Abstract
This study was designed to test the hypothesis that the transient receptor potential vanilloid type 1 (TRPV1) channel, expressed primarily in sensory nerves, and substance P (SP), released by sensory nerves, play a protective role against lipopolysaccharide (LPS)-induced hypotension. LPS (10 mg/kg, iv) elicited tachycardia and hypotension in anesthetized male Wistar rats, which peaked at 10 min and gradually recovered 1 hour after the injection. Blockade of TRPV1 with its selective antagonist, capsazepine (CAPZ, 3 mg/kg, iv), impaired recovery given that the fall in mean arterial pressure (MAP) was greater 1 hour after CAPZ plus LPS injections compared to LPS injection alone (45 ± 5 vs. 25 ± 4 mmHg, P < 0.05). Blockade of the neurokinin-1 (NK1) receptor with its selective antagonists, RP67580 (5 mg/kg, iv) or L-733,060 (4 mg/kg, iv), prevented recovery considering that falls in MAP were not different 1 hour after injections of NK1 antagonists plus LPS from their peak decreases (66 ± 9 vs. 74 ± 5 mmHg, or 60 ± 7 vs. 69 ± 3 mmHg, respectively, P>0.05). LPS increased plasma SP, norepinephrine (NE), and epinephrine (EPI) levels compared to vehicles, and the increases in plasma SP, NE, and EPI were significantly inhibited by CAPZ or RP67580, respectively. The survival rate at 24 or 48 hours after LPS injection (20 mg/kg, ip) was lower in conscious rats pretreated with CAPZ or RP67580 compared to rats treated with LPS alone (P<0.05). Thus, our results show that the TRPV1, possibly via triggering release of SP which activates the NK1 and stimulates the sympathetic axis, plays a protective role against endotoxin-induced hypotension and mortality, suggesting that TRPV1 receptors are essential in protecting vital organ perfusion and survival during the endotoxic condition.
Keywords: endotoxin, transient receptor potential vanilloid type1 channel, substance P
Introduction
The transient receptor potential vanilloid type 1 channel (TRPV1), also known as vanilloid receptor type 1, is a ligand-gated nonselective cation channel (4). Double immunohistochemical labeling studies have shown that in addition to the central nervous system, TRPV1 is abundantly expressed in sensory nerves including unmyelinated C-fibers and thinly myelinated Aδ-fibers innervating cardiovascular tissues including the heart and blood vessels (37, 45, 48). Sensory nerves not only function as afferent fibers sending information to the central nervous system, but they also have an efferent function to release a number of sensory neurotransmitters, commonly substance P (SP) and calcitonin gene-related peptide (CGRP). The TRPV1 acts as a molecular integrator of multiple chemical and physical stimuli including noxious heat, low pH, capsaicin, and lipid metabolites (23).
Endotoxic shock is a life-threatening cardiovascular depression with high mortality rate (33). It is caused mainly by an exaggerated systemic response to endotoxemia induced by gram-negative bacteria and their characteristic cell wall component, lipopolysaccharide (LPS) (12). Its evolution is characterized by an increased variety of biological mediators including cytokines, nitric oxide and free radicals (21, 33). Excessive production of these mediators may cause hypotensive shock and multiple organ failure. Therefore, the pathogenesis of endotoxic shock is multi-factorial and incompletely understood.
In this regard, an increased production of the endocannabinoid anandamide by macrophages has been reported to contribute to the endotoxin-induced hypotension (3, 40). Although it has been well documented that activation of cannabinoid receptor 1 (CB1) by endocannabinoid anandamide elicits the profound and long-lasting hypotension (24, 39), Zygmunt et al (49) have shown that activation of TRPV1 receptors acts as a predominant mechanism for anandamide-induced relaxation in the rat mesenteric arteries, indicating that TRPV1 receptors can be activated by anandamide. The possibility is further confirmed by our in vivo studies showing that the depressor response to methanandamide, a metabolically stable analog, is attenuated by the TRPV1 antagonist, capsazepine (CAPZ) (43). Therefore, the TRPV1 receptor might be important in regulating blood pressure during endotoxemia.
This study was designed to determine: (1) the potential action of TRPV1 receptors in endotoxin-induced hypotension; (2) whether TRPV1 mediated effects are attributed to activation of NK1 receptors by substance P released from sensory nerves upon TRPV1 activation.
Materials and Methods
Animals
The experiments were carried out on 7 to 8-week old male Wistar rats obtained from Charles River Laboratories (Wilmington, MA). The rats were housed in the animal facility for 1 week before the experiment and were allowed free access to regular rat chow and water ad libitum. All animal procedures were in accordance with the guidelines of the National Institutes of Health and approved by the University Animal Care and Use Committee.
