG. Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92:7001–7005. doi: 10.1073/pnas.92.15.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G, Samama P, Lohse M, Wang M, Codina J, Lefkowitz RJ. A constitutively active mutant β2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc Natl Acad Sci U S A. 1994;91:2699–2702. doi: 10.1073/pnas.91.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persani L, Lania A, Alberti L, Romoli R, Mantovani G, Filetti S, Spada A, Conti M. Induction of specific phosphodiesterase isoforms by constitutive activation of the cAMP pathway in autonomous thyroid adenomas. J Clin Endocrinol Metab. 2000;85:2872–2878. doi: 10.1210/jcem.85.8.6712. [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, Sleeman MW, Tschop MH. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- Porcellini A, Ciullo I, Laviola L, Amabile G, Fenzi G, Avvedimento VE. Novel mutations of thyrotropin receptor gene in thyroid hyperfunctioning adenomas.Rapid identification by fine needle aspiration biopsy. J Clin Endocrinol Metab. 1994;79:657–661. doi: 10.1210/jcem.79.2.8045989. [DOI] [PubMed] [Google Scholar]
- Porcellini A, Ruggiano G, Pannain S, Ciullo I, Amabile G, Fenzi G, Avvedimento EV. Mutations of thyrotropin receptor isolated from thyroid autonomous functioning adenomas confer TSH-independent growth to thyroid cells. Oncogene. 1997;15:781–789. doi: 10.1038/sj.onc.1201240. [DOI] [PubMed] [Google Scholar]
- Ramon E, del Valle LJ, Garriga P. Unusual thermal and conformational properties of the rhodopsin congenital night blindness mutant Thr-94 ⤍ Ile. J Biol Chem. 2003;278:6427–6432. doi: 10.1074/jbc.M210929200. [DOI] [PubMed] [Google Scholar]
- Rao VR, Cohen GB, Oprian DD. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994;367:639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- Rao VR, Oprian DD. Activating mutations of rhodopsin and other G protein-coupled receptors. Ann Rev Biophy Biomol Struct. 1996;25:287–314. doi: 10.1146/annurev.bb.25.060196.001443. [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Dumont J, Vassart G. Thyroid disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 4029–4075. [Google Scholar]
- Ren Q, Kurose H, Lefkowitz RJ, Cotecchia S. Constitutively active mutants of the β2-adrenergic receptor. J Biol Chem. 1993;268:16483–16487. [PubMed] [Google Scholar]
- Richter-Unruh A, Wessels HT, Menken U, Bergmann M, Schmittmann-Ohters K, Schaper J, Tappeser S, Hauffa BP. Male LH-independent sexual precocity in a 3.5-year-old boy caused by a somatic activating mutation of the LH receptor in a Leydig cell tumor. J Clin Endocrinol Metab. 2002;87:1052–1056. doi: 10.1210/jcem.87.3.8294. [DOI] [PubMed] [Google Scholar]
- Rim J, Oprian DD. Constitutive activation of opsin: interaction of mutants with rhodopsin kinase and arrestin. Biochemistry. 1995;34:11938–11945. doi: 10.1021/bi00037a035. [DOI] [PubMed] [Google Scholar]
- Ringkananont U, Van Durme J, Montanelli L, Ugrasbul F, Yu YM, Weiss RE, Refetoff S, Grasberger H. Repulsive separation of the cytoplasmic ends of transmembrane helices 3 and 6 is linked to receptor activation in a novel thyrotropin receptor mutant (M626I) Mol Endocrinol. 2006;20:893–903. doi: 10.1210/me.2005-0339. [DOI] [PubMed] [Google Scholar]
- Rivas M, Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol. 2003;213:31–45. doi: 10.1016/j.mce.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Robinson PR, Buczylko J, Ohguro H, Palczewski K. Opsins with mutations at the site of chromophore attachment constitutively activate transducin but are not phosphorylated by rhodopsin kinase. Proc Natl Acad Sci U S A. 1994;91:5411–5415. doi: 10.1073/pnas.91.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9:719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- Rodien P, Bremont C, Sanson ML, Parma J, Van Sande J, Costagliola S, Luton JP, Vassart G, Duprez L. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823–1826. doi: 10.1056/NEJM199812173392505. [DOI] [PubMed] [Google Scholar]
