Abstract
Ongoing clinical trials provide promise for the introduction of immunotherapy into the armamentarium against prostate cancer, but the precise role for immunotherapy remains to be determined. Combinations of immunotherapies may be needed to improve the response rates and the duration of response. Investigators have begun to examine the effect of immunotherapy in combination with other standard treatment, including as an adjuvant to chemotherapy or radiotherapy and as a neoadjuvant agent before prostatectomy. Although many studies examine efficacy in men with metastatic hormone-refractory prostate cancer, there is some evidence for improved responses at earlier stages of disease: the ability of the tumor to evade the immune system may be lessened with lower tumor burden, or the immune system may already be weakened in men with later stages of disease.
Key words: Prostate cancer, Immunotherapy, Vaccine
Prostate cancer is the most common tumor in the United States. In 2007 an estimated 218,890 cases of prostate cancer were diagnosed, with 27,050 deaths being attributed to the disease. Local therapy (surgery, external beam radiotherapy, brachytherapy) is effective in controlling local disease; however, a significant number of men develop disease recurrence after local therapy. Hormonal therapy, although effective in impacting prostate cancer, has numerous adverse effects. The median time to androgen independence is 14 to 30 months. Docetaxel-based chemotherapy has shown a survival benefit in randomized controlled trials of 2.4 months. Further therapies are needed to improve survival in men with hormone-resistant prostate cancer (HRPC), and a variety of potential avenues are under exploration to fill this void.
Immunotherapy has become standard treatment in a wide variety of tumors. Such therapy includes cytokine administration (eg, interleukin [IL] 2 in metastatic renal cell carcinoma), monoclonal antibody therapy (eg, trastuzumab in breast cancer), and local immune stimulation (eg, Bacillus Calmette-Guéerin [BCG] for carcinoma in situ of the bladder). In prostate cancer, effective immune strategies have been investigated for 25 years. Recent progress has been made in a variety of agents. This review outlines some of the recent advances in immunotherapy strategies for prostate malignancy.
Tumor Immunology
The immune system is divided into 2 components, innate and adaptive. The innate immune system includes neutrophils, macrophages/monocytes, mast cells, and natural killer cells. These cells are not specific to the invader and function by secreting cytokines, presenting antigens, and mediating cell lysis. Adaptive immunity includes lymphocytes, namely B cells and T cells, each of which responds to a specific antigen. Their activity is modulated by exposure to that specific antigen. This portion of the immune system can be amplified and develops memory. Activated B cells mature into plasma cells, which are responsible for antibody production. T cells exist in subsets based on cell-surface marker expression. CD8 cells are referred to as cytotoxic T cells, whereas CD4 cells are termed helper T cells. CD4 cells direct the immune response through the secretion of cytokines, the maturation of B-cell/antibody responses, the stimulation of CD8 T-cell cytotoxic responses, and antigen-presenting cell (APC) activity. In general, antitumor response is controlled by T cells, an overview of which is provided in Figure 1. Activation of T cells requires 2 signals, 1 signal through the T-cell receptor (TCR) and a second signal. The TCR interacts with major histocompatibility complex (MHC) class 1 and class 2 molecules (also termed human leukocyte antigen [HLA] 1 and 2) expressed on the cell surface. MHC 1 is expressed on all nucleated cells, presents peptide antigens from the cell itself, and interacts with the TCR of CD8 T cells. MHC 2 is expressed exclusively on APCs, presents peptide antigens taken up from the cellular environment, and interacts with the TCR on CD4 T cells. APCs include monocytes, macrophages, B cells, and dendritic cells. The second signal for T-cell activation often occurs through interaction of coreceptors between the two cells, the major one for the purposes of this review being an interaction between B7-1 on the APC and CD28 on the T cell.
Figure 1.
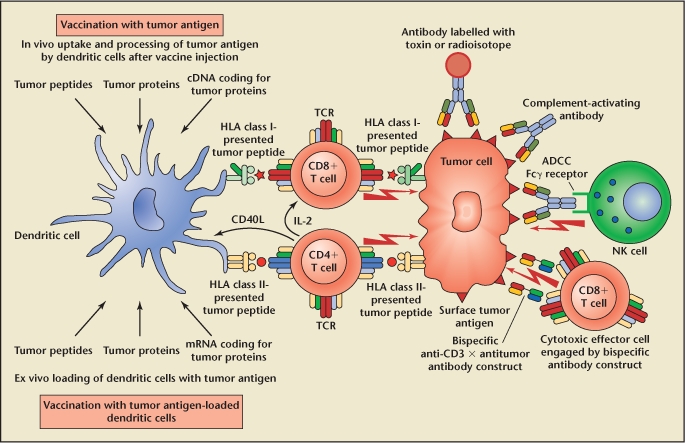
Schematic representation of the antitumor response and its modification by immunotherapy. CD4 and CD8 T cells are the cornerstone of this response, which is affected by tumor cell and dendritic cell antigen presentation. T cells in turn modulate some of the other host responses. Please refer to the text for further details. Reproduced with permission from Kiessling A et al, “Advances in specific immunotherapy for prostate cancer,” Eur Urol. 2008;53:694–708.
CD8 T cells induce apoptosis of the cell presenting appropriate antigen on MHC 1. APCs presenting appropriate antigen to CD4 T cells through MHC 2 lead to cytokine secretion, which further stimulates CD8 T-cell activation and proliferation, thus amplifying the immune response. Activated B cells are further stimulated by cytokines released by CD4 T cells. These cells produce antibodies that can mediate tumoricidal effects through complement-mediated cell lysis or natural killer cell-mediated antibody-dependent cellular cytotoxicity. Cytokines released by CD4 cells also alter dendritic cell activity, leading to increased antigen presentation. Thus, although CD8 T cells are the major effector in antitumor immunity, CD4 T cells play a vital role in amplifying the response. Additionally, a variety of cytokines and other molecules inhibit this cascade. Although such regulators prevent overactivation and autoimmune responses, they also aid in evasion of the antitumor response.
