Abstract
αIIbβ3 interaction with fibrinogen promotes Src-dependent platelet spreading in vitro. To determine the consequences of this outside-in signaling pathway in vivo, a “β3(Δ760-762)” knockin mouse was generated that lacked the 3 C-terminal β3 residues (arginine-glycine-threonine [RGT]) necessary for αIIbβ3 interaction with c-Src, but retained β3 residues necessary for talin-dependent fibrinogen binding. β3(Δ760-762) mice were compared with wild-type β3+/+ littermates, β3+/− heterozygotes, and knockin mice where β3 RGT was replaced by β1 C-terminal cysteine-glycine-lysine (EGK) to potentially enable signaling by Src kinases other than c-Src. Whereas β3+/+, β3+/− and β3/β1(EGK) platelets spread and underwent tyrosine phosphorylation normally on fibrinogen, β3(Δ760-762) platelets spread poorly and exhibited reduced tyrosine phosphorylation of c-Src substrates, including β3 (Tyr747). Unlike control mice, β3(Δ760-762) mice were protected from carotid artery thrombosis after vessel injury with FeCl3. Some β3(Δ760-762) mice exhibited prolonged tail bleeding times; however, none demonstrated spontaneous bleeding, excess bleeding after surgery, fecal blood loss, or anemia. Fibrinogen binding to β3(Δ760-762) platelets was normal in response to saturating concentrations of protease-activated receptor 4 or glycoprotein VI agonists, but responses to adenosine diphosphate were impaired. Thus, deletion of β3 RGT disrupts c-Src–mediated αIIbβ3 signaling and confers protection from arterial thrombosis. Consequently, targeting αIIbβ3 signaling may represent a feasible antithrombotic strategy.
Introduction
Integrins are αβ transmembrane heterodimers whose extracellular domains recognize a repertoire of adhesive ligands, some of which contain a canonical arginine-glycine-aspartic acid (RGD) recognition motif.1,2 The ligand binding affinity or “activation state” of integrins can be regulated by “inside-out” signals that induce conformational changes in the extracellular domains.3–5 Ligand binding also activates integrins and initiates “outside-in” signals that regulate many cellular functions, including cell spreading and migration.2,6,7 Integrin αIIbβ3 binds ligands such as fibrinogen, von Willebrand factor, and fibronectin and mediates platelet spreading and aggregation on vascular surfaces during hemostasis and thrombosis.8–11 αIIbβ3 provides a critical test of the clinical importance of integrin signaling. Bidirectional αIIbβ3 signaling is impaired and bleeding is observed in humans12,13 and mice14,15 with a point mutation (S752P or Y747A) or a sizeable deletion (Δ724) of the β3 cytoplasmic domain. In these examples, defective signaling is thought to result from a loss of β3 interactions with intracellular regulatory proteins, including talin and Src family protein tyrosine kinases (SFKs).9,16
Upon platelet activation by agonists, talin is recruited from the cytoplasm to αIIbβ3,17,18 its FERM domain interacting with specific β3 cytoplasmic domain residues, including membrane-proximal residues and N744PLY747 (Figure 1A).3,4 c-Src is constitutively associated with αIIbβ3 in a manner dependent on its SH3 domain and on β3 C-terminal residues R760GT762 (Figure 1A), and it is activated when fibrinogen binds to αIIbβ3.19–21 Talin and c-Src play key roles in inside-out and outside-in signaling, respectively, and their binding to β3 is not mutually exclusive.20 Conditional knockout of talin in mouse platelets impairs agonist-induced αIIbβ3 activation and causes a severe bleeding diathesis,22,23 while mouse platelets deficient in 4 SFKs (c-Src, Hck, Fgr, Lyn) exhibit impaired tyrosine phosphorylation and spreading on fibrinogen.24 Platelet spreading ex vivo is also defective if 760R or 762T in murine β3 is mutated to alanine,14 or if human platelets are treated with a membrane-permeable RGT peptide that blocks c-Src interaction with β3.25 While these data support a role for the β3 cytoplasmic domain C-terminus and its interaction with c-Src in αIIbβ3 signaling, there is as yet no information as to whether this interaction is involved in hemostasis or thrombosis in vivo. Interestingly, among the key substrates of c-Src in platelets is β3 itself, and double mutation of β3 Tyr747 and Tyr759 to phenylalanine in the mouse results in reduced platelet aggregate size, increased tendency to rebleed from tail wounds, and deficient fibrin clot retraction.26
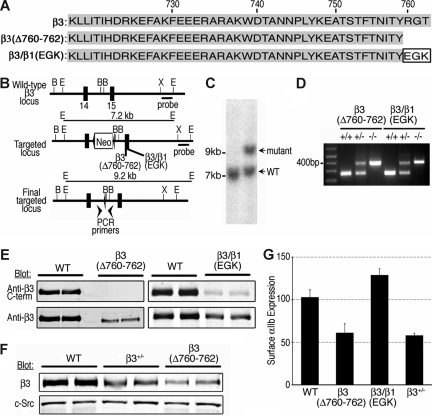
Figure 1.
Generation of β3 knockin mice. (A) Wild-type β3+/+ (WT) and mutant β3 cytoplasmic domain sequences. β3(Δ760-762) lacks the 3 C-terminal residues of β3 (arginine-glycine-threonine [RGT]), while in β3/β1(glutamic acid–glycine-lysine [EGK]) those residues have been replaced with the respective 3 C-terminal residues of β1. (B) Gene-targeting strategy. A 7.2-kb targeting vector for the mouse β3 gene contained a Neo cassette flanked by 2 lox P sequences between β3 exons 14 and 15. Either of the β3 mutations was introduced into exon 15. B, BamHI; E, EcoRI; X, XhoI. (C) Southern blot analysis with a 3′ probe of R1 embryonic stem (ES) cell genomic DNA transfected with the β3(Δ760-762) targeting vector and digested with EcoRI. (D) PCR genotyping of final floxed β3 cytoplasmic domain mutants. (E) Mouse platelet lysates blotted with antibodies recognizing the extracellular domain of β3 (anti-β3) or the β3 C-terminus (anti-β3 C-terminus). (F) Platelet lysate (10 μg) from β3+/+, β3+/−, and β3(Δ760-762) mice were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotted with antibody to β3 or c-Src. (G) Surface expression of αIIbβ3 was determined by flow cytometry using an antibody to αIIb. Data are expressed as mean fluorescence intensity in arbitrary units plus or minus standard error of the mean [SEM] (n = 5-13).