Surgery
Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (60 mg/kg). The trachea was cannulated and opened to room air to facilitate respiration. Catheters (PE-50) were inserted into the left carotid artery and jugular and femoral vein for the measurement of mean arterial pressure (MAP) and intravenous injection of drugs, respectively. MAP was monitored using a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instrument Systems, Valley View, OH). Heart rate (HR) was derived from the pulse pressure via a tachograph preamplifier. The rats were placed on heating pads that maintained core temperature at 36 ~ 37°C.
Rats were allowed to stabilize for 30 min following completion of cannulation. After baseline measurements were obtained, LPS (10 mg/kg) was intravenously administered in a volume of 0.2 ~ 0.3 ml over 1 min. Blood pressure was monitored for 1 h. To explore the role of the TRPV1 or neurokinin-1 (NK1) receptor in the acute hypotensive response to LPS, rats were pretreated with the TRPV1 antagonist capsazepnie (CAPZ, 3 mg/kg) or the NK1 antagonists, RP67580 (5 mg/kg) and L-733,060 (4 mg/kg), 10, 5, or 25 min, respectively, before injection of the similar dose of LPS. The doses and time frames for injection of these drugs were based on the results described previously (5, 11, 34, 44). Moreover, to determine whether CGRP contributes to the differences in MAP and HR between TRPV1 and NK1 receptor blockade during LPS injection, CGRP8-37, a CGRP receptor antagonist, was intravenously administered for 2 min at the rate of 1 mg/kg per min starting 10 min after LPS injection in rats pretreated with the NK1 receptor antagonist RP67580, and continued at the rate of 0.5 mg/kg per min for 48 min. The effectiveness of the dose and time frame for CGRP8-37 injection was verified by blockade of the hypotensive response to capsaicin (30 μg/kg), a specific TRPV1 agonist.
Survival experiment
For survival analysis, bolus injection of LPS (20 mg/kg) was intraperitoneally given into the unanesthetized rats with or without the TRPV1 antagonist, CAPZ, or the NK1 antagonist, RP67580. CAPZ (3 mg/kg) or RP67580 (5 mg/kg) was intraperitoneally administered 20 min before and 6 h after injection of LPS. After injection, rats were individually housed, and the number of surviving animals was counted at 24 and 48 h after LPS treatment. Surviving animals were euthanized by the overdose of anesthesia.
Sample collection
Blood samples were collected via the arterial catheter 1 h after injection of LPS with or without CAPZ or RP67580. Plasma samples were obtained by centrifugation at 1,700 g for 15 min at 4°C and stored at -80°C for measurements of plasma catecholamines including norepinephrine (NE) and epinephrine (EPI), and SP.
Analysis of plasma catecholamines
The catecholamines in plasma were extracted using an alumina extraction procedure and eluted with acetic acid, as described previously (10, 17). The volume of plasma used was 1 mL and the mass of activated alumina (MP Biomedicals Germany GmbH) was 10 mg. The alumina was activated prior to use by the procedure reported by Eisenhofer et al. (10). HPLC with coulometric detection was performed using a commercial system (ESA, Inc., Chelmsford, MA) consisting of a solvent delivery module (model 582), an autosampler (model 542) cooled to 4°C and a CoulArray detector (model 5600A) with a high sensitivity analytical cell (model 5011A). NE was detected at +0.3 V vs. a solid-state Pd reference electrode. At this potential, NE was oxidized at a mass transfer limited rate. The catecholamines were separated on a HR-80 (C18, 3 μm particle size, 80 mm × 4.6 mm I.D.) reverse-phase column (ESA Biosciences, Inc.). The mobile phase was a commercial Cat-A-Phase II (ESA Biosciences, Inc.) that consisted of a proprietary mixture of acetonitrile, methanol, phosphate buffer (ca. pH 3.2) along with an ion pairing agent. The optimum flow rate was found to be 1.1 mL/min. The injected sample volume was 20 μL. The separation column and detection cell were maintained at 30°C.
Radioimmunoassay
Peptides were extracted from plasma using Strata C18-E column (Phenomenex, Torrance, CA). A rabbit anti-rat SP radioimmunoassay kit (Peninsula Laboratories, San Carlos, CA) was used to determine SP. The assay was performed as recommended by the supplier. This antibody has 100% cross-reactivity with rat SP and 0.01% with rat neurokinin A. There was no cross-reactivity with rat neurokinin B.
Reagents
LPS, derived form Escherichia coli (serotype 0127:B8), was purchased from Sigma Chemicals (St. Louis, MO). CAPZ (Calbiochem, San Diego, CA), RP67580 (Tocris Cookson, Ellisville, MO) and L-733,060 (Tocris Cookson, Ellisville, MO) were dissolved in dimethyl sulfoxide (10%, v/v), Tween-80 (10%, v/v), and normal saline.