- Roess DA, Horvat RD, Munnelly H, Barisas BG. Luteinizing hormone receptors are self-associated in the plasma membrane. Endocrinology. 2000;141:4518–4523. doi: 10.1210/endo.141.12.7802. [DOI] [PubMed] [Google Scholar]
- Rong R, Tao YX, Cheung BM, Xu A, Cheung GC, Lam KS. Identification and functional characterization of three novel human melanocortin-4 receptor gene variants in an obese Chinese population. Clin Endocrinol. 2006;65:198–205. doi: 10.1111/j.1365-2265.2006.02573.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–1673. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- Rosenthal IM, Refetoff S, Rich B, Barnes RB, Sunthornthepvarakul T, Parma J, Rosenfield RL. Response to challenge with gonadotropin-releasing hormone agonist in a mother and her two sons with a constitutively activating mutation of the luteinizing hormone receptor-A clinical research center study. J Clin Endocrinol Metab. 1996;81:3802–3806. doi: 10.1210/jcem.81.10.8855841. [DOI] [PubMed] [Google Scholar]
- Rudolph J, Esler WP, O'Connor S, Coish PD, Wickens PL, Brands M, Bierer DE, Bloomquist BT, Bondar G, Chen L, Chuang CY, Claus TH, Fathi Z, Fu W, Khire UR, Kristie JA, Liu XG, Lowe DB, McClure AC, Michels M, Ortiz AA, Ramsden PD, Schoenleber RW, Shelekhin TE, Vakalopoulos A, Tang W, Wang L, Yi L, Gardell SJ, Livingston JN, Sweet LJ, Bullock WH. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem. 2007;50:5202–5216. doi: 10.1021/jm070071+. [DOI] [PubMed] [Google Scholar]
- Russo D, Arturi F, Schlumberger M, Caillou B, Monier R, Filetti S, Suarez HG. Activating mutations of the TSH receptor in differentiated thyroid carcinomas. Oncogene. 1995;11:1907–1911. [PubMed] [Google Scholar]
- Russo D, Arturi F, Suarez HG, Schlumberger M, Du Villard JA, Crocetti U, Filetti S. Thyrotropin receptor gene alterations in thyroid hyperfunctioning adenomas. J Clin Endocrinol Metab. 1996;81:1548–1551. doi: 10.1210/jcem.81.4.8636365. [DOI] [PubMed] [Google Scholar]
- Russo D, Tumino S, Arturi F, Vigneri P, Grasso G, Pontecorvi A, Filetti S, Belfiore A. Detection of an activating mutation of the thyrotropin receptor in a case of an autonomously hyperfunctioning thyroid insular carcinoma. J Clin Endocrinol Metab. 1997;82:735–738. doi: 10.1210/jcem.82.3.3838. [DOI] [PubMed] [Google Scholar]
- Russo D, Wong MG, Costante G, Chiefari E, Treseler PA, Arturi F, Filetti S, Clark OH. A Val 677 activating mutation of the thyrotropin receptor in a Hurthle cell thyroid carcinoma associated with thyrotoxicosis. Thyroid. 1999;9:13–17. doi: 10.1089/thy.1999.9.13. [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor: extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Sangkuhl K, Schulz A, Schultz G, Schoneberg T. Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J Biol Chem. 2002;277:47748–47755. doi: 10.1074/jbc.M203491200. [DOI] [PubMed] [Google Scholar]
- Schaison G, Young J, Pholsena M, Nahoul K, Couzinet B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1993;77:1545–1549. doi: 10.1210/jcem.77.6.8263139. [DOI] [PubMed] [Google Scholar]
- Schipani E, Jensen GS, Pincus J, Nissenson RA, Gardella TJ, Juppner H. Constitutive activation of the cyclic adenosine 3',5'-monophosphate signaling pathway by parathyroid hormone (PTH)/PTH-related peptide receptors mutated at the two loci for Jansen's metaphyseal chondrodysplasia. Mol Endocrinol. 1997a;11:851–858. doi: 10.1210/mend.11.7.9934. [DOI] [PubMed] [Google Scholar]
- Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- Schipani E, Langman C, Hunzelman J, Le Merrer M, Loke KY, Dillon MJ, Silve C, Juppner H. A novel parathyroid hormone (PTH)/PTH-related peptide receptor mutation in Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 1999;84:3052–3057. doi: 10.1210/jcem.84.9.6000. [DOI] [PubMed] [Google Scholar]
- Schipani E, Langman CB, Parfitt AM, Jensen GS, Kikuchi S, Kooh SW, Cole WG, Juppner H. Constitutively activated receptors for parathyroid hormone and 87parathyroid hormone-related peptide in Jansen's metaphyseal chondrodysplasia. N Engl J Med. 1996;335:708–714. doi: 10.1056/NEJM199609053351004. [DOI] [PubMed] [Google Scholar]
- Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci U S A. 1997b;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneberg T, Schulz A, Biebermann H, Hermsdorf T, Rompler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacology and Therapeutics. 2004;104:173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schoneberg T, Schulz A, Gudermann T. The structural basis of G-protein-coupled receptor function and dysfunction in human diseases. Rev Physiol Biochem Pharmacol. 2002;144:143–227. [PubMed] [Google Scholar]
- Schwab KO, Gerlich M, Broecker M, Sohlemann P, Derwahl M, Lohse MJ. Constitutively active germline mutation of the thyrotropin receptor gene as a cause of congenital hyperthyroidism. J Pediatr. 1997;131:899–904. doi: 10.1016/s0022-3476(97)70040-4. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors:cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Serby MD, Zhao H, Szczepankiewicz BG, Kosogof C, Xin Z, Liu B, Liu M, Nelson LT, Kaszubska W, Falls HD, Schaefer V, Bush EN, Shapiro R, Droz BA, Knourek-Segel VE, Fey TA, Brune ME, Beno DW, Turner TM, Collins CA, Jacobson PB, Sham HL, Liu G. 2,4-diaminopyrimidine derivatives as potent growth hormone secretagogue receptor antagonists. J Med Chem. 2006;49:2568–2578. doi: 10.1021/jm0510934. [DOI] [PubMed] [Google Scholar]
- Shenker A. G protein-coupled receptor structure and function: the impact of disease-causing mutations. Bailliere's Clin Endocrinol Metab. 1995;9:427–451. doi: 10.1016/s0950-351x(95)80519-2. [DOI] [PubMed] [Google Scholar]
- Shenker A. Disorders caused by mutations of the lutropin/choriogonadotropin receptor gene. In: Spiegel AM, editor. G Proteins and Disease. Totowa: Humana Press; 1998. pp. 139–152. [Google Scholar]
- Shenker A. Activating mutations of the lutropin choriogonadotropin receptor in precocious puberty. Receptors Channels. 2002;8:3–18. [PubMed] [Google Scholar]
- Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, Minegishi T, Cutler GB., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- Shinozaki H, Fanelli F, Liu X, Jaquette J, Nakamura K, Segaloff DL. Pleiotropic effects of substitutions of a highly conserved leucine in transmembrane helix III of the human lutropin/choriogonadotropin receptor with respect to constitutive activation and hormone responsiveness. Mol Endocrinol. 2001;15:972–984. doi: 10.1210/mend.15.6.0661. [DOI] [PubMed] [Google Scholar]
- Shinozaki H, Butnev V, Tao YX, Ang KL, Conti M, Segaloff DL. Desensitization of Gs-coupled receptor signaling by constitutively active mutants of the human lutropin/choriogonadotropin receptor. J Clin Endocrinol Metab. 2003;88:1194–1204. doi: 10.1210/jc.2002-021051. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Richards JE, Naarendorp F, Bingham EL, Scott K, Alpern M. Dark-light: model for nightblindness from the human rhodopsin Gly-90⤍Asp mutation. Proc Natl Acad Sci U S A. 1995;92:880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor:biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Leurs R, Alewijnse AE, Blauw J, Van Nieuw Amerongen GP, Van De Vrede Y, Roovers E, Timmerman H. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U S A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med. 2003;349:760–766. doi: 10.1056/NEJMoa030064. [DOI] [PubMed] [Google Scholar]
- Soegiarto DW, Kiachopoulos S, Schipani E, Juppner H, Erben RG, Lanske B. Partial rescue of PTH/PTHrP receptor knockout mice by targeted expression of the Jansen transgene. Endocrinology. 2001;142:5303–5310. doi: 10.1210/endo.142.12.8553. [DOI] [PubMed] [Google Scholar]
- Spambalg D, Sharifi N, Elisei R, Gross JL, Medeiros-Neto G, Fagin JA. Structural studies of the thyrotropin receptor and Gs alpha in human thyroid cancers: low prevalence of mutations predicts infrequent involvement in malignant transformation. J Clin Endocrinol Metab. 1996;81:3898–3901. doi: 10.1210/jcem.81.11.8923835. [DOI] [PubMed] [Google Scholar]
