Abstract
Mutations in the LMNA gene, encoding the nuclear envelope protein lamin A/C, are responsible for a number of distinct disease entities including Dunnigan-type familial partial lipodystrophy. Dunningan-type lipodystrophy is characterized by loss of subcutaneous adipose tissue, insulin resistance, dyslipidemia, and type 2 diabetes and shares many of the features of the metabolic syndrome. Furthermore, several genome-wide linkage scans for type 2 diabetes have found evidence of linkage at chromosome 1q21.2, the region that harbors the LMNA gene. Therefore, LMNA is a biological and positional candidate for type 2 diabetes susceptibility. Previous studies have reported association between a common LMNA variant (1908C>T; rs4641) and adverse metabolic traits in ethnically diverse populations from Asia and North America. In the present study, we characterized the common variation across the LMNA gene (including rs4641) and tested for association with type 2 diabetes in two large case-control studies (n = 2,052) and with features of the metabolic syndrome in a separate cohort study (n = 1,572). Despite our study being sufficiently powered to detect effects similar and even smaller in magnitude than those previously reported, none of the LMNA single nucleotide polymorphisms were statistically significantly associated with type 2 diabetes or the metabolic syndrome. Thus, it appears unlikely that variation at LMNA substantially increases the risk of type 2 diabetes or related traits in U.K. Europids.
Deleterious mutations in the LMNA gene, encoding the nuclear envelope protein lamin A/C, are responsible for a number of distinct disease entities known as “laminopathies” including Dunnigan-type familial partial lipodystrophy (FPLD; OMIM 151660) (1-5). Dunnigan-type FPLD is characterized by loss or absence of adipose tissue and severe metabolic derangements, including insulin resistance, dyslipidemia, and type 2 diabetes. Recent evidence has demonstrated that common variants in genes previously implicated in monogenic disorders of glucose imbalance increase the risk of common type 2 diabetes (6). These observations support the examination of common variants in LMNA for their effect on type 2 diabetes and metabolic syndrome risk. Furthermore, genome-wide linkage scans for type 2 diabetes have found evidence for linkage at chromosome 1q21-q24 (7-9), the region that harbors the LMNA gene. Therefore, LMNA is a biological and positional candidate for type 2 diabetes susceptibility.
The most frequently studied variant at the LMNA gene in relation to type 2 diabetes– and metabolic syndrome–related traits is the silent C>T substitution at nucleotide 1908 (1908C>T; rs4641). This variant affects the third base at codon 566 (His566His) in exon 10, just before the alternative splicing site that gives rise to the two distinct proteins, lamin A and C (10). Several studies have reported on the association between the 1908T allele and type 2 diabetes–related traits in ethnically diverse populations (11-17).
In the present study, which is the most comprehensive of its kind on the LMNA gene, we characterized the common variation at the LMNA gene (including 1908C>T) and tested for association with risk of type 2 diabetes and related phenotypes in three large population-based cohorts.
RESEARCH DESIGN AND METHODS
This study included two type 2 diabetes case-control studies of middle-aged U.K. Europid: the type 2 diabetes case-control European Prospective Investigation into Cancer (EPIC) Study and the Cambridgeshire Case-Control (CCC) Study. Genetic associations with the features of the metabolic syndrome were assessed in a separate cohort of middle-aged U.K. Europids (the Medical Research Council [MRC] Ely Study). Participants from these studies reside within a region that has traditionally had low admixture rates. Because of its genetic homogeneity, it is likely to be free from population stratification, which may be an important source of confounding in some cohorts. Descriptive characteristics are given in online appendix Table 1 (available at http://dx.doi.org/10.2337/db06-1055).
The EPIC Study is nested within the EPIC Norfolk Study, a population-based cohort study of Europid men and women aged 40–78 years. Both the case-control and full cohort (18,19) study have been previously described in detail. Briefly, the case-control study consists of 417 incident type 2 diabetic case subjects and two sets of 417 control subjects, each matched in terms of age, sex, general practice, and recruitment date, with one set additionally matched for BMI. A case was defined by a physician's diagnosis of type 2 diabetes, with no insulin prescribed within the 1st year following diagnosis, and/or A1C >7% at the health check. Control subjects were randomly selected from the EPIC-Norfolk cohort from among those without diabetes, cancer, stroke, or myocardial infarction at baseline and who had not developed diabetes by the time of selection. Potential control subjects with measured A1C levels >6% were excluded.