Statistical analysis
All values are expressed as means ± SE. Comparisons of MAP and HR in the different treatment groups were performed by using two-way ANOVA for repeated measurement with the Newman-Keuls test. The differences among groups were analyzed using one-way ANOVA followed by a Bonferroni's adjustment for multiple comparisons. Survival rate was evaluated by the Cox-Mantel test. Differences were considered statistically significant at P < 0.05.
Results
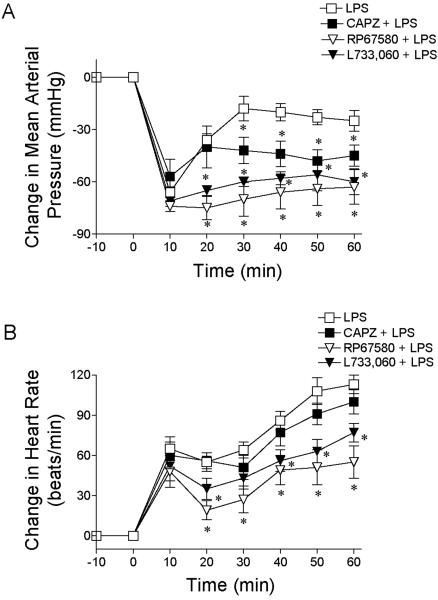
There were no significant differences in the baseline MAP and HR among the groups as presented in Table 1. As shown in Fig. 1, intravenous injection of LPS (10 mg/kg) produced a profound hypotension that lasted up to 1 hour in pentobarbital-anesthetized rats. The hypotensive response was associated with tachycardia. Although blockade of TRPV1 receptors with CAPZ (3 mg/kg) did not affect the maximal hypotensive response to injection of LPS, CAPZ delayed the recovery of MAP, which remained lower than that of LPS treated alone rats until 1 hour after injection of LPS (-45 ± 5 vs. -25 ± 4 mmHg, P<0.05). CAPZ did not affect the tachycardia response to LPS injection. Injection of CAPZ alone caused transient elevation of MAP but it returned to the baseline level 10 min after CAPZ injection (Table 1).
Table 1.
Effects of TRPV1 and NK1 receptor antagonists on baseline MAP and HR
| CAPZ |
RP67580 |
L-733,060 |
||||
|---|---|---|---|---|---|---|
| Baseline | 10 min after | Baseline | 5 min after | Baseline | 25 min after | |
| CAPZ | RP67580 | L-733,060 | ||||
| MAP (mmHg) |
109±6 | 115±4 | 115±5 | 118±6 | 112±5 | 120±6 |
| HR (beats/min) |
422± 10 | 417±8 | 413±11 | 357±7* | 419±9 | 427±13 |
Values are means ± SE (n = 6-7 rats). CAPZ (3 mg/kg), capsazepine (TRPV1 receptor antagonist); RP67580 (5 mg/kg) and L-733,060 (4 mg/kg), NK1 receptor antagonists; MAP, mean arterial pressure; HR, heart rate.
P < 0.05 vs. baseline value.
Figure 1.
LPS-induced changes in mean arterial pressure (A) and heart rate (B) in anesthetized rats with or without the TRPV1 receptor antagonist CAPZ or the NK1 receptor antagonist RP67580 and L-733,060. LPS (10 mg/kg) was intravenously injected at 0 min. CAPZ (3 mg/kg), RP67580 (5 mg/kg) or L-733,060 (4 mg/kg) was intravenously injected 10, 5 or 25 min prior to administration of LPS. Values are means ± SE (n = 6-7). *P < 0.05 versus LPS group.
To explore of the role of NK1 receptors in the hypotensive response to LPS, rats were pretreated with the NK1 receptor antagonist RP67580 (5 mg/kg) 5 min before injection of LPS. As illustrated in Fig. 1, the LPS-induced hypotensive response was exacerbated by RP67580. The initial decrease in MAP (~ -80 mm Hg) was sustained in the presence of the antagonist. MAP was not different 1 hour after injection of RP67580 from the peak decrease (66 ± 9 vs. 74 ± 5 mmHg, P>0.05). Moreover, RP67580 significantly attenuated the LPS-induced increase in HR. The administration of RP67580 alone had no significant effect on blood pressure but did decrease HR (Table 1).
The ability of RP67580 to sustain the hypotensive response to LPS suggested that the NK1 receptor may contribute to the recovery of LPS-induced hypotension. To further test this possibility, the hypotensive response to LPS was tested in rats pretreated with another NK1 antagonist L-733,060. L-733,060 (4 mg/kg) aggravated the LPS-induced hypotension, as shown in Fig. 1. During hypotension induced by LPS, L-733,060 significantly attenuated the increased HR response to LPS. L-733,060 alone did not affect the baseline MAP and HR (Table 1).
