Abstract
BACKGROUND
Despite the importance of medication adherence in heart failure (HF), clinically relevant cutpoints for distinguishing the level of adherence associated with outcomes are unknown.
OBJECTIVE
To determine the cutpoint above which there is a positive relationship between level of medication adherence and event-free survival.
METHODS
This was a longitudinal study of 135 patients with HF. Medication adherence was measured using a valid and objective measure, the Medication Event Monitoring System (MEMS). Two indicators of adherence were assessed by the MEMS: 1) dose-count, percentage of prescribed doses taken and 2) dose-days, percentage of days correct number of doses taken. Patients were followed up to 3.5 years to collect data on outcomes. A series of Kaplan-Meier plots with log-rank tests, Cox-survival analyses, and receiver operating characteristic (ROC) curves were assessed comparing event-free survival in patients divided at one point incremental cutpoints.
RESULTS
Event-free survival was significantly better when the prescribed number of doses taken (dose-count) or the correct dose (dose-day) was ≥ 88%. This level was confirmed in a Cox regression model controlling for age, gender, ejection fraction, NYHA, comorbidity, angiotensin-converting enzyme inhibitor use, and beta-blocker use. ROC curves showed that adherence rates above 88% produced the optimal combination of sensitivity and specificity with respect to predicting better event-free survival. With 88% as the adherence cutpoint, the hazard ratio for time to first event for the nonadherent group was 2.2 by dose-count (p=.021) and 3.2 by dose-day (p=.002).
CONCLUSION
The results of this study provide clinicians and researchers with an evidence-based recommendation about the level of adherence needed to achieve optimal clinical outcomes.
Keywords: medication adherence, heart failure, outcomes, MEMS
Introduction
Heart failure (HF) is a serious and costly cardiovascular disorder, but its natural history can be modified by appropriate, sustained drug therapy.1 Lifelong adherence to prescribed medications is an important determinant of optimal health outcomes. Poor adherence to prescribed medication therapy increases the risk of mortality and morbidity2 and leads to high health costs2, 3 and poor quality of life4 in patients with HF. A significant portion of the healthcare advice and prescriptions dispensed at HF medical encounters was not fully implemented due to nonadherence. The annual health care costs related to nonadherence are estimated to be as high as $300 billion per year.5
Despite the importance of adherence, the level of medication adherence that distinguishes clinically significant adherence vs. nonadherence is unknown.6 Although 100% medication adherence is the desired goal, it may be unrealistic to expect patients to achieve perfect medication adherence and there is no evidence in HF suggesting that 100% adherence is needed to achieve optimal outcomes. In prior investigations, adherence has been defined arbitrarily as taking between 70% and 100% of a medication as prescribed.7, 8 The reason for choosing these cutpoints is unclear and not based on empirical data.9-13 Further, levels of medication adherence may not be clinically relevant as they are not based on evidence that links frequency and dosing with clinical outcomes.14 An unambiguous cutpoint is needed to help researchers analyze data and to provide an objective adherence level for patients and clinicians. Accordingly, the purpose of this study was to determine the level of medication adherence associated with better health outcomes, specifically, time to the composite endpoint of emergency department (ED) visits for HF exacerbation, cardiac rehospitalizations and all-cause mortality.
Methods
Study Design
In this prospective study, we used the composite endpoint of time to first event (i.e., ED visit for HF exacerbation, cardiac rehospitalization, or all-cause mortality) as a criterion to determine the level of medication adherence required to achieve the longest time to event after controlling for demographic and clinical variables (i.e., age, gender, medication regimen, comorbidity, New York Heart Association [NYHA] functional class and left ventricular ejection fraction [LVEF]).
Samples and Setting
Detailed eligibility criteria and recruitment methods have been published previously.15 Briefly, patients were recruited from outpatient cardiology clinics in Central Kentucky. Patients with a confirmed diagnosis of chronic HF and stable doses of HF medications were enrolled. Patients were excluded if they had obvious cognitive impairment (i.e., not able to give informed consent or participate in an interview) or a co-existing terminal illness such as cancer.
