Abstract
Given the predicted increase in prevalence of Alzheimer’s disease (AD) in coming decades, early detection and intervention in persons with the pre-dementia condition known as Mild Cognitive Impairment (MCI) is of paramount importance. Recent years have seen remarkable advances in the application of neuroimaging and other biomarkers to the study of MCI. The article reviews the most recent developments in the use of MRI to characterize brain changes and to prognosticate clinical outcome of MCI patients. The review begins with description of methods and findings in structural MRI research, delineating findings regarding both gross atrophy and microstructural brain changes in MCI. Second, we describe the most recent findings regarding brain function in MCI, enumerating findings from functional MRI and brain perfusion studies. Third, we will make recommendations regarding the current clinical use of MRI in identification of MCI.As a conclusion, we will look to the future of neuroimaging as a tool in early AD detection.
Keywords: Alzheimer’s disease, Mild Cognitive Impairment, MRI
INTRODUCTION
The prevalence of Alzheimer’s disease (AD) is expected to increase rapidly in coming decades, emphasizing the importance of early detection and intervention in persons with mild memory symptoms who are at risk for progressing to AD 1. The pre-dementia condition known as Mild Cognitive Impairment (MCI) has become the focus of many studies of potential preventive strategies, as adults with MCI are at greater risk of developing AD. In order to better characterize patients with MCI, it is critical to develop tools that can be reliably used in the early detection of MCI and AD – initially for use in clinical research and ultimately for use in the clinical care of patients at risk for AD.
Recent years have seen remarkable advances in the application of neuroimaging and other biomarkers to the study of MCI. Current clinical practice guidelines, including NINCDS-ADRDA criteria, support the use of structural neuroimaging in the diagnosis of probable AD, primarily to rule out other competing causes of memory loss, such as brain neoplasm, subdural hematoma, vascular dementia, or normal pressure hydrocephalus 2, 3. However, with discovery of increasingly sensitive and specific diagnostic imaging biomarkers, magnetic resonance imaging (MRI) may soon play a more central role in AD diagnosis 4. Of paramount importance, some of these biomarkers show potential for use in accurate determination of at-risk individuals, such as individuals with MCI, who are most likely to develop AD.
Copious epidemiologic5–7, neuropathologic8–10, and neuroimaging data confirm that MCI represents a transitional phase between normal aging and AD for many individuals. However, MCI is heterogeneous in terms of clinical symptomatology and the course of symptom development and progression to AD, and the following discussion of MR imaging of MCI should be interpreted with this in mind. The amnestic MCI subtype, a condition in which patients show a prominent memory deficit but intact functional abilities, is most predictive of progression to AD11. Although it is estimated that up to 80% amnestic MCI patients who present in a memory clinic setting progress to AD over six years, others remain stable in their cognitive profile and yet others recover. However, MCI patients who show in cognitive domain(s) other than memory (non-amnestic MCI) are likely to show far more variability in terms of symptomatology and progression to various forms of dementia (e.g., frontotemporal dementia, primary progressive aphasia, etc.). Of note, the majority of studies presented in this review focus exclusively on amnestic MCI; however, some studies also include non-amnestic MCI patients (see Table 2 and Table 3 for a description of MCI samples in structural MRI studies). Although there are published general criteria for MCI, variability in characterization of this construct also relates to a lack of standard operational criteria defining MCI (e.g., neuropsychological cutoff scores). Although this is a necessary constraint of the clinical MCI diagnosis given that a patient’s cognitive performance and clinical presentation must be interpreted within the context of his/her psychosocial and medical history, the lack of operational criteria also translates into variable definition of MCI across studies. Despite the above concerns regarding heterogeneity of MCI, the study of MCI, especially the amnestic subtype, has provided an invaluable inroad to the early detection of AD.
Table 2.
Cross-Sectional Volumetric MRI Studies
| MRI Method: Region of Interest | |||||
|---|---|---|---|---|---|
| Participants | MCI definition | Regions Studied | Results | Interpretation | |
| Becker et al.(2006) | •20 Controls age 70.1 (8.4) •6 MCI-A age 74.8 (7.4) •20 MCI-MD age 71.4 (10.2) •20 AD age 69.1 (9.7) |
•MCI-A: verbal AND/OR nonverbal delayed recall >1.5 SD below average for age & education •MCI-MD: “deterioration” on 1 non-memory test OR 2 abnormal tests in different cognitive domains (1 domain could be memory) |
•Hippocampus | •Total hippocampal volume: Controls >AD ‡ Controls > MCI-A † MCI-A = AD Controls = MCI-MD MCI-MD > AD † |
•MCI-A associated with hippocampal atrophy at a similar level as AD •MCI-MD not associated with hippocampal atrophy |
| Bottino et al.(2006) | •20 controls age 69.2 (4.8) •21 MCI age 69.5 (5.6) •39 AD age 73.1 (7.2) |
•Subjective complaint of cognitive decline for ≥ 6 months •MMSE ≥ 24 •No functional impairment |
•3 Mesial Temporal Lobe Structures: Hippocampus, Parahippocampal gyrus & Amygdala | •Bilateral hippocampi, parahippocampal gyri, and amygdala volumes: MCI < AD. Volume of all 3 left-sided structures: MCI < Controls. •Left hippocampal volume correctly classified 80.5% of MCI vs. Controls •Combination of right hippocampal and amygdala volume correctly classified 81.7% of MCI vs AD patients. |
•Volume of mesial temporal lobe structures is useful for discriminating between MCI vs cognitive healthy adults as well as between MCI vs AD. |
| Giesel et al.(2006) | •21 controls age 66.5 (range 65–67) •21 MCI age 66.6 (range 65–67) •10 AD age 65.1 (range 54–74) |
•Subjective memory complaint •Impaired objective memory performance |
•Temporal horn volume (THV; temporal horns of the cerebral ventricles are adjacent to the hippocampi) •Temporal horn index (THV/lateral ventricular volume) |
•Temporal horn volume and temporal horn index: Controls = MCI Controls < AD* MCI <AD* |
•Measurements of temporal horn volume show differences between AD patients and other groups •Temporal horn volume nor index discriminates between MCI and controls |
| Kantarci et al.(2002) | •61 Controls age 80.6 (7.2) •24 MCI age 82.2 (5.1) •22 AD age 79.2 (6.1) |
•Subjective memory complaint •Objective memory impairment •Normal general cognition (WAIS-R FSIQ); •No difficulty with ADL’s •CDR = 0.5 |
•Hippocampus | •Total hippocampal volume: At fixed specificity of 80%: Sensitivity to distinguish control from MCI = 79% Sensitivity to distinguish MCI from AD = 45% | •Hippocampal volume is a good measure for discriminating between healthy controls and MCI patients |
| Pennanen et al. (2004) | •59 controls age 72.7 (4.3) •65 MCI age 72.8 (4.5) •48 AD age 71.1 (8.1) |
•Subjective memory complaint by patient, family, or physician •No difficulty with ADL’s •Impaired performance on cognitive testing (≥1SD; memory OR other cognitive domain) •No dementia •CDR=0.5 |
•Hippocampus and entorhinal cortex | •Hippocampal and entorhinal volumes: control > MCI > AD •Using discriminant analysis, entorhinal volume correctly classified 65.9% of MCI vs. controls; hippocampal volume correctly classified 82.3% of MCI vs. AD |
•Entorhinal cortex volume was more accurate in discriminating controls from MCI patients, whereas hippocampal volume was more accurate for discriminating MCI from AD. |
| Phillips et al. (2004) | •15 Controls age 75.2 (5.5) •16 MCI age 75.8 (6.4) •14 AD age 70.6 (7.0) |