Accordingly, we deleted R760GT762 from mouse β3 by knock-in technology and report here the in vivo consequences of this deletion. β3(Δ760-762) knockin mice were alive and fertile, exhibited normal blood counts, and, unlike mice lacking β3,27 did not display a significant bleeding diathesis. However, their platelets exhibited reduced tyrosine phosphorylation and spreading on fibrinogen, a reduction in fibrinogen binding in response to adenosine diphosphate (ADP), and the mice were protected from occlusive thrombosis after chemical injury to the carotid artery. Currently available antiplatelet drugs used for cardiovascular indications inhibit agonist-induced activation of αIIbβ3 and block fibrinogen binding to the integrin.11,28 The present results suggest a different antithrombotic approach, the targeting of αIIbβ3 signaling at the level of the extreme C-terminus of β3.
Methods
Generation of β3 cytoplasmic domain mutant mice
Cloning strategies for β3 knockin mutants were similar to those presented by Petrich et al.15 Briefly, a 6.1 kb mouse β3 cytoplasmic domain EcoRI-XhoI genomic fragment was cloned from R1 ES cells derived from 129 SvJ mice using genomic DNA and the polymerase chain reaction (PCR). The β3(Δ760-762) and β3/β1(EGK) mutations were introduced with overlap extension PCR using the following primers (mutated residues in lowercase): (a) for β3(Δ760-762): 5′-CTGCTTACTGGCTTATCGAAATTAATACGACTC-3′; 5′-CCTACtGataGACTTAATGAGACCATCTTCAGtTaACGCCAGG-3′; 5′-GTtAaCTGAAGATGGTCTCATTAAGTCtatCaGTAGGTGATATTGG-3′; 5′-TTCTCTAGATTTCTTGACATGGAGG-3′; and (b) for β3/β1(EGK): 5′-CTGCTTACTGGCTTATCGAAATTAATACGACTC-3′; 5′-CTACgaGGGGAaaTAATGAGACCATaTgCAGATGACGCC-3′; 5′-CTGcAtATGGTCTCATTAttTCCCCtcGTAGGTGATATTGG-3′; 5′-TTCTCTAGATTTCTTGACATGGAGG-3′. Silent mutations were introduced to generate new HpaI and NdeI restriction sites within the genomic locus of β3(Δ760-762) and β3/β1(EGK), respectively. Final targeting constructs contained a neomycin-resistance cassette between exons 14 and 15, flanked by 2 loxP sequences. NotI-linearized targeting constructs were electroporated into R1 ES cells by the Transgenic Mouse and Gene Targeting Core at University of California San Diego (UCSD, La Jolla, CA). Genomic DNAs from positive ES cells were analyzed by Southern blotting using a 3′ probe, where Neo in targeted ES cells introduced a 2 kb larger EcoRI fragment (∼9.2 kb) than the wild-type allele (7.2 kb). Five positive clones per mutation were karyotyped, and 2 clones per mutation were used for injections into C57BL/6 mouse host blastocysts. Neo was deleted in germ cells by crossing 2 independently derived heterozygous chimeric male mice for each mutation with EIIa-Cre mice (The Jackson Laboratory, Bar Harbor, ME),29 and the deletion was confirmed by genomic PCR using primers 5′-CAGTCCTCTACCTTACAGTG-3′ and 5′-CTCTGCCCTCAGTTTCCTTA-3′ (+/+ ∼ 240 bp, −/− ∼ 400 bp). All experiments were performed with independently derived lines that were backcrossed to the C57BL/6 strain for at least 3 generations. Mice were housed in the UCSD animal facility and experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Carotid injury and thrombosis
Assays were similar to those of Konstantinides et al.30 Each side of the adventitia of the left carotid artery was treated with 5% FeCl3 soaked filter paper for 3 minutes. Flow rate was monitored with a miniature ultrasound flow probe (T402 flowmeter, Transonic Systems, Ithaca, NY) for at least 2 minutes before and 30 minutes after application of FeCl3. The operator was blinded to the genotype of the mice, and each experiment included mutant and control mice.
Hemostasis assays
Tail bleeding assays were performed by resecting 2 to 3 mm of tail tip followed by tail immersion in isotonic saline at 37°C.27 Bleeding times were taken as the time at which bleeding from the tip ceased for at least 60 seconds; if bleeding restarted within 60 seconds, it was scored as a rebleed.