Measurement of Variables
Patients’ demographic and clinical data were collected by patient interview or medical record review and medication adherence monitoring with the MEMS was started. Data on ED visits, hospitalizations and survival were assessed monthly by phone and at the end of the study by examining the hospital administrative database.
Medication Adherence
Medication adherence was measured continuously for 3 months using an unobtrusive microelectronic monitoring device in the caps of a medication vial (AARDEX®). The Medication Event Monitoring System (MEMS) is an objective measure considered the gold-standard for the measurement of medication adherence.16, 17 An electronic chip in the medication cap records each date and time the cap is removed from the medication bottle. Patients were given a medication diary to record unscheduled cap openings, such as those to refill the bottle, so that unscheduled events could be removed from analysis.
In this study, MEMS data were collected from one HF medication for each patient. Prior research has demonstrated that monitoring one medication provides a valid indicator of all medication-taking behavior.16, 17 The medication chosen to be monitored was based on the following criteria. If the patient was taking a medication twice a day, this medication was chosen for monitoring using the MEMS. If all medications were taken twice or only once per day, then the beta-adrenergic blocking agent was chosen unless the patient was not prescribed one. In those cases, the angiotension-converting-enzyme (ACE) inhibitor or angiotensin receptor blocker was used. If no beta-blocker or ACE inhibitor was prescribed, digoxin or a diuretic was used in the MEMS device.
Two indicators of medication adherence from the MEMS were used in analysis: 1) dose-count, defined as the percentage of prescribed number of doses taken during the 3-month monitoring period; and 2) dose-day, defined as the percentage of days the correct number of doses taken.15 These two indicators were chosen because they were the best predictors of event-free survival in our prior study.15 Groups (above and below a given percentage of adherence) were created based on the medication adherence rate measured by the MEMS device.
Time to First Event
The dependent variable was the time to the first event. Events considered were ED visits for symptoms of decompensated HF, cardiac rehospitalizations and mortality. Patients were asked to keep a diary of events. In addition, data were determined by a combination of medical record review, review of hospital administrative records, and patient and family interview. Dates and reasons for events were noted after the medical record was carefully reviewed to confirm the visit date and reason. Patients/families were interviewed to obtain self-reports to augment automated data because the patient many have been admitted to EDs or hospitals outside of the system. If the admission was outside the system, a patient release was obtained and the medical record of the visit was reviewed. In all cases, conflicting data between patient report and administrative records were resolved with review of the medical record and interview of the patient and family.
Additional methods were used to track mortality. At enrollment, patients were asked for contact information on a relative or close friend to be used if they could not be contacted. If unable to reach patients by telephone, we contacted healthcare providers and checked automated hospital records to determine if the patient had died. If evidence was not found, we contacted the friend or relative. If neither the patient nor their contacts could be located during follow-up or if additional information was needed, the county death records were searched. Although death certificates were usually a valid source of data about the date of death, they were less valid for determining cause of death and supplemental data were always sought to establish whether the death was cardiac or non-cardiac.
In this study, NYHA functional class, age, gender, LVEF, medication regimen and comorbidity were collected as covariates. NYHA was determined by standardized patient interview.18 Patients’ age, gender, LVEF and medication regimen (i.e., ACE inhibitor [yes/no], β blocker [yes/no], diuretics [yes/no], digoxin [yes/no], aldosterone antagonist [yes/no]) were collected from the medical record, and patient interview.
Comorbidity was measured using the interview format of the Charlson Index.19, 20 At enrollment, patients were queried about preexisting diseases (e.g., ulcer disease, diabetes). Scores can range from 0 to 34 but because each patient had HF, they had a score of at least 1. Validity was supported in prior research in which comorbidity category predicted mortality, complications, health care resource use, length of hospital stay, and discharge disposition.19, 21
Protocol
Permission for the conduct of the study was obtained from the University of Kentucky (UK) Institutional Review Board (IRB). Patients were referred to this project by nurse practitioners in the HF clinic. Patient eligibility was confirmed by a trained research associate. The research associate then explained study requirements to eligible patients and obtained informed, written consent.