•Individual or family report of patient's gradual memory decline for ≥ 6 months •Objective memory impairment on testing (≥1.5 SD) •No difficulty with ADL’s •CDR = 0.5 |
•Hippocampus | •Total hippocampal volume: Controls > MCI and AD* MCI = AD •Discriminant analysis of 3 groups: delayed verbal memory significantly separated groups*;hippocampal volume did not •Discriminant analysis of MCI and AD groups only: delayed memory and hippocampal volume correctly classified 75% of MCI patients and 64% of controls |
•Together, memory performance and hippocampal volume provided relatively accurate classification of people as controls or MCI patients •Hippocampal volume did not provide significant information for separation of patient groups when controls,MCI, and AD were included |
| Singh et al. (2006) | •34 controls age 76 (7.0) •65 MCI age 75 (6.0) •42 AD age 76 (9.0) |
•Subjective memory complaint for ≥ 6 months •Impaired objective memory performance (≥1 SD) |
•Cortical Thickness | •Mean cortical thickness: Controls > MCI >AD*(with Bonferroni correction) •Largest difference among groups found in temporal lobe; least in occipital lobe |
•Cortical thickness, particularly in the inferior/ middle temporal lobes decreases systematically from controls to MCI to AD |
| Wolf et al. (2004) | •35 Controls •38 MCI • 32 Dementia Age ranged from 75–5; Age did not vary significantly across groups |
•No requirement of subjective memory complaint •Impaired performance (≥1SD) in one cognitive domain (memory or non-memory) •No dementia •CDR=0.5 |
•ROI analysis of Hippocampus •Automated segmentation of brain into Brain Volume (gray & white matter) & Intracranial Volume (brain volume & CSF) |
•All hippocampal volume measures: Controls > MCI‡Brain Volume: Control = MCI MCI > Dementia‡ At fixed specificity of 80% •Left hippocampal volume & education distinguished controls from MCI with 74% sensitivity •Brain Volume, age, & education distinguished MCI from dementia with 85%sensitivity |
•Hippocampal volume is less in MCI than control brains and is a good measure for distinguishing controls and MCI. Hippocampal volume does not distinguish MCI from dementia. •Brain volume is less in dementia than in MCI, accurately distinguishing MCI from dementia. |
| MRI Method: Voxel Based Morphometry | |||||
| Chetelat et al. (2002) | •22 controls age 66.6 (7.2) •22 MCI age 71 (8) •16 AD age 72.1 (5.8) |
•Subjective memory complaint •Objective memory impairment on testing (≥1.5 SD) •No dementia |
•Gray Matter | •Controls > MCI‡Bilateral mesial temporal lobe (hippocampus, parahippocampal gyrus, amygdala, periamygdaloid complex) and middle temporal gyrus; subcallosal anterior cingulate •MCI > AD‡parietal association cortex, superior parietal cortex, precuneus |
•MCI and controls showed brain volume differences in mesial temporal lobe and anterior cingulated •MCI and AD differences in more posterior brain regions. |
| Karas et al. (2004) | •14 controlsage 70.2 (9.8) •22 MCI age 71.4 (6.9) •33 AD age 73.9 (3.8) |
•Subjective memory complaint •Objective memory impairment (≥1.5 SD) •No non-memory cognitive impairment •No difficulty with ADL’s •No dementia •Global Deterioration Scale = 3 |
•Gray Matter |
Global gray matter: •Attenuation of global gray matter in MCI versus controls and in AD versus MCI not statistically significant Specific brain regions: •Controls > MCI‡bilateral thalamus, bilateral superior temporal cortex, left insula, bilateral hippocampus •MCI > AD‡parietal association cortex, retrosplenial cortex, left amygdala, hippocampus |
•Global gray matter measures did not accurately distinguish MCI from controls or AD •MCI and controls brain volume differences in mesial temporal lobe and other aspects of temporal cortex •MCI and AD differences in posterior cingulate, parietal association cortex & mesial temporal areas. |
| Pennanen et al. (2005) | •32 Controls Age 74 (4) •51 MCI Age 72 (5) |
•Subjective memory complaint made by patient, family, or physician about patient •Objective memory impairment on testing (>1.5) •Normal general cognition •No difficulties with ADL's •CDR = 0.5 •No dementia |
•Gray Matter | •MCI > AD‡right anterior hippocampus, amygdala, right hippocampal tail, thalamus | •Compared to controls, MCI patients show attenuated right-lateralized mesial temporal volume |
| Stoub et al. (2006) | •50 controls age 78.1 (6.0) •40 MCI age 77.9 (7.5) |
•Objective memory impairment on testing •Performance on other aspects of cognition not described •No dementia |
•White Matter (VBM) •Hippocampus and Entorhinal Cortex (ROI) |
White Matter •Controls > MCI-A‡Bilateral anterior-medial parahippocampal gyri (perforant path) •Hippocampal and parahippocampal white matter volume significantly predicted memory function* ROI analysis •Controls > MCI-A‡Hippocampus and entorhinal Cortex |
•MCI patients not only show gray matter MTL changes, but they also showed bilateral alterations in white matter that provides input to the entorhinal cortex and hippocampus |
| Trivedi et al. (2006) | •15 controls age 73.6 (7.1) •15 MCI age 73.3 |
•Subjective memory complaint; •Impaired objective memory performance (≥1.5 SD); •No difficulties with ADL's •No dementia |
•Gray Matter | •Controls > MCI†(uncorrected)Left anterior mesial temporal lobe, left fusiform gyrus, left posterior cingulate, right inferior and middle temporal gyri •Gray matter in anterior mesial temporal lobe and posterior cingulate gyrus discriminate between control and MCI (87% accuracy) |
•Compared to Controls, MCI patients gray matter attenuation in mesial and lateral temporal lobe as well as posterior cingulate |
“=” indicates no difference at p ≤ .05,
indicates significant difference at p ≤ .05,
p ≤ .01,
p ≤ .001.
Table 3.
Longitudinal Volumetric MRI Studies
| Baseline MRI; MRI Method: Region of Interest | |||||
|---|---|---|---|---|---|
| Participants | MCI definition | Regions Studied | Results | Interpretation | |
| de Toledo-Morrell et al. (2004) | •27 MCI (10 progressed to AD) Baseline Age: sMCI: 81.1 (8.1) pMCI: 82.7 (4.5) •Followed 3 yrs |
•Objective memory impairment on testing •No dementia |
•Hippocampal Formation •ERC |
•HF volume: pMCI < sMCI* ERC volume: pMCI < sMCI† •HF &ERC both predict progression to AD; ERC better predictor (overall classification accuracy: 90.6%) |
HF & ERC volumes are independent predictors of MCI progression to AD; ERC volume best differentiates progres-sive from stable MCI |
| Devanand et al. (2007) | •62 Controls (2 progressed to MCI) Baseline Age 65.6 (9.4) •139 MCI (37 progressed to AD) Baseline Age: sMCI: 64.8 (10) pMCI: 72.2 (7.1) •Followed 5 yrs (1 to 9) |
•Subjective memory complaint •Objective cognitive impairment (broadly defined) on testing •No dementia |
•Hippocampus •Parahippocampal gyrus •ERC |
•Hippocampal,parahippocampal & ERC volumes: Controls >sMCI > pMCI† •Hippocampal & ERC (with intracranial volume as a covariate) both predict progression to AD‡ •Overall classification accuracy using combined hippocampal + ERC volume: 86.7% |
•Hippocampal & ERC volumes are predictors of progression from MCI to AD |
| Jack et al. (1999) | •80 MCI (27 progressed to AD) Baseline Age 60–89 •Followed 32.6 months |
•Subjective memory complaint by patient or collateral source •Objective memory impairment on testing (≥1.5 SD) •Normal general cognition on testing •CDR = 0.5 •Normal ADL's;no dementia |
•Hippocampus | •Rate of progression from MCI to AD is greater in those patients with smaller hippocampi. | •Hippocampal volume is predictive of MCI progression to AD. |
| Killiany et al. (2002) | •24 Controls (0 progressed to MCI/AD) Baseline Age 71.8 •79 individuals with "mild memory difficulty"(19 progress to AD) Baseline Age sMCI: 71.5 pMCI: 72.8 •Followed 3 years |
•CDR = 0.5 | •ERC (ROI) •Banks of superior temporal sulcus (ROI) •3 portions of cingulate gyrus (ROI's) •6 cerebrospinal fluid spaces (automated measures) |