- Spiegel AM. Defects in G protein-coupled signal transduction in human disease. Annu Rev Physiol. 1996;58:143–170. doi: 10.1146/annurev.ph.58.030196.001043. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein-coupled receptors. Ann Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Weinstein LS, Shenker A. Abnormalities in G protein-coupled signal transduction pathways in human disease. J Clin Invest. 1993;92:1119–1125. doi: 10.1172/JCI116680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougneres P, Froguel P, Meyre D. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet. 2007;16:1837–1844. doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Scott KM, Falls HF, Richards JE, Sieving PA. A novel rhodopsin mutation at the retinal binding site (Lys296-Met) in ADRP. Invest Ophthalmol Vis Sci. 1993;34:1149. [Google Scholar]
- Takeshita A, Nagayama Y, Yokoyama N, Ishikawa N, Ito K, Yamashita T, Obara T, Murakami Y, Kuma K, Takamatsu J, Ohsawa N, Nagataki S. Rarity of oncogenic mutations in the thyrotropin receptor of autonomously functioning thyroid nodules in Japan. J Clin Endocrinol Metab. 1995;80:2607–2611. doi: 10.1210/jcem.80.9.7673402. [DOI] [PubMed] [Google Scholar]
- Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. 2005;239:1–14. doi: 10.1016/j.mce.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Tao YX. Inactivating mutations of G protein-coupled receptors and diseases: Structure-function insights and therapeutic implications. Pharmacol Ther. 2006;111:949–973. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Tao YX. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim Biophys Acta. 2007;1772:1167–1174. doi: 10.1016/j.bbadis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Tao YX, Abell AN, Liu X, Nakamura K, Segaloff DL. Constitutive activation of G protein-coupled receptors as a result of selective substitution of a conserved leucine residue in transmembrane helix III. Mol Endocrinol. 2000;14:1272–1282. doi: 10.1210/mend.14.8.0503. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J Biol Chem. 2004;279:5904–5914. doi: 10.1074/jbc.M311162200. [DOI] [PubMed] [Google Scholar]
- Tao YX, Mizrachi D, Segaloff DL. Chimeras of the rat and human FSH receptors (FSHRs) identify residues that permit or suppress transmembrane 6 mutation-induced constitutive activation of the FSHR via rearrangements of hydrophobic interactions between helices 6 and 7. Mol Endocrinol. 2002;16:1881–1892. doi: 10.1210/me.2001-0199. [DOI] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144:4544–4551. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89:3936–3942. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- Tao YX, Segaloff DL. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab. 2005;90:5632–5638. doi: 10.1210/jc.2005-0519. [DOI] [PubMed] [Google Scholar]
- Tassi V, Di Cerbo A, Porcellini A, Papini E, Cisternino C, Crescenzi A, Scillitani A, Pizzuti A, Ratti A, Trischitta V, Avvedimento VE, Fenzi G, De Filippis V. Screening of thyrotropin receptor mutations by fine-needle aspiration biopsy in autonomous functioning thyroid nodules in multinodular goiters. Thyroid. 1999;9:353–357. doi: 10.1089/thy.1999.9.353. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Caron MG. High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem. 1994;269:27925–27931. [PubMed] [Google Scholar]
- Tonacchera M, Agretti P, Rosellini V, Ceccarini G, Perri A, Zampolli M, Longhi R, Larizza D, Pinchera A, Vitti P, Chiovato L. Sporadic nonautoimmune congenital hyperthyroidism due to a strong activating mutation of the thyrotropin receptor gene. Thyroid. 2000;10:859–863. doi: 10.1089/thy.2000.10.859. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Chiovato L, Pinchera A, Agretti P, Fiore Eq, Cetani F, Rocchi R, Viacava P, Miccoli P, Vitti P. Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. J Clin Endocrinol Metab. 1998;83:492–498. doi: 10.1210/jcem.83.2.4559. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Van Sande J, Cetani F, Swillens S, Schvartz C, Winiszewski P, Dumont JE, Vassart G, Parma J. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. J Clin Endocrinol Metab. 1996;81:547–554. doi: 10.1210/jcem.81.2.8636266. [DOI] [PubMed] [Google Scholar]