At baseline, patients’ sociodemographic and clinical characteristics were collected by interview and medical record review. After interview, detailed written and verbal instructions on use of the MEMS bottle were given to patients. Patients were informed about the purpose of the MEMS and instructed to take the specified medicine from MEMS bottle for the next three months and close the lid after each use. They were trained to use their medication diary to record unscheduled cap openings. If patients opened the bottle for any reason not related to taking medication, the time and date were recorded in the diary, so that event could be excluded, if appropriate, when data were downloaded. Patients who used a pill box were asked to keep the MEMS bottle beside their pill box and take that medicine from the MEMS bottle.
After three months of continuous use of the MEMS bottle, patients returned the bottle. The data from the MEMS cap were downloaded using a manufacturer-supplied communicator and software installed on a personal computer. Unscheduled cap openings were excluded from analysis based on the medication diary recorded by patients. The MEMS data were then printed and entered into a data base for further analyses.
Data Management and Analysis
All data analyses were done using SPSS, version 15.0; a significance level of .05 was used throughout. The log-rank test was used to compare the time to event-free survival between groups formed by dividing the sample at varying levels of adherence. Because no standard cutpoint exists, patients were divided into groups (above and below a given percentage of adherence) based on their medication adherence rate measured by the MEMS using one point incremental cutpoints. Kaplan-Meier plots were used to graphically depict group differences in event-free survival. Cox proportional hazards regression modeling was used to assess the time to the composite endpoint, while controlling for the following potential covariates: age, gender, baseline NYHA, LVEF, comorbidity, ACE inhibitor use (yes/no), and any baseline variables upon which the groups differed. Baseline difference between groups were examined using either two-sample t-test (for continuous variables), Mann-Whitney U tests (for ordinal variables), or chi-square tests (for nominal variables). Receiver operating characteristic (ROC) curves were used to summarize the relationship between level of medication adherence and the prediction of negative clinical outcomes, as a function of differing levels of adherence.
Results
Patient Characteristics
We recruited 147 of the 301 eligible HF patients approached for the study; 152 patients refused to participate due to long travel distance, time concerns (e.g., have to take care of other family members), no interest in participating in research, or lack of energy. In this study, we only included data from the 135 for whom we have full data from the MEMS. MEMS data were missing in 12 patients because of malfunction of the MEMS cap (n = 2), loss of the MEMS cap or patient death (n = 6), or problems with the software interface (n = 4). Sample characteristics are presented in Table 1.
Table 1.