•Volumes of the banks of the superior temporal sulcus†and the anterior cingulate cortex†discriminated between sMCI and pMCI, with 75% overall classification accuracy. •Volumes of ERC‡& banks of the superior temporal sulcus†discriminated between Controls and pMCI, with 93% overall classification accuracy. |
•Non-mesial temporal structures (i.e., anterior cingulate) that are affected in early AD aid in the prediction of which individuals with MCI will have a faster rate of progression MCI will have a faster rate of progression. |
| Baseline MRI; MRI Method:Voxel Based Morphometry | |||||
| Bozzali et al.(2006) | •20 controls (cognition assessed once) Baseline age 65.8 (6.8) •22 MCI (14 progressed to AD) Baseline age 70.5 (10.5) •22 AD Baseline age 67.9 (7.6) •Followed 28.7 (5.7) months |
•Subjective memory complaint •CDR ≤ 0.5 •No difficulty with ADL’s •No dementia |
•Gray Matter (measured with Voxel Based Morphometry) | •pMCI < controls*corrected anterior cingulate, superior & medial frontal bilateral gyri, left insula, inferior frontal gyri, supramarginal gyrus, right superior & middle temporal gyri, left medial temporal & fusiform gyri. •pMCI <sMCI‡bilateral inferior frontal gyri, left supramarginal gyrus, right hippocampus |
•Individuals with pMCI show more decreased gray matter volume than sMCI in widespread cortical regions that show deterioration in Alzheimer's disease |
| Hämäläinen et al. (2007) | •22 controls Baseline age 72.9 (4.5) •56 MCI (13 progressed to dementia: 9 AD, 3 vascular dementia, 1 mixed dementia) Median Baseline Age sMCI: 72.7 (4.1) pMCI: 72.1 (4.2) •average follow-up for MCI: 32 (11.8) months; for controls: 48 (14) months |
•Subjective memory Complaint by patient, family or physician •Objective memory impairment on testing •Normal global cognitive function •No difficulty with ADL's •CDR = 0.5 •No dementia |
•Gray Matter (measured with Voxel Based Morphometry) | •pMCI (all dementias) < controls*correctedMTL, anterior cingulate, posterior cingulate, precuneus, temporoparietal cortex, lateral frontal and orbitofrontal cortex. •pMCI (all dementias)< sMCI*corrected posterior cingulate, precuneus, angular gyrus, left middle temporal lobe •pMCI (AD only) < sMCI*corrected posterior cingulate, precuneus, angular gyrus, left middle temporal lobe, right anterior hippocampus |
•Individuals with pMCI show more decreased gray matter volume than sMCI in widespread cortical regions that show deterioration in Alzheimer'sdisease |
| Whitwell et al. (2007) | •63 controls Median baseline age78(59–93) •63 MCI (42 progressed to AD) Median Baseline Age sMCI: 79 (range 61–97) pMCI: 79 (range 59–96) •follow-up: all pMCI patients showed progression within 18 months; sMCI patients followed over at least 3 years |
•Subjective memory complaint •Objective memory impairment on testing •Normal general cognitivefunction •No difficulty with ADL's •No dementia |
•Gray Matter (measured with Voxel Based Morphometry) •Hippocampal ROI measurement |
VBM: •sMCI = controls •pMCI < controls*corrected bilateral hippocampi, amygdalae, ERC, parahip-pocampi,fusiform gyri, lateral & anterior temporal lobes, temporoparietal cortex, basal forebrain, anterior insula, & frontal lobes. •pMCI < sMCI*correctedhippocampi, amygdalae, ERC, fusiform, lateral & anterior temporal, medial frontal lobes,basal forebrain, anterior insula, anterior & posterior cingulate, precuneus Hippocampal ROI analysis: Both sMCI & pMCI < controls‡ |
•Individuals with pMCI show more decreased gray matter volume than sMCI in widespread cortical regions that show deterioration in Alzheimer's disease |
| Baseline MRI; MRI Method: Medial Temporal Atrophy Scale | |||||
| DeCarli et al. (2007) | •187 MCI (66 progressed to dementia; 99% of these were AD) Baseline Age* sMCI: 72.3 (6.5) pMCI: 73.7 (7.0) •Followed 3 years |
•Subjective memory complaint •Objective memory impairment on testing •CDR = 0.5 •No difficulty with ADL's •No dementia |
•Medial temporal volume qualitatively assessed with use of a standardized 5-point (i.e., 0 to 5) Medial Temporal Atrophy scale (4 raters) | •A cutoff score of 2 on the MTA scale (i.e., moderately widened choroidal fissure, mildly widened width of temporal horn, mildly reduced hippocampal height) was not sensitive (14%) to progression, but was highly specific (98%) •Kappa statistics indicated moderate interrater agreement (.47 for "2" score) |
•A high MTA cutoff score in well-characterized MCI is relatively insensitive, but is highly specific to progression from MCI to AD |
| Korf et al. (2004) | •75 MCI (37 progressed to dementia; 92% possible/probable AD) Baseline Age sMCI: 60.6 (10.3) pMCI: 65.2 (7.4) •Followed 34 (20.8) mos |
•Objective cognitive impairment (memory or other domain) •CDR = 0.5 •No impairment in social or occupational function •No dementia |
•MTA score (1 rater) | •Higher MTA score associated with greater risk of progression •Predictive value of MTA was over and beyond that of other variables (e.g., age, memory recall, white matter hyperintensities) •Using a dichotomized MTA score (i.e., no atrophy (0–2) vs atrophy (3–5), overall classification accuracy: 69% |
•MTA score was predictive of progression from MCI to dementia, independent of other clinical variables |
| Visser et al.(2006) | •30 individuals with "minor cognitive impairment" (7 progressed to AD) Baseline Age 64.9 (9.5) •Followed 1.9 (0.7) years |
•Global deterioration scale score of 2 or 3 •Memory impairments were very mild |
•Score on MTA scale (1 rater) •Hippocampal ROI •Parahippocampal ROI |
•Overall accuracy in predicting progression of MCI to AD when either hippocampal ROI volume or MTA was entered in predictive model with age & memory recall = 81% | •Hippocampal volume & MTA were significant predictors of progression from MCI to AD; parahippocampal volume was not |
| Atrophy on MRI over time; MRI Method: Region of Interest | |||||
| Erten-Lyons et al. (2006) | •18 controls (0 progressed) age at baseline: 82.6 (4.65) •37 MCI (23 progressed to dementia: 22 possible/probable AD, 1 vascular dementia) Baseline Age pMCI: 87.7 (5.2) sMCI: 85.7 (6.8) •Followed 7.6 (2.7) years |
•2 CDR’s of 0.5 separated by at least 6 months •No dementia |
•Measurements of baseline volume & atrophy rate ➢ whole brain ➢ ventricle ➢ hippocampus ➢ temporal horn ➢ white matter "high signal" •Baseline MRI done when CDR = 0. •In MCI, follow-up MRI done before 2nd 0.5 CDR rating |
•At Baseline: ➢ Hippocampal volume: pMCI < Controls† pMCI < sMCI† sMCI = Controls ➢ No group differences in other brain regions •Atrophy rate: ➢ Percent rate of change in whole brain, ventricles, & temporal horn volume predicted group membership. ➢Post hoc analysis: rate of temporal horn change: sMCI <pMCI* |
•Progressive MCI is associated with smaller hippocampal volume at baseline (prior to symptom onset) and higher rates of temporal horn atrophy over time when compared to Stable MCI. |
| Stoub et al.(2005) | •35 controls (3 progressed to AD) •23 MCI (11 progressed to AD) •Baseline age across control & MCI groups Participants who progressed: 80 (6.0) Stable participants:81(6.0) •Followed 5 years with annual scans |
•Objective memory impairment on testing •No impairments in other cognitive domains •No dementia |
•Hippocampus •ERC |
•Note: analyses combine controls & MCI into one group and include baseline group status as an independent variable •At Baseline: ➢ ERC volume & diagnosis of MCI were significant predictors of rogression •Atrophy rate: ➢ ERC, but not hippocampal, slope of decline significantly predicted progression |
•Baseline ERC volume and the ERC's slope of decline over 5 annual measurements was predictive of progression to AD. •Neither hippocampal baseline nor atrophy was predictive of progression |
| Atrophy on MRI over time; Inclusion of an Automated MRI Analysis | |||||
| Chetelat et al. (2005) | •18 MCI (7 progressed to AD) Baseline Age pMCI: 74.0 (4.6) sMCI: 67.1 (8.9) •Followed 18 months |
•Subjective memory complaint •Objective memory impairment on testing (≥1.5 SD) •Normal general cognition on testing •No dementia |