Blood counts, platelet isolation, and platelet function assays
Peripheral blood collected in EDTA (ethylenediaminetetraacetic acid) was analyzed with the Hemavet 850FS Multi Species Hematology System (Drew Scientific, Waterbury, CT). Mouse blood was collected by cardiac or inferior vena cava puncture, anticoagulated 5:1 with acid-citrate-dextrose and diluted 1:1 with wash buffer (150 mM NaCl, 20 mM Pipes, pH 6.5) supplemented with 2 U/mL apyrase.31 After centrifugation, platelets were resuspended at 108/mL in Walsh buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2·6H2O, 3.3 mM NaH2PO4.H2O, 20 mM HEPES [N-2-hydroxyethylpoperazine-N′-2-ethanesulfonic acid], pH 7.4, 0.1% glucose, 0.1% bovine serum albumin), incubated with 150 μg/mL fluorescein isothiocyanate (FITC)–fibrinogen (Enzyme Research Laboratories, South Bend, IN) for 20 minutes, and analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Specific fibrinogen binding was taken as total binding minus nonspecific binding in the presence of 5 mM EDTA. αIIbβ3 expression was measured using an FITC-conjugated anti–mouse αIIb antibody (anti-CD41; BD Biosciences). Platelet surface P-selectin expression was measured with a biotin-conjugated anti–mouse P-selectin antibody (BD Biosciences) and phycoerythrin-conjugated streptavidin (Invitrogen, Carlsbad, CA). For platelet aggregation and fibrin clot retraction assays, blood was anticoagulated 9:1 with 3.8% sodium citrate, and diluted either 1:1 or 1:2.3 with Walsh buffer, respectively. Typically, blood from 4 mice of each genotype was pooled for aggregation assays (Chrono-log, Havertown, PA). Fibrin clot retraction was quantified as previously described.32 To analyze platelet spreading, washed cells were plated for 45 minutes on glass coverslips precoated with 100 μg/mL fibrinogen, fixed for 10 minutes with 4% formaldehyde in PBS, and stained with rhodamine-phalloidin (Invitrogen) and antibody to vinculin (VIN-11-5; Sigma-Aldrich, St Louis, MO) or phosphotyrosine.18 Images were captured by a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA) equipped with a Photometrics Sony Coolsnap HQ charged-coupled camera system attached to an inverted wide-field fluorescence microscope (Nikon TE-200; 100× Nikon oil-immersion objective; Nikon Instruments, Melville, NY). Cell area was quantified using Image J software (http://rsb.info.nih.gov/ij/).
Immunoprecipitations and Western blotting
Platelets in suspension were incubated with 250 μg/mL fibrinogen in the presence or absence of 0.5 mM MnCl2 for 20 minutes at room temperature, and lysed in buffer containing 1% NP-40, 150 mM NaCl, 50 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.4, 1 mM sodium vanadate, 0.5 mM sodium fluoride, 1 mM leupeptin, and complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). After clarification, whole-cell lysates were analyzed by Western blotting with antibodies to phosphotyrosine (4G10 and PY20),19 and immunoreactive signals were detected and quantified by infrared emission spectrometry (Odyssey; LI-COR Biosciences, Lincoln, NE). Phosphorylation of β3 Y747 was analyzed by immunoprecipitating αIIbβ3 with antibody 1B5,33 followed by Western blotting with a phospho-specific antibody (44-876G; Invitrogen). Immunoprecipitates were also probed with rabbit antibodies to full-length β3 (sc6627; Santa Cruz Biotechnology, Santa Cruz, CA) and to the C-terminal 20 residues of the β3 cytoplasmic domain (Rb8275).34
Affinity chromatography with recombinant integrin cytoplasmic domains
Recombinant integrin cytoplasmic domains were bound to His-Bind Resin (Novagen, Gibbstown, NJ) and used to pull down proteins from platelets as previously described.19,35,36 Samples were resolved on 4% to 20% SDS-PAGE (Novex; Invitrogen) and transferred for Western blotting with antibodies to talin (8d4; Sigma-Aldrich) and c-Src (ab16885; Abcam, Cambridge, MA).
Statistical analyses
Statistical significance was determined by calculating P values using a 2-sample t test for independent samples with unequal variances.
Results and discussion
Generation of β3 cytoplasmic domain knockin mice
β3(Δ760-762) knockin mice were developed because (1) β3 R760GT762 is required for β3 interaction with the SH3 domain of c-Src (and some other SFKs) in vitro and in αIIbβ3-CHO cell lines19,20; and (2) SFKs are necessary for platelet spreading on fibrinogen.24 However, replacement of R760GT762 in β3 with the corresponding C-terminal residues in β1(EGK) rescues αIIbβ3-CHO cell spreading on fibrinogen, presumably because this β3/β1(EGK) chimera can interact with one or more SFKs other than c-Src.20 Therefore, in addition to β3(Δ760-762) knockin mice, we developed β3/β1(EGK) knockin mice (Figure 1A), reasoning that the latter might “rescue,” at least in part, a platelet phenotype displayed by β3(Δ760-762) mice.
The gene targeting strategy for generating β3 knockin mice is shown in Figure 1B. β3(Δ760-762) and β3/β1(EGK) knockin mutations were confirmed by Southern blot analysis (Figure 1C), genomic PCR (Figure 1D), and sequencing cDNA from spleen RNA templates. Indirect confirmation of the mutations at the protein level was obtained by Western blotting of platelet lysates with an anti–β3 cytoplasmic domain antibody whose epitope is disrupted by deletion or replacement of the β3 C-terminus. Thus, in contrast to platelets from β3+/+ littermates, lysate from β3(Δ760-762) platelets failed to react with this antibody, and lysate from β3/β1(EGK) platelets showed diminished reactivity (Figure 1E). Platelet lysates from all 3 mouse strains reacted with an antibody to the β3 extracellular domain. However, the amount of immunoreactive β3 in lysates from β3(Δ760-762) platelets appeared lower than normal, closer to that observed with platelets from β3+/− heterozygotes (Figure 1F). Indeed, when surface expression of αIIbβ3 was quantified by flow cytometry with an antibody to αIIb, β3(Δ760-762) platelets expressed 59.3% plus or minus 3.7% (SEM; n = 11) the level of αIIbβ3 expressed by β3+/+ platelets, similar to levels in β3+/− platelets (56.3 ± 1.5%; n = 5). αIIbβ3 expression by β3/β1(EGK) platelets was 125.1% plus or minus 3.0% (n = 6) that of β3+/+ platelets (Figure 1G). Therefore, in addition to age- and sex-matched controls, both β3+/+ and β3+/− mice were used as controls throughout subsequent studies.