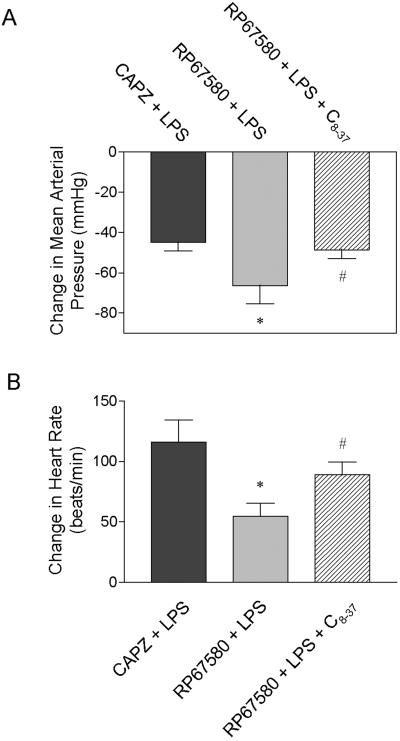
Blockade of the TRPV1 or NK1 receptors with CAPZ or RP67580 delayed the recovery of MAP and HR. The delay was significantly greater in rats pretreated with RP67580 than in rats treated with CAPZ (Fig. 1). As shown in Fig. 2, the difference in MAP and HR between rats treated with RP67580 and CAPZ was prevented by blockade of the CGRP receptor by its antagonist, CGRP8-37. CGRP8-37 infusion alone led to very brief increases in MAP and HR, which reached the peak 60-90 seconds after its administration and vanished (MAP and HR returned to the baseline levels) 150-180 seconds after CGRP8-37 injection.
Figure 2.
Changes in mean arterial pressure (A) and heart rate (B) 60 min after injection of LPS in anesthetized rats pretreated with the TRPV1 receptor antagonist CAPZ or the NK1 receptor antagonist RP67580 with or without the CGRP receptor antagonist CGRP8-37 (C8-37). CAPZ (3mg/kg) or RP67580 (5 mg/kg) was intravenously injected 10 or 5 min prior to injection of LPS (10 mg/kg). The dose and time frame for C8-37 were stated in the materials and methods. Values are mean ± SE (n = 5-7). *P < 0.05 versus CAPZ + LPS group; #P < 0.05 versus RP67580 + LPS group.
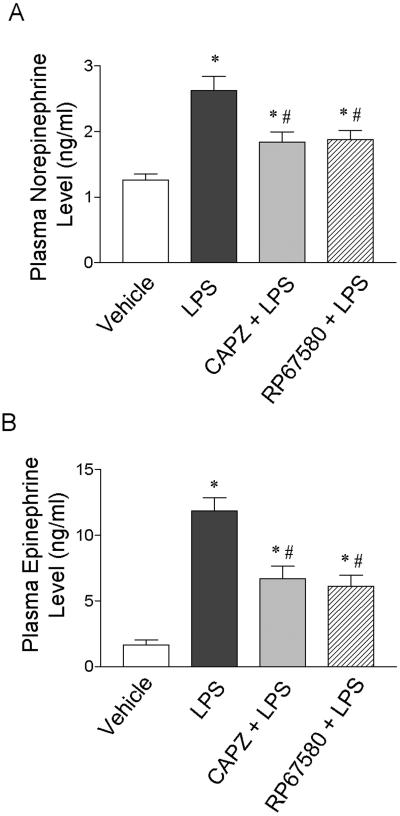
To investigate the role of the sympathetic nervous system in LPS-induced hypotension, we determined the plasma catecholamine levels using HPLC. As shown in Fig. 3, the plasma NE and EPI levels significantly increased 1 hour after injection of LPS compared to vehicle (P<0.05). The increased plasma NE and EPI levels were significantly attenuated by pretreatment with the TRPV1 receptor antagonist, CAPZ, or the NK1 receptor antagonist, RP67580. Injection of CAPZ or RP67580 alone did not affect the plasma NE and EPI levels as compared to the vehicle (data not shown).
Figure 3.
Plasma norepinephrine (A) and epinephrine (B) responses to LPS (10 mg/kg) in anesthetized rats with or without the TRPV1 receptor antagonist CAPZ (3 mg/kg) or the NK1 receptor antagonist RP67580 (5 mg/kg). Values are means ± SE (n = 7-9). *P < 0.05 versus vehicle group; #P < 0.05 versus LPS group.
In addition, we found that intravenous injection of LPS (10 mg/kg) significantly increased the plasma SP levels compared to vehicle (P<0.05), as presented in Fig. 4. The enhanced plasma SP level was prevented by pretreatment with CAPZ. However, pretreatment with the NK1 receptor antagonist RP67580 did not affect LPS-induced increases in the plasma SP level. In addition, injection of CAPZ or RP67580 alone did not affect the plasma SP levels compared to the vehicle (data not shown).