•Gray Matter (measured with Voxel Based Morphometry) | •Brain change over time: ➢ Both pMCI and sMCI: loss in lateral & medial temporal areas, orbitofrontal cortex, inferior parietal cortex, left thalamus ➢ MCI: greater loss in middle & inferior temporal gyri, hippocampus, parahippocampal area, fusiform gyrus, posterior cingulate, precuneus •At Baseline: (age & MMSE) ➢ pMCI < sMCI*one cluster in right posterior hippocampus, parahippocampal gyrus, fusiform gyrus |
•A fully-automated longitudinal VBM technique was sensitive gray matter loss occurring in MCI patients •Compared to nonprogressors, pMCI patients showed accelerated loss in right temporal and medial parietal regions |
| Jack et al. (2004) | •55 Controls (13 progressed to MCI; 2 to AD) Baseline Median Age (min/max) pControl: 80 (70, 89) sControl: 79 (56, 93) •41 MCI (26 progressed to AD) Baseline Median Age pMCI: 77 (64, 94) sMCI: 76 (56, 92) •64 AD Baseline Median Age slow progressors: 76 (58,86) fast progressors: 79 (52,92) •Followed 1–5 years |
•Subjective memory complaint documented by patient and
collateral source •Objective memory impairment on testing •Normal general cognition on testing •No difficulty with ADL's •No dementia |
•Measurements of baseline volume &annualized % change ➢ whole brain ➢ ventricle ➢ hippocampus ➢ ERC •Annualized % change of whole brain and ventricles measured in automated fashion with home-built software algorithm •Other measures were ROI's |
•Brain change over time: ➢ Rates of atrophy in all brain tissues measured and expansion of ventricles were greater in pMCI than sMCI; same relationship in progressing vs stable controls ➢ Whole brain measures (computed via automated methods) were as sensitive as ROI measures to differences in rate of brain change in progressors vs non-progressors |
•All four measures were sensitive to rates of brain change that were greater in pMCI than sMCI. •This finding highlights the potential utility of such automated volumetric methods in assessing risk in MCI patients. |
| Jack et al. (2005) | •91 Controls (11 progressed to MCI, 2 to AD) Age (2nd scan) 81.9 (7.5) Followed 1.4 years (range 0.9–2.0) •72 MCI (39 progressed to AD) Age (2nd scan) 80.0 (7.7) Followed 1.3 years (range 0.7–2.2) |
•Subjective memory complaint •Objective memory impairment on testing •Normal general cognition on testing •No difficulty with ADL's •No dementia |
•Measurements of baseline volume & annualized % change ➢ Whole brain ➢ Ventricle ➢ Hippocampus ➢ ERC •Annualized % change of whole brain and ventricles measured with the boundary shift integral technique •Other measures were ROI's |
•Brain change over time results: ➢ Greater hazard of progression of MCI to AD predicted by greater annualized % change of whole brain & ventricle. ➢ Greater hazard of progression from control to MCI/AD with larger ventricle annual percent change •Baseline results: ➢ Smaller hippocampal and whole brain volumes were associated with progression from MCI to AD. |
•Measures of whole brain and ventricle annual percent change, the two change measures that were calculated using an automated (BSI) method, were most predictive of progression from MCI to AD. •This finding highlights the potential utility of such automated volumetric methods in assessing risk in MCI patients. |
Ages are specified as mean group age (standard deviation) unless otherwise indicated; “=” indicates no difference at p ≤ .05,
indicates significant difference at p ≤.05,
p ≤.01,
p ≤.001.
The article reviews the most recent developments in the use of MRI to characterize brain changes in MCI and to prognosticate clinical outcome of MCI patients. Table 1 overviews the MRI terminology used in this paper. The review begins with a description of methods and findings in structural MRI research, delineating findings regarding both gross atrophy and microstructural brain changes in MCI. Second, we describe the most recent findings regarding brain function in MCI, enumerating findings from functional MRI and brain perfusion studies. Third, we will make recommendations regarding the current clinical use of MRI in identification of MCI. As a conclusion, we will look to the future of neuroimaging as a tool in early AD detection.
Table 1.
MRI Terminology Covered in Paper
| Scan Type | Indication | Measurement/Indices |
|---|---|---|
| Structural (T1-weighted) MRI | Used to index brain volume because of the clear distinction of gray/white matter boundaries | Hand-traced volume: • Region of interest Semi-Automated Techniques: • Voxel-Based Morphometry (VBM) • Boundary Shift Integral • Cortical Thickness Measures |
| Functional MRI | Used to depict regions of brain “activation” during a motor, sensory, affective, or cognitive task. | Blood Oxygen Level Dependent (BOLD) signal • Ratio of oxygenated (paramagnetic) to deoxygenated (diamagnetic) blood under specific task conditions.BOLD signal change reflects brain response to an active experimental task relative to a baseline task. |
| Diffusion MRI | Used to assess microstructural integrity of brain tissues through measurement of water diffusion in multiple directions (apparent diffusion coefficient or ADC) or preferentially in one direction (functional anisotropy or FA) | Diffusion Weighted Imaging (DWI): • Apparent Diffusion Coefficient (ADC) Diffusion Tensor Imaging (DTI): • ADC and Functional Anisotropy (FA) |
| Perfusion MRI | Used to assess blood flow. Also yields indices of blood volume and mean transit time. Most studies assess blood flow during a resting state | Dynamic Susceptibility Contrast • Signal change following injection of a paramagnetic MRI contrast agent (e.g., gadolinium) Arterial Spin Labeling • Uses magnetic labeling of protons in blood; endogenous tracer of blood flow |
WHAT DOES BRAIN STRUCTURE TELL US ABOUT MCI?
The cognitive changes that characterize MCI are thought to have their roots in an early phase of neurodegeneration due to Alzheimer's pathology. Neuropathologic studies corroborate this notion, showing that people with MCI have levels of plaques and tangles that are intermediate between those seen in cognitively-healthy older adults and those with Alzheimer's disease 10. Case-control and longitudinal studies employing a number of MR analysis techniques have substantially augmented our knowledge about volumetric brain changes that characterize MCI and predict conversion to AD.
Cross-sectional studies of Volumetric MRI: Identifying different patient groups
Cross-sectional studies have added to our knowledge about which brain structures are most vulnerable to change in MCI, which volumetric MR techniques provide the most sensitive indices of brain alterations in MCI, and how well we can accurately classify individuals into clinically-diagnosed patient groups based on various MRI measures. In the vast majority of MCI studies, a gold standard pathological diagnosis of AD is not available. Therefore the aim is to evaluate the concordance between MRI volumetric measures and clinical diagnosis. Two main MR analysis techniques employed in these studies include the region of interest (ROI) method and more automated methods such as voxel-based-morphometry (VBM).
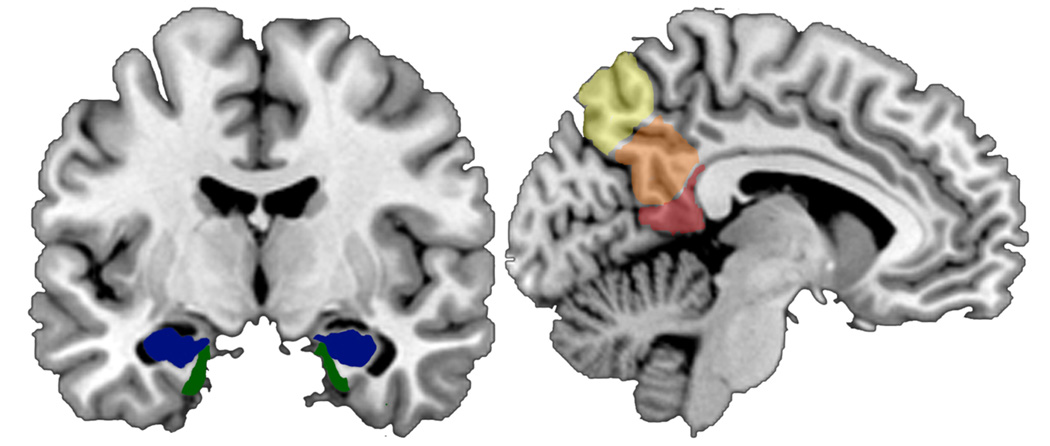
Medial temporal lobe (MTL) structures have long been known to play a critical role in memory, and some of the earliest AD-associated brain alterations gain their first foothold in this brain region 12. (see Figure 1) Therefore, many volumetric MCI studies measure hand-traced regions of interest (ROI's) of specific MTL structures; most of these focus on the hippocampus (see Table 2 for a detailed description of cross-sectional studies). The majority of published studies comparing group data indicate that MCI patients have less hippocampal volume than cognitively-healthy controls 13–19, and AD patients have less volume than those with MCI 16–18. Other studies show that entorhinal cortex (ERC) volume in MCI patients is less than that of cognitively-healthy controls. Evidence that ERC differs between individuals with MCI and AD is mixed 18,20.