Sample Characteristics and Comparison of Patients’ Characteristics in Adherent and Nonadherent Groupsa (N = 135)
| Characteristics | Total Sample | Nonadherent n = 60 | Adherent n = 75 | P Value* |
|---|---|---|---|---|
| Age, years | 61 ± 11 | 61 ± 12 | 61 ± 11 | .815 |
| Female | 41 (30.4) | 20 (33.3) | 21 (28.0) | .574 |
| Black race | 14 (10.4) | 8 (13.3) | 6 (8.0) | .398 |
| Education, years | 12.7 ± 3.3 | 12.3 ± 3.3 | 12.7 ± 3.2 | .502 |
| Marital status | .096 | |||
| Single | 13 (9.6) | 9 (15.0) | 4 (5.3) | |
| Married | 84 (62.2) | 31 (51.7) | 53 (70.7) | |
| Divorced | 16 (11.9) | 9 (15.0) | 7 (9.3) | |
| Widowed | 22 (16.3) | 11 (18.3) | 11 (14.7) | |
| Living alone | 40 (29.6) | 18 (30.0) | 22 (29.3) | 1.0 |
| Financial status | .915 | |||
| Comfortable | 32 (24.1) | 13 (22.4) | 19 (25.3) | |
| Enough to make ends meet | 71 (53.4) | 32 (55.2) | 39 (52.0) | |
| Not enough to make ends meet | 30 (22.6) | 13 (22.4) | 17 (22.7) | |
| LVEF, % | 34.6 ± 14.2 | 35.3 ± 14.0 | 34.1 ± 14.5 | .646 |
| NYHA functional class | .595 | |||
| I/II | 51 (38.9) | 20 (35.1) | 31 (41.9) | |
| III | 61 (46.6) | 27 (47.4) | 34 (45.9) | |
| IV | 19 (14.5) | 10 (17.5) | 9 (12.2) | |
| Charlson comorbidity index | 3.3 ± 1.7 | 3.7 ± 1.7 | 3.1 ± 1.6 | .038 |
| ACEI | .127 | |||
| Yes | 97 (71.9) | 39 (65.0) | 58 (77.3) | |
| BB | .584 | |||
| Yes | 120 (88.9) | 52 (86.7) | 68 (90.7) |
P value for comparison of adherent and nonadherent groups
Patients were classified as adherent when 88% or above of days they took correct dose.
Patients were classified as nonadherent if less than 88% of days they took correct dose. Data are presented as means ± SD, or N (%); ACEI = angiotensin-converting-enzyme inhibitor; BB = beta blocker; LVEF = Left Ventricular Ejection Fraction; NYHA = New York Heart Association
Survival Analyses
Kaplan-Meier plots with log-rank tests were used to compare patients’ time to first occurrence of the composite endpoint between groups created by various medication adherence cutpoints. Using this method, we determined that 88% was the first cutpoint at which patients’ times to first event were significantly different. That is, when patients were divided into two groups that consisted of those taking ≥ 88% of the prescribed number of doses in the time period examined (dose-count) and those taking less than 88%, adherence level predicted the composite endpoint (Figure 1). The 88% cutpoint was also predictive of the composite endpoint using the dose-day indicator (percentage of days the patient took the correct dose (Figure 1).
Figure 1.
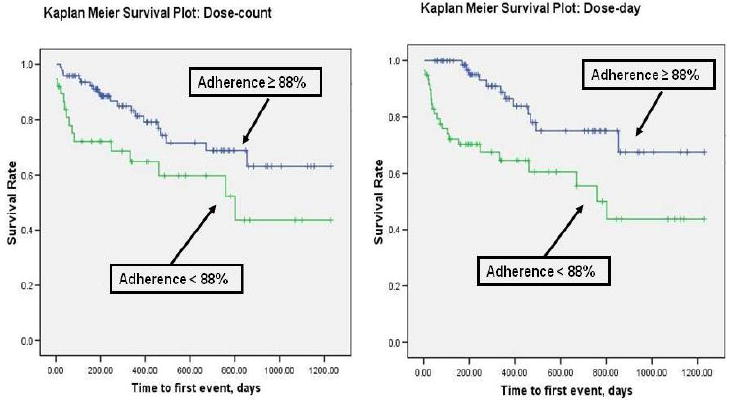
Medication Adherence and Time to First Event of Emergency Department Visits, Rehospitalization, or Mortality
When the 88% cutpoint was identified we compared patient characteristics by groups formed by this cutpoint (Table 1). Groups did not differ on sociodemographic characteristics; however, adherent patients had a lower comorbidity index. Using this cutpoint, 44% of patients were considered to be nonadherent, while the remaining 56% had adequate adherence.
The result from the Kaplan-Meier analysis was confirmed in Cox regression modeling (Table 2) after adjusting for potential confounding factors. The composite endpoint was consistently predicted by medication adherence after controlling for sociodemographic and clinical factors regardless of whether the dose-day or dose-count indicator was used. The hazard ratio for time to the composite endpoint for patients with inadequate adherence was 2.2 (by dose-count) to 3.2 (by dose-day; p = .021 and .002, respectively). In addition to medication adherence for both dose-count and dose-day models, being on a beta-blocker was an independent predictor of the composite endpoint (p = .04 and .03, respectively).