Figure 4.
Plasma substance P responses to LPS (10 mg/kg) in anesthetized rats with or without the TRPV1 receptor antagonist CAPZ (3 mg/kg). Values are means ± SE (n = 7-9). *P < 0.05 versus vehicle group; #P < 0.05 versus LPS group.
Since the TRPV1 and NK1 receptors were likely to be involved in LPS-induced hypotension, we investigated their effects on the survival in the model of LPS-induced septic shock. The survival rates among groups are presented in Fig. 5. Pretreatment with CAPZ or RP67580 significantly decreased the survival rate at 24 or 48 h after injection of LPS (20 mg/kg) as compared to injection of LPS alone. Injection of CAPZ or RP67580 without LPS had no effect on animal survival or mortality (data not shown).
Figure 5.
Effects of the TRPV1 receptor antagonist CAPZ and the NK1 receptor antagonist RP67580 on survival of rats challenged with LPS. CAPZ (3 mg/kg) or RP67580 (5 mg/kg) was intraperitoneally administered 20 min before and 6 h after intraperitoneal injection of LPS (20 mg/kg). Survival was monitored for 48 h after injection of LPS. Fifteen rats were used in each group.
Discussion
Our current study investigated the effects of the TRPV1 receptor on LPS-induced changes in blood pressure. This study provides evidence showing that pretreatment with CAPZ, a selective TRPV1 receptor antagonist, exaggerated LPS-induced hypotension, suggesting that TRPV1 predominately expressed in sensory nerves participates in the regulation of blood pressure during septic shock. The results are consistent with recent studies by Clark et al (7) demonstrating that a new role of TRPV1 in conferring resistance to hypotension in endotoxemia. Additionally, we further demonstrated that SP released by sensory nerves appeared to be involved, since radioimmunoassay studies revealed increased plasma SP levels during septic shock, and the NK1 receptor antagonists sustained the depressor response induced by LPS. Moreover, the increased plasma catecholamine levels during septic shock were attenuated by the TRPV1 and NK1 receptor antagonists. These observations support the notion that the effects of TRPV1 receptors mainly expressed in sensory nerves on blood pressure during endotoxemia may be attributed to the activation of NK1 receptors by increasing SP release, leading to activation of the sympathetic axis.
Our present results showed that LPS-induced hypotension was exaggerated by the TRPV1 receptor antagonist, CAPZ, suggesting that TRPV1 may be an important target for LPS. It has been well established that the TRPV1 can be activated by multiple stimuli including low pH and prostanoids (23). In addition, Zygmunt et al (49) demonstrated that in rat mesenteric arterial preparation in vitro, anandamide-induced relaxation was almost completely blocked by a selective TRPV1 receptor antagonist, CAPZ, but not by a selective CB1 receptor antagonist, SR141716A. Considering that endotoxemia may be associated with tissue acidification and increased production of several eicosanoids, including anandamide and arachidonic acid metabolites (30, 40, 46), it is likely that TRPV1 receptors are activated during endotoxemia leading to CGRP and SP release. However, the seemly opposite effect of TRPV1 on preventing LPS-induced hypotension indicates that different sequences of events or pathways mediate distinct effects of TRPV1 in vitro and in vivo, which is discussed below.
It is known that the death in septic shock is attributed to refractory hypotension or progressive multiple organ failures. The hallmark of septic shock is marked peripheral arteriolar vasodilation, which results in low systemic vascular resistance, high cardiac output, severe hypotension, and inadequate tissue perfusion (3, 33). The important compensatory response to hypotension includes an immediate baroreceptor sensing of the hypotension which initiates an autonomic response; i.e., sympathetic outflow to both heart and peripheral vessels is increased and serves to restore blood pressure to normal. Experimental models of septic shock indicate that the baroreflex, which regulates peripheral sympathetic nerve activity, is increased (2, 26). Moreover, human studies have provided convincing evidence showing that septic shock is characterized by an increased sympathetic nerve activity (13, 25). In agreement with these studies, we found that plasma catecholamine levels were increased during LPS-induced hypotension, suggesting that an increased sympathetic tone occurred in the pathological condition.
In addition, LPS initiates the release of nitric oxide, free radicals, and cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF- α)(33, 41, 47), which contribute to LPS-induced vasodilation and/or decreased cardiac contractility (33, 41). Thus, the net effect of LPS on vascular tone and cardiac contractility depends on the balance between sympathetically mediated vasoconstriction and positive inotrophy and the opposing vasodilatory and negative inotrophy actions of local and circulating vasoactive mediators. Based on the fact that blockade of the TRPV1 inhibits the rise of plasma catecholamine levels and exaggerates LPS-induced hypotension, we proposed that removal of enhanced sympathetic nerve activity by blockade of the TRPV1 would leave vasodilatory and negative inotrophy actions of local and circulating vasoactive factors unopposed, leading to exaggeration of LPS-induced hypotension.