Figure 1.
Brain regions that are vulnerable to structural and functional changes in Mild Cognitive Impairment and Alzheimer’s disease. A) Coronal depiction of mesial temporal structures that are critical for learning and memory. Hippocampus is blue; Entorhinal cortex is green. B) Midsagittal depiction of medial parietal regions important for memory and self-awareness. Retrosplenial Cortex is red; Ventral posterior cingulate cortex is orange; Precuneus is yellow
More automated analytic methods such as voxel-based morphometry (VBM) allow for an unbiased examination of anatomical group differences in controls, MCI, and AD across the whole brain. This statistical parametric mapping technique allows the researcher to evaluate group differences in gray matter, white matter, and CSF volume at a fine degree of spatial resolution. The advantage of whole-brain VBM is that it does not require priori assumptions about the size, location or shape of the brain region(s) of interest. Furthermore, VBM allows quantification of brain changes that are not easily apprehended by visual inspection (e.g., atrophy not fully encompassed by sulcal boundaries between structures). Results of several VBM studies corroborate the finding of statistically significant MTL volume differences between controls and MCI (see Table 2). The most common finding is that of attenuated hippocampal volume in MCI 21–24. Other regions of attenuated MTL volume in MCI have found been in the amygdala 21–23, 25 ERC 24, parahippocampal gyrus 21, and fusiform gyrus25. A VBM analysis constrained to white matter measurement showed that the MCI group had less volume in the anterior-medial aspects of both parahippocampal gyri than controls 24, 25. VBM studies comparing brain volume in individuals with MCI versus AD also find differences in the MTL 21, 22, 25.
Use of VBM has engendered more examination of non-MTL structures in MCI. Findings show regions of attenuated volume in MCI compared to controls include posterior cingulate 21, 25, and lateral temporal regions 21, 22, 25 Brain volume differences in structures outside of the MTL are more striking and consistent in comparisons of MCI and AD groups. Mild AD patients show less brain volume in medial parietal (i.e., retrosplenial cortex, posterior cingulate, and precuneus) and parietal association cortex 21, 22. AD patients also show less global cortical volume than those with MCI 16.
Several studies examine the concordance between determining diagnostic classification based on brain volume data and the clinically-determined diagnostic category of participants. Because information regarding the pathology of AD (i.e., the true "gold standard" of detecting AD-like changes in MCI) is not available without autopsy, the gold standard reference in these studies is a diagnosis of MCI based on careful clinical examination and/or neuropsychological testing. Measures of MTL volume generally yield the most promising results, but accuacy rates are highly variable across studies (see Table 2). Reports of accuracy in classifying individuals as either healthy controls and MCI using MTL volume vary widely, ranging from 66% to 83% overall accuracy 14–20,25. Both MTL and more global volumetric measures have been found to discriminate between MCI and AD, with accuracy of classification ranging from 77% to 82% 16–18.
Longitudinal studies of Volumetric MRI: Predicting progression of MCI
Not all individuals with MCI subsequently develop AD, and individuals with MCI who do develop AD vary in their rate of clinical progression 26. Therefore, several longitudinal studies have examined the accuracy with which volumetric MRI measures predict those MCI participants who will soon progress to AD (pMCI) versus those MCI participants who show functional stability over the time to follow-up (sMCI). The focus is increasingly on finding those measures that not only predict group differences, but also are sensitive and specific in their classification of individuals.
Longitudinal studies: Baseline scan as predictor of progression
Several studies have examined the accuracy of a single baseline brain MRI in predicting the conversion or stability of clinical status at longitudinal follow-up (see Table 3). Several MCI studies constraining analysis to the MTL find that lower baseline hippocampal volume is related to progression to AD, although rates of accurate prediction are not reported 27–29. Likewise, studies using VBM for whole-brain analysis also find that lower baseline hippocampal predict progression to MCI 30–33. However, others studies using manual tracing methods do not find this same association 34, 35, and report the ERC as a more sensitive predictor of progression 35–37. One study designed to directly compare baseline ERC and hippocampal volumes as predictors of progression from MCI to AD found that ERC was superior, predicting with 90.6% accuracy. Right ERC volume at baseline was the best index, predicting conversion with a concordance rate of 93.5%.
Investigations using VBM have identified areas outside the MTL in which lower baseline volume is related to later progression to MCI. The handful of studies using VBM show some consistency in finding that progressive MCI patients show less baseline volume than stable MCI patients in fusiform gyrus30, 31, medial and inferior frontal regions30, 32, and the posterior cingulate and precuneus30, 33. Significant findings in other extra-MTL brain regions have not been replicated.
Longitudinal studies: Atrophy rate as a predictor of progression
Several longitudinal studies have examined the relationship between brain atrophy rates and progression from MCI to AD (see Table 3). Although the studies reviewed in this section find significant group differences between MCI patients who do versus do not rapidly progress to AD, there is not evidence that these measures are clinically useful in predicting an individual patient’s course of disease and symptomatology. Most such studies collect MRI data and conduct coincident clinical/neuropsychological assessment at two time points spaced several years apart. Most studies show that the hippocampus shows higher annual rates of atrophy in pMCI than sMCI 28, 31, 38, 39. Estimates of annualized hippocampal atrophy in pMCI are generally about 3.7%; for sMCI estimates are about 2.5–2.8% 28, 38. However, one group finds that rate of atrophy of the ERC, but not hippocampus, is faster for individuals with pMCI 37. Evidence for accelerated MTL atrophy in pMCI than sMCI is reinforced by the finding of faster increase in temporal horn size over time 40. Furthermore, assessment of medial temporal lobe atrophy using a standardized visual scale (i.e., MTA), used in conjunction with assessment of delayed recall, aids prediction of individuals with MCI who will quickly progress to AD 41–43. Studies conducting analyses of the whole brain indicate that faster atrophy in pMCI occurs in wide-spread cortical regions 39. Specific non-MTL cortical regions that show evidence of faster atrophy in pMCI include the posterior cingulate, precuneus, and inferior and middle temporal gyri 31.
Another type of longitudinal design has been used to investigate whether brain changes prior to cognitive baseline are predictive of later cognitive decline from MCI to AD. In a study using this design, two scans were acquired over a period of 1–2 years, and baseline cognitive performance was measured at the time of the second scan 29. Results indicated that pre-cognitive baseline changes in whole brain and ventricular volume were predictive of progression from MCI to AD. Hippocampal volume on the initial scan, but not hippocampal atrophy, was also predictive.
Clinical Utility of Volumetric MRI
The last two decades have seen a surge in our knowledge regarding structural brain changes in MCI and those changes that predict progression to AD. Taken together, results of longitudinal studies indicate that hippocampal atrophy on baseline scan and/or widespread neocortical atrophy may be the best predictors of progression from MCI to AD. Given such advances in knowledge regarding brain regions most vulnerable to early AD-like changes, the research has become increasingly focused on determining the predictive value of imaging indices for the individual MCI patient. However, several issues continue to limit the use of brain volume as a clinically-viable prognostic index in MCI.
