Abstract
The afferent innervation contacting the type I hair cells of the vestibular sensory epithelia form distinct calyceal synapses. The apposed presynaptic and postsynaptic membranes at this large area of synaptic contact are kept at a remarkably regular distance. Here, we show by freeze-fracture electron microscopy that a patterned alignment of proteins at the calyceal membrane resembles a type of intercellular junction that is rare in vertebrates, the septate junction (SJ). We found that a core molecular component of SJs, Caspr, colocalizes with the K+ channel KCNQ4 at the postsynaptic membranes of these calyceal synapses. Immunolabeling and ultrastructural analyses of Caspr knock-out mice reveal that, in the absence of Caspr, the separation between the membranes of the hair cells and the afferent neurons is conspicuously irregular and often increased by an order of magnitude. In these mutants, KCNQ4 fails to cluster at the postsynaptic membrane and appears diffused along the entire calyceal membrane. Our results indicate that a septate-like junction provides structural support to calyceal synaptic contact with the vestibular hair cell and that Caspr is required for the recruitment or retention of KCNQ4 at these synapses.
Introduction
Vestibular organs are responsible for sensing head movement and head tilt, and the mechanosensory signal is transduced by hair cells that synapse with the vestibular afferent nerve. There are two types of vestibular hair cells that differ in shape and innervation morphology. Type II hair cells are innervated by bouton synapses, whereas type I hair cells are almost fully enveloped by a calyx-shaped postsynaptic terminal, and are found only in higher vertebrates (Eatock et al., 2008). The hair cell–calyx contact exhibit features that are characteristic of intercellular junctions including a regular spacing (Gulley and Bagger-Sjöbäck, 1979). Although the presence of intercellular junctional features was recognized decades ago, no subsequent information on the molecular characterization of this junction is available.
Functionally, calyceal synapses also exhibit features that are distinct from the regular bouton synapses. These include comparatively weak synaptic input to the postsynaptic terminal and persistent postsynaptic potential after inhibition of glutamate receptors (Yamashita and Ohmori, 1990; Holt et al., 2007a). Although these features have been the focus of several studies in the last decade, the mechanisms driving these differences are unknown. Theoretical models have correlated the unusual properties of calyceal synapses with the geometry of the contacts between the calyx and the type I hair cells (Goldberg, 1996; Soto et al., 2002). A common parameter emphasized in these models is the intercellular space between the membranes. Consensus is that the accumulation of K+ ions in the intercellular space greatly contributes to the peculiar properties of calyceal synapses. Interestingly, the potassium channel KCNQ4, a key protein in homeostasis of K+, is highly concentrated at calyceal synapses (Kharkovets et al., 2000; Hurley et al., 2006; Holt et al., 2007b).
We investigated the structure of calyceal contacts by freeze fracture and observed a pattern of intramembrane particle (IMP) distribution that resembles the pattern observed in septate junctions (SJs). Thus far, SJs in vertebrates have only been described at the paranodal region of myelinated axons, based on their morphological septa-like appearance and the localization of the vertebrate homologues of Drosophila SJ proteins (Banerjee et al., 2006). Caspr is the vertebrate homolog of Drosophila neurexin IV. Caspr is involved in the targeting of paranodal proteins and segregation of membrane domains, working as a fence for segregation of Na+ and K+ channels at the nodes of Ranvier and juxtaparanodes, respectively (Bhat et al., 2001). We show that Caspr is localized at the calyceal membrane at the region of the junctional contact with the type I hair cell. Using Caspr knock-out mice, we show that Caspr is essential for maintaining the junctional contact and for retaining KCNQ4 at the junctional site.
Materials and Methods
Animals.
Experiments were performed according to University of North Carolina and National Institutes of Health (NIH) guidelines for ethical treatment of laboratory animals. Rats younger than postnatal day 10 (P10) were killed by decapitation, whereas adult rats were anesthetized with CO2 and decapitated. Caspr−/− mice (Bhat et al., 2001; Garcia-Fresco et al., 2006) were anesthetized, perfusion fixed, and postfixed for 12 h. Fixations were performed for 2 h at room temperature using 4% paraformaldehyde in PBS for immunoassays, or 2.5% glutaraldehyde, 4% paraformaldehyde in 0.1 m sodium cacodylate buffer, pH 7.2, for thin section and freeze fracture.