The CCC Study consists of 552 patients with type 2 diabetes aged 45–76 years, randomly sampled from a population-based diabetes register, and 552 control subjects recruited from the same population and individually matched for age, sex, and geographical location. The study design and methods have been described in detail elsewhere (20). Briefly, case subjects were defined by onset of diabetes after the age of 30 years, without insulin treatment in the 1st year following diagnosis. Diabetes was excluded in control subjects by medical record search and by an A1C measurement <6%. In total, 2,052 individuals (41.5% with type 2 diabetes) from both the EPIC Study and CCC Study were genotyped for the 13 LMNA variants.
The MRC Ely Study commenced in 1990 as a prospective population-based cohort study of the etiology and pathogenesis of type 2 diabetes and related metabolic disorders. Part of the study design and methods have been described in detail elsewhere (21,22). At baseline (phase 1), 1,122 individuals (aged 40–67 years) were randomly selected from Ely (a town in East Anglia, U.K.). Of these, 937 were followed-up 4.5 years later, and an additional 183 young adults (aged 30–40 years) were recruited for the same clinical examinations (phase 2). Phase 3 was carried out 10 years after the start of the study. Of all phase 1 and 2 participants, 936 attended phase 3 examinations and 714 were newly recruited from the same geographical area. The current study examines the individuals of phase 3 (n = 1,572 men and women aged 35–79 years) for whom genotypic and phenotypic data (metabolic traits) were available. Of all participants, 30.2% had the metabolic syndrome as previously defined (based on obesity, dyslipidemia, elevated blood pressure, glucose intolerance, and insulin resistance) (23). Ethical permission for the three studies was granted by their respective local research ethics committee, and study participants provided written informed consent.
Single nucleotide polymorphism selection
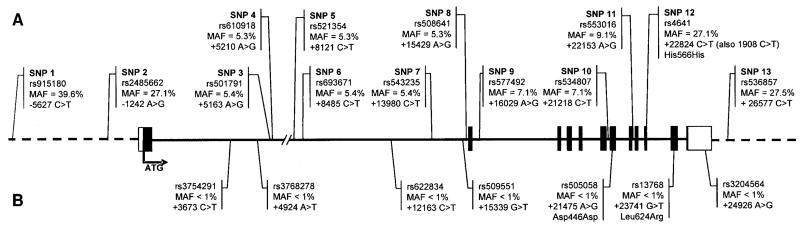
Single nucleotide polymorphism (SNP) selection was undertaken before the release of the International HapMap (24) and was based on literature reports and on the dbSNP database. The selection was performed in a stepwise manner to ensure even-spaced coverage of the gene, including 10 kb up- and downstream of the gene. First, we selected all common SNPs, as well as all polymorphic missense variants, that were reported in the literature. Subsequently, we selected all common SNPs (minor allele frequency [MAF] ≥5%) and missense variants that were catalogued in the dbSNP database. Furthermore, SNPs from the dbSNP database for which no allele frequency was reported but for which validation from multiple sources was available were also included. Finally, to ensure coverage of at least one SNP every 3 kb, we also included SNPs from the dbSNP database for which no allele frequency was available. Eventually, 20 SNPs were genotyped, of which 13 SNPs that had a MAF >1% were included in the analyses (Fig. 1).
FIG. 1.
Position (relative to the ATG methionine initiation codon) and MAF of the LMNA SNPs selected for genotyping. A: Polymorphic (MAF ≥ 1%) SNPs included in the analyses. B: SNPs with a MAF <1%, not analyzed.
The International HapMap Phase 2 (24) reports 14 common SNPs (MAF ≥0.05) for the Caucasian population (CEU), which can be tagged by four tag SNPs. Of the 13 SNPs we genotyped, 3 (SNP1, SNP2, and SNP9) were in common with HapMap SNPs. These 3 SNPs capture the genetic variation of 8 (57%) of the 14 HapMap SNPs (r2 threshold at 0.80). The six HapMap SNPs that were not captured by SNP1, SNP2, and SNP9 are in perfect linkage disequilibrium (LD) (r2 = 1) and can be tagged by one SNP. The remaining 10 SNPs we genotyped were not typed in the HapMap project, such that we cannot accurately assess the coverage of the genetic variation in LMNA. However, on the basis of their physical location on the gene, we can expect that at least four of our SNPs may represent the remaining six HapMap SNPs, as these four SNPs are located within the six-SNP block that shows perfect LD. Therefore, we can conclude that our 13 SNPs adequately represent the common genetic variation in the LMNA gene (online appendix Fig. 1 and Table 2). All genotypes conformed to expectations under Hardy-Weinberg equilibrium (P > 0.01).