- Trulzsc B, Krohn K, Wonerow P, Chey S, Holzapfel HP, Ackermann F, Fuhrer D, Paschke R. Detection of thyroid-stimulating hormone receptor and Gsα mutations: in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. J Mol Med. 2001;78:684–691. doi: 10.1007/s001090000170. [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli-Jaakola K, Lipsanen-Nyman M, Oksanen L, Hollenberg AN, Kontula K, Bjorbaek C, Schalin-Jantti C. Identification and characterization of melanocortin-4 receptor gene mutations in morbidly obese Finnish children and adults. J Clin Endocrinol Metab. 2004;89:940–945. doi: 10.1210/jc.2003-031182. [DOI] [PubMed] [Google Scholar]
- Van Sande J, Parma J, Tonacchera M, Swillens S, Dumont J, Vassart G. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab. 1995;80:2577–2585. doi: 10.1210/jcem.80.9.7673398. [DOI] [PubMed] [Google Scholar]
- VanLeeuwen D, Steffey ME, Donahue C, Ho G, MacKenzie RG. Cell surface expression of the melanocortin-4 receptor is dependent on a C-terminal di-isoleucine sequence at codons 316/317. J Biol Chem. 2003;278:15935–15940. doi: 10.1074/jbc.M211546200. [DOI] [PubMed] [Google Scholar]
- Vasseur C, Rodien P, Beau I, Desroches A, Gerard C, de Poncheville L, Chaplot S, Savagner F, Croue A, Mathieu E, Lahlou N, Descamps P, Misrahi M. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med. 2003;349:753–759. doi: 10.1056/NEJMoa030065. [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-[2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Geller F, Dempfle A, Schauble N, Friedel S, Lichtner P, Fontenla-Horro F, Wudy S, Hagemann S, Gortner L, Huse K, Remschmidt H, Bettecken T, Meitinger T, Schafer H, Hebebrand J, Hinney A. Ghrelin receptor gene:identification of several sequence variants in extremely obese children and adolescents,healthy normal-weight and underweight students, and children with short normal stature. J Clin Endocrinol Metab. 2004;89:157–162. doi: 10.1210/jc.2003-031395. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008 doi: 10.1038/nature07101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SG, Radford AD, Kipar A, Ibarrola P, Blackwood L. Somatic mutations of the thyroid-stimulating hormone receptor gene in feline hyperthyroidism:parallels with human hyperthyroidism. J Endocrinol. 2005;186:523–537. doi: 10.1677/joe.1.06277. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Morgan PH, Lutz MW, Kenakin TP. The cubic ternary complex receptor-occupancy model. III. resurrecting efficacy. J Theor Biol. 1996;181:381–397. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Gerber BO, Meng EC, Baranski TJ, von Zastrow M, Bourne HR. Constitutive activation and endocytosis of the complement factor 5a receptor:evidence for multiple activated conformations of a G protein-coupled receptor. Traffic. 2002;3:866–877. doi: 10.1034/j.1600-0854.2002.31203.x. [DOI] [PubMed] [Google Scholar]
- Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- Wonerow P, Chey S, Fuhrer D, Holzapfel HP, Paschke R. Functional characterization of five constitutively activating thyrotrophin receptor mutations. Clin Endocrinol (Oxf) 2000;53:461–468. doi: 10.1046/j.1365-2265.2000.01119.x. [DOI] [PubMed] [Google Scholar]
- Wonerow P, Schoneberg T, Schultz G, Gudermann T, Paschke R. Deletions in the third intracellular loop of the thyrotropin receptor. A new mechanism for constitutive activation. J Biol Chem. 1998;273:7900–7905. doi: 10.1074/jbc.273.14.7900. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Leschek EW, Brain C, Chan WY. A novel luteinizing hormone receptor mutation in a patient with familial male-limited precocious puberty: effect of the size of a critical amino acid on receptor activity. Mol Genet Metab. 1999;66:68–73. doi: 10.1006/mgme.1998.2780. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry. 2006;45:7277–7288. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- Xin Z, Serby MD, Zhao H, Kosogof C, Szczepankiewicz BG, Liu M, Liu B, Hutchins CW, Sarris KA, Hoff ED, Falls HD, Lin CW, Ogiela CA, Collins CA, Brune ME, Bush EN, Droz BA, Fey TA, Knourek-Segel VE, Shapiro R, Jacobson PB, Beno DW, Turner TM, Sham HL, Liu G. Discovery and pharmacological evaluation of growth hormone secretagogue receptor antagonists. J Med Chem. 2006;49:4459–4469. doi: 10.1021/jm060461g. [DOI] [PubMed] [Google Scholar]