SP is an important member of the family of structurally related peptides named tachykinins. Tachykinin receptors are G protein-coupled receptors, termed NK1, NK2, and NK3. Although SP may activate all three tachykinin receptors, its potency is greatest when binding to the NK1 receptor subtype (18, 27). The nucleus tractus solitarius (NTS) plays a crucial role in the control of cardiovascular function via baroreflex. High densities of NK1 receptors have been identified in the NTS areas, which are involved in the transmission of cardiovascular reflexes (19, 29). Several lines of evidence show that microinjections of SP or neurokinin receptor agonists into the NTS increase blood pressure and baroreflex sensitivity (1, 14, 31), although the opposite response has also been reported (19). These functional studies confirm anatomical evidence that suggests that NK1 receptors modulate baroreflex control.
In addition, SP has a direct action on postganglionic sympathetic nerve activity. Immunohistochemical and receptor autoradiographic studies indicate that SP-positive sensory nerves and post-ganglionic sympathetic neurons may constitute a peripheral reflex mechanism (8, 22, 28). Moreover, several studies have shown that sympathetic ganglia stimulated by SP in situ increase renal sympathetic nerve activity, blood pressure, and heart rate (15, 16). The increases in these parameters can be attenuated by the selective NK1 receptor antagonist, GR-82334, indicating that SP increases postganglionic sympathetic nerve activity via activation of NK1 receptors (35). On the other hand, SP has a direct action on endothelial NK1 receptors to cause vasodilation by release of endothelium-derived relaxant factor (42). Thus, the actions of SP on blood pressure are determined by the balance between vasodilation and increased sympathetic nerve activity stimulated by SP. We found that the NK1 receptor antagonist RP67580 or L-733,060 aggravated LPS-induced hypotension in the present study, suggesting that the increased sympathetic nerve activity stimulated by SP activation of NK1 is intense enough to override the vasodilator action.
The recent development of selective, non-peptide NK1 receptor antagonists has enabled investigation of the role of tachykinin. RP67580 and L-733,060, both non-peptide antagonists, are generally more potent, selective, and stable than the previous peptide tachykinin NK1 receptor antagonist (11, 36). Our results, obtained with the use of these two antagonists, point to a protective role of NK1 receptors in LPS-induced hypotension. L-733,060 readily crosses the blood-brain barrier to interact with central NK1 receptors after systemic administration (9, 34). In contrast, RP67580 has poor brain penetration in rats (20). Based on the effects of RP67580 and L-733,060 on the LPS-induced hypotension in the present study, we speculate that peripheral NK1 receptors may primarily contribute to the recovery of LPS-induced hypotension. However, further studies are required to discriminate between the central and peripheral effects mediated by tachykinin NK1 receptor on the LPS-induced hypotension.
In patients with severe septic shock, profoundly low peripheral vascular resistance often persists in the presence of high levels of circulating endogenous catecholamines (13, 25). Hypotension that ensues is frequently resistant to high doses of α-adrenergic agonists, and may lead to inadequate perfusion of vital organs and death (6). Nevertheless, it is possible that removal of TRPV1 or its neuropeptide-mediated sympathetic vasoconstriction would further compromised tissue perfusion. This notion was supported by the fact that TRPV1 or tachykinin NK1 antagonist exaggerated LPS-induced hypotension. Furthermore, our findings showed that pretreatment with antagonists of TRPV1 or tachykinin NK1 lowers the survival rate of endotoxemic rats.
Taken together, these data point to the conclusion that SP released from sensory nerve terminals upon TRPV1 activation plays a protective role against LPS-induced hypotension and mortality via action of the NK1 receptors. It appears that, however, the changes in blood pressure and HR by blockade of TRPV1 and NK1 during LPS administration were not fully the same as shown in Figure 1, in which blockade of NK1 prevented recovery of both blood pressure and heart rates whereas heart rates but not blood pressure recovered in the case of TRPV1 blockade. Indeed, in addition to SP, CGRP is a common neuropeptide released from sensory nerves when TRPV1 is activated. CGRP is a potent vasodilator and has been shown to inhibit sympathetic nerve activity (32, 38). It is likely that the apparent discrepancies between blockade of TRPV1 and NK1 were that, when TRPV1 was blocked, both SP-induced stimulation and CGRP-induced inhibition of the sympathetic nervous system were removed; whereas in the case of NK1 blockade, CGRP-mediated action was left unopposed leading to lower blood pressure and HR than that of TRPV1 blockade as shown in Figure 1. This notion is supported by the findings showing that the differences in MAP and HR between TRPV1 and NK1 receptor blockade during LPS injection were prevented by blockade of the CGRP receptor by CGRP8-37.
