Abstract
Prostate biopsy and needle-directed prostate therapies are currently performed free-handed or with needle external templates under ultrasound guidance. Direct image-guided intervention robots are modern instruments that have the potential to substantially enhance these procedures. These may increase the accuracy and repeatability with which needles are placed in the gland. The authors’ group has developed a robot for precise prostate targeting that operates remotely alongside the patient in the magnetic resonance imaging scanner, as guided according to the image.
Key words: Robot, Prostate, Direct image-guided intervention (DIGI), MRI compatible, Transrectal ultrasound
It is estimated that the number of prostate biopsy procedures performed each year in the United States is on the order of 1 million. These yield the detection of approximately 230,000 new prostate cancers yearly.1 The difference is explained not only by tests with true-negative results but also unfortunately by those in which the biopsies missed sampling the cancer. With the current stage migration,1,2 tumors are becoming ever more difficult to sample; yet the staging of the disease becomes increasingly dependent on biopsy results. Moreover, wider treatment options are currently under evaluation for the management of localized prostate cancer, including needle-directed therapy methods such as cryotherapy,3 high-intensity focused ultrasound,4 and photodynamic therapy.5,6
The routine clinical modality for imaging the prostate is transrectal ultrasound (TRUS), for either diagnosis or needle therapy. Transrectal or transperineal needle access is performed either free-handed or with the use of a needle guide template. Systematic sampling protocols and gland-distributed treatment-planning algorithms have been developed to cope with the reduced accuracy of the ultrasound in cancer detection. The concept of focal therapy by the delivery of ablative therapy proximal to gland locations where positive biopsies were sampled is being tested,7,8 but neither the biopsies nor therapies are yet directly targeting imaged tumor foci.
New needle delivery mechanisms are therefore needed to increase the accuracy and repeatability with which needles can be placed in the gland. If accurate prostate cancer imaging were commonly used and accepted, such instruments could be immediately used for targeted biopsies or focal therapy. Today, prostate cancer image maps are still controversial, but accurate needle delivery mechanisms could be used to advance current procedures. For example, primary biopsies could better follow systematic plans, repeat biopsies could be tailored to target regions unsampled in previous biopsies, and expectant management biopsies could resample critical regions within the prostate. For treatment, these could potentially improve the execution and outcome of treatment plans and reduce side effects.
Significant research is currently concentrated on the development of new prostate cancer markers and imaging methods. Their local validation could benefit from a precise biopsy mechanism. If correlated with pathology findings, cancer image maps could advance the field of targeted biopsies and focal therapies.
The true potential of needle delivery mechanisms relies on their ability to operate with, be guided by, and use feedback from medical imaging equipment. Image guidance and navigation has been traditionally performed free-handed on preacquired images and with the use of spatial localizers, such as optical9 and magnetic trackers.10 The current trend, however, is for embedded systems that allow for re-imaging during the intervention for relocalization, treatment-planning updates, and quality control. We call these direct image-guided interventions (DIGI). The performance of DIGI is not new: the routine TRUS biopsy is performed under direct guidance. However, the new term is essential for distinguishing this important class of image-guided interventions from navigation based on preacquired imaging data.
A DIGI needle delivery mechanism is best implemented by a computercontrolled device. This is a robot of special design and control architecture. DIGI robots are substantially different from the common surgeon-driven da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA), and neither could take the other’s place. This article reviews clinical considerations and technical challenges for DIGI prostate robots and presents several such systems under development.
Clinical Considerations
Puncture Paths
Three paths have been used to reach the prostate with a needle: the transrectal, transperineal, and transgluteal. The transrectal path is extensively used in routine clinical practice for TRUS biopsies and is typically limited to biopsy because of the infection concerns associated with more extensive or invasive procedures. However, the transrectal approach presents a simplicity advantage because it is well tolerated with minimal anesthesia. Currently, interest in performing biopsies transperineally is increasing owing to the potential increase in accuracy of localization and the ability to sample more tissue from the peripheral zone. Prostate modeling11 has theoretically demonstrated that 98.5% of the peripheral zone volume may be sampled with the transperineal approach, compared with only 64.9% using the transrectal approach. Needle therapies are commonly performed transperineally. The transgluteal path is very rarely used in clinical practice because of the larger depth of insertion required.12,13
Target Size
The size of prostate cancer tumors varies significantly. A study on 1832 radical prostatectomy specimens evaluated the mean volume of the 5 largest cancers in each gland.14 Results show that volumes were 2.13, 0.39, 0.17, 0.09, and 0.04 cm3. The gold standard for clinically insignificant prostate cancer is determined on the basis of a radical prostatectomy specimen containing less than 0.5 mL (0.5 cm3) of prostate cancer with a Gleason score of 6 or less.15,16 Considering that after resection the volume of the prostate shrinks approximately 20%,17 the preoperative size of an insignificant tumor is calculated to be a sphere of approximately 5.2-mm radius. Even though the shapes of the tumors are irregular, this sphere size sets the upper limit of the accuracy required for needle targeting. For treatment, the accuracy may be even more stringent for avoiding adjacent structures, such as the rectum and neurovascular bundles.