Immunomodulatory Therapy
A variety of studies have examined methods to stimulate the immune system to augment the immune reaction to prostate cancer. The earliest clinical trials in the use of immunotherapy in prostate cancer involved injection of BCG, with a limited though statistically significant improvement in overall survival.1–3 More recent strategies use immunomodulatory agents (granulocyte-macrophage colony-stimulating factor [GM-CSF], Flt3 ligand, IL-2) to stimulate antitumor response. The advantage of this approach is the relative ease of production and administration of cytokines as compared with the immunotherapies described later. A disadvantage to such therapy is a global stimulation of immune responses rather than a tumor-specific response.
Granulocyte-Macrophage Colony-Stimulating Factor
Granulocyte-macrophage colony-stimulating factor has been used in a number of clinical trials, both alone and with concomitant administration of conventional treatment, with varied results. GM-CSF has a number of functions, including stimulation of antigen uptake and processing by dendritic cells, thus recruiting more T cells in the antitumor response. Small and colleagues4 initially examined the efficacy of GM-CSF administration in a staged trial on 35 men with HRPC. The first cohort of 22 men was treated in 28-day cycles consisting of 250 µg/m2 of GM-CSF daily for 14 days, followed by 14 days off. Ten of the 22 patients in this cohort demonstrated prostate-specific antigen (PSA) level declines at the end of each 14-day treatment, followed by a return to baseline in an oscillating manner. Median time to disease progression in this group was 3.5 months. Cohort 2 consisted of 13 men who were treated with the same initial 14-day treatment period, followed by maintenance therapy with thrice-weekly injections of GM-CSF until disease progression. PSA decreased in all but 1 patient; however, only 1 patient had a sustained decrease in PSA greater than 50%, and this patient dropped from a PSA level of 77 ng/mL to 0.1 ng/mL with objective radiographic improvement.
Subsequent studies have shown improved response rates to GM-CSF in earlier-stage disease. Dreicer and coworkers5 administered 250 µg of GM-CSF thrice weekly for a total of 24 weeks to 16 men with prostate cancer in a phase II trial. Treatment was halted for biochemical or objective disease progression. Four of 6 hormone-naive patients completed the trial with stable disease, compared with only 3 of 9 with androgen-independent disease. Another phase II trial examined the effect of GM-CSF in a group of 30 patients with biochemical recurrence after localized therapy.6 Patients received 250 µg/m2 of GM-CSF daily for 14 days, followed by 14 days off. Three of 29 evaluable patients had a greater than 50% decline in PSA levels during treatment, whereas 16 of 29 had a 2-fold or greater increase in PSA doubling time. Eight of 29 patients remained on study at the time of publication, with at least stable disease for 20 to 32 months. In a follow-up study, 7 of 29 remained on treatment a median of 5.1 years from initiation of therapy.7 Patients with a long-term response had lower tumor stage, Gleason score, and pretreatment PSA level.
Granulocyte-macrophage colony-stimulating factor has been used with other therapies to evaluate overall benefit. Ryan and colleagues8 published a study on 30 men with HRPC, in which all the patients were given ketoconazole, hydrocortisone, and 250 µg/m2 of GM-CSF daily on days 15 to 28 of a 28-day cycle. Treatment was continued until disease progression was confirmed. Interestingly, those without radiographic disease on study initiation had longer times to progression (15.4 months vs 6.9 months).
Thalidomide is another agent that has undergone trials in HPRC, in part owing to its purported antiangiogenic activity in vitro. Dreicer and associates9 looked at a combination of GM-CSF and thalidomide in 22 patients with HRPC. All patients had a decreased PSA level at 2 weeks, and 5 had a greater than 50% drop. Seven patients completed the 6 months on protocol.
Flt3 Ligand
Flt3 ligand is a stimulant of a variety of hematopoietic cell types, including dendritic cells. Preclinical and human studies have demonstrated the ability of Flt3 ligand to increase circulating levels of dendritic cells. Higano and others10 performed a clinical trial of Flt3 ligand in 31 patients with bone scan-negative HRPC. The treatment involved 6 28-day cycles, with administration of the agent daily for the first 14 days of each cycle. The first cycle was divided into a placebo and Flt3 ligand arms to examine safety, and only injection-site reactions were noted. All 21 patients who completed the study had elevations in circulating dendritic cells, and 11 patients had disease stabilization marked by stable or slight decreases in PSA levels. PSA velocity was significantly decreased in the study group during treatment (0.007/d before treatment vs 0.002/d during treatment; P < .0001).
Interleukin 2
IL-2 is an essential cytokine for T-cell recruitment and activation. Its role in therapy for renal cell carcinoma is well studied. A recent trial11 has examined a novel therapy (zoledronate) targeting stimulation of the γδ T-cell subset to treat metastatic HRPC in combination with IL-2. The γδ T cells are unique in that they recognize antigens not seen by αβ T cells. The γδ T cells are not restricted to MHC presentation for recognition. In this phase I trial, Dieli and colleagues treated 18 patients with late-stage metastatic HRPC with either zoledronate or zoledronate and low-dose IL-2 for 12 months or until progression. Only 3 of 9 patients who received zoledronate alone survived during the entire 12-month trial, and only 2 remained free from progression. In comparison, 7 of 9 survivors and 6 progression-free patients received zoledronate plus IL-2 (P < .05 for survival). Additionally, clinical responses correlated well with immunologic response as seen by circulating γδ T-cell levels, which increased and/or stabilized in the responders, compared with the precipitous drop often seen in the nonresponders.