Antibodies.
The antibodies used were guinea pig and rabbit anti-Caspr antibodies (Bhat et al., 2001), rabbit anti-KCNQ4 (Beisel et al., 2005), and rabbit anti-βIII tubulin (Covance). Alexa Fluor 568, 488, and 647 secondary antibodies were from Invitrogen. Colloidal gold-conjugated antibody (15 nm) was from BB International.
Immunofluorescence.
Fixed tissues were permeabilized for 20 min with 0.5% Triton X-100 (Sigma-Aldrich) in PBS and blocked overnight at 4°C in 2% bovine serum albumin (Sigma-Aldrich) in PBS. Tissue was incubated with primary antibody for 2 h, washed in PBS, and incubated with secondary antibody for 1 h and counterstained with Alexa Fluor 568 phalloidin (Invitrogen), mounted using Prolong anti-fade (Invitrogen), viewed with a confocal microscope (PerkinElmer) and analyzed using ImageJ (NIH).
Thin sections.
Fixed tissues were postfixed with 1% osmium tetroxide (Sigma-Aldrich) for 40 min, dehydrated in acetone, and embedded in PolyBed 812 (EMS Acquisition Corp). Thin sections were stained with 2% uranyl acetate (EMS Acquisition Corp) and lead citrate (EMS Acquisition Corp), imaged with a JEOL 1010 or a Zeiss 922 [electron microscope and analyzed with ImageJ (NIH)]. The gap between apposed membranes at the calyceal contacts was measured in Caspr+/+ and Caspr−/− (three mice per genotype). Images corresponding to regions at the basal half of the calyceal contacts with the hair cells (14 cells of each genotype) were selected, and 20 measurements were performed per image. Measurements, evenly distributed along the extension of the contacts, consisted of the distance between the centers of well defined adjacent lipid bilayers in a perpendicular angle to the membranes. We avoided invaginations of calyx, where the intercellular space is narrower (Bagger-Sjöbäck and Gulley, 1979).
Immunoelectron microscopy.
After fixation, the samples were glycerinated (30%) and plunge-frozen in Freon 22 cooled in liquid nitrogen. Frozen tissues were freeze-substituted in a Leica freeze-substitution apparatus with 1% uranyl acetate in methanol at −90°C for 2 d, embedded in Lowicryl (EMS Acquisition Corp) at −20°C for 1 d, and polymerized in UV light at −20°C for 2 d. Immunogold labeling was performed on thin sections as described previously (Dumont et al., 2001). For the quantification of the Caspr labeling, gold particles at the contacts between the hair cells and the calyx were counted in 12 images of different cells from two mice. For KCNQ4 labeling, gold particles were counted along the hair cell membrane and neurons at the inner face of the calyx.
Freeze fracture.
After fixation, samples were glycerinated (30%), placed on gelatin-covered aluminum stubs, and slam-frozen on a copper block cooled with liquid nitrogen in a LifeCell apparatus. Samples were freeze fractured as described previously (Kachar et al., 1986).
Results
The contacts between the membranes of type I hair cells and the neuronal calyx appear in thin-section electron micrographs as two conspicuously electron-dense membranes separated by a very regular space (Fig. 1a,b). In close-up views of these calyceal contacts, we observe periodic electron-dense elements that project from the juxtaposed surfaces of the contacting plasma membranes into the intercellular space and often appear to form cross-bridges (Fig. 1b, inset). The regular spacing, enhanced electron density of the membranes, and the periodic cross bridges, are all common structural features observed in intercellular junctions (Furuse and Tsukita, 2006). Freeze-fracture images of the plasma membrane of vestibular type-I hair cells show IMPs organized in parallel linear arrays (Fig. 1c, arrows). These parallel linear arrays of IMPs resemble the IMP organization of SJs (Kachar et al., 1986), but an optimal alignment of the section with the orientation of this linear array of particles is necessary for visualization of the cross-bridges in thin-section electron micrographs. We tested by immunofluorescence for the presence of Caspr. Caspr immunoreactivity was observed at the contacts between the calyx and type I hair cells in mouse, rat (Fig. 1d), and guinea pig. The Caspr immunofluorescence at the calyx was even stronger than that at the hemiparanodes (Fig. 1). To quantify the cellular distribution of Caspr immunolabeling in the calyceal contacts with the hair cells, we counted gold particles along the membranes of both sides of the contacts and found that 86% of the total gold particles (n = 398) was associated with the nerve membrane (Fig. 1f).