Movement and Deformation of the Prostate
The motion of the prostate is significantly less than that of other organs owing to its distal location from the diaphragm and its support provided by the pelvic structures. This makes the prostate a good target candidate for image-guided robots. However, small respiratory motion of a few millimeters has been reported in radiotherapy studies.18–20 The motion depends on the position of the patient and is larger for the ventral decubitus than for the supine position.21
The prostate may also move or deform as a function of rectal peristalsis and bladder filling.22,23 During a 20-minute radiotherapy session the prostate was found to move as much as 3 mm.24
Prostate motion and deformations may also be induced by the use of endorectal instruments. TRUS end-fire probes can easily induce movement of the prostate and put it out of its shape in a significant manner. Magnetic resonance imaging (MRI) endorectal coils may also induce changes. Inflatabletype coils may cause larger distortions compared with rigid coils, compressing the prostate in the anterior-posterior direction by 4.1 ± 3.0 mm versus 1.2 ± 2.2 mm.25
The insertion of the needle may also affect the shape of the prostate, depending on the access path, the size and type of the needle, and the way that the needles are inserted (ie, manual vs biopsy firing gun). For the transperineal approach with manually inserted 18-gauge needles, the force of insertion was reported to be lower than 10 N.26 During brachytherapy the prostate was found to rotate up to 13.8° in the coronal plane27 and 10.2° in the sagittal plane. Studies also found that the shape of the needle point may also be a factor in insertion force and prostate deformation and that diamond-tipped needles in general have a lower resistive force than beveled, asymmetric tips.28
Mechanical laws applied to needle insertion establish that a reduction of the axial force along the needle minimizes tissue deformation and target deflection.29 So, decreasing the axial force by implementing some special movements, such as spinning the needle30 or fast insertions,31 can increase the accuracy for reaching the target.
Imaging Used for Guiding Prostate Interventions
Three imaging methods are used for the prostate,32 with TRUS being the most popular for both biopsy and treatment. Its main advantages are its availability, simplicity, and real-time imaging, and its main drawback is its poor sensitivity and specificity for imaging prostate cancer.33 TRUS shows the contour of the gland and is mainly used to guide the punctures inside the global shape of the prostate on the basis of systematic methods and dosimetry plans. However, the accuracy of the distribution of the biopsies seems to be inadequate. Preliminary work with a 3-dimensional (3D) TRUS fusion system used on 15 patients by the same clinician showed that only 63% of planned targets were reached under 2-dimensional (2D) TRUS guidance.34 Three-dimensional real-time (called 4D) systems could potentially help the physician improve the ability to reproduce a biopsy protocol.35
Computed tomography (CT) is used to control seed implantation after brachytherapy, and an acquisition can be made simultaneously with 3D TRUS to capitalize on the advantages of each modality.36
Multiple groups have reported 85% sensitivity with various MRI-based methods for detecting prostate cancer tumors that were larger than 1 cm.37 It seems promising that anatomic MRI with multiparametric examinations, such as spectroscopy, perfusion, and diffusion-weighted or dynamic contrast imaging, may soon be able to offer a reliable way of imaging prostate cancer. But performing TRUS-guided interventions on the basis of these MRI images requires complicated, error-prone, image-to-image registration methods.38 MRI/TRUS fusion studies investigated ways to correlate the 2 image sets according to the correspondence of several landmarks detected by ultrasound and MRI.39 The correlation is even more difficult because TRUS typically deforms the prostate, so deformable registration methods are investigated.40
An alternative is to guide the intervention according to the MRI itself, without the TRUS.41–43 But this requires special instrumentation, which faces significant MRI compatibility restrictions, as discussed below.
Technical Considerations
Image-guided robots have stringent requirements for imager compatibility, precision, sterility, and safety, as well as size and ergonomics.44 A robot’s compatibility with a medical imager refers to the capability of the robot to safely operate within the confined space of the imager while performing its clinical function, without interfering with the functionality of the imager.45 Among all types of imagers the MRI is the most demanding, and the development of MRI robots is a very challenging engineering task.46,47 But this also makes MRI multi-imager compatible, if care is taken for the selection of radiolucent materials for the components in immediate proximity to the imaging site.45
MRI Compatibility
MRI scanners use magnetic fields of very high density (3 T is becoming common), with pulsed magnetic and radiofrequency fields. Within the imager, ferromagnetic materials are exposed to very high magnetic interaction forces, and heating may occur in conductive materials by electromagnetic induction. The use of electricity may cause interference leading to signal-to-noise attenuation, signal distortions, and image artifacts. For these, most of the components commonly used in robotics may not be used in close proximity to the MRI. For example, the most common type of motor used in robots is electromagnetic, being clearly incompatible with the MRI because it works on the basis of magnetism.
Several nonferrous metals, such as titanium and nitinol, have been found to be acceptable for small parts and are being used in commercial MRI instrumentation. However, for noninterference with electromagnetism the ideal materials should be nonmagnetic but also dielectric (eg, plastics, ceramics, rubbers, and glasses). From the energetic point of view, electricity is not MRI compatible because currents generate electromagnetic waves and require conductor materials in which electricity is being induced from the scanner. On the other hand, pressure and light are ideal choices because these are decoupled from electromagnetism.
Actuators
Commonly, previous research has used piezoelectric motors,46 also called ultrasonic motors. The piezoelectric effect is the ability of some crystals and certain ceramic materials to generate a voltage in response to applied mechanical stress. The effect is reversible, so in motors piezoelectric crystals change shape and produce motion when subjected to an externally applied voltage. The problem with the piezo effect is that the change in the shape of the piezo crystal is very small (approximately 0.1%) and is only achieved under high voltages. To create usable motion the voltage needs to be pulsed at high frequencies, which create distortions if operated closer than 0.5 m from the image isocenter.48 Piezo actuation is a compromise with limited applicability that has been used in the absence of a better solution.