Vaccine-Based Therapy
As opposed to broad stimulation across the immunologic panacea, vaccine-based therapies seek to stimulate a specific immune reaction against 1 or multiple tumor antigens. The methods used to do this vary widely. At their core, these therapies seek to drive a specific antitumor response with little collateral damage to normal tissues. As such, vaccine therapies often utilize prostate-specific (PSA, prostatic acid phosphatase [PAP], prostate-specific membrane antigen [PSMA], prostate stem cell antigen [PSCA]) or tumor-specific antigens to direct the response. The delivery methods vary widely; however, few trials exist comparing delivery methods directly.
Peptide/Carbohydrate Vaccines
Although there have been preclinical investigations related to direct antigen injection for immunization, relatively few clinical trials exist for this modality in prostate cancer. Perambakam and colleagues12 used a PSA peptide known to bind HLA-A2 and to elicit T-cell responses in vitro. PSA makes an attractive target because its expression is primarily limited to the prostate and is increased in most prostate cancers. In this study 28 patients were assessed. Group A consisted of 14 patients with high-risk disease (T3–4 or PSA level > 10 ng/mL or Gleason score ≥ 7) having completed local therapy. Group B consisted of 14 patients with metastatic, hormone-naive prostate cancer. Patients were randomized to receive either PSA peptide and GM-CSF or PSA-pulsed autologous dendritic cells. Delayed-type hypersensitivity to the PSA peptide could be detected in 50% of the patients during the 52-week study period (9 of 14 received PSA peptide plus GM-CSF, 5 of 14 received pulsed dendritic cells), suggesting feasibility of the mechanism for immunotherapy.
Noguchi and associates13 tested an individualized method of peptide vaccination based on preexisting cytotoxic T-cell and immunoglobulin (Ig)G reactivity and combined this with low-dose estramustine. Each patient was tested for reactivity among 16 immunogenic peptides known to bind to HLA-A24. Peptides were derived from a number of targets, including PSA, PAP, PSMA, multidrug resistance protein, and a variety of other epithelial tumor antigens. Each patient was immunized with 4 peptides on the basis of his reactivity panel. Sixteen patients with metastatic HRPC were enrolled, of whom 13 were available for assessment. All 13 had a decrease in serum PSA level, including 6 (46%) with decreases of 50% or more, for a median duration of 7.5 months.
Although most therapies have been focused on peptide antigens derived from proteins, early investigations have also used carbohydrate antigens as potential targets. To elicit an immune response, the carbohydrate antigens in these trials are conjugated to a carrier protein (keyhole limpet hemocyanin [KLH]) and administered with an immunologic adjuvant (QS-21). An early trial examined globo H, a hexasaccharide found on the secretory border of epithelial cells of the breast, pancreas, small bowel, and prostate. Nonmalignant tissues have limited exposure to immunologic surveillance owing to their position in the lumen; however, in prostate cancer their expression is increased, and exposure is more pronounced. Slovin and colleagues14 injected 20 men with advanced prostate cancer, of whom 18 were evaluable, with differing doses of globo H conjugated to KLH along with QS-21. Four groups were defined according to dose (3, 10, 30, or 100 µg) and injected on weeks 1, 2, 3, 7, and 19. Nine patients were given a boost at 50 weeks in light of declining antibody titers. Adverse events were minimal, most commonly grade 2 local site reactions. All doses seemed to be effective according to IgM and IgG antibody titers. Nine patients had radiographic evidence of metastatic disease at entry to the trial, and all with bone metastases progressed. One patient with nodal disease only remained without evidence of progression at 110 weeks, and the lymph node had decreased in size by 50%. Two patients with biochemical recurrence demonstrated a prolonged decreased PSA velocity.
Other carbohydrate antigens vaccines have been used in phase I trials, with mixed results. Using Tn antigen, it was demonstrated that KLH conjugate generated more robust antibody responses than conjugation to palmitic acid. This correlated with improved overall PSA responses in the groups receiving the KLH conjugate.15 Further studies in men with biochemical relapse using TF antigen16 and a bivalent MUC2 and globo H vaccine17 demonstrated good antibody responses and temporary decreases in PSA velocity in a majority of patients. A study attempting to combine multiple carbohydrate antigens into a single polyvalent vaccine was less successful in terms of both antibody and PSA responses.18 Further studies of these agents in prostate cancer treatment are ongoing.
Tumor Cell Vaccines
Rather than administering single or multiple antigens to target an antitumor reaction, tumor vaccines use autogenic or allogenic tumor cells to generate an immune response. Such vaccines stimulate multiple antigens, potentially minimizing the ability of the tumor cells to evade detection. Before their use in prostate cancer, tumor cell vaccines were investigated in renal cell carcinoma, lung cancer, and melanoma. Typically adjuvants are used with the tumor vaccine to stimulate the immune response to break tolerance to tumor antigens.
An early trial involved the use of autologous tumor cells taken at the time of radical prostatectomy.19 The cells were then expanded ex vivo, transfected with GM-CSF complementary DNA, lethally irradiated, and administered intradermally. Cells were evaluated for GM-CSF secretion and DNA integration before injection. Eleven patients with advanced prostate cancer had cells harvested. Eight of these patients had successful primary cultures and were eligible for analysis. The majority of patients had temporary irritation at the injection site, with occasional low-grade fevers and malaise. Two of the 8 patients had delayed-type hypersensitivity before therapy, whereas 7 had delayed-type hypersensitivity during and after therapy, indicating induction of a T-cell response to tumor antigens. Three patients developed new antibodies to tumor cell antigens, indicating a B-cell response as well. The investigators concluded that this approach was feasible and safe, though 3 of 11 subjects were unable to participate owing to difficulties with cell expansion.