Figure 1.
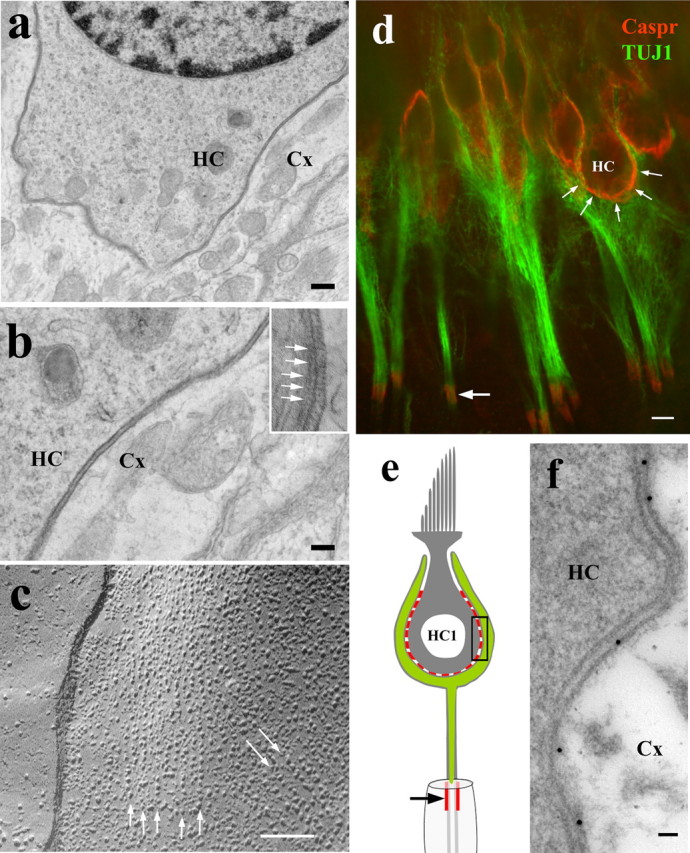
Septate junction-like features and localization of Caspr at type I hair cell calyceal synapses. a, Thin-section electron microscopy of mouse vestibular epithelia showing regular intercellular gap between the conspicuously electron-dense membranes of the calyx (Cx) and the type I hair cells (HC). b, Higher magnification shows the presence of periodic elements lining up the extracellular surface of the membranes (inset, arrows). Some of these periodic elements seem to be connected to form cross-bridges. c, Freeze-fracture image of the protoplasmic fracture face of a rat vestibular type I hair cell membrane shows parallel linear arrays of intramembrane particles (arrows). d, Confocal immunofluorescence image of type I hair cells of adult rat (red) shows localization of Caspr (arrows, red) at the internal part of the calyx (counterstained with anti-βIII tubulin; green). The first hemiparanode (arrow) shows Caspr immunoreactivity (green). e, Schematic diagram showing in red the regions that are positive for Caspr in the calyceal innervation (green) of the type I hair cell. f, Caspr immunogold-labeled thin section (of adult rat ampula) showing the contact between the Cx and the HC equivalent to the region shown by the rectangle in e. Majority of the gold particles line up with the nerve calyx membrane. Scale bars: a, 500 nm; b, c, 50 nm; d, 5 μm; e, 50 nm.