Although research shows that baseline and atrophy rates can statistically separate progressive MCI from stable MCI, too much overlap in these groups limits accurate prognosis for the individual patient. Thus, volumetric MRI is currently too variable to be used as a reliable and valid clinical measure. Furthermore, comparing results across studies is difficult due to differences in patient samples, MRI acquisition, and image processing methods. Sample size of studies also varies widely, and the small samples used in some studies limits statistical power to show true group differences (see Table 2 and Table 3 for details of the volumetric MRI studies). Despite these limitations, research on volumetric brain measures of pre-AD is becoming increasingly refined, and there is promise of providing useful information regarding risk of progression in older individuals with MCI. Currently, the clinical use of volumetric MRI measures as a measure of risk is significantly limited by the time-intensity and skill required to conduct manual ROI measurements. However, continuing development of visual rating scales of atrophy (e.g., MTA) and more automated methods of assessing brain volume (e.g., VBM) may permit faster and more reliable brain volume measurement.
The imaging methods reviewed here have been validated against a clinical diagnosis of MCI, a diagnosis typically based on the results of neuropsychological testing and medical examination. Thus, although cross-sectional volumetric MRI studies provide useful information regarding the brain correlates of cognitive dysfunction, and they do not currently propose to improve upon the clinical diagnosis of MCI. Furthermore, structural imaging currently provides little predictive value beyond that achieved by neuropsychological testing. However, much research is also focused on combining multiple potentially useful indices (e.g., CSF measures, select neuropsychological indices, various imaging modalities) to improve evaluation of prognosis in MCI 44.
Diffusion MRI: Detecting differences among patient groups
MRI measures of microstructural water diffusion include Diffusion Weighted Imaging (DWI) and Diffusion Tensor Imaging (DTI). DWI gives a measure of average non-direction dependent (i.e., isotropic) diffusivity of water in tissue that is described in terms of the apparent diffusion coefficient (ADC). Increased ADC likely reflects neuronal loss and an increase in extracellular space (where diffusion of water is faster) 45, and can reflect integrity of gray or white matter. DTI provides an index of the differential permeability of tissues in varying directions (i.e., anisotropy of tissue). Mean diffusivity (MD; i.e., isotropic diffusivity) can be assessed with DTI. Another measure derived from DTI, fractional anisotropy (FA), is a particularly sensitive measure of the microstructural integrity of cerebral white matter, given that water preferentially diffuses along the long axis of white-matter tracts.
Studies of DWI indicate that MCI participants show increased ADC values compared to controls in both hippocampus and corpus callosum 46, 47. Furthermore, one DWI study indicated that the ADC of hippocampus and corpus callosum is increased in individuals with MCI who soon progress to AD compared to those who do not progress 45. DTI studies have corroborated the finding of increased water diffusion (MD) in the hippocampus 48, 49. Findings regarding FA have been more variable. However, the replicated finding of decreased FA in the posterior cingulate bundle is consistent with evidence of structural and functional changes in the posterior cingulate cortex.
Diffusion imaging may augment volumetric methods, increasing the rate of accurate prediction of progression in MCI 45. However, research regarding usefulness of diffusion measurements in predicting progression from MCI to AD is still in its infancy. Further empirical study will determine the clinical utility of this method in MCI and AD.
WHAT DOES BRAIN FUNCTION TELL US ABOUT MCI?
Investigations of brain physiology in MCI have great potential to augment our understanding of brain changes provided by volumetric studies. Functional studies may be particularly sensitive to the earliest changes associated with AD pathology, and indices of brain physiology are likely to be critical outcome measures in studies of drug development. Functional case-control studies indexing brain activity with the blood oxygen level dependent response (BOLD) and perfusion arterial spin labeling (ASL) measures are providing an exciting window into how brain function differs among healthy older individuals, individuals with MCI, and those with AD. These studies also further our understanding of how changes in brain function relate to the clinical symptomatology of MCI.
BOLD fMRI studies: Detecting differences among patient groups
Functional MRI offers a method of examining brain regions while those regions are functionally engaged in a specific cognitive, affective, or motor task. In most fMRI studies of MCI, brain activity has been indexed by the BOLD response, an increase in the ratio of oxygenated to deoxygenated hemoglobin that is correlated with neural activity. Although the neural response to stimulation is rapid (<100 ms), the BOLD response is much slower. Following an increase in neural activity, the typical BOLD signal gradually increases to its peak magnitude over approximately 6 seconds and decreases back to baseline over the next 6–8 seconds.
Neuropathology underlying amnestic MCI and AD originates in brain structures responsible for memory, and impaired episodic memory (i.e., conscious recollection and/or re-experiencing of past events ) is the hallmark feature of both amnestic MCI and Alzheimer's disease. Not surprisingly, most fMRI studies of MCI have examined brain activity accompanying an episodic memory task. Given evidence that many individuals with MCI have limited awareness of their cognitive deficits, studies are also beginning to focus on the brain substrate of anosognosia in MCI. Research attention is also turning to the pattern of "default mode" activity shown by people with MCI.
Memory Encoding
Clinical and experimental studies of MTL lesions provide copious evidence of this brain region's role in encoding of memories (i.e., learning), and functional imaging evidence indicates that the intact hippocampus is responsive to new information 50, 51. Specifically, the intact hippocampus shows an adaptation response over learning trials (i.e., hippocampal response is seen with the first presentation of a new item, and the magnitude of activation attenuates with each subsequent presentation of this new item). Although this adaptation response is robust in cognitively-healthy adults, it is significantly reduced in MCI 52. Other investigations of the brain response to novel items have found less MTL response in well-characterized amnestic MCI participants compared to controls 53–55. In a study requiring participants to encode face-name associations, MCI participants showed attenuated activity most prominently in bilateral frontal regions 56. MCI participants in all of the above studies had notable memory decline as reported by someone who knows them well as well as on objective memory testing.
There is also evidence that the timing of the BOLD response during encoding may differ among controls and those with MCI and AD 57. One study comparing early- and late-phase BOLD responses found that controls show a robust early BOLD response in a number of cortical regions. MCI participants showed a significantly smaller early response in occipital regions. AD participants showed a significantly smaller early response across more wide-spread cortical regions. No group differences were found in the late BOLD response.
Although the aforementioned studies have shown that fMRI activation during encoding declines in a linear fashion from cognitive health to MCI to AD, other studies indicate that an early stage of MCI is characterized by an increase in activation which decreases as MCI-AD symptoms progress 58, 59. The focus of these studies was to examine individuals in a very early stage of MCI. Although participants had a global rating of 0.5 on the Clinical Dementia Rating (CDR) scale and subjective memory complaint, there was no requirement that participants show memory impairment on objective testing. In one study comparing controls and MCI participants, performance in the MCI group was not significantly different than controls and delayed recall was nearly identical to controls. The metric used was extent of activation (# of significant voxels) in the hippocampus during response to new information. Results indicated that early MCI participants show significantly greater extent of activation than either older controls or AD patients 59. Another study investigated MTL function in early MCI. Results indicated that the extent of MTL activity in MCI was positively correlated with successful encoding. Paradoxically, extent of activation was also correlated with clinical impairment. A post-hoc analysis of the magnitude of MTL activity showed no difference between controls and MCI. In a longitudinal follow-up, individuals who declined by at least 1 point on the CDR sum of ratings had shown greater extent of fMRI activation 58. A third study of MCI individuals with a more pronounced memory deficit found a similar increase in activation in posterior hippocampus and parahippocampal and fusiform regions, together with atrophy of more anterior MTL. The results of these studies showing an upsurge in extent of activity during MCI have been interpreted by the authors as a compensatory brain response reflecting recruitment of supplementary neural resources to counteract the effects of AD pathology, change in cognitive strategy, or cognitive reserve.
Memory Retrieval
Medial parietal regions (i.e., retrosplenial cortex, posterior cingulate cortex (PCC), and precuneus) have only recently been recognized as important in memory. Functional neuroimaging research demonstrates medial parietal cortex, a region that has anatomical connections with the MTL, is active during the retrieval of previously-learned information 60–62. Numerous FDG PET studies have shown this region to be hypometabolic in AD, and retrosplenial cortex and PCC show volumetric and metabolic decline in MCI that discriminates between individuals with MCI who soon develop AD and those who do not 63, 64.