Subsequent trials using tumor cell vaccines transfected with GM-CSF have been performed using allogenic prostate cancer cell lines, namely LnCaP and PC3 cells. This has been developed as the GVAX® vaccine for prostate cancer (Cell Genesys, South San Francisco, CA). For this vaccine, each cell line is transfected with GM-CSF, irradiated, and examined for GM-CSF production and DNA integration as with the autologous study described above. Simons and others20 described the first phase I/II trial with the GVAX vaccine in 21 men with biochemical recurrence within 4 years of radical prostatectomy. Patients received weekly injections of the vaccine (6 × 107 cells from each cell line) for 8 weeks with minimal side effects. The injection sites were found on histopathology to have invasion of inflammatory cells and APCs. Sixteen of 21 patients had decreasing PSA velocities at 5 months (P < .001), and 1 patient had a greater than 50% PSA reduction for 7 months. In a second phase II trial of GVAX,21 55 men with HRPC (34 with asymptomatic metastasis, 21 with no detectable metastasis) were treated with a 500 × 106-cell priming dose and either a 100 × 106-cell (low-dose) or 300 × 106-cell (high-dose) boost every 2 weeks for 6 months. PSA levels decreased more than 25% in 6 of 55 patients (11%) and more than 50% in 1 patient, whose response lasted nearly 9 months and included resolution of a lesion on bone scan. PSA velocity was decreased in 74% of those with metastases and 52% of those with only biochemical recurrence. In those with metastases, the high-dose group (n = 10) compared with the low-dose group (n = 24) had improved PSA velocity response (80% response vs 67% response), time to progression (3.7 months vs 2.3 months), and overall survival (34.9 months vs 24.0 months; P = .33) (Figure 2). Although this survival advantage was not statistically significant, the expected median survival of the metastasis group was 19.5 months. Subsequently, the GVAX program was halted when a phase III study demonstrated no survival benefit in the GVAX arm when compared with decetaxol in patients with metastatic hormone refractory disease.
Figure 2.
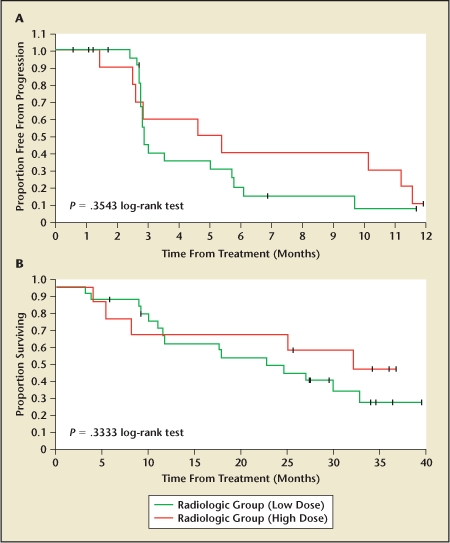
(A)Progression-free survival and (B) overall survival in hormone-refractory prostate cancer patients receiving high-dose versus low-dose GVAX prostate cancer vaccine. Reproduced with permission from Small EJ et al.21
Other allogenic tumor cell vaccines have been evaluated in phase I trials. Michael and colleagues22 used 3 cell lines (LnCaP, P4E6, and OnyCap23) to vaccinate 26 men with HRPC and no detectable metastases. Each patient received 8 × 106 irradiated cells from each cell line combined in 1 vaccine, with the first 3 doses occurring at 2-week intervals and then monthly for a total of 12 months. BCG was used as an adjuvant for the first 2 doses. The vaccine was well tolerated, with grade 1 to 2 local site reactions, arthralgias, rash, and gastrointestinal symptoms. Eleven patients (42%) had statistically significant decreases in PSA level. Median time to disease progression was 58 weeks, compared with 28 weeks in historical controls. More recently, Brill and coworkers23 performed a dose-escalating phase I trial using LnCaP cells transfected with IL-2 and interferon γ. A total of 6 men with metastatic HRPC were treated in 2 dose groups. Of the 3 high-dose patients, 2 had a greater than 50% decrease in PSA. The time to PSA progression in these 2 patients was 322 and 693 days. The promising results in these initial investigations support the utility of tumor cell vaccines in prostate cancer treatment.
Viral Vaccines
Gene therapy has merged with immunotherapy to induce immunoreactivity and antitumoral response in patients with prostate cancer. The approaches used in this merger have included both DNA and viral vaccines, and often immunomodulatory agents have been added to amplify the response.
The most extensive studies in this area have been PSA-based vaccines. The fact that PSA has localized expression to the prostate and increased levels in most prostatic adenocarcinomas makes it a prime target, though its expression in normal tissues leads to tolerance. Such tolerance needs to be overcome to develop an effective immune reaction. Initial studies with a recombinant vaccinia virus modified to express PSA (rV-PSA; PROSTVAC®; Therion Biologics, Cambridge, MA) in vitro and in animal models demonstrated the ability to induce cytotoxic and delayed-type hypersensitivity reactions to PSA peptides. Safety was confirmed in a small trial of 6 patients with biochemical recurrence after primary therapy.24
Early phase I trials included using rV-PSA in men with biochemical recurrence after local therapy and in men with nodal or bone metastasis.25 Thirty-three men were divided into 4 groups. All groups received 3 vaccinations at 4-week intervals. Group 1 (n = 6) received rV-PSA at 2.65 × 106 plaque-forming unit (pfu) with each vaccination, group 2 (n = 6) received 2.65 × 107 pfu, group 3 (n = 11) received 2.65 × 108 pfu, and group 4 (n = 10) was treated with 2.65 × 108 pfu + 250 μg/m2 GM-CSF in each vaccination. The rV-PSA was well tolerated. Disease stabilization, defined as a PSA level 80% below to 50% above baseline, occurred for greater than 6 months in 14 of 33 patients, with 6 patients (2 from group 3 and 4 from group 4) remaining progression free at 11 to 21 months after treatment. Enzyme-linked immunosorbent spot tests were performed on 7 patients, and 5 developed PSA-specific T-cell populations. The greatest increase in these T cells was seen after the first vaccination, with little subsequent response. This suggests that repeated doses of rV-PSA were ineffective, possibly owing to the immune response against the vaccinia virus itself.