The structural features of the contact between the type I hair cells and the calyx and the presence of Caspr suggest that this contact is supported by a septate-like junction. Previous ultrastructural studies have shown disruption of paranodal SJs in Caspr−/− mouse (Bhat et al., 2001). To explore the role of Caspr in calyceal contacts, we performed thin-section electron microscopy of utricle and ampullae of Caspr−/−. When comparing with Caspr+/+, we do not detect changes in the overall ultrastructural morphology of Caspr−/− sensory epithelium, including the calyceal terminals and the hair cells (data not shown). However, in the Caspr−/−, the membranes of the calyceal contact do not exhibit the characteristic electron-dense appearance, and the gap between the membranes is very irregular and often significantly enlarged (Fig. 2). This enlargement of the intercellular space was specific to the calyceal synapses, as we did not observe swellings at the contacts between the same calyx and the supporting cells (Fig. 2b). We measured the gap between the membranes at the calyceal synapses; it averaged 28 ± 4 nm (n = 279) in Caspr+/+, but this value increased to 64 ± 55 nm (n = 436) in Caspr−/−. The box plot of the data (Fig. 2c) shows the increased variability in the separation between the membranes. These results indicate that Caspr is required for maintaining the regular separation between hair cells and the calyx membranes and for recruiting other proteins that produce the characteristic electron-dense profile of these membranes.
Figure 2.
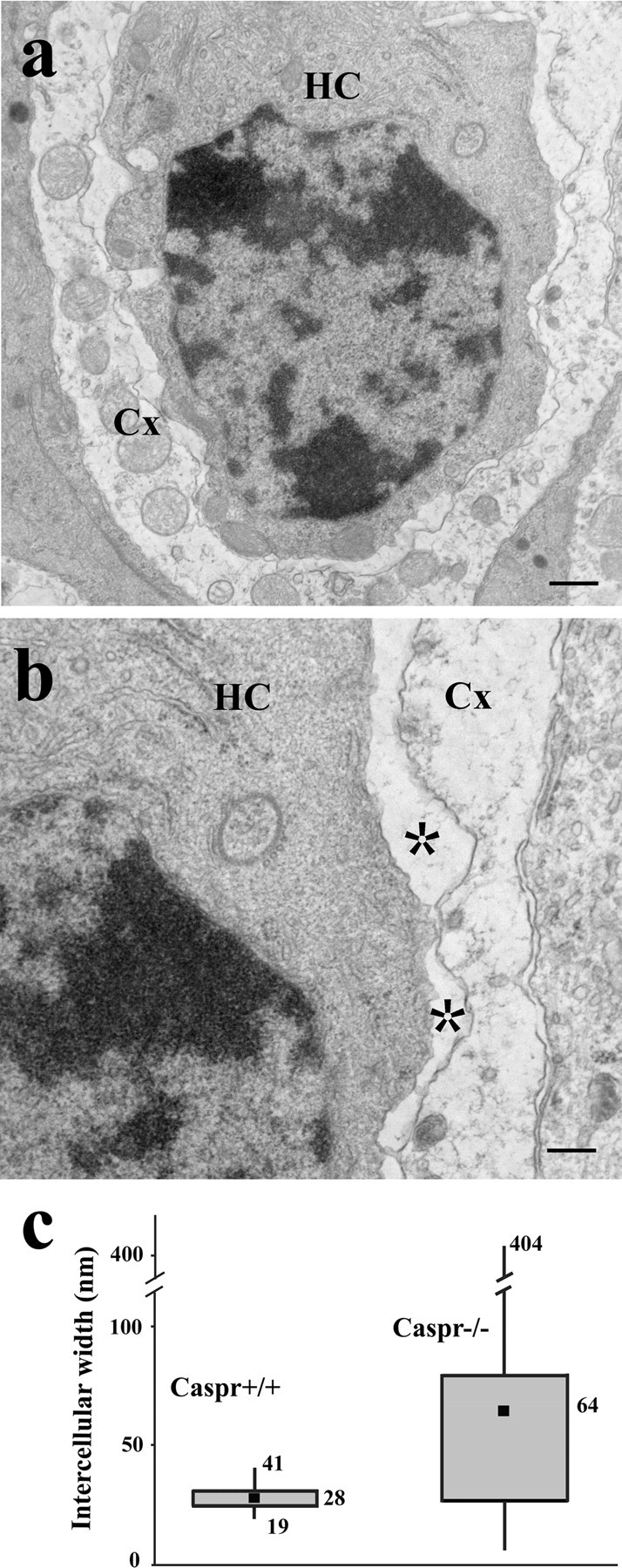
Ablation of Caspr leads to disruption of the calyceal synaptic contact. a, b, Low (a) and high (b) magnification thin-section micrographs showing the irregular and often enlarged gap between the membranes of the calyx (Cx) and the type I hair cell (HC) from a Caspr−/− mouse ampula (asterisk). The contacting membranes also lost their characteristic electron density as seen in Figure 1. c, Box plot of the distance between the calyx and the type I hair cell membranes measured in Caspr+/+ (average, 28 ± 4 nm; n = 279) and Caspr−/− (average, 64 ± 55 nm; n = 436) mice; filled square, mean; middle line of the box, median; top line of the box, 75th percentile; bottom line of the box, 25th percentile; top and bottom whiskers, maximum and minimum, respectively. Scale bars: a, 400 nm; b, 200 nm.