Given that the medial parietal metabolic changes during resting FDG PET correlate with MCI participants' ability on psychometric tests of memory retrieval 65, it was of interest to investigate the integrity of PCC activity while completing an fMRI episodic retrieval paradigm. Consistent with the PET results, two functional MRI studies to date have found that PCC activity during episodic retrieval is attenuated in MCI when compared to controls 54, 66. However, another study of episodic retrieval did not find that MCI participants show decreased medial parietal activity. Instead, these authors found decreased activity in bilateral frontal regions and left hippocampus 56.
Self-Awareness
Awareness of cognitive dysfunction shown by MCI patients is quite variable, ranging from clear insight and marked concern about cognitive difficulties to severe anosognosia 67. Importantly, the ability of a person with MCI to accurately appraisal his/her cognitive abilities may be a valuable prognostic index, with research indicating that MCI patients who lack accurate awareness show greater conversion to AD than those who show awareness 68.
A number of functional MRI studies have implicated medial frontal and parietal regions as part of network of cortical midline structures important in self-referential processing 69. Neuropsychological and functional research indicates that these regions are altered in MCI, and a hypothesis proposed in the literature held that impaired awareness in MCI relates to functional decline in cortical midline structures. However, results of one fMRI study suggested that MCI participants and age-matched controls show comparable activity during self-appraisal in a PCC location 70. In that study, the PCC was the sole area where control participants showed common activation during self-appraisal and episodic recognition. However, MCI participants did not show common activation across tasks. Further investigation of this PCC location indicated that MCI participants showed reduced BOLD response during recognition; however, activity was similar to controls during self-appraisal. Given evidence that healthy young adults show reliable PCC activity during self-referential processing, together with evidence that individuals with MCI are quite variable in their ability to make accurate self appraisals 67, a possible explanation for this result was that PCC activity during self-appraisal is variable in MCI participants. A follow-up study assessed the hypothesis that MCI participants were variable in their ability to accurately rate their own level of cognitive problems (indexed by a discrepancy score between patient and informant ratings of cognitive decline in each MCI participant), and that activation of cortical midline structures during self-appraisal would covary with level of insight into cognitive difficulties. Results revealed a highly significant relationship between cortical midline BOLD response during self-appraisal and self-awareness of deficit in MCI. This result suggests that impaired awareness shown by a subset of MCI patients, perhaps those more likely to progress to AD, is accompanied by a decrease in activity in structures that support self-referential cognition.
Default Mode
The primary outcome variable reported in fMRI BOLD studies is mean signal change. This index is a relative measure, typically assessing brain activity during an experimental task relative to a comparison task. A number of studies have shown that a conglomerate of structures (i.e., medial frontal cortex, medial parietal cortex, and PCC) show greater activity during low-level comparison tasks (i.e. looking at a fixation cross) than during experimental tasks in which a high-level of effort must be put into the task. This heightened activity during low-level comparison tasks has been replicated several times and has been termed the "default mode."
Interpretation of the meaning of “default mode” activity is controversial. The term “default mode” implies that this state of activity can be interpreted as physiological or cognitive baseline state of the brain. However, many argue that the experimental setting in which the “default mode” is evoked (often one in which participants “rest” with eyes closed) is cognitively and behaviorally uncontrolled, and is more likely to reflect a self-monitoring cognitive state than neural quiescence. Despite this controversy, resting activation may be ideal for assessment of MCI, given the ease of acquisition and low-task demands on the clinical patient. Thus, although assessing default mode in MCI may not provide greater understanding of neural mechanisms of cognitive dysfunction, it may have diagnostic or prognostic value.
Empirical evidence indicates that AD patients show diminished default mode activity. Results of a recent study compared individuals with MCI with controls and individuals with AD 71 indicate that MCI participants showed evidence of diminished default mode activity, particularly in frontal regions. In AD, diminishment of the default mode activity was found in more widespread cortical regions.
Brain perfusion: Arterial Spin Labeling
Although the majority of studies examine brain function in MCI with the BOLD response, perfusion MRI using Arterial Spin Labeling (ASL) offers another non-invasive method. ALS uses magnetically-labeled water protons as an endogenous tracer. In comparison to BOLD, which reflects changes in cerebral blow flow (CBF), cerebral blood volume (CBV), and cerebral metabolic rate of oxygen uptake oxygenation, ASL is an absolute measure of temporal changes in CBF.
Most ASL studies investigate CBF while subjects lie quietly in the MRI. An initial ASL study of AD showed CBF decreases in temporal cortex, and both lateral and medial aspects of frontal and parietal cortex 72. The one study of MCI found hypoperfusion of the right inferior parietal lobe in MCI participants relative to controls. AD patients showed attenuated CBF in posterior cingulate, precuneus, and bilateral inferior parietal gyri compared to MCI participants 73. This finding is highly consistent with PET literature on AD and suggests that perfusion MRI may be advantageous for examining crucial brain regions.
It is possible to examine perfusion not only during rest, but also during the completion of motor or cognitive tasks, much as is done with fMRI. A recent ASL study examined CBF while both control subjects and MCI patients completed a memory encoding task. Results of this study showed that control participants demonstrate a 23% increase in CBF during memory encoding; however, MCI patients do not show this encoding-induced increase in perfusion.74
Clinical utility of MRI measures of function
The application of MRI measures of brain function to the study of AD is a relatively new venture, with the initial BOLD study published in 1999 55 and the initial ASL study published in 2000 72. Since then, a handful of studies have shown that functional MRI BOLD methods detect differences among cognitively healthy older adults, MCI patients, and AD patients at a group level. The one ASL study also detected a difference between MCI patients and controls. However, in this early stage of development, the clinical utility of these methods remain limited by a number of factors (for in-depth review, see 75) .
Although BOLD fMRI shows group differences, similar studies yield variable results with respect to the degree, directionality, and location of functional differences (e.g., Is MCI is associated with hypo or hyperactivation relative to older controls during memory encoding, and where are these changes localized?) The variability in task design (in both experimental and comparison conditions), definitions of MCI, and imaging parameters across studies make comparison of findings quite difficult. Without more standardized and clinically-appropriate methods 76 applied in larger studies, it will be difficult to ascertain group differences in a reliable fashion. Furthermore, this provides a barrier to determination of meaningful differences within individuals. Other issues that currently obfuscate the interpretability of BOLD fMRI findings relate to variability of measurement (both between and within subjects), finding adequate methods to account for the presence of tissue atrophy in fMRI analysis77, 78, and limited understanding of how neurovascular coupling (i.e., the relationship between neural activity and the hemodynamic response) compares between MCI patients and healthy older adults79.
The application of ASL methods to the study of MCI is quite new, and a greater understanding of its clinical utility will emerge with its increased application to clinical research questions. However, many factors augur well for its clinical utility. Because ASL is an absolute measure of CBF, signal changes are quantitative and can be interpreted without reference to a baseline. ASL also has the advantage over BOLD of being more reproducible (over time and across participants), and given its sensitivity to capillary-level CBF, it may localize regions of activation more accurately. In terms of potential limitations, ASL has a reduced signal-to-noise ratio and a slower sampling rate relative to BOLD.
NEUROIMAGING AS IT STANDS NOW: WHAT SCANS ARE CLINICALLY USEFUL IN THE WORK-UP OF AN MCI PATIENT?
Similar to its role in the diagnosis of Alzheimer’s disease, neuroimaging is critical to rule out non-neurodegenerative conditions that may mimic Mild Cognitive Impairment, such as brain neoplasm, subdural hematoma, vascular dementia, or normal pressure hydrocephalus. MRI has several advantages over computed-assisted tomography (CT), especially improved tissue characterization. Use of T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR) scans and diffusion-weighted imaging (DWI) allow confident detection of ischemic lesions, with DWI showing only recent events. Remote cerebral ‘microbleeds’ related to amyloid angiopathy cannot be detected by CT, but are well seen on gradient echo MRI sequences. Contrast is used to exclude meningiomas, primary glial tumors or metastatic disease which can sometimes present with cognitive impairment. Although MRI usually takes longer than CT, most patients tolerate it well, and fast MRI scan techniques can reduce exam time to under 10 minutes. However, MRI use may not be possible in patients who have cardiac pacemakers or those who are severely claustrophobic. In these cases, CT allows for exclusion of important structural lesions.