- Xin Z, Zhao H, Serby MD, Liu B, Schaefer VG, Falls DH, Kaszubska W, Colins CA, Sham HL, Liu G. Synthesis and structure-activity relationships of isoxazole carboxamides as growth hormone secretagogue receptor antagonists. Bioorg Med Chem Lett. 2005;15:1201–1204. doi: 10.1016/j.bmcl.2004.11.075. [DOI] [PubMed] [Google Scholar]
- Yang T, Snider BB, Oprian DD. Synthesis and characterization of a novel retinylamine analog inhibitor of constitutively active rhodopsin mutants found in patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1997;94:13559–13564. doi: 10.1073/pnas.94.25.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Hidaka A, Saji M, Polymeropoulos MH, Okuno A, Kohn LD, Cutler GB. A sporadic case of male-limited precocious puberty has the same consitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J Clin Endocrinol Metab. 1994;79:1818–1823. doi: 10.1210/jcem.79.6.7527413. [DOI] [PubMed] [Google Scholar]
- Yano K, Kohn LD, Saji M, Kataoka N, Okuno A, Cutler GB., Jr A case of male-limited precocious puberty caused by a point mutation in the second transmembrane domain of the luteinizing hormone choriogonadotropin receptor gene. Biochem Biophys Res Commun. 1996;220:1036–1042. doi: 10.1006/bbrc.1996.0528. [DOI] [PubMed] [Google Scholar]
- Yano K, Saji M, Hidaka A, Moriya N, Okuno A, Kohn LD, Cutler GB., Jr A new constitutively activating point mutation in the luteinizing hormone/choriogonadotropin receptor gene in cases of male-limited precocious puberty. J Clin Endocrinol Metab. 1995;80:1162–1168. doi: 10.1210/jcem.80.4.7714085. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- Young EH, Wareham NJ, Farooqi S, Hinney A, Hebebrand J, Scherag A, O'Rahilly S, Barroso I, Sandhu MS. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. Int J Obes (Lond) 2007;31:1437–1441. doi: 10.1038/sj.ijo.0803609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mizrachi D, Fanelli F, Segaloff DL. The formation of a salt bridge between helices 3 and 6 is responsible for the constitutive activity and lack of hormone responsiveness of the naturally occurring L457R mutation of the human lutropin receptor. J Biol Chem. 2005;280:26169–26176. doi: 10.1074/jbc.M502102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Tao YX, Ryan GL, Feng X, Fanelli F, Segaloff DL. Intrinsic differences in the response of the human lutropin receptor versus the human follitropin receptor to activating mutations. J Biol Chem. 2007;282:25527–25539. doi: 10.1074/jbc.M703500200. [DOI] [PubMed] [Google Scholar]
- Zhao H, Xin Z, Liu G, Schaefer VG, Falls HD, Kaszubska W, Collins CA, Sham HL. Discovery of tetralin carboxamide growth hormone secretagogue receptor antagonists via scaffold manipulation. J Med Chem. 2004;47:6655–6657. doi: 10.1021/jm0491750. [DOI] [PubMed] [Google Scholar]
- Zhao H, Xin Z, Patel JR, Nelson LT, Liu B, Szczepankiewicz BG, Schaefer VG, Falls HD, Kaszubska W, Collins CA, Sham HL, Liu G. Structure-activity relationship studies on tetralin carboxamide growth hormone secretagogue receptor antagonists. Bioorg Med Chem Lett. 2005;15:1825–1828. doi: 10.1016/j.bmcl.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Zhao XM, Hauache O, Goldsmith PK, Collins R, Spiegel AM. A missense mutation in the seventh transmembrane domain constitutively activates the human Ca2+ receptor. FEBS Lett. 1999;448:180–184. doi: 10.1016/s0014-5793(99)00368-3. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvyaga TA, Fahmy K, Sakmar TP. Characterization of rhodopsin-transducin interaction: a mutant rhodopsin photoproduct with a protonated Schiff base activates transducin. Biochemistry. 1994;33:9753–9761. doi: 10.1021/bi00198a046. [DOI] [PubMed] [Google Scholar]