KCNQ4 is a potassium channel enriched at the calyceal synapses. In the adult rat, its major expression is in the calyx, but early in development it is expressed in both hair cells and the calyx (Hurley et al., 2006). To determine if KCNQ4 localization is related or dependent on Caspr, we performed a coimmunofluorescence localization assay. Confocal images tangential to the calyceal junction show a remarkable colocalization of these two proteins in adult rat (Fig. 3a), but clustering of Caspr at these junctions preceded that of KCNQ4 in the first postnatal days (data not shown). Previous studies have shown that ion channels at the paranodal membranes are mislocalized in Caspr−/− (Bhat et al., 2001). Using immunofluorescence, we analyzed the distribution of KCNQ4 in the vestibular system of adult Caspr−/−. Whereas Caspr colocalizes with KCNQ4 at the calyceal synapses of Caspr+/+ (Fig. 3b), Caspr was not detected in the Caspr−/− with the same experimental conditions, and the distribution of KCNQ4 in the calyx in Caspr−/− was notably faint and with a more disperse pattern of localization (Figs. 3c, 4). We analyzed the distribution of KCNQ4 labeling in the calyceal contacts by quantifying the number of KCNQ4 immunogold particles at these contacts and determining the percentage of particles associated with the membranes of hair cells and the nerve calyx for both genotypes. In Caspr+/+, the frequency of gold particles associated with the calyx inner face (0.54 particles per micrometer, n = 100) was higher than that associated with the hair cell (0.11 particles per micrometer) or the calyx outer face (0.09 particles per micrometer). In contrast, in Caspr−/−, the frequency of gold particles associated with the calyx outer face (0.69 particles per micrometer, n = 90) was higher than that associated with the calyx inner face (0.47 particles per micrometer) and the hair cells (0.17 particles per micrometer). These data confirm that KCNQ4 is indeed redistributed to outer face of the calyx of Caspr−/− (Fig. 3d,e), as illustrated in the diagrams (Fig. 3f,g). Together, these results demonstrate that Caspr is necessary for recruiting or retaining KCNQ4 at the calyceal synaptic contacts.
Figure 3.