Genetic analyses
Samples were arrayed on 96-well plates with three replicates and one water control per plate. For the case-control populations, case and control samples were randomly distributed across each 96-well plate, with approximately the same number of cases and controls per plate. Genotyping of samples was performed in 384-well plates at the Wellcome Trust Sanger Institute, Cambridge, using an adaptation of the homogenous MassExtend protocol supplied by Sequenom for the MassArray system (25). Call rates ≥95% were observed for all SNPs, except SNP 11 that had an 86% call rate. Concordance rates between duplicate samples were ≥98%.
Statistical analysis
Analyses were performed using Stata SE 8.2 for Windows (StataCorp, College Station, TX) and SPSS 12.0 for Windows (SPSS, Chicago, IL). Genotype frequencies were tested for Hardy-Weinberg equilibrium using the χ2 test. LD was assessed using the expectation-maximization algorithm. We calculated the effect of LMNA variants on discrete outcomes (type 2 diabetes and metabolic syndrome) using logistic regression. Before combining the two type 2 diabetes case-control cohorts, we tested for heterogeneity between the populations. For one SNP (rs536857), evidence (P = 0.04) of heterogeneity was observed, which was adjusted for in a random effects model. To test association between LMNA variants and continuous metabolic traits, we used generalized linear models adjusted for age, sex, BMI, and study. We first tested for an additive effect of the SNP on the risk of type 2 diabetes and the metabolic traits. If these results showed evidence for a dominant or recessive inheritance, the respective models were tested as well. Because the number of rare homozygotes was small (n = 1–5) for SNP3 to SNP11, a dominant model was also tested. We tested for sex-specific effects by including genotype-by-sex interaction terms in the models. Since no evidence for genotype-by-sex interaction was found (P > 0.24), we do not present stratified results. Haplotype blocks were defined using Gabriel's method (26), as implemented in Haploview (27). Haplotype frequencies were inferred using the expectation-maximization algorithm as implemented in the HelixTree Genetic Analyses Software (version 4.4.1; Golden Helix, Bozeman, MT). Haplotypes prevalent at >5% were retained for further analysis. A χ2 test was used to compare haplotype frequencies of case and control samples. Haplotype trend regression analysis was performed to test for association between the haplotypes and continuous metabolic traits. Power calculations were performed using Quanto version 1.1.1 (http://hydra.usc.edu/gxe).
RESULTS
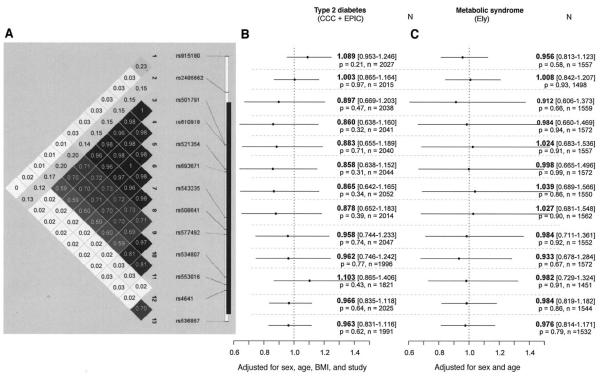
Figure 2A shows the extent of LD (r2) between SNPs. In the EPIC Study and CCC Study, none of the SNPs were associated with diabetes risk after adjustment for age, sex, BMI, and study (Fig. 2B). In the MRC Ely Study, none of the SNPs were associated with the metabolic syndrome after adjustment for age and sex (Fig. 2C). Results for the unadjusted models were similar (data not shown).
FIG. 2.
A: Pairwise LD estimates (r2 values) assessed in control subjects for the 13 polymorphic LMNA SNPs included in the study. (The black solid line represents the gene region.) B: Adjusted OR of LMNA variants for type 2 diabetes incidence for the additive model. C: Adjusted OR of LMNA variants for metabolic syndrome incidence for the additive model. Values in B and C represent ORs (increased risk per additional minor allele) and 95% CIs (OR [95 CI]), P values for the additive model, and number of participants with full genotype and phenotype data.