In an attempt to circumvent this issue, a heterologous prime/boost approach was devised. Fowlpox virus will infect but will not replicate in mammalian cells and can transduce gene expression in infected cells for a longer period than vaccinia virus. Additionally, the lack of replication produces less immune response to the virus, allowing for repeated vaccinations with the same agent. Thus, recombinant fowlpox virus expressing PSA was generated (rF-PSA) and used in an Eastern Cooperative Oncology Group phase II trial in men with biochemically recurrent prostate cancer.26 Sixty-four patients with no evidence of metastatic disease were randomized to 1 of 3 arms: group 1 (n = 23) received 4 rF-PSA injections, group 2 (n = 20) received 3 rF-PSA vaccinations followed by an rV-PSA vaccination, and group 3 (n = 21) received 1 rV-PSA vaccination followed by 3 rF-PSA vaccinations. Of the 64 patients, 29 (45%) were free of biochemical progression (defined as a PSA level more than 50% above baseline) 2 years after treatment. Median time to PSA progression among the 3 arms was 13.6 months, with a trend toward prolonged time to PSA progression in Group 3. This suggests that the regimen used in group 3, using a prime/boost approach, was an improvement over rV-PSA alone.
The next advancement of the vaccine model was the addition of virally expressed T-cell costimulatory molecules. B7-1, intercellular adhesion molecule 1, and lymphocyte function-associated antigen 3 are all coreceptors involved in the interaction between APCs and T cells. A viral vaccine expressing these 3 costimulatory molecules (TRICOM) was generated and used in combination with recombinant virus expressing carcinoembryonic antigen to treat carcinoembryonic antigen-expressing tumors with good results. Arlen and colleagues27 performed a phase I study using TRICOM with rV-PSA and rF-PSA in 15 patients with metastatic HRPC. The study examined 5 different regimens with 3 patients in each arm: all received rF-PSA/TRICOM, 4 arms (arms 2 through 5) received prime rVPSA/TRICOM followed by 3 boosts with rF-PSA/TRICOM, 2 arms (arms 4 and 5) received a rF-GM-CSF vaccine in addition, and 1 arm (arm 3) received recombinant GM-CSF protein as an adjuvant. Overall, 9 of 15 patients had decreased PSA velocity after vaccination. Median time to clinical progression was 20.5 weeks. Large, prospective, randomized trials using this regimen with GM-CSF are ongoing.
Others have examined DNA vaccines with PSA to induce an immune response. DNA vaccines have the advantage of ease of production and administration, as well as lack of viral antigens that may generate an immune response. The disadvantage is that the rate of cell transfection is low; thus the ability to produce an immune response is weakened. In a phase I dose-escalation trial on 9 patients with HRPC, varying doses (100, 300, and 900 µg) of a DNA plasmid engineered to express PSA were administered to men 5 times at 4-week intervals along with GM-CSF and IL-2 around the time of vaccine administration.28 The treatment was well tolerated, and T-cell and IgG antibody production were robust. Three patients had decreased PSA levels after treatment.
Preliminary studies for a number of other immunotherapies based on viral and DNA vaccines have been performed, including PSMA as a target in both DNA and viral vaccines,29 IL-2 delivery as a transgene in viral vaccines,30 and others. Ongoing research will assist in determining the best targets, vectors, immunization strategies, and adjuvants to mature this area of potential prostate cancer therapy.
Dendritic Cell Therapy
Dendritic cells are APCs present in nearly all tissues. Dendritic cells present antigens through their MHC class 1 and 2 receptors and thus can induce immune responses by activating both CD8 and CD4 T cells to develop a potent antitumor response. Autologous dendritic cells can be grown in vitro and transfected with antigen, cytokines, or other agents before reintroduction to the patient to direct an immune response.
Numerous experimental immunologic regimens have adopted dendritic cells as the basis of their protocol. Sipuleucel-T (APC8015; Dendreon, Seattle, WA) is one of the most extensively studied dendritic cell modalities. It consists of autologous dendritic cells, which are harvested by leukophoresis. The cells are loaded by coculture with PA2024, a recombinant fusion protein of PAP and GM-CSF. PAP is an enzyme localized to the prostate and expressed in 95% of all prostate cancers. Thus, similar to PSA, it represents an excellent target to direct the antitumor response. GM-CSF, as noted above, stimulates dendritic cell maturation and activity. Once loaded, the dendritic cells are washed and infused to the patient.
The initial phase I trial of sipuleucel-T used both infusion of autologous dendritic cells loaded with PA2024 as well as subcutaneous (SC) injection of PA2024.31 This study demonstrated that cytotoxic T-cell responses could be induced through the dendritic cells; however, SC injections of antigen were needed to produce a humoral immune response. Thirteen patients with HRPC were enrolled, and the treatment regimen included sipuleucel-T at weeks 0 and 4, followed by SC injection of PA2024 at weeks 8, 12, and 16. A dose-escalation analysis was performed because patients were treated with different doses of PA2024. The treatment was well tolerated, with adverse events consisting of grade 1 injection-site reactions and grade 1 to 2 fevers and myalgias. In all evaluated patients the dendritic cells induced a T-cell response as determined by in vitro proliferation assays. The SC injections did not affect the T-cell response. The dendritic cells and SC injections both contributed to humoral immunity; however, the majority of this reaction was directed at GM-CSF. PSA responses, determined by a greater than 50% decrease in PSA level from baseline, occurred in 3 of 12 patients.