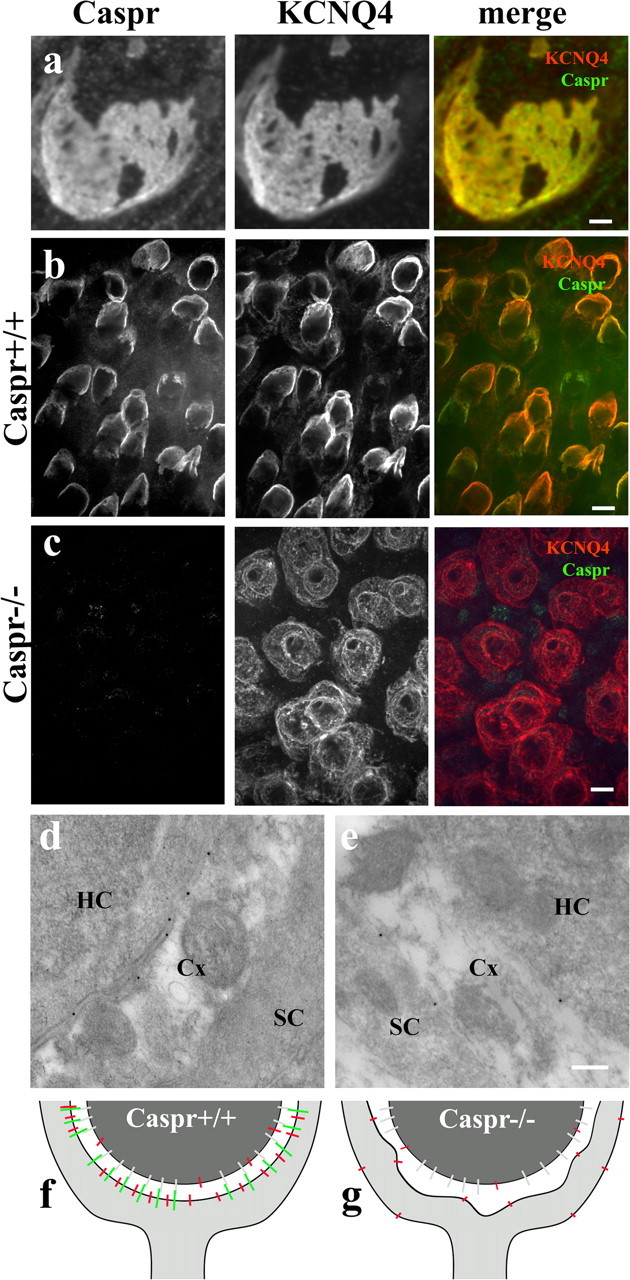
Localization of KCNQ4 at the calyceal membrane depends on Caspr. a, Confocal optical section grazing the calyx-type I hair cell (from adult rat ampulla); the contact region shows perfectly matched patterns of distribution of the immunofluorescence for Caspr (red) and KCNQ4 (green), indicating that these two proteins are colocalized. b, c, Caspr immunoreactivity in ampulla of Caspr+/+ and Caspr−/− at P18. b, Caspr and KCNQ4 colocalize at the calyceal synapses in Caspr+/+ mice. c, The labeling for KCNQ4 in the membranes of the Caspr−/− calyx is faint and sparsely distributed. d, e, Immunogold labeling of KCNQ4 confirms its localization at the internal membranes of the nerve calyx in Caspr+/+ and along both internal and external membranes of the calyx in Caspr−/−. f, g, Schematic diagram shows the colocalization of Caspr and KCNQ4 in Caspr+/+ (f) and the redistribution of KCNQ4 in Caspr−/− (g). A yet unidentified binding partner for the Caspr complex on the hair cell membrane is represented in gray (f, g). Scale bars: a, 2 μm; b, c, 10 μm; d, e, 200 nm.
Figure 4.