A group from Switzerland has recently reported developments using hydraulic actuation49 and is advocating the advantages of hydraulic master-slave coupling for MRI actuation.46 This is a very promising solution if leakage can be perfectly controlled.
Pneumatic actuation is a fundamentally flawless option for MRI compatibility.45 A research group from Karlsruhe, Germany, was the first to realize this after multiple unsuccessful attempts with piezo actuation.50,51 But the major limitation of pneumatic actuators in general has been their reduced precision in controlled motion.52 Pneumatics is traditionally used for free-spinning motion, such as drills (MRI compatible53), or in industrial automation, such as opening and closing gates. No classic pneumatic motor could collectively satisfy the reliability, precision, and safety required for a medical robot. For this, we have developed a new type of motor,54 which will be discussed later.
Manual Devices for MRI-Guided Prostate Interventions
Because of the very stringent requirements of MRI, the development of MRI robots is highly challenging. Instead, a few groups have explored the feasibility and clinical utility of guiding the intervention based on the MRI not with robots but rather with simple, manually actuated devices.
Menard and colleagues55 at the National Institutes of Health investigated the feasibility of image-guided interventions for high-dose prostate brachytherapy in a closed-bore MRI scanner using a needle template guide registered to the MRI. This study demonstrated both the feasibility and advantages of MRI in guidance but also revealed the need for improved instrumentation because numerous scanner table moves were required to access the patient.
The D’Amico and Tempany group56 at Brigham and Women’s Hospital in Boston, Massachusetts, has performed multiple clinical interventions involving both transperineal biopsy and brachytherapy using a 0.5-T open MRI scanner. Specifically, they have reported their experience with MRI-guided transperineal prostate biopsy in a patient with recurrent prostate cancer after brachytherapy.57 They demonstrated that 0.5-T MRI guidance was useful for targeting recurrent prostate cancer and that the open scanner facilitated the access.
In Germany, 2 studies reported MRI-guided biopsies in patients with elevated prostate-specific antigen levels and without previous TRUS-guided biopsies58 and for repeat biopsies.59
Another clinical study performed at the National Institutes of Health demonstrated the feasibility of performing transrectal MRI-guided prostate biopsies and brachytherapy in a standard 1.5-T MR scanner.60 The approach used a custom endorectal MR probe, which incorporated an imaging coil, a biopsy needle, and tracking coils. The tracking coils provided the real-time location of the endorectal probe in 3D space, and the imaging coil was used to acquire the anatomic images that were used to target the biopsy.61–63 A recent report showed improved cancer detection in MRI-guided biopsies but only for patients for whom the repeat biopsies were not immediately following the TRUS.64,65 This could be explained by the advancing disease when biopsies were performed at a later time and the problems created by bleeding of previous biopsies, but also by the limitations of the manual device used for guiding the biopsy.
Image-Guided Robots for the Prostate
A robot is a motorized mechanical device controlled by a computer. A first type of medical robot is surgeon driven, such as the da Vinci robot. These are remotely controlled and do not perform autonomous actions, being controlled by the surgeon under enhanced visual feedback and with better dexterity. Yet clinical performance may be augmented with the addition of other information. Image-guided robots take advantage of medical imaging for guiding the interventions. Needle robots, or robots for interventions with needles or other slender probes or instruments, are connected to an imaging modality (eg, CT, MRI, ultrasound, fluoroscopy). Targets and paths are defined in the image according to planning algorithms, and the robot aligns and may insert the needle accordingly. This article focuses on image-guided needle robots for the prostate.
Direct Image-Guided Intervention
Direct image-guided intervention refers to interventions for which the imaging and intervention are performed in a single session, to distinguish these from interventions using preacquired images. These include real-time imaging such as TRUS or x-ray fluoroscopy-guided procedures, but also include volumetric imaging that is not real time, such as CT or MRI. The main concern with preacquired images is the change that occurs over time and with the repositioning of the patient, and by contrast this defines the main advantages of DIGI.
A DIGI robot is an image-guided robot that is integrated with the imager and operates within the environment of the imager alongside the patient so that the intervention is performed on the basis of the image without moving the patient and reimaging can be immediately used for verifications and additional guidance. The imager and the robot are integrated in a digital system. When the robot is embedded in the imaging system, the effect of the treatment can be controlled and the planning updated. For example, for thermal ablation under MRI, the distribution of the temperature in the prostate can be monitored in real time,66 and the planning could be updated in case of modification of the shape of the gland. Indeed, there are numerous challenges and current technology limitations to the DIGI approach, such as the temporal frequency and spatial resolution of the imagers, the mechanical performance of the robot, and other imager compatibility constraints, but innovative research has already produced a few promising prototypes.