We tested for association between the 13 SNPs and continuous metabolic traits (i.e., plasma HDL, LDL, and total cholesterol and BMI) in a combined nondiabetic population comprising the MRC Ely Study, as well as control subjects from both the EPIC and CCC Studies. We also examined the associations with fasting glucose and insulin, which was only available in the MRC Ely Study. None of the LMNA variants were significantly associated with any of the continuous metabolic traits, adjusted for age, sex, BMI, and study (online appendix Table 3). However, carriers of the 1908T allele tended to have lower triglyceride (P = 0.06) and total cholesterol (P = 0.05) levels than C1908C homozygotes, and SNP1 T-allele carriers tended to have lower glucose levels (P = 0.05).
None of the haplotypes were significantly associated with risk of type 2 diabetes or the metabolic syndrome. Also, for the continuous metabolic traits, haplotypes did not provide any additional information beyond the individual marker associations (online appendix Tables 6 and 7).
Both case-control and cohort studies had sufficient power to detect similar and even smaller effect sizes than those previously reported; i.e., for the previously studied 1908C>T (rs4641) variant, our study was powered at 95% to detect an odds ratio (OR) ≥1.35 for type 2 diabetes per copy of the minor allele, at a significance of 1%. For SNPs 1, 2, 12, and 13, we had sufficient power (>80%) to detect ORs >1.25 in the case-control study and differences of >2% in the cohort study at a significance of 5%. SNP3 to SNP11 had a lower MAF and are therefore slightly less powered; i.e., we had 69–83% power to detect ORs >1.4 in the case-control study, and differences as small as 3% could be detected in the cohort study at a significance of 5% (online appendix Tables 4 and 5).
DISCUSSION
In the present study, the largest of this kind on the LMNA gene, we were unable to detect association between variants in the LMNA gene and risk of type 2 diabetes or the metabolic syndrome, despite our study being sufficiently powered. We observed tentative associations between 1908C>T and lipid levels. However, the direction of these trends contrast with those reported in other studies, and we conclude that our observations are likely to be false positive due to the number of statistical comparisons undertaken. Given the appropriate design of this study and the fact that no other associations were detected strongly suggests that, in this population, LMNA variants are unlikely to play a major role in the development of type 2 diabetes or metabolic syndrome. However, small effects (i.e., OR <1.25 or <3% for quantitative traits) cannot be excluded.
The LMNA gene encodes two nuclear proteins, lamin A and C, which are essential structural components of most differentiated mammalian cells. Rare mutations in exon 8 of LMNA cause the autosomal-dominant Dunnigan-type FPLD (2-5). At birth, individuals with FPLD have normally distributed adipose tissue but experience a progressive loss of subcutaneous fat from the extremities, gluteals, and torso following puberty. In later life, individuals with FPLD often experience severe metabolic derangements, including insulin resistance, dyslipidemia, heart disease, and type 2 diabetes. Mutations elsewhere in the LMNA gene are associated with several additional autosomal-dominant diseases (1).
Common variants in the LMNA gene, the 1908C>T variant in particular, have been associated with the risk of type 2 diabetes and with related metabolic phenotypes in several ethnically distinct populations (11-17). Hegele and colleagues (16,17) first reported that in two genetically distinct aboriginal populations, Oji-Cree and Inuit, the T1908T homozygotes had significantly higher measures of obesity-related traits than C-allele carriers. In a case-control study of Japanese men, Murase et al. (15) reported an association of borderline statistical significance (P = 0.08) between the 1908C>T variant and type 2 diabetes, with the 1908T allele being more frequent (44%) in case than control subjects (32%). In addition, T-allele carriers of both case and control subjects had significantly higher fasting insulin, cholesterol, and triglyceride levels than C/C homozygotes. In the case group from the same study, Liang et al. (14) reported a modest (P = 0.06) increased risk of diabetic nephropathy in 1908T allele carriers compared with 1908C allele homozygotes. In 908 Old Order Amish (13), six LMNA SNPs were identified and tested for association with type 2 diabetes and the metabolic syndrome. Only the 1908C>T variant was modestly (P < 0.03) associated with the metabolic syndrome and triglyceride and HDL cholesterol levels but not with diabetes. In a small cohort of Pima Indians, weak evidence of linkage (logarithm of odds 1.73) for subcutaneous abdominal adipocyte size was localized to the LMNA region of chromosome 1 (1q21-q23), and association analyses showed that adipocytes of C1908C homozygotes were larger than those of T1908T homozygotes (12). As enlarged subcutaneous abdominal adipocyte size is an independent predictor of type 2 diabetes in Pima Indians (28), these data suggest that C1908C homozygotes may be at increased risk of diabetes, which contradicts the findings by Steinle et al. (13). In a subsequent study of 1,338 Pima Indians, no evidence of association was observed between the 1908C>T variant and type 2 diabetes or any related quantitative traits (11). Taken together, although the LMNA gene appears to be a strong functional and positional candidate for type 2 diabetes and related pheno-types, the associations reported thus far have been weak and inconsistent.