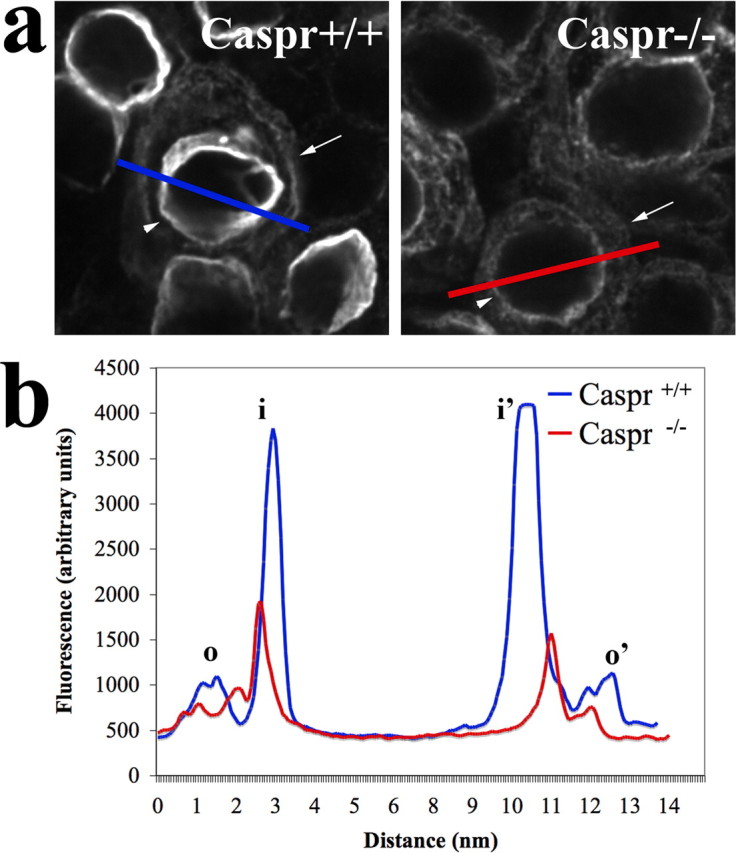
Ablation of Caspr leads to decreases in KCNQ4 expression and its redistribution in the calyx membranes. a, Representative image showing the different distribution of fluorescence intensity between inner (arrowheads) and outer (arrows) faces of the calyceal region of two KCNQ4-labeled cells from Caspr+/+ (blue) and Caspr−/− (red). A line crossing hair cells and calyx indicates the sampling plane used in the profile plots shown in b. Maximum values for peak, corresponding to inner (i, i′) and outer (o, o′) faces of the calyx, were used to calculate the ratio of fluorescence distribution at the calyx membranes.
To characterize the redistribution of KCNQ4, we quantitatively compared the distribution of KCNQ4 immunofluorescence at the inner and outer faces of Caspr+/+ and Caspr−/− calyces. Although we detected KCNQ4 immunofluorescence at the outer face of virtually all Caspr−/− calyces, KCNQ4 immunofluorescence was detected in only ∼15% of Caspr+/+ calyces (n = 82 cells). This result, per se, indicates a redistribution of KCNQ4 immunofluorescence in the calyx membranes. To evaluate the redistribution of KCNQ4 immunoreactivity, we compared profiles of fluorescence intensity spanning inner and outer faces of calyces in Caspr+/+ and Caspr−/−. For that, we subtracted the fluorescence background and used as a parameter the ratio (R) between the maximum values for the fluorescence peaks corresponding to the inner (i) and outer (o) faces of each measured calyx (for each individual cell, r = [i + i′]/[o + o′]) and found that Caspr+/+, indeed, displays much higher R values (Rwt = 9.8 ± 7.8, n = 10) than Caspr−/− (Rko = 1.3 ± 0.4, n = 28). A representative example of this comparative analysis is shown in Figure 4. The profiles clearly illustrate that KCNQ4 immunofluorescence is highly enriched at the inner face of Caspr+/+ calyces, whereas it is much reduced in the Caspr−/− calyces. The quantitative immunofluorescence and immunoelectron microscopy data further support the model depicted in Figure 3, in which ablation of Caspr leads to decreases in KCNQ4 expression accompanied by the redistribution of these channels at the membranes of the calyx.
Discussion
Our results show that a septate-like junction supports the contact between the neuronal calyx and the vestibular type 1 hair cell. Based on immunolocalization and electron microscopy experiments using Caspr+/+ and Caspr−/− mice, we show that Caspr, a SJ core protein found in myelin paranodal contacts, is essential for the formation of the calyceal contact. Caspr is not only required for structural support of the calyceal contact but also for recruiting or retaining KCNQ4, a key physiological component of the calyx synapse.
SJs are common in invertebrates where they form selective permeability barriers and also segregate membrane domains (Laval et al., 2008). Tight junctions have replaced SJs in vertebrates, and the only previously know example of SJ in vertebrates is the paranodal junction in myelinated axons (Banerjee et al., 2006). The discovery of a calyceal SJ-like structure indicates that there are unrecognized parallels between myelinated axons and vestibular calyces, which are vertebrate-specific systems. The reason for SJs preservation at the paranodes is unclear, but it has been suggested that a leaky diffusion barrier might be necessary in specific physiologic contexts (Skaer and Maddrell, 1987). In addition to promoting the close apposition between the axonal and glial membranes, paranodal SJs restrict ion movement between the extracellular space confined in the insulated portion of the myelinated axons (internodes) and the extracellular space surrounding the nodes of Ranvier (Salzer, 2003).