Prostate DIGI Robots
From a historic point of view, the first image-guided robot intervention on a human was performed in the late 1980s to obtain a brain biopsy in a CT scanner.67 The first robot for the prostate and in fact for urology was developed at the Imperial College in London in 1989 for transurethral resection of the prostate.68
The earliest work for MRI-guided prostate intervention robots was performed at Brigham and Women’s Hospital in collaboration with the Agency of Industrial Science and Technology/Ministry of International Trade and Industry (AIST-MITI, Ibaraki, Japan).69 A robotic intervention assistant was constructed for open MRI to provide a guide for needles and probes.70 To minimize image interference from the piezoelectric motors, the robot had to be located distally, at the top of the imager between the vertical coils of the MRI. To operate at the isocenter, long arms had to be extended, which made them flexible. The system assists the physician by positioning a needle guide for manual needle intervention. Applications included prostate biopsy and brachytherapy.71,72 A new graphic planning interface has been recently tested with phantom experiments,73 but resolution and functional imaging are limited by the low magnetic field of the open MR.
The Institute for Medical Engineering and Biophysics (Karlsruhe, Germany) reported several versions of a robotic system for breast lesion biopsy and therapy under MR guidance.50,51 Their last version used a cylinder for driving an end-effector axis,48 and their report gives a well-reasoned presentation of these advantages. This German institute is no longer active, but fortunately a spin-off company was created. The company (Innomedic, Berlin, Germany) is developing a pneumatic robot for general CT- or MRI-guided needle procedures.74 The robot orients the needle about the axial-sagittal planes for interventions targeting abdominal organs. However, a group from Frankfurt, Germany, has recently used the Innomotion system (Innomedic) for targeting the prostate.75,76 The limitations of the robot restricted access to the transgluteal path (prone patient with needle pointing down), for which the needle path is much deeper than normally used (approximately 14 cm reported in the cadaver experiment).76 A 15-gauge needle was used to prevent deflections. Manual needle insertion was performed through the guide after retracting the table from the scanner. The Innomedic system is not approved by the US Food and Drug Administration, and its designed application range does not include the prostate, but it is approved for clinical use in Europe and is a commercial DIGI robot.
Professor Brian Davies of the Imperial College in London, who pioneered the application of robots in urology, has also reported the development of a simple robot that performs similar to but replaces the brachytherapy template.77 Rotation about the axis of the needle is added to reduce needle deflections. The system uses 2D TRUS guidance, and the report describes successful preclinical testing.
Three-dimensional reconstruction from a regular 2D TRUS probe is also being investigated for image guidance.78,79 This was integrated with a robot in a system for prostate brachytherapy or biopsy. Mock-up tests demonstrated a precision on the order of 1 mL, and a clinical study for biopsy is in progress.79
The Thomas Jefferson University (Philadelphia, PA) is pursuing a multichannel concept for percutaneous prostate interventions. Unlike the typical single-channel robotic system or conventional manual technique, the designed multichannel robotic system is capable of inserting a large number of needles concurrently80 for the purpose of increasing targeting accuracy with better prostate stabilization.
MrBot Robot for Direct MRI-Guided Prostate Interventions
Our team developed an “MRI Stealth,” fully automated robot for transperineal prostate access, the MrBot. It is mounted alongside the patient (Figure 1) in the MR imager and can be precisely operated from the control room under image feedback.81 The Internet site for the device (http://urology.jhu.edu/urobotics/projects/MrBot/) shows several movies of the robot.
Figure 1.
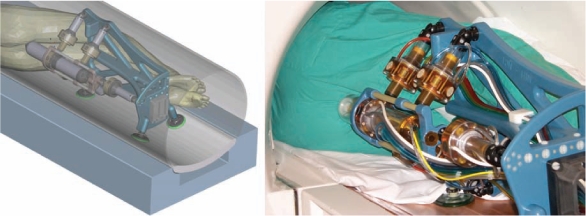
MrBot robot alongside the man on the magnetic resonance imaging table: computer-aided design rendering (left), and photo (right).
The robot presents 6 degrees of freedom (DOF): 5 for positioning and orienting the injector and 1 for setting the depth of needle insertion. The needle is inserted with high velocity to prevent soft tissue deflections. Various needle drivers can be mounted in the robot for performing various needle interventions. The first driver was developed for fully automated low-dose (seed) brachytherapy.31 Compared with the classic template of needle guide holes commonly used in TRUS interventions, the robot gives additional freedom of motion for better targeting. For example, the skin entry point may be chosen ahead of time, and targeting can be performed with angulations, which is impossible with the template. As such, multiple needle insertions can be performed through the same skin entry point. Moreover, angulations also allow reduction of pubic arch interference, thus allowing for targeting otherwise inaccessible regions of the prostate.
The robot is controlled from a unit remotely located outside the imager’s room, either in the control room of the imager or another proximal space. The robot is connected to the control cabinet by a bundle of hoses (Figure 2). This allows for all MRI-incompatible components of the system to be located outside the MRI room.
Figure 2.

MrBot robot connected with 7-m-long hoses to its control cabinet.
The MrBot robot was constructed to be multi-imager compatible, which includes compatibility with all classes of medical imaging equipment (ultrasound-, x-ray-, and MR-based imagers). All robotic components are constructed of nonmagnetic and dielectric materials. To overcome MRI incompatibilities, a new type of motor was purposely designed for the robot. This motor, the PneuStep, is a pneumatic motor using optical feedback with fail-safe operation,54 and it is the only fully MRI compatible motor (see how the motor works at http://urology.jhu.edu/urobotics/projects/PneuStep/).
