Abstract
Multiple antimuscarinic agents are available for the treatment of overactive bladder. Many of the agents have undergone reformulation in an attempt to improve patient adherence and drug tolerability. Oxybutynin evolved from an immediate-release pill to a once-daily oral preparation, and is now available as a transdermal patch and gel. This article discusses the clinical impact of oxybutynin reformulation and reviews the evolution and benefits of transdermal therapy.
Key words: Oxybutynin, Reformulation, Transdermal therapy
Overactive bladder syndrome (OAB), defined by the International Continence Society as the presence of urinary urgency, with or without urge incontinence, usually associated with frequency and nocturia, affects millions of Americans.1 According to the National Overactive Bladder Evaluation study, OAB prevalence in the United States is 16.9% in women and 16.0% in men.2 The negative impact of OAB on quality of life is significant and should not be underestimated; OAB may result in impaired mobility, social isolation, impaired work-related productivity, depression, disturbed sleep, and impaired domestic and sexual function.3
Several US Food and Drug Administration (FDA)-approved antimuscarinic agents are available in both oral and transdermal formulations. Oxybutynin, the most widely prescribed antimuscarinic agent for over 30 years, evolved from an immediate-release pill to an extended-release oral preparation, and is now available as a transdermal patch and gel. Reformulation of antimuscarinic agents has consistently resulted in improved tolerability and enhanced patient adherence.4
This article assesses the reformulation of oxybutynin and its beneficial effects on efficacy and tolerability. In addition, it discusses the evolution of transdermal/topical treatment of OAB, as well as the benefits of transdermal delivery over oral therapy.
Immediate-Release Oxybutynin
Immediate-release oxybutynin (OXY-IR) is a tertiary amine that has anticholinergic, smooth muscle relaxant, and local anesthetic properties. The acetylenic amino ester has both R- and S-chirality, and its anticholinergic activity has been predominantly attributed to its R isomer. It undergoes extensive first-pass liver metabolism that generally limits its bioavailability to about 6%.
N-desethyloxybutynin (N-DEO) is the primary liver metabolite of oxybutynin. It is an active metabolite shown in vitro to be equivalent in activity to the parent compound. The half-life of OXY-IR is 2 to 5 hours, and the maximum plasma concentration (Cmax) values are achieved at 0.5 to 1.5 hours for the parent and 0.5 to 2 hours for the N-desethyl metabolite.5 Due to variations in the elimination pattern of the parent compound versus N-DEO, there is an approximately 5-fold higher area under the curve (AUC) for the metabolite than the parent. There is general acceptance that the dry mouth associated with oxybutynin is largely due to its metabolite N-DEO. This may be partially explained by its higher affinity for the salivary gland M3 muscarinic receptors compared with the detrusor.6
OXY-IR, most commonly prescribed as a 5-mg oral dose 3 times daily, has been the gold standard pharmacotherapy for OAB, and its clinical efficacy has been well documented.7 A summary of 15 randomized, controlled studies showed that OXY-IR produced a 52% mean reduction in urge incontinence episodes.8 In comparative studies, OXY-IR has also shown similar efficacy to immediate-release tolterodine.9
The most common side effect associated with OXY-IR is dry mouth, which is reported in 17% to 93% of patients.8 Although the incidence of side effects associated with OXY-IR can be reduced by using lower dosages, poor tolerability and 3 times daily dosing has limited its acceptance in clinical practice.
Extended-Release Oxybutynin
A once-daily, orally administered, extended-release oxybutynin (Ditropan XL®; Ortho-McNeil Pharmaceutical, Raritan, NJ) (OXY-ER) received FDA approval for the treatment of OAB in 1999. The drug utilizes a patented, push-pull, osmotic-release oral system that delivers steady-state serum levels of oxybutynin over a 24-hour time frame, avoiding the peaks and troughs associated with OXY-IR.10 Plasma levels of oxybutynin rise over a 4- to 6-hour period and steady-state concentrations are achieved after 3 days of ingestion.
N-DEO, the primary metabolite of oxybutynin, appears to be responsible for the anticholinergic side effects associated with the oxybutynin ingestion. Sathyan and colleagues11 demonstrated that the incidence of dry mouth correlated with the plasma concentration of N-DEO. In the same group of patients, parent drug serum concentration did not correlate with the presence of dry mouth or the reduction in salivary gland output.