Deletion of β3 R760GT762 confers protection from thrombosis without major bleeding
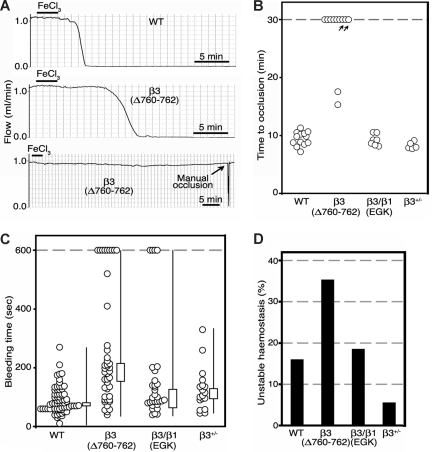
β3(Δ760-762) and β3/β1(EGK) mouse lines had no obvious gross developmental abnormalities, enabling studies of thrombosis and hemostasis in mice at 6 to 8 weeks of age. To determine whether modifications in the β3 cytoplasmic domain affect platelet function in thrombosis, mice were evaluated in a platelet-dependent model of carotid artery injury. After surgical exposure, a carotid artery was treated with 5% FeCl3 for 3 minutes. Under these conditions, platelets from normal mice typically form a stable occlusive thrombus within 12 minutes, as monitored with a flow probe.15 Similarly, all 14 β3+/+ littermates in the present study formed an occlusive thrombus within this time period (9.4 ± 1.2 minutes; Figure 2B). In contrast, β3(Δ760-762) mice exhibited either no thrombotic occlusion of the carotid for up to 30 minutes (7 of 11 mice), delayed occlusion (2 mice; 16.4 ± 1.1 minutes) or partial occlusion (2 mice; Figure 2B). Furthermore, in 1 of the 2 β3(Δ760-762) mice that showed partial occlusion, the thrombus was unstable and embolized completely within a few minutes, a response never observed with the other mouse strains. Like β3+/+ mice, all 7 β3/β1(EGK) mice and all 5 β3+/− mice tested exhibited complete carotid artery occlusion within 12 minutes of injury (β3+/− mice, 8.3 ± 0.6 minutes; β3/β1(EGK) mice, 9.2 ± 1.0 minutes), indicating that differences in αIIbβ3 expression cannot account for the antithrombotic phenotype of β3(Δ760-762) mice. The carotid artery/FeCl3 injury model is one of several complementary thrombosis models that have been developed for the mouse. While the relative importance of various platelet stimuli, receptors, and rheologic conditions may vary with the particular thrombosis model,37 these results establish that deletion of the C-terminal 3 amino acid residues from the β3 cytoplasmic domain can confer protection from occlusive arterial thrombosis. Because β3(Δ760-762) in our knockin model would be paired with αV as well as with αIIb, it is theoretically possible that αVβ3(Δ760-762) in endothelial or vascular smooth muscle cells might contribute to the antithrombotic phenotype of β3(Δ760-762) mice. This seems unlikely given the acute nature of the thrombotic response (minutes), the low expression levels of αVβ3 in nonproliferative endothelial cells, and the lack of evidence for involvement of smooth muscle cell αVβ3 in acute platelet thrombus formation.
Figure 2.
β3(Δ760-762) mice are resistant to carotid artery thrombosis. (A) Carotid artery blood flow profiles from 1 representative WT and 2 β3(Δ760-762) mice. Arteries were exposed to FeCl3 for 3 minutes beginning at t = 0, and blood flow was measured for at least 30 minutes. (B) Times to thrombotic occlusion for each mouse studied: WT, n = 13; β3(Δ760-762), n = 11; β3/β1(EGK), n = 7; β3+/−, n = 5. Two β3(Δ760-762) mice (arrows) showed either an approximately 90% reduction in initial flow rate, or restoration of flow within 6 minutes after complete occlusion. (C) Tail bleeding times for initial cessation of bleeding. Each circle indicates 1 animal: WT, n = 56; β3(Δ760-762), n = 46; β3/β1(EGK), n = 31; β3+/−, n = 18. Box graphs indicate median plus or minus SEM, and the total distribution for each genotype. (D) Frequency of rebleeding from tail wounds: WT, n = 56; β3(Δ760-762), n = 34; β3/β1(EGK), n = 27; β3+/−, n = 18.
Next, β3(Δ760-762) mice were evaluated for evidence of defects in hemostasis. In contrast to the reported gross hemorrhagic phenotype of mice lacking β3 entirely,15,27 β3(Δ760-762) mice displayed no spontaneous hemorrhages for up to 6 months of observation. In addition, compared with β3+/+ littermates, there was no excess surgical bleeding at the time of carotid artery exposure, no gastrointestinal blood loss as monitored by random stool guaiac testing (21 mice studied), and no evidence of acute or chronic blood loss anemia as evidenced by normal values for blood hemoglobin, hematocrit, and mean corpuscular red blood cell volume (Table 1). In the absence of thrombocytopenia (Table 1), tail bleeding times in the mouse may reveal a subtle defect in hemostasis due to platelet dysfunction.38 The tail bleeding times of wild-type β3+/+ littermates showed a distribution pattern similar to those we and others have reported for normal mice15,23,27 (Figure 2C). The median bleeding time for β3(Δ760-762) mice was longer (188 ± 30 seconds; n = 43) than that of β3+/+ controls (79 ± 6 seconds; n = 69), β3+/− heterozygotes (110 ± 13 seconds; n = 18), and β3/β1(EGK) mutants (98 ± 31 seconds; n = 31). In addition, 9 of the 43 β3(Δ760-762) mice that were tested failed to stop bleeding from their tail wounds after 10 minutes, a response never observed in the β3+/+ or β3+/− controls (Figure 2C). Of the β3(Δ760-762) mice that did stop bleeding spontaneously, a greater proportion than normal showed a tendency to rebleed after transient hemostasis had been achieved (Figure 2D). Thus, protection from arterial thrombosis afforded by deletion of β3 R760GT762 is associated with a subtle defect in platelet-dependent hemostasis, but not with a clinically apparent bleeding diathesis. Because the phenotype of β3(Δ760-762) mice was not observed in β3/β1(EGK) mice, β1(EGK) appears to have restored a functionality inherent in wild-type β3. Therefore, to understand that functionality in more detail, platelet αIIbβ3 signaling was evaluated ex vivo.