Precision tests in tissue mock-ups yielded a mean seed placement error of 0.72 ± 0.36 mm.82 With different needle drivers, the MrBot applies to various automated DIGI, such as biopsy, therapy injections, and thermal or radiofrequency ablations. The system is presently in preclinical testing with cadaver and animal experiments, but tests show very promising results, and clinical trials are expected to commence in the near future.
Conclusions
Image-guided robots not only augment the physician’s manipulation capabilities but establish a digital platform for integrating medical imaging data. This gives robots abilities unattainable to humans because, unlike humans, robots and imagers are digital devices. Direct image guidance allows for monitoring, updating the intervention according to recently acquired images, and performing quality control at the end of the procedure. These robots bring new dimensions to typical vision-based surgeries and diagnosis imaging. The physician does not directly control the robot but defines its tasks and monitors its actions on the basis of the image. Clinical performance no longer depends on the physician’s 3D cognition and motor skills; thus he or she is free to concentrate on the critical clinical aspects of the intervention. These new characteristics have the potential to improve how current procedures are performed and allow for new, advanced diagnostic and therapeutic methods to be developed. Image-guided robots are expected to become a new generation of robots in medicine. No commercial system exists yet, but several robots that allow prostate interventions to be performed under direct guidance are under development. Most promising are MRI-guided robots that can take advantage of the high-resolution anatomic and special functional imaging and operate alongside the man in the MRI scanner to remotely access the prostate with special needles for biopsy and therapeutic interventions.
Main Points.
Accurate needle delivery mechanisms could be used to advance current prostate biopsy procedures. For example, primary biopsies could better follow systematic plans, repeat biopsies could be tailored to target regions unsampled in previous biopsies, and expectant management biopsies could resample critical regions within the prostate.
A direct image-guided intervention (DIGI) needle delivery mechanism is best implemented by a computer-controlled device—a robot of special design and control architecture.
The authors’ team has developed the MrBot, a fully automated robot for transperineal prostate access. It is mounted alongside the patient in the magnetic resonance imager and can be precisely operated from the control room under image feedback.
With the MrBot, multiple needle insertions can be performed through the same skin entry point. Moreover, angulations also allow reduction of pubic arch interference, thus allowing for targeting otherwise inaccessible regions of the prostate.
The MrBot robot was constructed to be multi-imager compatible, which includes compatibility with all classes of medical imaging equipment. All robotic components are constructed of nonmagnetic and dielectric materials, and to overcome MRI incompatibilities, a new type of motor (the PneuStep) was purposely designed for the robot.
With a DIGI robot, the physician does not directly control the robot but defines its tasks and monitors its actions on the basis of the image. Clinical performance no longer depends on the physician’s three-dimensional cognition and motor skills; thus he or she is free to concentrate on the critical clinical aspects of the intervention.
Acknowledgments
Acknowledgment: The development of the MrBot robot presented in this article was supported by the National Cancer Institute (NCI) of the National Institutes of Health under grant CA88232. Preclinical tests of the robot were sponsored by the Prostate Cancer Foundation (PCF) and the Patrick C. Walsh Foundation (PCW). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, PCF, or PCW.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Taylor SH, Merriman KW, Spiess PE, Pisters L. Inadequacies of the current American Joint Committee on cancer staging system for prostate cancer. Cancer. 2006;106:559–565. doi: 10.1002/cncr.21605. [DOI] [PubMed] [Google Scholar]
- 3.Shelley M, Wilt TJ, Coles B, Mason MD. Cryotherapy for localised prostate cancer. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005010.pub2. CD005010. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JH, Loeb S. The role of high-intensity focused ultrasound in prostate cancer. Curr Oncol Rep. 2007;9:222–225. doi: 10.1007/s11912-007-0025-0. [DOI] [PubMed] [Google Scholar]
- 5.Haider MA, Davidson SR, Kale AV, et al. Prostate gland: MR imaging appearance after vascular targeted photodynamic therapy with palladium-bacteriopheophorbide. Radiology. 2007;244:196–204. doi: 10.1148/radiol.2441060398. [DOI] [PubMed] [Google Scholar]
- 6.Trachtenberg J, Bogaards A, Weersink RA, et al. Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. J Urol. 2007;178:1974–1979. doi: 10.1016/j.juro.2007.07.036. discussion 1979. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed HU, Pendse D, Illing R, et al. Will focal therapy become a standard of care for men with localized prostate cancer? Nat Clin Pract Oncol. 2007;4:632–642. doi: 10.1038/ncponc0959. [DOI] [PubMed] [Google Scholar]