OXY-IR undergoes extensive first-pass proximal gut wall and liver P450 metabolism, producing high plasma levels of N-DEO. In contrast, as a result of its rapid small bowel transit time of 3 to 5 hours, OXY-ER is primarily absorbed in the large intestine, where there is a lower concentration of p450 isomers. The reduced first-pass effect from decreased absorption in the proximal gut results in more parent oxybutynin being absorbed and comparatively less metabolite. Lower N-DEO levels results in fewer anticholinergic side effects and improved tolerability.
The efficacy and tolerability of OXY-ER (available in 6 strengths, from 5–30 mg) is well documented in the literature. Clinical phase III studies demonstrated an 83% to 90% reduction in urge incontinence episodes and efficacy similar to OXY-IR.12 OPERA (which stands for Overactive Bladder: Performance of Extended Release Agents), a study comparing the efficacy and tolerability of 10 mg of OXY-ER to long-acting 4 mg of tolterodine, demonstrated statistical superiority in favor of oxybutynin in reducing micturition frequency and achieving total dryness.13
In a randomized, double-blind, active control study, Anderson and colleagues14 demonstrated a lower incidence of anticholinergic side effects associated with OXY-ER. Dry mouth was reported in 68% and 87% (P = .04) of the patients receiving OXY-ER and OXY-IR, respectively. In OPERA, the incidence of dry mouth in patients treated with 10 mg of OXY-ER was 30%.13
Historically, a low percentage of patients remain on long-term (> 6 month) therapy with OXY-IR. However, more recent data suggest much better compliance with OXY-ER. An open-label study15 evaluating 1069 patients demonstrated that 60% of patients remained on the drug at 12 months. Efficacy was maintained throughout the 12-month study period in responding patients. Sixteen percent of patients discontinued therapy due to adverse events, with an additional 3.8% stopping therapy due to lack of efficacy. The results of the study appear to closely resemble the author’s experience in clinical practice.
Transdermal Drug Delivery
Advances in polymer science and drug formulation have resulted in the development of transdermal medications for the treatment of a number of medical conditions, including estrogen and androgen deficiency syndromes, contraception, analgesia, smoking cessation, and OAB. In general, transdermal delivery is convenient and offers a number of advantages over oral drug therapy, including improved pharmacokinetics, a more convenient dosing schedule, and a lower incidence of adverse events.
Skin Science and Drug Absorption
The skin is broadly divided into the epidermis, dermis, and subcutaneous tissue. Drugs must penetrate the relatively avascular epidermis and reach the rich capillary system located in the underlying dermis to be absorbed into the systemic circulation. Drug absorption is affected by biologic and physiochemical properties of the various skin layers, the nature of the medication, and the design of the drug delivery system.16
The stratum corneum of the epidermis is the primary rate-limiting barrier to drug absorption. Lipophilic substances transit the stratum corneum through the lipid-rich intercellular spaces, whereas more hydrophilic molecules dissolve and diffuse through the cell cytoplasm. Absorption through the skin can be influenced by a number of factors, including radiation, solvents, exfoliative diseases, and dermal blood flow.16 Drug absorption is also influenced by the presence of cutaneous cytochrome 450 enzymes that have the potential to oxidize drugs, resulting in approximately 10% to 20% first-pass metabolism.17
The properties of the drug and its vehicle also influence skin permeation and absorption. Lipophilic drugs such as oxybutynin are better suited for transdermal delivery because of their increased solubility and ability to diffuse through the cutaneous layers. Penetration enhancers increase skin permeability by interacting with intercellular lipids and/or denaturing cutaneous proteins.16
Drug delivery is a function of the type of device used to store and release the medication. Matrix patches combine the drug and rate-controlling permeation enhancer into a single layer. These systems are smaller and thinner, and drug release is controlled by diffusion through the polymeric matrix.16 In contrast, reservoir/-membrane-controlled systems contain the drug in a polymeric membrane that controls the rate at which the drug is released.
Oxybutynin Transdermal Delivery System
Oxybutynin transdermal delivery system (OXY-TDS) is a skin patch and transdermal delivery of oxybutynin to patients with OAB. OXY-TDS (Oxytrol®; Watson Pharma, Corona, CA) offers a number of advantages over oral drug administration, including improved pharmacokinetics, enhanced adherence, and a lower incidence of anticholinergic side effects.
OXY-TDS is a matrix-type system composed of 3 layers; the middle layer contains oxybutynin and a skin permeation enhancer called triacetin. Triacetin controls the rate of drug absorption through the stratum corneum by its physiochemical interaction with skin lipids. Once through the stratum corneum, oxybutynin enters the systemic circulation via small capillaries located in the dermis.