Table 1.
Blood cell parameters in mouse strains
| β3+/+ | β3(Δ760-762) | β3/β1(EGK) | Normal range | |
|---|---|---|---|---|
| Platelets, ×109/L | 661 ± 48 | 827 ± 63 | 544 ± 97 | 473-1220 |
| Hemoglobin, g/dL | 15.3 ± 0.4 | 15.2 ± 0.6 | 14.3 ± 0.8 | 12.8-16.4 |
| Hematocrit, % | 55.9 ± 1.9 | 58.2 ± 4.7 | 50.0 ± 3.6 | 38.0-59.3 |
| Mean corpuscular volume, fL | 47.4 ± 0.4 | 50.8 ± 3.4 | 47.0 ± 3.1 | 43.0-54.3 |
Values are expressed as mean plus or minus SEM; n = 4 for β3+/+, 3 for β3(δ760-762), and 6 for β3/β1(EGK).
Deletion of β3 R760GT762 interferes with outside-in αIIbβ3 signaling in platelets
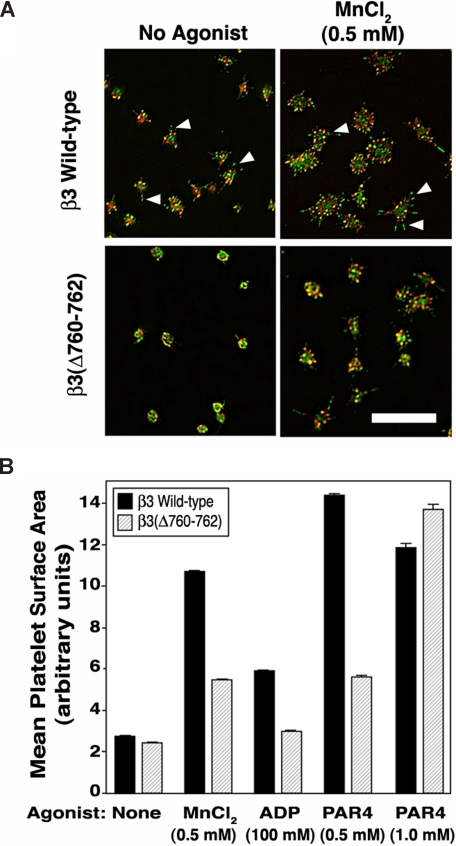
Mouse platelets adherent to fibrinogen undergo filopodial protrusion and limited lamellipodial extension.39 These responses can be accentuated by costimulation of platelets with agonists and, to a certain extent, by extrinsic activation of αIIbβ3 with MnCl2.15 When β3(Δ760-762) platelets were allowed to adhere to fibrinogen for 45 minutes and then examined by confocal microscopy, they failed to undergo an extent of filopodial protrusion exhibited by β3+/+ platelets, and this discrepancy was even more apparent after αIIbβ3 activation by MnCl2 (Figure 3A). Thus, whereas β3+/+ platelets exhibited multiple, prominent filopodia staining positive for F-actin and phosphotyrosine-containing proteins, β3(Δ760-762) platelets exhibited fewer, blunted filopodia (Figure 3A). When spreading of MnCl2-treated platelets was quantified by computerized image analysis, β3(Δ760-762) platelets spread less well than β3+/+ platelets (P < .01; Figure 3B). Also, β3(Δ760-762) platelets spread less well than β3+/+ platelets after agonist stimulation by ADP (100 μM) or a subsaturating concentration of a PAR4 thrombin receptor-activating peptide (alanine-tyrosine-proline-glycine-lysine-phenylalanine [AYPGKF], 0.5 mM; P < .01). However, platelets stimulated with a higher concentration of AYPGKF (1 mM) appeared to spread normally (Figure 3B and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast to β3(Δ760-762) platelets, β3/β1(EGK) and β3+/− platelets spread normally under all conditions (not shown). Thus, β3(Δ760-762) platelets exhibit a fundamental defect in spreading on fibrinogen, a response known to be dependent on outside-in αIIbβ3 signaling.
Figure 3.
Platelet spreading on immobilized fibrinogen. (A) Platelets were incubated for 45 minutes on fibrinogen-coated coverslips in the absence or presence of 0.5 mM MnCl2. Platelets were fixed and stained with rhodamine-phalloidin (red) to label F-actin or with an antibody to phosphotyrosine (green). Wild-type platelets showed filopodia (arrowheads) and minimal lamellipodial extension, both of which were accentuated by extrinsic activation of αIIbβ3 with MnCl2. Scale bar = 10 μm. (B) Platelets were plated on fibrinogen as in panel A, in the absence or presence of MnCl2, ADP, or PAR4 peptide. After fixation and staining, platelet spreading (mean surface area) was quantified by image analysis (see “Methods” for details). *P < .01. Data represent means plus or minus SEM of 349 to 859 platelets analyzed on 6 to 10 separate coverslips for each experimental condition.