In the present study, participants were exclusively Europid, living in the same geographical area in eastern England. Although our current and previous findings suggest that this is an ethnically homogeneous population, we cannot completely exclude the existence of underlying genetic heterogeneity. It should also be highlighted that the current study was aimed at examining the effect of common genetic variation in the LMNA gene, and we cannot exclude the possibility that rare SNPs (MAF <0.05) may affect the risk of type 2 diabetes or related metabolic traits.
The control subjects of the case-control studies were not tested for type 2 diabetes, which may introduce misclassification in the control sample and, as a consequence, bias the observation toward the null. We have attempted to diminish this misclassification using A1C. At an A1C level of 6%, previous reports have suggested sensitivity to be 100% and specificity 91% (29). Thus, by using this cutoff, we would exclude all the cases of undiagnosed diabetes in the control subjects but would only be excluding <10% of the potential control subjects who in fact had normal glucose tolerance.
We conclude that although common genetic variation at LMNA may underlie adverse metabolic phenotypes in Asian and North American populations, our results in large population-based cohorts indicate that it is unlikely that common variation in the LMNA gene increases the susceptibility to type 2 diabetes or features of the metabolic syndrome in U.K. Europids.
Supplementary Material
ACKNOWLEDGMENTS
The Ely Study was funded by the MRC and Diabetes U.K., and the EPIC-Norfolk Study is supported by program grants from the MRC U.K. and Cancer Research U.K. Additional support was provided by the Stroke Association, the British Heart Foundation, the Department of Health, Food Standards Agency, and the Wellcome Trust.
I.B. is funded by the Wellcome Trust. S.O. and I.B. also acknowledge support from EU FP6 funding (contract no LSHM-CT-2003-503041) and J.L.M. from the Spanish Ministry of Science and Education (AP-2002-2920).
We are grateful to the volunteers in the CCC Study, the EPIC-Norfolk Study, and the Ely Study who gave their time to take part in these studies. We thank S. Bumpstead for genotyping all the samples.
Glossary
- CCC
Cambridgeshire Case-Control
- EPIC
European Prospective Investigation into Cancer
- FPLD
familial partial lipodystrophy
- LD
linkage disequilibrium
- MAF
minor allele frequency
- MRC
Medical Research Council
- SNP
single nucleotide polymorphism
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Broers JL, Hutchison CJ, Ramaekers FC. Laminopathies. J Pathol. 2004;204:478–488. doi: 10.1002/path.1655. [DOI] [PubMed] [Google Scholar]
- 2.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Shackleton S, Lloyd DJ, Jackson SNJ, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O'Rahilly S, Trembath RC. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- 4.Speckman RA, Garg A, Du F, Bennett L, Veile R, Arioglu E, Taylor SI, Lovett M, Bowcock AM. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66:1192–1198. doi: 10.1086/302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegele RA, Anderson CM, Wang J, Jones DC, Cao H. Association between nuclear lamin A/C R482Q mutation and partial lipodystrophy with hyper-insulinemia, dyslipidemia, hypertension, and diabetes. Genome Res. 2000;10:652–658. doi: 10.1101/gr.10.5.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh WC, Jean PL, Mitchell BD, Pollin TI, Knowler WC, Ehm MG, Bell CJ, Sakul H, Wagner MJ, Burns DK, Shuldiner AR. Genome-wide and fine-mapping linkage studies of type 2 diabetes and glucose traits in the Old Order Amish: evidence for a new diabetes locus on chromosome 14q11 and confirmation of a locus on chromosome 1q21–q24. Diabetes. 2003;52:550–557. doi: 10.2337/diabetes.52.2.550. [DOI] [PubMed] [Google Scholar]