Perspectives
On the basis of results of the present study, it appears that TRPV1 and SP play a protective role against endotoxin-induced hypotension and mortality. These findings may have broader perspective and significance. Although the pathophysiological changes of septic shock have been studies extensively and for decades, the mortality rate is still unacceptably high and effective treatment strategies yet to be developed. The traditional treatment approaches and available therapeutic means have been focused on altering the activity of the sympathetic nervous systems. The data presented in the present study indicate that TRPV1 expressed in the sensory nerves and TRPV1-mediated sensory neuropeptide release involves in the septic shock process and regulate sympathetic nervous activity. It is conceivable that modulation of TRPV1 function may serve as an effective means and may be beneficial in treating hypotension as well as in reducing the complications and mortality resulting from septic shock.
Acknowledgment
This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
References
- 1.Abdala AP, Haibara AS, Colombari E. Cardiovascular responses to substance P in the nucleus tractus solitarii: microinjection study in conscious rats. Am J Physiol Heart Circ Physiol. 2003;285:H891–H898. doi: 10.1152/ajpheart.00869.2002. [DOI] [PubMed] [Google Scholar]
- 2.Andrew PS, Kaufman S. Splenic denervation worsens lipopolysaccharide-induced hypotension, hemoconcentration, and hypovolemia. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1564–R1572. doi: 10.1152/ajpregu.2001.280.5.R1564. [DOI] [PubMed] [Google Scholar]
- 3.Bátkai S, Pacher P, Járai Z, Wagner JA, Kunos G. Cannabinoids antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Cattaruzza F, Cenac N, Barocelli E, Impicciatore M, Hyun E, Vergnolle N, Sternini C. Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents. Am J Pathol. 2006;169:177–188. doi: 10.2353/ajpath.2006.051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernow BL, Roth B. Pharmacological manipulation of the peripheral vasculature in shock: clinical and experimental approaches. Circ Shock. 1986;18:141–155. [PubMed] [Google Scholar]
- 7.Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21:1–9. doi: 10.1096/fj.06-7460com. [DOI] [PubMed] [Google Scholar]
- 8.Dalsgaard CJ, Hökfelt T, Elfvin LG, Skirboll L, Emson P. Substance P-containing primary sensory neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience. 1982;7:647–654. doi: 10.1016/0306-4522(82)90070-7. [DOI] [PubMed] [Google Scholar]
- 9.Duffy RA, Varty GB, Morgan CA, Lachowicz JE. Correlation of neurokinin (NK) 1 receptor occupancy in gerbil striatum with behavioral effects of NK1 antagonists. J Pharmacol Exp Ther. 2002;301:536–542. doi: 10.1124/jpet.301.2.536. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 11.Garret C, Carruette A, Fardin V, Moussaoui S, Peyronel JF, Blanchard JC, Laduron PM. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci USA. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 13.Groves AC, Griffiths J, Leung F, Meek RN. Plasma catecholamines in patients with serious postoperative infection. Ann Surg. 1973;178:102–107. doi: 10.1097/00000658-197307000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall ME, Miley FB, Stewart JM. Cardiovascular effects of substance P peptides in the nucleus of the solitary tract. Brain Res. 1989;497:280–290. doi: 10.1016/0006-8993(89)90273-4. [DOI] [PubMed] [Google Scholar]
- 15.Hancock JC, Lindsay GW. Pressor and tachycardiac responses to intravenous substance P in anesthetized rats. Peptides. 1995;16:1439–1445. doi: 10.1016/0196-9781(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 16.Hancock JC, Lindsay GW. Enhanced ganglionic responses to substance P in spontaneously hypertensive rats. Peptides. 2000;21:535–541. doi: 10.1016/s0196-9781(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 17.He H, Stein CM, Christman B, Wood AJ. Determination of catecholamines in sheep plasma by high-performance liquid chromatography with electrochemical detection: comparison of deoxyepinephrine and 3,4-dihydroxybenzylamine as internal standard. J Chromatogr B Biomed Sci Appl. 1997;701:115–119. doi: 10.1016/s0378-4347(97)00343-5. [DOI] [PubMed] [Google Scholar]
- 18.Helke CJ, Krause JE, Mantyh PW, Couture R, Bannon MJ. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. [PubMed] [Google Scholar]