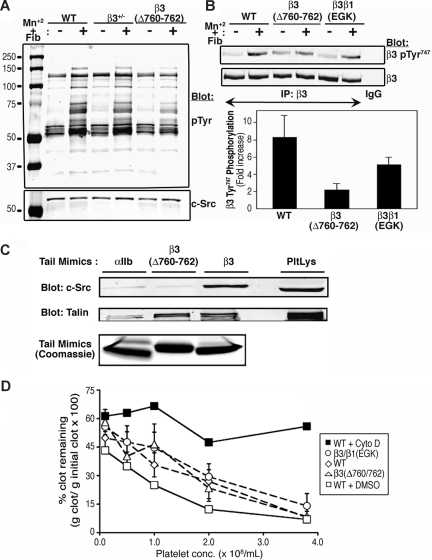
During outside-in αIIbβ3 signaling, platelets undergo SFK-dependent protein tyrosine phosphorylation, including phosphorylation of the β3 cytoplasmic domain itself at Tyr747 and Tyr759 (Figure 1A).9,26 To study this response, β3(Δ760-762) platelets were incubated with MnCl2 to activate αIIbβ3 and induce soluble fibrinogen binding, and tyrosine phosphorylation in platelet lysates was examined by Western blotting. Control β3+/+ and β3+/− platelets exhibited the expected increase in tyrosine phosphorylation of numerous proteins, particularly at approximately 60 to 75, 85 to 100, and 130 kDa. However, the magnitude of this response was diminished in β3(Δ760-762) platelets (Figure 4A), but normal in β3/β1(EGK) platelets (not shown). When a phospho-specific antibody to β3 Y747 was used as a probe, β3(Δ760-762) platelets exhibited less inducible tyrosine phosphorylation than did β3+/+ and β3/β1(EGK) platelets (2.3-fold vs 8.5-fold and 5.2-fold, respectively; Figure 4B). These results indicate that deletion of β3 R760GT762 reduces, but does not eliminate, fibrinogen- and SFK-dependent tyrosine phosphorylation in platelets.
Figure 4.
Platelet tyrosine phosphorylation, fibrin clot retraction, and binding properties of β3 cytoplasmic domain mutants. (A) Platelets were incubated in suspension in the presence of 250 μg/mL fibrinogen plus or minus 0.5 mM MnCl2 for 20 minutes at room temperature and then lysed. Protein tyrosine phosphorylation was assessed by immunoblotting with antiphosphotyrosine antibodies (pTyr). Blots were stripped and reprobed with an antibody to c-Src. (B) Phosphorylation of β3 Tyr747 was detected with a phospho-specific antibody. Blots were reprobed with an antibody to total β3 and phosphorylation data were normalized for β3 expression. The bar graph depicts the fold-increase in β3 Tyr747 phosphorylation induced by MnCl2 + fibrinogen. Data are means (± range) for 2 independent experiments. (C) Pull-down of c-Src and talin from mouse platelet lysate (PltLys) by recombinant wild-type and mutant β3 cytoplasmic domain model proteins. An αIIb cytoplasmic domain protein was used as a negative control. Bound c-Src and talin were detected by immunoblotting. Loading of pull-down beads with recombinant cytoplasmic domains was monitored by staining with Coomassie brilliant blue. (D) Fibrin clot retraction using platelet-rich plasma from wild-type or mutant mice. Clotting was initiated with 9.4 U/mL thrombin and 1.9 mM CaCl2. Data represent mean (± SEM) for least 3 separate experiments performed in duplicate on at least 4 animals with each genotype. Experiments with wild-type platelets in the presence of 10 μM cytochalasin D (cyto D) or vehicle (dimethyl sulfoxide [DMSO]) were performed in duplicate on platelets from 4 animals to confirm that clot retraction was dependent on actin polymerization in this assay.
A potential explanation for these results is that the β3 R760GT762 deletion may reduce or eliminate interaction of β3 with c-Src.20,21,25 This interaction can be monitored by a pull-down assay using recombinant integrin cytoplasmic domain model proteins.19,20 Indeed, unlike the recombinant wild-type β3 cytoplasmic domain, the recombinant β3(Δ760-762) cytoplasmic domain was unable to retain c-Src from platelet lysate (Figure 4C). We speculate that the residual tyrosine phosphorylation observed in β3(Δ760-762) platelets after their interaction with fibrinogen (Figure 4A) may be triggered by another SFK, such as Fyn, whose mode of binding to the β3 cytoplasmic domain may differ from that of c-Src.21 Even if this were the case, such an interaction is not sufficient to rescue the spreading defect demonstrated by β3(Δ760-762) platelets (Figures 3 and S1). Since platelets normally express 6 SFKs in addition to c-Src,24 the relatively normal spreading and tyrosine phosphorylation responses of β3/β1(EGK) platelets may be explained by interaction of one or more of these with the chimeric β3/β1(EGK) cytoplasmic domain.19,20
Fibrin clot retraction in whole blood depends on thrombin-mediated cleavage of fibrinogen, fibrin interaction with activated αIIbβ3, and myosin-mediated contraction of the platelet actin cytoskeleton.40 Fibrin clot retraction is impaired in platelets expressing the double mutation of β3 Tyr747 and Tyr759 to phenylalanine.41 On the other hand, β3(Δ760-762) platelets and β3/β1(EGK) platelets supported clot retraction normally (Figure 4D). Thus, β3(Δ760-762) platelets appear to be defective in some, but not all, responses that depend, at least in part, on outside-in αIIbβ3 signaling.