The 39 cm2 patch containing 36 mg of oxybutynin delivers 3.9 mg of oxybutynin daily. Steady-state plasma concentrations are maintained for approximately 96 hours, eliminating the peaks and troughs associated with oral OXY-IR and allowing for twice-weekly application.18 Bioequivalence has been demonstrated when applied to the abdomen, buttock, and thigh, enabling the patient to rotate sites and lower adverse site reactions.
Patient adherence with prescribed therapy is affected by a number of factors, including pill burden, complexity of dosing schedule, memory lapses, and adverse events.19 In studies, patients have been shown to fail to take less than 50% of their prescribed dose of medication,20 and adherence has been improved by less-frequent dosing intervals.21 OXY-TDS applied twice weekly has the potential to improve patient adherence, especially in older polypharmacy patients.
Transdermal delivery of oxybutynin results in a lower incidence of anticholinergic side effects by avoiding first-pass gastrointestinal and hepatic metabolism associated with oral administration. Avoidance of first-pass metabolism dramatically reduces the amount of N-DEO present in the systemic circulation, resulting in improved tolerability, with reported dry mouth and constipation rates similar to placebo.22 The most common treatment-related systemic adverse events experienced with OXY-TDS in integrated phase III studies include dry mouth (7.0%), constipation (2.1%), dizziness (0.8%), dysuria (1.2%), nausea (2.1%), and abnormal vision (1.2%).22,23 The lower levels of N-DEO relative to parent drug have similarly been shown to have smaller declines in saliva output.18
The literature supports the efficacy of OXY-TDS in treating patients with OAB. In a randomized, placebo-controlled, phase III trial, OXY-TDS significantly reduced the number of weekly incontinence episodes (median change, −19.0 vs −14.5; P = .0165), 24-hour frequency of urination (mean change, −2.3 vs −1.7; P = .0457), and increased the mean volume voided (median change, 24 vs 6 mL; P = .0063).22 In a subsequent head-to-head, placebo-controlled trial comparing OXY-TDS to extended-release tolterodine, both medications were equally effective in reducing incontinence episodes and urinary frequency, and superior to placebo23 (Figure 1).
Figure 1.
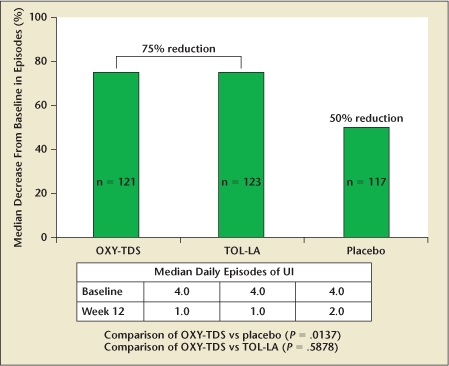
Reduction in daily incontinence episodes: oxybutynin transdermal delivery system (OXY-TDS) and extended-release tolterodine (TOL-LA) versus placebo. UI, urinary incontinence. Data from Dmochowski RR et al.23
A potential limitation of transdermal drug delivery devices is the risk for application site reactions (ASE). Skin adverse events can result from the drug, adhesive, permeation enhancer, or can be due to the occlusive nature of the device. The most common ASEs are allergic contact dermatitis, irritant dermatitis, alterations in skin pigmentation, redness, pruritis, or local edema.24
Erythema (8.3%) and itchiness (14.0%) are the most commonly reported skin adverse events associated with OXY-TDS and are usually mild or moderate in severity.22,23 Erythema usually resolves spontaneously within days and requires no treatment. Itchiness is usually due to skin dryness and can be alleviated by liberal usage of skin moisturizers and application site rotation. The “ring around the patch” residue can be removed with warm soap and water, or, in some cases, baby oil. Nail polish remover (acetone) can irritate the skin and should be avoided. Simple patch and skin care instructions given to patients have been shown to significantly decrease the incidence and severity of local skin reactions in phase IV studies.25
Oxybutynin Chloride Topical Gel
Oxybutynin chloride topical gel (OTG) was recently approved by the FDA for the treatment of overactive bladder. The once-daily gel formulation (Gelnique™; Watson Pharma) uses a small application volume (1.14 mL/dose; 1 g) that is applied to the abdomen, thigh, shoulder, or upper arm. OTG is quick drying, colorless, and leaves no residue. Its hydroalcoholic system utilizes ethanol as a skin permeation enhancer and a glycerin emollient to soften the skin and to minimize dryness.