Table 2.
Impact of Medication Adherence on Event-free Survival
| Variables | Hazard Ratio | Wald | Significance |
|---|---|---|---|
| *Dose-count a Cox regression model | |||
| Adherent vs. nonadherent based on cutpoint of 88% | 2.208 | 4.289 | .038 |
| Age | 1.001 | .005 | .946 |
| Gender | .888 | .074 | .785 |
| LVEF | .981 | 1.383 | .240 |
| NYHA | 1.015 | .003 | .953 |
| Comorbidity | 1.070 | .227 | .634 |
| Med_ACEI | .912 | .042 | .838 |
| Med_BB | .349 | 4.210 | .040 |
| **Dose-day b Cox regression model | |||
| Adherent vs. nonadherent based on cutpoint of 88% | 3.165 | 8.8770 | .003 |
| Age | 1.004 | .055 | .815 |
| Gender | 1.013 | .001 | .977 |
| LVEF | .976 | 2.299 | .129 |
| NYHA | .965 | .018 | .892 |
| Comorbidity | 1.045 | .088 | .766 |
| Med_ACEI | .934 | .023 | .879 |
| Med_BB | .330 | 4.729 | .030 |
χ2 = 16.526, P = .035;
χ2 = 21.473, P = .006
Dose-count: % of prescribed number of doses taken;
Dose-day: % of days the correct number of doses taken
Receiver Operating Characteristic Curves
From ROC curves, we confirmed that time to the composite endpoint between those who adhered and did not adhere was different when a medication adherence cutoff rate = 88% was used to dichotomize patients (p < .05). An 88% adherence rate resulted in an optimal combination of sensitivity (.770 and .610, respectively) and specificity (.0.486 and 0.686, respectively) in the prediction of the composite endpoint.
Discussion
This was the first study to use patients’ composite endpoint of time to ED visit for HF exacerbation, cardiac hospitalization and mortality as a criterion to determine the level of medication adherence required to achieve the best clinical outcomes in patients with HF. To date, levels of medication adherence have been defined based on expert opinion and varied widely. Our study demonstrated that a medication adherence rate of 88% is positively associated with a composite endpoint of time to ED visit, hospitalization and mortality outcomes and provides a clinically relevant cutpoint for clinicians. Over a 30-day period, a dose-count of 88% means that patients need to take at least 53 of 60 doses of a drug prescribed twice a day. A dose-day of 88% means that patients must take the correct number of doses for at least 26 days within a 30-day cycle. These results demonstrate that a high level of adherence is necessary to achieve a longer event-free survival period.
There are two ways medication adherence has been reported in the adherence research: 1) data used as a continuous variable, and 2) data used as a dichotomous variable. When investigators used medication adherence as continuous data, they commonly found a significant relationship between medication adherence and outcomes, even though measures of medication adherence differed (i.e., self-report,22-24 pharmacy refill,25-27 pill count28 and the MEMS15, 29) and patient populations differed (i.e., patients with HF,15, 22, 29, myocardial infarction,23 diabetes,24, 25 or coronary heart disease26, 28).
However, the most common method of using adherence data in the literature has been to choose a cutpoint to dichotomize patients as adherent or nonadherent. Several investigators have grouped patients by arbitrarily chosen cutpoints, and examined the relationship of medication adherence with ED visits, rehospitalization and mortality.2, 9, 12 In some of the studies, medication adherence predicted health outcomes;2, 9, 12 while in others it did not.30, 31 The different results from previous studies may reflect the manner in which adherence was operationalized. For example, in one study,9 investigators used clinician estimates to place patients in a adherent or nonadherent group (i.e., 80% used as the cutpoint to form the two groups) in a sample of 7599 patients with HF. Investigators found that good adherence was associated with lower all-cause mortality. However, Billups, et al.30 studied the relationship between drug therapy nonadherence and health outcomes in 1054 patients at high risk for drug-related problems. Eighty percent was also chosen as the cutpoint. The investigators found that adherence was not a predictor of concurrent or future hospitalization, mortality, or health care costs. Without an evidence-based cutpoint, it is hard to judge which result is more trustworthy.