- 19.Helke CJ, Seagard JL. Substance P in the baroreceptor reflex: 25 years. Peptides. 2004;25:413–423. doi: 10.1016/j.peptides.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Holzer-Petsche U, Rordorf-Nikolic T. Central versus peripheral site of action of the tachykinin NK1 antagonist RP67580 in inhibiting chemonociception. Br J Pharmacol. 1995;115:486–490. doi: 10.1111/j.1476-5381.1995.tb16359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 22.James S, Burnstock G. Autoradiographic localization of binding sites for 125I-substance P on neurons from cultured rat superior cervical ganglia. Brain Res. 1988;458:205–211. doi: 10.1016/0006-8993(88)90462-3. [DOI] [PubMed] [Google Scholar]
- 23.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 24.Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertesion. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- 25.Leinhardt DJ, Arnold J, Shipley KA, Mughal MM, Little RA, Irving MH. Plasma NE concentrations do not accurately reflect sympathetic nervous system activity in human sepsis. Am J Physiol Endocrinol Metab. 1993;265:E284–E288. doi: 10.1152/ajpendo.1993.265.2.E284. [DOI] [PubMed] [Google Scholar]
- 26.MacNeil BJ, Jansen AH, Janz LJ, Greenberg AH, Nance DM. Peripheral endotoxin increases splenic sympathetic nerve activity via central prostaglandin synthesis. Am J Physiol Regul Integr Comp Physiol. 1997;273:R609–R614. doi: 10.1152/ajpregu.1997.273.2.R609. [DOI] [PubMed] [Google Scholar]
- 27.Maggio JE. Tachykinins. Ann Rev Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- 28.Mantyh PW, Catton MD, Allen CJ, Labenski ME, Maggio JE, Vigna SR. Receptor binding sites for cholecystokinin, galanin, somatostatin, substance P and vasoactive intestinal polypeptide in sympathetic ganglia. Neuroscience. 1992;46:739–754. doi: 10.1016/0306-4522(92)90160-4. [DOI] [PubMed] [Google Scholar]
- 29.Mantyh PW, Gates T, Mantyh CR, Maggio JE. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J Neurosci. 1989;9:258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978;93:526–617. [PMC free article] [PubMed] [Google Scholar]
- 31.Nagashima A, Takano Y, Tateishi Y, Matsuoka T, Hamaoka T, Kamiya H. Cardiovascular roles of tachykinin peptides in the nucleus tractus solitarii of rats. Brain Res. 1989;487:392–396. doi: 10.1016/0006-8993(89)90848-2. [DOI] [PubMed] [Google Scholar]
- 32.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, Nagai R, Kuwaki T, Kurihara H. Elevated sympathetic nervous activity in mice deficient in αCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 33.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 34.Rupniak NM, Carlson E, Boyce S, Webb JK, Hill RG. Enantioselective inhibition of the formalin paw late phase by the NK1 receptor antagonist L-733,060 in gerbils. Pain. 1996;67:189–195. doi: 10.1016/0304-3959(96)03109-0. [DOI] [PubMed] [Google Scholar]
- 35.Schoborg RV, Hoover DB, Tompkins JD, Hancock JC. Increased ganglionic responses to substance P in hypertensive rats due to upregulation of NK1 receptors. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1685–R1694. doi: 10.1152/ajpregu.2000.279.5.R1685. [DOI] [PubMed] [Google Scholar]
- 36.Seabrook GR, Shepheard SL, Williamson DJ, Tyrer P, Rigby M, Cascieri MA, Harrison T, Hargreaves RJ, Hill RG. L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]i mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- 37.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 38.Takenaga M, Kawasaki H. Endogenous calcitonin gene-related peptide suppresses vasoconstriction mediated by adrenergic nerves in rat mesenteric resistance blood vessels. Eur J Pharmacol. 1999;367:239–245. doi: 10.1016/s0014-2999(98)00949-2. [DOI] [PubMed] [Google Scholar]
- 39.Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- 40.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 41.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 42.Wallerstedt SM, Bodelsson M. Endothelium-dependent relaxation by substance P in human isolated ometal arteries and veins: relative contribution of prostanoids, nitric oxide and hyperpolarization. Br J Pharmacol. 1997;120:25–30. doi: 10.1038/sj.bjp.0700879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Kaminski NE, Wang DH. Endocannabinoid regulates blood pressure via activation of the TRPV1 in Wistar rats fed a high salt diet. J Pharmacol Exp Ther. 2007;321:763–769. doi: 10.1124/jpet.106.112904. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Wang DH. Prevention of endothelin-1-induced increases in blood pressure: role of endogenous CGRP. Am J Physiol Heart Circ Physiol. 2004;287:1868–1874. doi: 10.1152/ajpheart.00241.2004. [DOI] [PubMed] [Google Scholar]
- 46.West MA, Wilson C. Hypoxic alterations in cellular signal transduction in shock and sepsis. New Horiz. 1996;4:168–178. [PubMed] [Google Scholar]
- 47.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 48.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vailloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]