NEUROIMAGING: A LOOK INTO THE FUTURE OF EARLY DETECTION OF ALZHEIMER’S DISEASE
Clearly, remarkable research gains are being made regarding detection of brain changes in MCI and prediction of those MCI patients most likely to progress to AD. Volumetric measures of T1-weighted MRI scans have been the most long-standing focus of empirical work and show the most immediate promise in terms of providing information on an individual MCI patient’s risk of progression to dementia. However, no imaging measure currently provides a reliable prediction of which patients with MCI will rapidly progress to develop AD.
In addition to MRI, careful neuropsychological assessment, CSF markers, and PET imaging each provide data that is useful in solving the AD puzzle. One of the most remarkable new advances relates to the developing ability to assess Alzheimer's pathology in vivo. In vivo imaging of amyloid has been demonstrated with the use of the radiotracer Pittsburgh Compound B (PIB) 80. In vivo imaging of both plaques and tangles with 18F-FDDNP has also been reported 81. The latest findings suggest that combining MRI with clinical assessment and other biomarkers such as these will have greatest prognostic value for MCI patients.
One of the barriers to gaining a clear understanding of the value of various diagnostic and prognostic methods relates to difficulty in comparing results across various studies using different methodologies and participant groups. Given the somewhat fractionated nature of results from multiple small studies, the largest gains will result from large-scale multi-site studies using standard methods. The Alzheimer's Disease Neuroimaging Initiative (ADNI) is one such effort recruiting healthy controls and individuals with MCI and AD. This study of potential AD biomarkers involves the collection of volumetric MRI data, FDG PET, and CSF measures using standardized methods across 50 sites in the U.S. Furthermore, a recent addition to ADNI involves PIB imaging at a subset of study sites. Efforts such as the ADNI promise to speed the progression toward finding sensitive and specific approaches to the early detection of AD.
ACKNOWLEDGMENT
Conflict of Interest Disclosures: Below is a checklist for all authors to complete and attach to their papers during submission.
| Elements of Financial/Personal Conflicts | M. Ries | C. Carlsson | H. Rowley | C. Gleason | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
For “yes”×mark(s): give brief explanation below:
| Elements of Financial/Personal Conflicts | M. Sager | S. Asthana | S.Johnson | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||
| Grants/Funds | X | X | X | |||
| Honoraria | X | X | X | |||
| Speaker Forum | X | X | X | |||
| Consultant | X | X | X | |||
| Stocks | X | X | X | |||
| Royalties | X | X | X | |||
| Expert Testimony | X | X | X | |||
| Board Member | X | X | X | |||
| Patents | X | X | X | |||
| Personal Relationship | X | X | X | |||
Sponsor’s Role: N/A
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 3.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA workgroup under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 4.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–877. doi: 10.1016/j.nic.2005.09.008. xi–xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 6.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:383–389. doi: 10.1159/000084709. [DOI] [PubMed] [Google Scholar]
- 8.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic Outcome of Mild Cognitive Impairment Following Progression to Clinical Dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic Features of Amnestic Mild Cognitive Impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. European Neurology. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 13.Becker JT, Davis SW, Hayashi KM, et al. Three-dimensional Patterns of Hippocampal Atrophy in Mild Cognitive Impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 14.Kantarci K, Xu Y, Shiung MM, et al. Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:198–207. doi: 10.1159/000066021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips NA, Chertkow H, Leblanc MM, Pim H, Murtha S. Functional and anatomical memory indices in patients with or at risk for Alzheimer's disease. J Int Neuropsychol Soc. 2004;10:200–210. doi: 10.1017/S1355617704102063. [DOI] [PubMed] [Google Scholar]
- 16.Wolf H, Hensel A, Kruggel F, et al. Structural correlates of mild cognitive impairment. Neurobiol Aging. 2004;25:913–924. doi: 10.1016/j.neurobiolaging.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bottino CM, Castro CC, Gomes RL, Buchpiguel CA, Marchetti RL, Neto MR. Volumetric MRI measurements can differentiate Alzheimer's disease, mild cognitive impairment, and normal aging. Int Psychogeriatr. 2002;14:59–72. doi: 10.1017/s1041610202008281. [DOI] [PubMed] [Google Scholar]
- 18.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 20.Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 21.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 22.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Pennanen C, Testa C, Laakso MP, et al. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. PNAS. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trivedi MA, Wichmann AK, Torgerson BM, et al. Structural MRI discriminates individuals with mild cognitive impairment from age-matched controls: A combined neuropsychological and voxel based morphometry study. Alzheimer's and Dementia. The Journal of the Alzheimer's Association. 2006;2:296–302. doi: 10.1016/j.jalz.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Do all patients with mild cognitive impairment progress to dementia? J Am Geriatr Soc. 2006;54:1008–1010. doi: 10.1111/j.1532-5415.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2007 doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chetelat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: A longitudinal MRI study. NeuroImage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Bozzali M, Filippi M, Magnani G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67:453–460. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- 33.Hamalainen A, Tervo S, Grau-Olivares M, et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37:1122–1131. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 34.De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 35.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 36.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: A longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 38.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of Mild Cognitive Impairment to Alzheimer Disease Predicted by Hippocampal Atrophy Maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 39.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erten-Lyons D, Howieson D, Moore MM, et al. Brain volume loss in MCI predicts dementia. Neurology. 2006;66:233–235. doi: 10.1212/01.wnl.0000194213.50222.1a. [DOI] [PubMed] [Google Scholar]
- 41.Visser PJ, Verhey FRJ, Hofman PAM, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72:491–497. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korf ESC, Wahlund L-O, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 43.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 44.de Leon MJ, DeSanti S, Zinkowski R, et al. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med. 2004;256:205–223. doi: 10.1111/j.1365-2796.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 45.Kantarci K, Petersen RC, Boeve BF, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64:902–904. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray KM, Wang H, Chu Y, et al. Mild cognitive impairment: apparent diffusion coefficient in regional gray matter and white matter structures. Radiology. 2006;241:197–205. doi: 10.1148/radiol.2411051051. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Su MY. Regional pattern of increased water diffusivity in hippocampus and corpus callosum in mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22:223–229. doi: 10.1159/000094934. [DOI] [PubMed] [Google Scholar]
- 48.Muller MJ, Greverus D, Dellani PR, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28:1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 49.Fellgiebel A, Wille P, Muller MJ, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004;18:101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- 50.Saykin AJ, Johnson SC, Flashman LA, et al. Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: an fMRI study. Brain. 1999;122(Pt 10):1963–1971. doi: 10.1093/brain/122.10.1963. [DOI] [PubMed] [Google Scholar]
- 51.Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–4049. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson S, Baxter L, Susskind-Wilder L, Connor D, Sabbagh M, Caselli R. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 56.Petrella JR, Krishnan S, Slavin MJ, Tran TT, Murty L, Doraiswamy PM. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240:177–186. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- 57.Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer's disease. Neuroimage. 2005;26:1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 2005;17:183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- 61.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrella JR, Wang L, Krishnan S, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- 63.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 64.Huang C, Wahlund LO, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002;2:9. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chetelat G, Desgranges B, de la Sayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 66.Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17:181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 68.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 69.Johnson S, Baxter L, Wilder L, Pipe J, Heiserman J, Prigatano G. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 70.Ries ML, Schmitz TW, Kawahara TN, Torgerson BM, Trivedi MA, Johnson SC. Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease An fMRI study. Hum Brain Mapp. 2005 doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- 73.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu G, Antuono PG, Jones J, et al. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007 doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- 75.Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23:808–815. doi: 10.1002/jmri.20585. [DOI] [PubMed] [Google Scholar]
- 76.Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging. 2006;23:816–826. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- 77.Krishnan S, Slavin MJ, Tran T-TT, Doraiswamy PM, Petrella JR. Accuracy of spatial normalization of the hippocampus: Implications for fMRI research in memory disorders. NeuroImage. 2006;31:560–571. doi: 10.1016/j.neuroimage.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 78.Johnson S, Saykin A, Baxter L, et al. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11:179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- 79.D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 80.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 81.Shoghi-Jadid K, Small GW, Agdeppa ED, et al. Localization of Neurofibrillary Tangles and Beta-Amyloid Plaques in the Brains of Living Patients With Alzheimer Disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]