Eighty percent is the most commonly used cutpoint to dichotomize patients into adherent or nonadherent groups,9-11, 32-40 although other investigators chose 75%12, 13 or 90%41, 42 as cutpoints. The rationale for choosing 75%, 80% or 90% was either not given or arbitrarily chosen by prior investigators. Many investigators used pharmacy computer databases to retrieve patients’ prescription refill history and calculate the refill rate to define medication adherence rates. In such studies, patients who refilled 80%-120% of the mediations were defined as adherent and those who refilled less than 80% or greater than 120% as nonadherent.2, 43 Again, the cutpoint is not based on empirical evidence. The current study is the first to generate empirical evidence on the level of adherence needed to achieve a longer time to event in people with HF.
In addition to medication adherence, we demonstrated that beta-blocker use was an important independent predictor of event-free survival. Along with medication adherence, beta-blocker use predicted a longer time to the composite endpoint. Multiple large scale, multicenter, randomized controlled trials have demonstrated the importance of beta-blocker therapy to HF patients outcomes.44 Our data are consistent with these data and current consensus guidelines.
A strength of our study was use of the MEMS to measure medication adherence. The MEMS is objective, non-invasive and accurate for measuring medication adherence in research settings.45 The MEMS is superior to clinician-estimate, self-report or pharmacy refill methods of measuring medication adherence.2, 9, 12, 13 The clinician-estimate method is a poor measure of assessing actual patient adherence as it is subjective and indirect.46 The self-report method is subject to recall bias and social desirability and lacks consistency in the detection of patients who are nonadherent.15 Pharmacy refill measures are dissociated from actual medication consumption.47 Obtaining serum drug levels is an objective and direct measure of adherence, but such biological assays are invasive, not feasible for most settings, not affordable, and not available for all drugs used.48
To our knowledge, there has been only one study conducted, in HIV-infected patients, in which an evidence-based method was used to identify the cutpoint for medication adherence.49 In that study, the investigators used the MEMS to measure medication adherence and cluster analysis to determine what level of adherence to use to differentiate adherent patients from nonadherent. Patients categorized as adherent had a larger drop in viral load and rise in CD4+ cell count, demonstrating the importance of defining medication adherence systematically.
Conclusion
In this study, we identified an evidence-based cutpoint by which to define medication adherence in patients with HF. Patients who take 88% of their prescribed medication doses and on 88% of days take the correct dose experience a longer event-free survival than patients who are less adherent. These findings can be used by researchers in future studies of adherence and by clinicians in evaluating their patients’ adherence levels.
Acknowledgments
Funding Sources: This study was supported by funding from the Philips Medical-American Association of Critical Care Nurses Outcomes Grant, University of Kentucky General Clinical Research Center (M01RR02602), grant # R01 NR008567 from the National Institute of Nursing Research and a Center grant to the University of Kentucky College of Nursing from NIH, NINR, 1P20NR010679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
Disclaimer Statement: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Air Force or the Department of Defense.
Disclosures: No conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jia-Rong Wu, University of Kentucky, College of Nursing.
Debra K. Moser, University of Kentucky, College of Nursing.
Terry A. Lennie, University of Kentucky, College of Nursing.
Marla J. De Jong, DoD Blast Injury Research Program Coordinating Office, U.S. Army Research and Materiel Command.
Mary Kay Rayens, University of Kentucky, College of Nursing.
Misook L. Chung, University of Kentucky, College of Nursing.