Other studies were performed to evaluate the safety and efficacy of sipuleucel-T.32 A phase I trial evaluated 12 men with metastatic HRPC, with sipuleucel-T administered in a dose-escalation format. A phase II trial comprised 19 men with HRPC and no evidence of metastasis. All patients in both phases developed T-cell responses to PA2024; however, only 10 (38%) developed T-cell responses to PAP. Additionally, 16 patients (52%) developed antibodies to PAP. Overall, 3 patients had a greater than 50% decline in PSA levels, and another 3 had a 25% to 49% reduction in PSA levels. Median time to progression correlated with development of either a T- or B-cell response to PAP (34 weeks vs 13 weeks; P < .027).
A subsequent phase II trial was performed on 21 patients with HRPC, with 17 having detectable metastases.33 The treatment consisted of sipuleucel-T at weeks 0 and 2, followed by SC injections of PA2024 at weeks 4, 8, and 12. Nineteen patients were evaluable, and 3 had a greater than 25% drop in PSA levels after treatment. One of these patients had a dramatic response, with a PSA drop from 221 ng/mL at baseline to undetectable, and this persisted for 52 months. This patient also had resolution of metastatic adenopathy on computed tomographic imaging.
On the basis of the results of these trials, a phase III randomized, double-blind, placebo-controlled trial was performed to evaluate the efficacy of sipuleucel-T, with time to clinical disease progression as a primary endpoint.34 All 127 patients enrolled had metastatic HRPC, 82 and 45 patients were randomized to sipuleucel-T and placebo, respectively. Entry criteria included greater than 25% of cancer cells staining positive for PAP. Dendritic cell infusions were performed on weeks 0, 2, and 4. Placebo patients were infused with autologous dendritic cells that had not been loaded with PA2024. At progression, the placebo patients were offered the opportunity to cross over. With regard to the primary endpoint, patients in the sipuleucel-T group had longer time to disease progression, though this did not reach statistical significance (11.7 vs 10.0 weeks; P = .052). The investigators found a significant improvement of 4.5 months in overall survival in the treatment group (25.9 months vs 21.4 months; P = .01) (Figure 3). A 36-month survival analysis found 34% 3-year survival in the sipuleucel-T group, compared with 11% in the placebo group (P < .005). An additional randomized phase III study is currently underway examining the efficacy of sipuleucel-T in men with metastatic HRPC.
Figure 3.
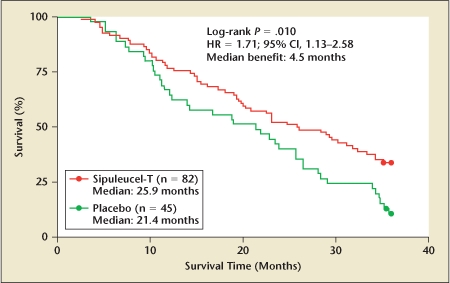
Overall survival improvement in phase III trial of sipuleucel-T. 95% CI, 95% confidence interval; HR, hazard ratio. Reproduced with permission from Small EJ et al.34
A variety of other approaches using dendritic cells have been studied, including evaluation of dendritic cells pulsed with antigenic PSMA peptides. A phase II trial examined the efficacy of the vaccine in 33 men with HRPC, and a second trial enrolled 37 men with biochemical recurrence after primary therapy.35,36 The results of the trials demonstrated 6 partial responses and 2 complete responses. In a follow-up study of the responders from these 2 studies, median response duration was 144 days in the HRPC group and 187 days in the biochemically recurrent group.37
Other trials using dendritic cells have evaluated targets such as PSA,38 PAP,39 PSCA,40 and telomerase.41 To expand the antitumor reaction and prevent tumor evasion from the immune system, investigators have used dendritic cells engineered to express a wider range of antigens. Strategies in this endeavor have included pulsing dendritic cells with multiple peptides,42,43 tumor cell lysates,44 and cell line messenger RNA.45
Antibody-Based Therapy
Antibody therapies are also undergoing extensive investigation. Antibodies can be used to induce cellular cytotoxicity-in which the antibody directs lysis of tumor cells by macrophages and neutrophils—or they can be conjugated to deliver toxins or radioactive substances that result in cell death. Many different antibody-based modalities have undergone trials.
Prostate-Specific Membrane Antigen
Monoclonal antibodies to PSMA have been used for several years diagnostically in the form of the ProstaScint® (Cytogen Corporation, Princeton, NJ) scan. The ProstaScint scan utilizes a monoclonal antibody to PSMA, 7E11 (capromab), which targets an intracellular segment of PSMA. The ProstaScint scan is limited by its poor imaging of bone metastasis. This failure has been linked to the fact that its target is intracellular, thus only seen in necrotic tumors with lysed cells. A second monoclonal antibody developed to the extracellular domain of PSMA (J591) has been used in phase I radioimmunotherapy trials. J591, when complexed to PSMA, is internalized; thus toxins or radioactive substances coupled to the antibody can be delivered to the targeted cells. Initial studies in patients with metastatic prostate cancer demonstrated the ability of J591 coupled to radiometals to target metastatic lesions.46 Subsequently, phase I clinical trials have been published to examine safe and effective dosing regimens.
Each trial used different radiometals (111I, 177Lu, 111I, and 90Y) to induce antibody-dependent cellular cytotoxicity. Overall, the only significant morbidity was dose-limiting myelotoxicity, controlled with titration. In the trial using 177Lu-J591, 35 patients with progressive HRPC were treated. Four had a greater than 50% decrease in PSA lasting 3 to 8 months, and another 16 of 35 had disease stabilization for a median of 60 days.47 The 90Y trial enrolled 29 patients with HRPC; 2 had PSA decreases greater than 50%, and another 6 experienced disease stabilization. Fourteen men with metastatic HRPC were treated with 111I-J591 plus unlabeled J591; 1 had a 90% decrease in PSA levels, and a second patient had disease stabilization. J591 radioconjugates are presently in phase II trials.