- 8.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Lepretre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiltshire S, Hattersley AT, Hitman GA, Walker M, Levy JC, Sampson M, O'Rahilly S, Frayling TM, Bell JI, Lathrop GM, Bennett A, Dhillon R, Fletcher C, Groves CJ, Jones E, Prestwich P, Simecek N, Rao PV, Wishart M, Bottazzo GF, Foxon R, Howell S, Smedley D, Cardon LR, Menzel S, McCarthy MI. A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet. 2001;69:553–569. doi: 10.1086/323249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 11.Wolford JK, Hanson RL, Bogardus C, Prochazka M. Analysis of the lamin A/C gene as a candidate for type II diabetes susceptibility in Pima Indians. Diabetologia. 2001;44:779–782. doi: 10.1007/s001250051688. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Wolford JK, Hanson RL, Foley JE, Tataranni PA, Bogardus C, Pratley RE. Subcutaneous abdominal adipocyte size, a predictor of type 2 diabetes, is linked to chromosome 1q21–q23 and is associated with a common polymorphism in LMNA in Pima Indians. Mol Genet Metab. 2001;72:231–238. doi: 10.1006/mgme.2001.3147. [DOI] [PubMed] [Google Scholar]
- 13.Steinle NI, Kazlauskaite R, Imumorin IG, Hsueh WC, Pollin TI, O'Connell JR, Mitchell BD, Shuldiner AR. Variation in the lamin A/C gene: associations with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:1708–1713. doi: 10.1161/01.ATV.0000136384.53705.c9. [DOI] [PubMed] [Google Scholar]
- 14.Liang H, Murase Y, Katuta Y, Asano A, Kobayashi J, Mabuchi H. Association of LMNA 1908C/T polymorphism with cerebral vascular disease and diabetic nephropathy in Japanese men with type 2 diabetes. Clin Endocrinol. 2005;63:317–322. doi: 10.1111/j.1365-2265.2005.02344.x. [DOI] [PubMed] [Google Scholar]
- 15.Murase Y, Yagi K, Katsuda Y, Asano A, Koizumi J, Mabuchi H. An LMNA variant is associated with dyslipidemia and insulin resistance in the Japanese. Metabolism. 2002;51:1017–1021. doi: 10.1053/meta.2002.34030. [DOI] [PubMed] [Google Scholar]
- 16.Hegele RA, Cao H, Harris SB, Zinman B, Hanley AJ, Anderson CM. Genetic variation in LMNA modulates plasma leptin and indices of obesity in aboriginal Canadians. Physiol Genomics. 2000;3:39–44. doi: 10.1152/physiolgenomics.2000.3.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Hegele RA, Huff MW, Young TK. Common genomic variation in LMNA modulates indexes of obesity in Inuit. J Clin Endocrinol Metab. 2001;86:2747–2751. doi: 10.1210/jcem.86.6.7550. [DOI] [PubMed] [Google Scholar]
- 18.Day NE, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham NJ. EPIC-Norfolk: study design and characteristics of the cohort: European Prospective Investigation of Cancer. Br J Cancer. 1999;80:95–103. [PubMed] [Google Scholar]
- 19.Harding AH, Day NE, Khaw KT, Bingham S, Luben R, Welsh A, Wareham NJ. Dietary fat and the risk of clinical type 2 diabetes: the European Prospective Investigation of Cancer-Norfolk Study. Am J Epidemiol. 2004;159:73–82. doi: 10.1093/aje/kwh004. [DOI] [PubMed] [Google Scholar]
- 20.Rathmann W, Haastert B, Icks A, Giani G, Hennings S, Mitchell J, Curran S, Wareham NJ. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroenterol. 2001;36:1056–1061. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- 21.Williams DR, Wareham NJ, Brown DC, Byrne CD, Clark PM, Cox BD, Cox LJ, Day NE, Hales CN, Palmer CR, Shackleton JR, Wang TWM. Undiagnosed glucose intolerance in the community: the Isle of Ely Diabetes Project. Diabet Med. 1995;12:30–35. doi: 10.1111/j.1464-5491.1995.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 22.Wareham NJ, Byrne CD, Williams R, Day NE, Hales CN. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care. 1999;22:262–270. doi: 10.2337/diacare.22.2.262. [DOI] [PubMed] [Google Scholar]
- 23.Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, Halsall I, O'Rahilly S, Wareham NJ. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [DOI] [PubMed] [Google Scholar]
- 24.The International HapMap Consortium A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittaker P, Bumpstead S, Downes K, Ghori J, Deloukas P. SNP analysis by MALDI-TOF mass spectrometry. In: Celis J, Carter N, Simons K, Small JV, Hunter T, editors. Cell Biology: A Laboratory Handbook. 3rd ed. Academic Press; San Diego, CA: 2005. [Google Scholar]
- 26.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 28.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 29.Peters AL, Davidson MB, Schriger DL, Hasselblad V. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels: meta-analysis research group on the diagnosis of diabetes using glycated hemoglobin levels. JAMA. 1996;276:1246–1252. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.