Barbara Riegel, University of Pennsylvania, School of Nursing.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Hope CJ, Wu J, Tu W, et al. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004;61:2043–2049. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Morrow-Howell N, Proctor EK. Post-acute home care and hospital readmission of elderly patients with congestive heart failure. Health Soc Work. 2004;29:275–285. doi: 10.1093/hsw/29.4.275. [DOI] [PubMed] [Google Scholar]
- 4.Cote I, Farris K, Feeny D. Is adherence to drug treatment correlated with health-related quality of life? Qual Life Res. 2003;12:621–633. doi: 10.1023/a:1025180524614. [DOI] [PubMed] [Google Scholar]
- 5.DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 6.Wu JR, Moser DK, Lennie TA, et al. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin North Am. 2008;43:133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart Lung. 2001;30:294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 8.Gwadry-Sridhar FH, Arnold JM, Zhang Y, et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150:982, e981–e989. doi: 10.1016/j.ahj.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Kindmalm L, Melander A, Nilsson JL. Refill adherence of antihyperglycaemic drugs related to glucose control (HbA1c) in patients with type 2 diabetes. Acta Diabetol. 2007;44:209–213. doi: 10.1007/s00592-007-0006-7. [DOI] [PubMed] [Google Scholar]
- 12.Gehi AK, Ali S, Na B, et al. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454, e451–458. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder K, Fahey T, Ebrahim S, et al. Adherence to long-term therapies: recent WHO report provides some answers but poses even more questions. J Clin Epidemiol. 2004;57:2–3. doi: 10.1016/j.jclinepi.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Wu JR, Moser DK, Chung ML, et al. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008;14:203–210. doi: 10.1016/j.cardfail.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunbar-Jacob J, Bohachick P, Mortimer MK, et al. Medication adherence in persons with cardiovascular disease. J Cardiovasc Nurs. 2003;18:209–218. doi: 10.1097/00005082-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Bouvy ML, Heerdink ER, Urquhart J, et al. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: A randomized controlled study. J Card Fail. 2003;9:404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Mills RM, Jr, Haught WH. Evaluation of heart failure patients: Objective parameters to assess functional capacity. Clin Cardiol. 1996;19:455–460. doi: 10.1002/clc.4960190603. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian U, Eckert G, Yeung A, et al. A single health status question had important prognostic value among outpatients with chronic heart failure. J Clin Epidemiol. 2007;60:803–811. doi: 10.1016/j.jclinepi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Laramee AS, Levinsky SK, Sargent J, et al. Case management in a heterogeneous congestive heart failure population: A randomized controlled trial. Arch Intern Med. 2003;163:809–817. doi: 10.1001/archinte.163.7.809. [DOI] [PubMed] [Google Scholar]
- 23.Ziegelstein RC, Fauerbach JA, Stevens SS, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 24.Piette JD, Heisler M, Krein S, et al. The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165:1749–1755. doi: 10.1001/archinte.165.15.1749. [DOI] [PubMed] [Google Scholar]
- 25.Balkrishnan R, Rajagopalan R, Camacho FT, et al. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25:2958–2971. doi: 10.1016/s0149-2918(03)80347-8. [DOI] [PubMed] [Google Scholar]
- 26.Jackson JE, Doescher MP, Saver BG, et al. Prescription drug coverage, health, and medication acquisition among seniors with one or more chronic conditions. Med Care. 2004;42:1056–1065. doi: 10.1097/00005650-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Skrepnek GH, Abarca J, Malone DC, et al. Incremental effects of concurrent pharmacotherapeutic regimens for heart failure on hospitalizations and costs. Ann Pharmacother. 2005;39:1785–1791. doi: 10.1345/aph.1G124. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. Jama. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 29.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Billups SJ, Malone DC, Carter BL. The relationship between drug therapy noncompliance and patient characteristics, health-related quality of life, and health care costs. Pharmacotherapy. 2000;20:941–949. doi: 10.1592/phco.20.11.941.35266. [DOI] [PubMed] [Google Scholar]