- 8.Eggener SE, Scardino PT, Carroll PR, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178:2260–2267. doi: 10.1016/j.juro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 9.Bucki M, Dauliac B, Daanen V, et al. ProNav: a navigation software for prostate biopsies. Montpellier, France: Sauramps Medical; 2005. pp. 479–483. [Google Scholar]
- 10.Xu S, Kruecker J, Guion P, et al. Closed-loop control in fused MR-TRUS image-guided prostate biopsy. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10:128–135. doi: 10.1007/978-3-540-75757-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan P, Ho H, Yuen J, et al. A 3D computer simulation to study the efficacy of transperineal versus transrectal biopsy of the prostate. Int J Comput Assist Radiol Surg. 2007;1:351–360. [Google Scholar]
- 12.Papanicolaou N, Eisenberg PJ, Silverman SG, et al. Prostatic biopsy after proctocolectomy: a transgluteal, CT-guided approach. AJR Am J Roentgenol. 1996;166:1332–1334. doi: 10.2214/ajr.166.6.8633443. [DOI] [PubMed] [Google Scholar]
- 13.Zangos S, Eichler K, Engelmann K, et al. MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: technique and preliminary results. Eur Radiol. 2005;15:174–182. doi: 10.1007/s00330-004-2458-2. [DOI] [PubMed] [Google Scholar]
- 14.Ohori M, Eastham JA, Koh H, et al. Is focal therapy reasonable in patients with early stage prostate cancer (CAP)—an analysis of radical prostatectomy (RP) specimens; Presented at: American Urological Association Annual Meeting; May 20–25, 2006; Atlanta, GA. Abstract 1574. [Google Scholar]
- 15.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 16.Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71(3 suppl):933–938. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Egevad L, Engström K, Busch C. A new method for handling radical prostatectomies enabling fresh tissue harvesting, whole mount sections, and landmarks for alignment of sections. J Urol Pathol. 1998;9:17–28. [Google Scholar]
- 18.Keros L, Bernier V, Aletti P, et al. Qualitative estimation of pelvic organ interactions and their consequences on prostate motion: study on a deceased person. Med Phys. 2006;33:1902–1910. doi: 10.1118/1.2198190. [DOI] [PubMed] [Google Scholar]
- 19.Malone S, Crook J, Kendal W, Szanto J. Respiratory-induced prostate motion: quantification and characterization. Int J Radiat Oncol Biol Phys. 2000;48:105–109. doi: 10.1016/s0360-3016(00)00603-9. [DOI] [PubMed] [Google Scholar]
- 20.Onishi H, Kuriyama K, Komiyama T, et al. CT evaluation of patient deep inspiration selfbreath-holding: how precisely can patients reproduce the tumor position in the absence of respiratory monitoring devices? Med Phys. 2003;30:1183–1187. doi: 10.1118/1.1570372. [DOI] [PubMed] [Google Scholar]
- 21.van Herk M, Bruce A, Kroes AG, et al. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int J Radiat Oncol Biol Phys. 1995;33:1311–1320. doi: 10.1016/0360-3016(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 22.Melian E, Kutcher G, Leibel S, et al. Variation in prostate motion: quantification and implications for three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 1993;27:137. [Google Scholar]
- 23.Schild S, Casale H, Bellefontaine L. Movements of the prostate due to rectal and bladder distension: implication for radiotherapy. Med Dosim. 1993;18:13–15. doi: 10.1016/0958-3947(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 24.Artignan X, Rastkhah M, Balosso J, et al. [Quantification of prostate movements during radiotherapy] Cancer Radiother. 2006;10:381–387. doi: 10.1016/j.canrad.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Noworolski S, Pouliot J, et al. Analysis of prostate deformation due to different MRI/MRS endorectal coils for image fusion and brachytherapy treatment planning. Med Phys. 2004;31:1728. [Google Scholar]
- 26.Podder T, Clark D, Sherman J, et al. Vivo motion and force measurement of surgical needle intervention during prostate brachytherapy. Med Phys. 2006;33:2915–2922. doi: 10.1118/1.2218061. [DOI] [PubMed] [Google Scholar]
- 27.Lagerburg V, Moerland MA, Lagendijk JJ, Battermann JJ. Measurement of prostate rotation during insertion of needles for brachytherapy. Radiother Oncol. 2005;77:318–323. doi: 10.1016/j.radonc.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Okamura AM, Simone C, O’Leary MD. Force modeling for needle insertion into soft tissue. IEEE Trans Biomed Eng. 2004;51:1707–1716. doi: 10.1109/TBME.2004.831542. [DOI] [PubMed] [Google Scholar]
- 29.Podder T, Clark DP, Fuller D, et al. Effects of velocity modulation during surgical needle insertion. Conf Proc IEEE Eng Med Biol Soc. 2005;6:5766–5770. doi: 10.1109/IEMBS.2005.1615798. [DOI] [PubMed] [Google Scholar]
- 30.Abolhassani N, Patel RV, Ayazi F. Minimization of needle deflection in robot-assisted percutaneous therapy. Int J Med Robot. 2007;3:140–148. doi: 10.1002/rcs.136. [DOI] [PubMed] [Google Scholar]
- 31.Patriciu A, Petrisor D, Muntener M, et al. Automatic brachytherapy seed placement under MRI guidance. IEEE Trans Biomed Eng. 2007;54:1499–1506. doi: 10.1109/TBME.2007.900816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hricak H, Choyke PL, Eberhardt SC, et al. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 33.Muntener M, Ursu D, Patriciu A, et al. Robotic prostate surgery. Expert Rev Med Devices. 2006;3:575–584. doi: 10.1586/17434440.3.5.575. [DOI] [PubMed] [Google Scholar]