Steady-state plasma concentrations of both oxybutynin and N-DEO are achieved within 1 week of OTG application and with similar plasma concentrations to OXY-TDS.26 A parallel group study demonstrated equal bioavailability and steady-state pharmacokinetics of oxybutynin and N-DEO following gel applications to the abdomen, upper arm/shoulder, and thigh (Figure 2). In addition, its pharmacokinetic profile is not adversely affected by sunscreen application or showering.27
Figure 2.
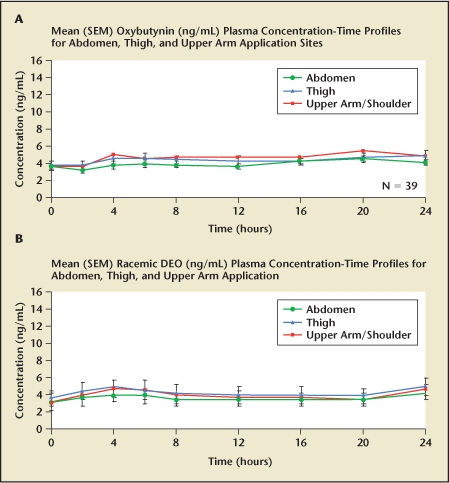
Oxybutynin transdermal gel bioequivalence for (A) oxybutynin and (B) N-desethyloxybutynin (DEO), when applied to abdomen, thigh, and upper arm.
Transference is a potential issue with transdermal gels and creams. Minor person-to-person transference of OTG occurs with skin contact, but it is minimal, likely not clinically important, and largely eliminated by covering the application site with clothing.27
The OTG formulation further improves the favorable metabolite-to-parent (N-DEO/oxybutynin) plasma concentration ratio that is seen with OXY-TDS.28,29 The mean DEO:oxybutynin AUC0−96 ratios achieved with OTG and OXY-TDS were 0.77 and 1.07, respectively.
The efficacy and tolerability of OTG has been evaluated in a 12-week, phase III, randomized, placebo-controlled, double-blind study and a 14-week extension study. The approval of OTG by the FDA was largely attributed to the results of this study, which have not yet been published.
The Evolution of Oxybutynin
The evolution of oxybutynin has resulted in new formulations that offer patients improved tolerability with a lower incidence of anticholinergic side effects and enhanced dosing options. OXY-ER significantly reduces the incidence of dry mouth associated with OXY-IR, and provides convenient once daily dosing. OXY-TDS, applied twice weekly, gives patients a transdermal option and anticholinergic side effects similar to placebo. Oxybutynin chloride topical gel similarly provides a topical therapy for OAB, which may be preferable to a pill for many patients. In addition, the favorable metabolite-to-parent (N-DEO/oxybutynin) plasma concentration associated with OTG is expected to be associated with excellent tolerability. The less occlusive gel is also expected to have reduced adverse skin events due to the presence of glycerin and the absence of the skin permeation enhancer triacetin.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the Standardization Sub-Committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooven JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6(11 suppl):S580–S590. [PubMed] [Google Scholar]
- 4.MacDiarmid S, Sandage BW , Jr, Malhotra BK. The effects of reformulation: improved therapeutic index. Curr Urol Rep. 2008;9:465–471. doi: 10.1007/s11934-008-0080-6. [DOI] [PubMed] [Google Scholar]
- 5.Guay DR. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacokinet. 2003;42:1243–1285. doi: 10.2165/00003088-200342140-00004. [DOI] [PubMed] [Google Scholar]
- 6.Waldeck K, Larrson B, Andersson KE. Comparison of oxybutynin and its active metabolite N-desethyloxybutynin in the human detrusor and parotid gland. J Urol. 1997;157:1093–1097. [PubMed] [Google Scholar]
- 7.Yarker YE, Goa KL, Fitton A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–262. doi: 10.2165/00002512-199506030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Thüroff JW, Chartier-Kastler E, Corcus J, et al. Medical treatment and medical side effects in urinary incontinence in the elderly. World J Urol. 1998;16(suppl 1):S248–S261. doi: 10.1007/pl00014139. [DOI] [PubMed] [Google Scholar]
- 9.Appell RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: a pooled analysis. Urology. 1997;50(6A suppl):90–96. doi: 10.1016/s0090-4295(97)00599-2. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol. 1999;39:289–296. [PubMed] [Google Scholar]