Deletion of β3 R760GT762 causes partial disruption of inside-out signaling
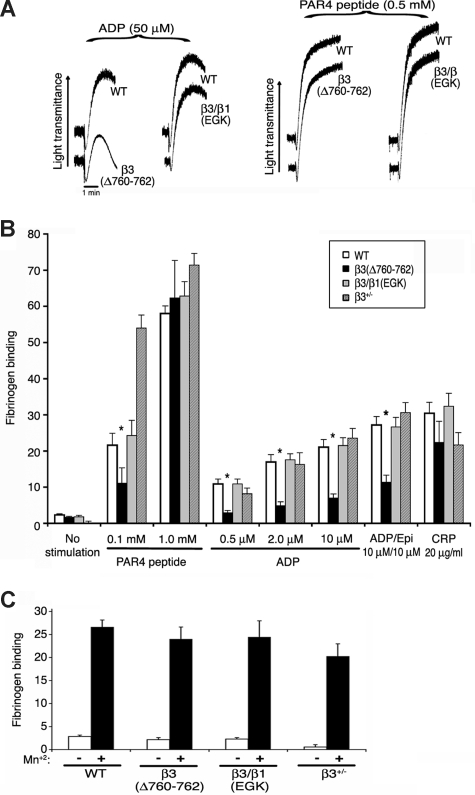
The size of platelet aggregates depends on inside-out signaling, fibrinogen binding, and outside-in signaling.9,10 Therefore, platelet aggregation and fibrinogen binding were studied in the mutant mice. Shape change, an initial agonist response independent of αIIbβ3, was normal in β3(Δ760-762) and β3/β1(EGK) platelets stimulated with 50 μM ADP or 0.5 mM PAR4 peptide, as were aggregation and fibrinogen binding responses to PAR4 peptide (0.5 mM, 1 mM) or CRP (20 μg/mL), a collagen-related peptide agonist for GP VI (Figure 5A). However, aggregation and/or fibrinogen binding responses of β3(Δ760-762) platelets were impaired at a lower input concentration of PAR4 peptide (0.1 mM), and at all ADP concentrations tested (0.5-50 μM), even in the presence of a second agonist, epinephrine (Figure 5A,B). Fibrinogen binding to β3(Δ760-762) platelets was normal in response to MnCl2 (Figure 5C), indicating that the extracellular domains of this integrin were inherently capable of undergoing conformational changes required for ligand binding. Without stirring, platelets typically undergo submaximal granule secretion in response to ADP and epinephrine in a manner dependent on fibrinogen binding to αIIbβ3. Under these conditions, β3(Δ760-762) platelets exhibited reduced α-granule secretion compared with wild-type controls, as monitored by surface P-selectin expression (Figure S2).
Figure 5.
Platelet aggregation and fibrinogen binding. (A) Platelet aggregation in platelet-rich plasma was stimulated with ADP or PAR4 receptor-activating peptide (AYPGKF). Results are representative of at least 3 experiments, performed in duplicate on at least 4 animals from each genotype. (B) Specific FITC-fibrinogen binding to platelets was assessed by flow cytometry. Fibrinogen binding is reported as mean fluorescence intensity in arbitrary fluorescence units and was normalized for αIIbβ3 surface expression measured with antibody to αIIb. *P ≤ .05 compared with wild-type platelets. (C) Specific fibrinogen binding in the absence (□) and presence (■) of 0.5 mM MnCl2. Results in B and C are representative of at least 6 independent experiments for each genotype.
The reduced fibrinogen binding to β3(Δ760-762) platelets in response to ADP and epinephrine or to a relatively low concentration of AYPGKF was somewhat unexpected because previous work has suggested that deletion of β3 R760GT762 may not interfere with the binding of β3 to talin,4,20,42,43 a necessary step for inside-out activation of αIIbβ3 in cell lines, megakaryocytes, and platelets.15,43,44 Furthermore, treatment of platelets with a cell-permeable RGT peptide disrupts c-Src, but not talin, interaction with β3.25 Thus, rather than disrupt β3 interaction with talin, the β3 R760GT762 deletion may disrupt outside-in signals that feedback to regulate αIIbβ3 activation or it may affect the function of proteins that serve as coactivators of talin-dependent αIIbβ3 activation. Candidate integrin coactivators of the kindlin gene family have been identified recently in many cell types including platelets,45–47 and one family member, kindlin-2, has been shown to interact with the membrane-distal portion the β3 cytoplasmic domain.47 Accordingly, β3(Δ760-762) mice may be useful in clarifying the precise role of kindlins and other coactivators in integrin signaling.
Perspective
This work establishes that the C-terminal 3 amino acids of integrin β3 (R760GT762) are involved in promoting platelet-dependent arterial thrombosis in the mouse, likely due, in part, to disruption of SFK-dependent outside-in αIIbβ3 signaling and, in part, to a reduction in ADP-induced fibrinogen binding. The protection from thrombosis afforded by the β3 (R760GT762) deletion without spontaneous hemorrhage or blood loss anemia is similar to the phenotype of mice with a β3 knockin mutation (β3 L746A) that selectively disrupts talin-dependent inside-out αIIbβ3 signaling.15 This contrasts with the variable hemorrhagic phenotypes observed with some reported β3 mutations that completely disrupt bidirectional αIIbβ3 signaling in mice14,15 or humans,12,13 and with the severe hemorrhagic phenotype associated with marked deficiency or loss of αIIbβ3.27,48
Current pharmacologic approaches to prevent platelet-dependent thrombotic complications of cardiovascular disorders include αIIbβ3 antagonism with parenteral inhibitors of ligand binding, and partial blockade of inside-out αIIbβ3 signaling by inhibitors of cyclooxygenase (aspirin) or P2Y12 ADP receptors (eg, clopidogrel). However, there is an unmet need for more effective and safe antiplatelet drugs for chronic indications.11,28 While this need may be met by new drugs currently in the pipeline, we speculate that interactions of the αIIbβ3 cytoplasmic domains with specific regulatory proteins during αIIbβ3 signaling might provide new therapeutic targets for antiplatelet drug development. However, disruption of integrin signaling at the level of the β3 cytoplasmic domain might also affect the function of αVβ3, either adversely or for therapeutic gain, in endothelial cells, osteoclasts, and others cells.49–51 It will therefore be of interest to study processes ranging from pathologic angiogenesis to bone remodeling in the β3(Δ760-762) mice.
Supplementary Material
Acknowledgments
We thank the UCSD Transgenic Core Facility for assistance in generating gene-targeted mice.
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants HL56595, HL57900 (S.J.S), and HL78784 (S.J.S., M.H.G.), and NIH training grant T32AR007608 (A.J.A.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.J.A., B.G.P., M.H.G., and S.J.S. designed the research; A.J.A., J.K., and B.G.P. performed the research; A.J.A., M.H.G., and S.J.S. analyzed data; and A.J.A., M.H.G., and S.J.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ararat J. Ablooglu, Department of Medicine, University of California San Diego, 9500 Gilman Dr, Rm 180, Leichtag Biomedical Research Building, La Jolla, CA 92093-0726; e-mail: aablooglu@ucsd.edu.