- 34.Mozer P, Baumann M, Chevreau G, et al. Accuracy evaluation of prostate biopsies under endorectal 2D ultrasound; Abstract presented at: 22nd Annual Meeting of the Engineering and Urology Society; May 17, 2007; Anaheim, CA. [Google Scholar]
- 35.Long JA, Daanen V, Moreau-Gaudry A, et al. Prostate biopsies guided by three-dimensional realtime (4-D) transrectal ultrasonography on a phantom: comparative study versus two-dimensional transrectal ultrasound-guided biopsies. Eur Urol. 2007;52:1097–1105. doi: 10.1016/j.eururo.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 36.Steggerda M, Schneider C, van Herk M, et al. The applicability of simultaneous TRUS-CT imaging for the evaluation of prostate seed implants. Med Phys. 2005;32:2262–2270. doi: 10.1118/1.1940147. [DOI] [PubMed] [Google Scholar]
- 37.Kirkham APS, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–1175. doi: 10.1016/j.eururo.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Lattouf JB, Grubb RL, Lee SJ, et al. Magnetic resonance imaging-directed transrectal ultrasonography-guided biopsies in patients at risk of prostate cancer. BJU Int. 2007;99:1041–1046. doi: 10.1111/j.1464-410X.2006.06690.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan I, Oldenburg NE, Meskell P, et al. Real time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging. 2002;20:295–299. doi: 10.1016/s0730-725x(02)00490-3. [DOI] [PubMed] [Google Scholar]
- 40.Baumann M, Mozer P, Daanen V, Troccaz J. Towards 3D ultrasound image based soft tissue tracking: a transrectal ultrasound prostate image alignment system. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10:26–33. doi: 10.1007/978-3-540-75759-7_4. [DOI] [PubMed] [Google Scholar]
- 41.Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int. 2008;101:181–185. doi: 10.1111/j.1464-410X.2007.07219.x. [DOI] [PubMed] [Google Scholar]
- 42.Anastasiadis AG, Lichy MP, Nagele U, et al. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006;50:748–749. doi: 10.1016/j.eururo.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Engelhard K, Hollenbach HP, Kiefer B, et al. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–1243. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RH, Stoianovici D. Medical robotics in computer-integrated surgery. IEEE Trans Robot Automation. 2003;19:765–781. [Google Scholar]
- 45.Stoianovici D. Multi-imager compatible actuation principles in surgical robotics. Int J Med Robot. 2005;1:86–100. doi: 10.1002/rcs.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gassert R, Yamamoto A, Chapuis D, et al. Actuation methods for applications in MR environments. Concepts Magn Reson. 2006;29B:191–209. [Google Scholar]
- 47.DiMaio S, Kapur T, Cleary K, et al. Challenges in image-guided therapy system design. Neuroimage. 2007;37:S144–S151. doi: 10.1016/j.neuroimage.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hempel E, Fischer H, Gumb L, et al. An MRI-compatible surgical robot for precise radiological interventions. Comput Aided Surg. 2003;8:180–191. doi: 10.3109/10929080309146052. [DOI] [PubMed] [Google Scholar]
- 49.Gassert R, Moser R, Burdet E, Bleuler H. MRI/fMRI-compatible robotic system with force feedback for interaction with human motion. IEEE-ASME Trans Mechatronics. 2006;11:216–224. [Google Scholar]
- 50.Kaiser WA, Fischer H, Vagner J, Selig M. Robotic system for biopsy and therapy of breast lesions in a high-field whole-body magnetic resonance tomography unit. Invest Radiol. 2000;35:513–519. doi: 10.1097/00004424-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Felden A, Vagner J, Hinz A, et al. ROBITOM—robot for biopsy and therapy of the mamma. Biomed Tech (Berl) 2002;47(suppl 1 pt 1):2–5. doi: 10.1515/bmte.2002.47.s1a.2. [DOI] [PubMed] [Google Scholar]
- 52.Choi HS, Han CS, Lee KY, Lee SH. Development of hybrid robot for construction works with pneumatic actuator. Automation in Construction. 2005;14:452–459. [Google Scholar]
- 53.Neuerburg J, Adam G, Bucker A, et al. [A new MR-(and CT-) compatible bone biopsy system: first clinical results] Rofo. 1998;169:515–520. doi: 10.1055/s-2007-1015330. [DOI] [PubMed] [Google Scholar]
- 54.Stoianovici D, Patriciu A, Mazilu D, et al. A new type of motor: pneumatic step motor. IEEE/ASME Trans Mechatronics. 2007;12:98–106. doi: 10.1109/TMECH.2006.886258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menard C, Susil RC, Choyke P, et al. MRI-guided HDR prostate brachytherapy in standard 1.5T scanner. Int J Radiat Oncol Biol Phys. 2004;59:1414–1423. doi: 10.1016/j.ijrobp.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hata N, Jinzaki M, Kacher D, et al. MR imaging-guided prostate biopsy with surgical navigation software: device validation and feasibility. Radiology. 2001;220:263–268. doi: 10.1148/radiology.220.1.r01jl44263. [DOI] [PubMed] [Google Scholar]
- 57.Barnes AS, Haker SJ, Mulkern RV, et al. Magnetic resonance spectroscopy-guided transperineal prostate biopsy and brachytherapy for recurrent prostate cancer. Urology. 2005;66:1319. doi: 10.1016/j.urology.2005.06.105. [DOI] [PubMed] [Google Scholar]
- 58.Beyersdorff D, Winkel A, Hamm B, et al. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology. 2005;234:576–581. doi: 10.1148/radiol.2342031887. [DOI] [PubMed] [Google Scholar]