- 11.Sathayan G, Chancellor MB, Gupta SK. Effect of OROS controlled-release delivery on the pharmacokinetics and pharmacodynamics of oxybutynin chloride. Br J Clin Pharmacol. 2001;52:409–417. doi: 10.1046/j.0306-5251.2001.01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleason DM, Susset J, White C, et al. Evaluation of a new once-daily formulation of oxybutynin for the treatment of urinary urge incontinence. Ditropan XL Study Group. Urology. 1999;54:420–423. doi: 10.1016/s0090-4295(99)00259-9. [DOI] [PubMed] [Google Scholar]
- 13.Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687–695. doi: 10.4065/78.6.687. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RU, Mobley D, Blank B, et al. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. OROS Oxybutynin Study Group. J Urol. 1999;161:1809–1812. [PubMed] [Google Scholar]
- 15.Appell R, Diokno A, Antoci J, et al. One year prospective, open label trial of controlled release oxybutynin for overactive bladder in a community based population [abstract] Neurourol Urodyn. 2000;19:528. [Google Scholar]
- 16.Panchagnula R. Transdermal delivery of drugs. Indian J Pharmacol. 1997;29:140–156. [Google Scholar]
- 17.Guy RH, Burks DA. Transdermal drug delivery and cutaneous metabolism. Xenobiotica. 1987;17:325–343. doi: 10.3109/00498258709043943. [DOI] [PubMed] [Google Scholar]
- 18.Zobrist RH, Quan D, Thomas HM, et al. Pharmacokinetics and metabolism of transdermal oxybutynin: in vitro and in vivo performance of a novel delivery system. Pharm Res. 2003;20:103–109. doi: 10.1023/a:1022259011052. [DOI] [PubMed] [Google Scholar]
- 19.Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102:43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 20.Irvine J, Baker B, Smith J, et al. Poor adherence to placebo or amiodarone therapy predicts mortality: results from the CAMIAT study. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial. Psychosom Med. 1999;61:566–575. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;2 doi: 10.1002/14651858.CD000011. CD000011. [DOI] [PubMed] [Google Scholar]
- 22.Dmochowski RR, Davila GW, Zinner NR, et al. for the Transdermal Oxybutynin Study Group, authors. Efficacy and safety of transdermal oxybutynin in patients with urge and mixed incontinence. J Urol. 2002;168:580–586. [PubMed] [Google Scholar]
- 23.Dmochowski RR, Sand PK, Zinner NR, et al. for the Transdermal Oxybutynin Study Group, authors. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology. 2003;62:237–242. doi: 10.1016/s0090-4295(03)00356-x. [DOI] [PubMed] [Google Scholar]
- 24.Nitti VW, Sanders S, Staskin DR, et al. Transdermal delivery of drugs for urologic applications: basic principles and applications. Urology. 2006;67:657–664. doi: 10.1016/j.urology.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Sand P, Zinner N, Newman D, et al. Oxybutynin transdermal system improves quality of life in adults with overactive bladder: a multicentre, community-based, randomized study. BJU Int. 2007;99:836–844. doi: 10.1111/j.1464-410X.2006.06658.x. [DOI] [PubMed] [Google Scholar]
- 26.Caramelli KE, Staskin DR, Volinn W. Steady-state pharmacokinetics of an investigational oxybutynin gel in comparison with oxybutynin transdermal system; Poster presented at: Annual Meeting of the American Urological Association; May 17–22, 2008; Orlando, FL. Abstract 1508. [Google Scholar]
- 27.Caramelli KE, Stanworth S, Volinn W, Hoel G. Pharmacokinetics of oxybutynin topical gel: effects of showering, sunscreen application, and personto-person transference; Poster presented at: Annual Meeting of the American College of Clinical Pharmacy; October 19–22, 2008; Louisville, KY. [Google Scholar]
- 28.Oki T, Toma-Okura A, Yamada S. Advantages for transdermal over oral oxybutynin to treat overactive bladder: muscarinic receptor binding, plasma drug concentration, and salivary secretion. J Pharmacol Exp Ther. 2006;316:1137–1145. doi: 10.1124/jpet.105.094508. [DOI] [PubMed] [Google Scholar]
- 29.Reitz AB, Gupta SK, Huang Y, et al. The preparation and human muscarinic receptor profiling of oxybutynin and N-desethyloxybutynin enantiomers. Med Chem. 2007;3:543–545. doi: 10.2174/157340607782360353. [DOI] [PubMed] [Google Scholar]