HER-2/neu
Antibody therapy directed against HER-2/neu (trastuzumab) in patients with advanced breast cancer has shown clinical benefit. HER-2/neu is expressed in some advanced prostate cancers and has undergone trials in HER-2/neu-positive prostate cancer patients, with limited benefit.48,49 MDXH210 is a chimeric antibody that recognizes HER-2/neu and the IgG Fc receptor. The strategy is to bring Fc-expressing cells (monocytes, neutrophils) to the HER-2/neu-expressing cancer cells. In a phase I trial on 6 patients with HRPC, 5 patients demonstrated disease stabilization for at least 2 months after therapy.50 Another group used MDXH210 in combination with GM-CSF in 20 men with HRPC.51 Seven patients had a greater than 50% drop in PSA levels, and 15 of 18 evaluable patients had a decrease in PSA velocity after treatment.
CTLA-4
Whereas the goal of most antibody-based therapies is induction of cell death, CTLA-4 antibody therapy is aimed at improving the immune response. CTLA-4 is a receptor expressed in T cells that competes with CD28 in binding to B7-1 on the APC. This blocks the second costimulatory signal required for T-cell activation, and antibodies to CTLA-4 strive to prevent this interference. A potential adverse effect of this therapy is autoimmune responses. Small and others52 investigated anti-CTLA-4 antibodies (ipilimumab) in 14 patients with HRPC. At a dose of 3 mg/kg, 2 of 14 patients had a greater than 50% decline in PSA lasting 60 and 135 days, and an additional 8 patients had decreases in PSA below 50%. One grade 3 reaction occurred, an autoimmune dermatitis requiring steroid treatment. Preliminary results of trials combining CTLA-4 with other treatments (GM-CSF, GVAX) have been reported, with promising results. Further studies are ongoing to determine the role of anti-CTLA-4 in prostate cancer immunotherapy, possibly as an adjunct to other vaccine-based modalities.
Conclusions
Immunotherapy for prostate cancer has made great strides. Ongoing clinical trials provide promise for the introduction of immunotherapy into the armamentarium against prostate cancer, but the precise role for immunotherapy remains to be determined. Combination of immunotherapies may be needed to improve the response rates and the duration of response. Investigators have begun to examine the effect of immunotherapy in combination with other standard treatment, including as an adjuvant to chemotherapy or radiotherapy and as a neoadjuvant agent before prostatectomy. Although many studies examine efficacy in men with metastatic HRPC, there is mounting evidence for improved responses at earlier stages of disease: the ability of the tumor to evade the immune system may be lessened with lower tumor burden, or the immune system may already be weakened in men with later stages of disease. With mounting evidence of the impact of immune therapy upon prostate cancer, including modest survival benefits, the field remains an active area of investigation for therapy.
Main Points.
In prostate cancer, effective immune strategies have been investigated for 25 years, and recent progress has been made in a variety of agents.
Immunotherapy regimens under investigation include immunomodulatory cytokines/effectors, peptide and cellular immunization, viral vaccines, dendritic cell vaccines, and antibody therapies.
A variety of studies have examined methods to stimulate the immune system to augment the immune reaction to prostate cancer; recent strategies use immunomodulatory agents (granulocyte-macrophage colony-stimulating factor, Flt3 ligand, and IL-2) to stimulate antitumor response.
Vaccine-based therapies often utilize prostate-specific (prostate-specific antigen, prostatic acid phosphatase, prostate-specific membrane antigen, prostate stem cell antigen) or tumor-specific antigens to direct the response.
Gene therapy has merged with immunotherapy to induce immunoreactivity and antitumoral response in patients with prostate cancer. The approaches used in this merger have included both DNA and viral vaccines, and often immunomodulatory agents have been added to amplify the response.
Numerous experimental immunologic regimens have adopted dendritic cells as the basis of their protocol. Sipuleucel-T is one of the most extensively studied dendritic cell modalities.
Antibodies can be used to induce cellular cytotoxicity—in which the antibody directs lysis of tumor cells by macrophages and neutrophils—or they can be conjugated to deliver toxins or radioactive substances that result in cell death. Antibodies may also be used to stimulate the immune response through blockade of its normal negative feedback mechanisms (anti-CTLA-4), which in the future may improve responses to other immunotherapy regimens.