References
- 1.Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 2.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Ma YQ, Yang J, Pesho MM, Vinogradova O, Qin J, Plow EF. Regulation of integrin αIIbβ3 activation by distinct regions of its cytoplasmic tails. Biochemistry. 2006;45:6656–6662. doi: 10.1021/bi060279h. [DOI] [PubMed] [Google Scholar]
- 4.Wegener KL, Partridge AW, Han J, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:1–8. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 9.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 11.Phillips DR, Conley PB, Sinha U, Andre P. Therapeutic approaches in arterial thrombosis. J Thromb Haemost. 2005;3:1577–1589. doi: 10.1111/j.1538-7836.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-P, Djaffar I, Pidard D, et al. Ser-752 –> Pro mutation in the cytoplasmic domain of integrin subunit and defective activation of platelet integrin αIIbβ3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci U S A. 1992;89:10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Shattil SJ, Ambruso DR, Newman PJ. Truncation of the cytoplasmic domain of β3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin αΙΙbβ3complex. J Clin Invest. 1997;100:2393–2403. doi: 10.1172/JCI119780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Z, Chen H, Schmaier AA, Hynes RO, Kahn ML. Structure-function analysis reveals discrete β3 integrin inside-out and outside-in signaling pathways in platelets. Blood. 2007;109:3284–3290. doi: 10.1182/blood-2006-10-051664. [DOI] [PubMed] [Google Scholar]
- 15.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin αIIbβ3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leisner TM, Yuan W, DeNofrio JC, Liu J, Parise LV. Tickling the tails: cytoplasmic domain proteins that regulate integrin αIIbβ3 activation. Curr Opin Hematol. 2007;14:2552–2561. doi: 10.1097/MOH.0b013e3280dce543. [DOI] [PubMed] [Google Scholar]
- 17.Goksoy E, Ma YQ, Wang X, et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol Cell. 2008;31:124–133. doi: 10.1016/j.molcel.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe N, Bodin L, Pandey M, et al. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin αIIbβ3. J Cell Biol. 2008;181:1211–1222. doi: 10.1083/jcb.200803094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias-Salgado EG, Lizano S, Sarker S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by a novel and direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias-Salgado EG, Lizano S, Shattil SJ, Ginsberg MH. Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. J Biol Chem. 2005;280:29699–29707. doi: 10.1074/jbc.M503508200. [DOI] [PubMed] [Google Scholar]
- 21.Reddy KB, Smith DM, Plow EF. Analysis of Fyn function in hemostasis and αIIbβ3-integrin signaling. J Cell Sci. 2008;121:1641–1648. doi: 10.1242/jcs.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieswandt B, Moser M, Pleines I, et al. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obergfell A, Eto K, Mocsai A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su X, Mi J, Yan J, et al. RGT, a synthetic peptide corresponding to the integrin β3 cytoplasmic C-terminal sequence, selectively inhibits outside-in signaling in human platelets by disrupting the interaction of integrin αIIbβ3 with Src kinase. Blood. 2008;112:592–602. doi: 10.1182/blood-2007-09-110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 27.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov. 2003;2:15–28. doi: 10.1038/nrd985. [DOI] [PubMed] [Google Scholar]
- 29.Lakso M, Pichel JG, Gorman JR, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103:576–583. doi: 10.1161/01.cir.103.4.576. [DOI] [PubMed] [Google Scholar]
- 31.Prevost N, Kato H, Bodin L, Shattil SJ. Platelet integrin adhesive functions and signaling. Methods Enzymol. 2007;426:103–115. doi: 10.1016/S0076-6879(07)26006-9. [DOI] [PubMed] [Google Scholar]
- 32.Arias-Salgado EG, Haj F, Dubois C, et al. PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol. 2005;170:837–845. doi: 10.1083/jcb.200503125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengweiler S, Smyth SS, Jirouskova M, et al. Preparation of monoclonal antibodies to murine platelet glycoprotein IIb/IIIa (αIIbβ3) and other proteins from hamster-mouse interspecies hybridomas. Biochem Biophys Res Commun. 1999;262:167–173. doi: 10.1006/bbrc.1999.1172. [DOI] [PubMed] [Google Scholar]
- 34.Du XP, Saido TC, Tsubuki S, Indig FE, Williams MJ, Ginsberg MH. Calpain cleavage of the cytoplasmic domain of the integrin β3 subunit. J Biol Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin β cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- 36.Calderwood DA, Zent R, Grant R, Rees DJG, Hynes RO, Ginsberg MH. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 37.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- 39.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cγ2 in αIIbβ3-mediated platelet spreading. J Biol Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins AL, Nannizzi-Alaimo L, Silver D, et al. Tyrosine phosphorylation of the β3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 41.Law DA, Nannizzi-Alaimo L, Cowan KJ, Prasad KS, Ramakrishnan V, Phillips DR. Signal transduction pathways for mouse platelet membrane adhesion receptors. Thromb Haemostas. 1999;82:345–352. [PubMed] [Google Scholar]
- 42.Xi X, Bodnar RJ, Li Z, Lam SC, Du X. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin β3 cytoplasmic domain in inside-out and outside-in signaling. J Cell Biol. 2003;162:329–339. doi: 10.1083/jcb.200303120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin β cytoplasmic tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 44.Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 47.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of β3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- 49.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huveneers S, van den Bout I, Sonneveld P, Sancho A, Sonnenberg A, Danen EH. Integrin αVβ3 controls activity and oncogenic potential of primed c-Src. Cancer Res. 2007;67:2693–2700. doi: 10.1158/0008-5472.CAN-06-3654. [DOI] [PubMed] [Google Scholar]
- 51.Zou W, Kitaura H, Reeve J, et al. Syk, c-Src, the αVβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.