- 31.Rich MW, Gray DB, Beckham V, et al. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am J Med. 1996;101:270–276. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 32.Burnier M, Schneider MP, Chiolero A, et al. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens. 2001;19:335–341. doi: 10.1097/00004872-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Monane M, Bohn RL, Gurwitz JH, et al. Compliance with antihypertensive therapy among elderly Medicaid enrollees: the roles of age, gender, and race. Am J Public Health. 1996;86:1805–1808. doi: 10.2105/ajph.86.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalansky SJ, Levy AR. Effect of number of medications on cardiovascular therapy adherence. Ann Pharmacother. 2002;36:1532–1539. doi: 10.1345/aph.1C044. [DOI] [PubMed] [Google Scholar]
- 35.Chisholm MA, Williamson GM, Lance CE, et al. Predicting adherence to immunosuppressant therapy: a prospective analysis of the theory of planned behaviour. Nephrol Dial Transplant. 2007;22:2339–2348. doi: 10.1093/ndt/gfm149. [DOI] [PubMed] [Google Scholar]
- 36.Schectman JM, Bovbjerg VE, Voss JD. Predictors of medication-refill adherence in an indigent rural population. Med Care. 2002;40:1294–1300. doi: 10.1097/00005650-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. Jama. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 38.McGinnis B, Olson KL, Magid D, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41:1805–1811. doi: 10.1345/aph.1K209. [DOI] [PubMed] [Google Scholar]
- 39.Ekman I, Andersson G, Boman K, et al. Adherence and perception of medication in patients with chronic heart failure during a five-year randomised trial. Patient Educ Couns. 2006;61:348–353. doi: 10.1016/j.pec.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Li WW, Wallhagen MI, Froelicher ES. Hypertension control, predictors for medication adherence and gender differences in older Chinese immigrants. J Adv Nurs. 2008;61:326–335. doi: 10.1111/j.1365-2648.2007.04537.x. [DOI] [PubMed] [Google Scholar]
- 41.Marhefka SL, Farley JJ, Rodrigue JR, et al. Clinical assessment of medication adherence among HIV-infected children: examination of the Treatment Interview Protocol (TIP) AIDS Care. 2004;16:323–338. doi: 10.1080/09540120410001665330. [DOI] [PubMed] [Google Scholar]
- 42.George J, Shalansky SJ. Predictors of refill non-adherence in patients with heart failure. Br J Clin Pharmacol. 2007;63:488–493. doi: 10.1111/j.1365-2125.2006.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen DB, Williams B, Goldberg HI, et al. Assessing compliance to antihypertensive medications using computer-based pharmacy records. Med Care. 1997;35:1164–1170. doi: 10.1097/00005650-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Bouzamondo A, Hulot JS, Sanchez P, et al. Beta-blocker treatment in heart failure. Fundam Clin Pharmacol. 2001;15:95–109. doi: 10.1046/j.1472-8206.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 45.De Geest S, Schafer-Keller P, Denhaerynck K, et al. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20:359–368. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 46.Wagner JH, Justice AC, Chesney M, et al. Patient- and provider-reported adherence: toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol. 2001;54(Suppl 1):S91–98. doi: 10.1016/s0895-4356(01)00450-4. [DOI] [PubMed] [Google Scholar]
- 47.Murray MD, Morrow DG, Weiner M, et al. A conceptual framework to study medication adherence in older adults. Am J Geriatr Pharmacother. 2004;2:36–43. doi: 10.1016/s1543-5946(04)90005-0. [DOI] [PubMed] [Google Scholar]
- 48.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Van Wijngaerden E, De Saar V, De Graeve V, et al. Nonadherence to highly active antiretroviral therapy: clinically relevant patient categorization based on electronic event monitoring. AIDS Res Hum Retroviruses. 2002;18:327–330. doi: 10.1089/088922202753519098. [DOI] [PubMed] [Google Scholar]