References
- 1.Guinan P, Crispen R, Baumgartner G, et al. Adjuvant immunotherapy with bacillus Calmette-Guérin in prostatic cancer. Urology. 1979;14:561–565. doi: 10.1016/0090-4295(79)90523-5. [DOI] [PubMed] [Google Scholar]
- 2.Guinan P, Toronchi E, Shaw M, et al. Bacillus Calmette-Guerin (BCG) adjuvant therapy in stage D prostate cancer. Urology. 1982;20:401–403. doi: 10.1016/0090-4295(82)90464-2. [DOI] [PubMed] [Google Scholar]
- 3.Guinan PD, John T, Baumgartner G, et al. Adjuvant immunotherapy (BCG) in stage D prostate cancer. Am J Clin Oncol. 1982;5:65–68. [PubMed] [Google Scholar]
- 4.Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 5.Dreicer R, See WA, Klein EA. Phase II trial of GM-CSF in advanced prostate cancer. Invest New Drugs. 2001;19:261–265. doi: 10.1023/a:1010637105066. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Weinberg V, Bok R, Small EJ. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol. 2003;21:99–105. doi: 10.1200/JCO.2003.04.163. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Fong L, Weinberg V, et al. Clinical and immunological characteristics of patients with serologic progression of prostate cancer achieving long-term disease control with granulocyte-macrophage colony-stimulating factor. J Urol. 2006;175:2087–2091. doi: 10.1016/S0022-5347(06)00261-8. [DOI] [PubMed] [Google Scholar]
- 8.Ryan CJ, Weinberg V, Rosenberg J, et al. Phase II study of ketoconazole plus granulocyte-macrophage colony-stimulating factor for prostate cancer: effect of extent of disease on outcome. J Urol. 2007;178:2372–2376. doi: 10.1016/j.juro.2007.08.011. discussion 2377. [DOI] [PubMed] [Google Scholar]
- 9.Dreicer R, Klein EA, Elson P, et al. Phase II trial of GM-CSF + thalidomide in patients with androgen-independent metastatic prostate cancer. Urol Oncol. 2005;23:82–86. doi: 10.1016/j.urolonc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Higano CS, Vogelzang NJ, Sosman JA, et al. Safety and biological activity of repeated doses of recombinant human Flt3 ligand in patients with bone scan-negative hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:1219–1225. doi: 10.1158/1078-0432.ccr-1404-02. [DOI] [PubMed] [Google Scholar]
- 11.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perambakam S, Hallmeyer S, Reddy S, et al. Induction of specific T cell immunity in patients with prostate cancer by vaccination with PSA146–154 peptide. Cancer Immunol Immunother. 2006;55:1033–1042. doi: 10.1007/s00262-005-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi M, Mine T, Yamada A, et al. Combination therapy of personalized peptide vaccination and low-dose estramustine phosphate for metastatic hormone refractory prostate cancer patients: an analysis of prognostic factors in the treatment. Oncol Res. 2007;16:341–349. doi: 10.3727/000000006783980955. [DOI] [PubMed] [Google Scholar]
- 14.Slovin SF, Ragupathi G, Adluri S, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci U S A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slovin SF, Ragupathi G, Musselli C, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. J Clin Oncol. 2003;21:4292–4298. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slovin SF, Ragupathi G, Musselli C, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slovin SF, Ragupathi G, Fernandez C, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semisynthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–3122. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 18.Slovin SF, Ragupathi G, Fernandez C, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother. 2007;56:1921–1930. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 20.Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 21.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor—secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 22.Michael A, Ball G, Quatan N, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005;11:4469–4478. doi: 10.1158/1078-0432.CCR-04-2337. [DOI] [PubMed] [Google Scholar]
- 23.Brill TH, Kubler HR, von Randenborgh H, et al. Allogeneic retrovirally transduced, IL-2- and IFN-gamma-secreting cancer cell vaccine in patients with hormone refractory prostate cancer—a phase I clinical trial. J Gene Med. 2007;9:547–560. doi: 10.1002/jgm.1051. [DOI] [PubMed] [Google Scholar]
- 24.Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 25.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 26.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 27.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 28.Pavlenko M, Roos AK, Lundqvist A, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mincheff M, Tchakarov S, Zoubak S, et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 30.Trudel S, Trachtenberg J, Toi A, et al. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 2003;10:755–763. doi: 10.1038/sj.cgt.7700626. [DOI] [PubMed] [Google Scholar]
- 31.Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 32.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 33.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 34.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 35.Murphy GP, Tjoa BA, Simmons SJ, et al. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39:54–59. doi: 10.1002/(sici)1097-0045(19990401)39:1<54::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Murphy GP, Tjoa BA, Simmons SJ, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate. 1999;38:73–78. doi: 10.1002/(sici)1097-0045(19990101)38:1<73::aid-pros9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 37.Tjoa BA, Simmons SJ, Elgamal A, et al. Follow-up evaluation of a phase II prostate cancer vaccine trial. Prostate. 1999;40:125–129. doi: 10.1002/(sici)1097-0045(19990701)40:2<125::aid-pros8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Barrou B, Benoit G, Ouldkaci M, et al. Vaccination of prostatectomized prostate cancer patients in biochemical relapse, with autologous dendritic cells pulsed with recombinant human PSA. Cancer Immunol Immunother. 2004;53:453–460. doi: 10.1007/s00262-003-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong L, Brockstedt D, Benike C, et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 40.Thomas-Kaskel AK, Zeiser R, Jochim R, et al. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119:2428–2434. doi: 10.1002/ijc.22097. [DOI] [PubMed] [Google Scholar]
- 41.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 42.Fuessel S, Meye A, Schmitz M, et al. Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial. Prostate. 2006;66:811–821. doi: 10.1002/pros.20404. [DOI] [PubMed] [Google Scholar]
- 43.Waeckerle-Men Y, Uetz-von Allmen E, Fopp M, et al. Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma. Cancer Immunol Immunother. 2006;55:1524–1533. doi: 10.1007/s00262-006-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandha HS, John RJ, Hutchinson J, et al. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94:412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 45.Mu LJ, Kyte JA, Kvalheim G, et al. Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer. 2005;93:749–756. doi: 10.1038/sj.bjc.6602761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bander NH, Trabulsi EJ, Kostakoglu L, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170:1717–1721. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 47.Bander NH, Milowsky MI, Nanus DM, et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 48.Lara PN , Jr, Chee KG, Longmate J, et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer. 2004;100:2125–2131. doi: 10.1002/cncr.20228. [DOI] [PubMed] [Google Scholar]
- 49.Ziada A, Barqawi A, Glode LM, et al. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60:332–337. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 50.Schwaab T, Lewis LD, Cole BF, et al. Phase I pilot trial of the bispecific antibody MDXH210 (anti-Fc gamma RI X anti-HER-2/neu) in patients whose prostate cancer overexpresses HER-2/neu. J Immunother. 2001;24:79–87. doi: 10.1097/00002371-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 51.James ND, Atherton PJ, Jones J, et al. A phase II study of the bispecific antibody MDX-H210 (anti-HER2 × CD64) with GM-CSF in HER2+ advanced prostate cancer. Br J Cancer. 2001;85:152–156. doi: 10.1054/bjoc.2001.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]