- 59.Anastasiadis AG, Lichy MP, Nagele U, et al. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol. 2006;50:738–748. doi: 10.1016/j.eururo.2006.03.007. discussion 748–749. [DOI] [PubMed] [Google Scholar]
- 60.Susil RC, Menard C, Krieger A, et al. Transrectal prostate biopsy and fiducial marker placement in a standard 1.5T magnetic resonance imaging scanner. J Urol. 2006;175:113–120. doi: 10.1016/S0022-5347(05)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krieger A, Susil RC, Menard C, et al. Design of a novel MRI compatible manipulator for image guided prostate interventions. IEEE Trans Biomed Eng. 2005;52:306–313. doi: 10.1109/TBME.2004.840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Susil RC, Krieger A, Derbyshire JA, et al. System for MR image-guided prostate interventions: canine study. Radiology. 2003;228:886–894. doi: 10.1148/radiol.2283020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Susil RC, Camphausen K, Choyke P, et al. System for prostate brachytherapy and biopsy in a standard 1.5 T MRI scanner. Magn Reson Med. 2004;52:683–687. doi: 10.1002/mrm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lattouf JB, Grubb RL , 3rd, Lee SJ, et al. Magnetic resonance imaging-directed transrectal ultrasonography-guided biopsies in patients at risk of prostate cancer. BJU Int. 2007;99:1041–1046. doi: 10.1111/j.1464-410X.2006.06690.x. [DOI] [PubMed] [Google Scholar]
- 65.Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int. 2008;101:181–185. doi: 10.1111/j.1464-410X.2007.07219.x. [DOI] [PubMed] [Google Scholar]
- 66.Rieke V, Kinsey AM, Ross AB, et al. Referenceless MR thermometry for monitoring thermal ablation in the prostate. IEEE Trans Med Imaging. 2007;26:813–821. doi: 10.1109/TMI.2007.892647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 68.Davies BL, Hibberd RD, Coptcoat MJ, Wickham JE. A surgeon robot prostatectomy—a laboratory evaluation. J Med Eng Technol. 1989;13:273–277. doi: 10.3109/03091908909016201. [DOI] [PubMed] [Google Scholar]
- 69.Chinzei K, Hata N, Jolesz FA, Kikinis R. MR compatible surgical assist robot: system integration and preliminary feasibility study. Medical Image Computing and Computer-Assisted Intervention — Miccai 2000. 2000;1935:921–930. [Google Scholar]
- 70.Chinzei K, Miller K. Towards MRI guided surgical manipulator. Med Sci Monit. 2001;7:153–163. [PubMed] [Google Scholar]
- 71.Koseki Y, Koyachi N, Arai T, Chinzei K. Remote actuation mechanism for MR-compatible manipulator using leverage and parallelogram—workspace analysis, workspace control, and stiffness evaluation. Proceedings of the International Conference on Robotics and Automation, 2003. 2003;1:652–657. [Google Scholar]
- 72.Koseki Y, Kikinis R, Jolesz FA, Chinzei K. Precise evaluation of positioning repeatability of MR-compatible manipulator inside MRI. Medical Image Computing and Computer-Assisted Intervention — Miccai 2004, Pt 2, Proceedings. 2004;3217:192–199. [Google Scholar]
- 73.DiMaio SP, Pieper S, Chinzei K, et al. Robot-assisted needle placement in open MRI: system architecture, integration and validation. Comput Aided Surg. 2007;12:15–24. doi: 10.3109/10929080601168254. [DOI] [PubMed] [Google Scholar]
- 74.Cleary K, Melzer A, Watson V, et al. Interventional robotic systems: applications and technology state-of-the-art. Minim Invasive Ther Allied Technol. 2006;15:101–113. doi: 10.1080/13645700600674179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zangos S, Eichler K, Thalhammer A, et al. MR-guided interventions of the prostate gland. Minim Invasive Ther Allied Technol. 2007;16:222–229. doi: 10.1080/13645700701520669. [DOI] [PubMed] [Google Scholar]
- 76.Zangos S, Herzog C, Eichler K, et al. MR-compatible assistance system for punction in a high-field system: device and feasibility of transgluteal biopsies of the prostate gland. Eur Radiol. 2007;17:1118–1124. doi: 10.1007/s00330-006-0421-0. [DOI] [PubMed] [Google Scholar]
- 77.Davies BL, Harris SJ, Dibble E. Brachytherapy—an example of a urological minimally invasive robotic procedure. Int J Med Robot. 2004;1:88–96. doi: 10.1002/rcs.10. [DOI] [PubMed] [Google Scholar]
- 78.Wei Z, Ding M, Downey D, Fenster A. 3D TRUS guided robot assisted prostate brachytherapy. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2005;8:17–24. doi: 10.1007/11566489_3. [DOI] [PubMed] [Google Scholar]
- 79.Yu Y, Podder T, Zhang Y, et al. Robot-assisted prostate brachytherapy. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2006;9:41–49. doi: 10.1007/11866565_6. [DOI] [PubMed] [Google Scholar]
- 80.Podder TK, Ng WS, Yu Y. Multi-channel robotic system for prostate brachytherapy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1233–1236. doi: 10.1109/IEMBS.2007.4352520. [DOI] [PubMed] [Google Scholar]
- 81.Stoianovici D, Song D, Petrisor D, et al. “MRI Stealth” robot for prostate interventions. Minim Invasive Ther Allied Technol. 2007;16:241–248. doi: 10.1080/13645700701520735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muntener M, Patriciu A, Petrisor D, et al. Magnetic resonance imaging compatible robotic system for fully automated brachytherapy seed placement. Urology. 2006;68:1313–1317. doi: 10.1016/j.urology.2006.08.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]