In type I hair cells, the apposed postsynaptic and presynaptic membranes at the calyceal contacts span ∼100× the surface of a typical bouton end seen in type II hair cells. The postsynaptic potential in a calyx receiving input of ∼10 synaptic ribbons (type I hair cell) is only approximately three times larger than the postsynaptic potential of a single bouton receiving input from one synaptic ribbon (type II hair cell), suggesting that calyceal synapses are rather inefficient (Eatock et al., 1998). In addition, experiments using pharmacological inhibitors of glutamate receptors show calyx depolarization in the absence of formal synaptic transmission (Yamashita and Ohmori, 1990; Holt et al., 2007a). Goldberg has proposed a theoretical model correlating the narrow gap and the extent of the contact between the calyx and the type I hair cells to the unexplained properties of calyceal synapses, where the K+ ions in the intercellular space affect calyceal transmission (Goldberg, 1996). For instance, an accumulation of neurotransmitters and ions in the calyceal synaptic contacts could explain the observed decreases in synaptic gain and slow decay in postsynaptic potential after termination of mechanical stimulation (Goldberg, 1996). The role of Caspr in the calyceal contact would be to form a septate-like junction and define the narrow space between the apposed membranes and at the same time provide a leaky diffusion barrier. That would parallel a known function of paranodal SJs, which provides a diffusion barrier for K+ (Salzer, 2003). Caspr is a transmembrane glycoprotein that contains an extracellular domain rich in laminin G-like domains and a cytoplasmic domain that binds to the axonal cytoskeleton. Different lines of evidence point to Caspr as a scaffolding protein that recruits other proteins to the paranodes (Girault et al., 2003). The large extracellular domain and the scaffolding properties of Caspr might contribute to the electron-dense profile observed in calyceal junctions and support the idea that the extracellular gap in calyceal synapses is a crowded molecular environment that can limit free diffusion of solutes.
Intercellular adhesion molecules have been implicated in recruiting other proteins to the sites of cell contacts. For example, neuroligin-1 has been implicated in recruiting postsynaptic proteins to the synapses (Shapiro et al., 2007). K+ channels have been reported to transiently cluster at SJs in paranodes during developmental myelination and during remyelination (Rasband and Trimmer, 2001). The characteristic electron-dense profile of the membranes at the calyceal contacts suggests that there is a high density of proteins recruited and maintained at the contact site. Interestingly, when Caspr is absent (in the Caspr−/−), the membranes no longer display the electron-dense appearance, suggesting that Caspr is essential for the targeting and retention of other proteins in the calyceal contact. The colocalization of Caspr and KCNQ4 in the calyceal contacts is striking and together with the data showing dispersion of KCNQ4 in the Caspr−/−, strongly suggests that the localization of these two proteins is interdependent. We also observed that Caspr expression in the calyceal contact precedes by several days the localization of KCNQ4 (data not shown), suggesting that this interdependency takes place during normal development. KCNQ4 is believed to contribute to the homeostasis of K+ at the calyceal synapses (Kharlovets et al., 2000). A dependence of KCNQ4 on Caspr for targeting and retention is certainly a reliable way to target KCNQ4 specifically to the extensive area of calyceal contact. One possible scenario where the colocalization of these two proteins could be advantageous to synaptic function is if the cell is trying to limit the diffusion of the neurotransmitter out of the synaptic cleft, but at the same time, it needs to buffer the concentration of K+. We could not test for the in vivo effect of ablation of Caspr because the Caspr−/− mice develop severe ataxia, eventual paralysis, and premature death at about postnatal day 20 (Bhat et al., 2001).
Intriguingly, like the calyceal synapses in vestibular epithelia, the myelinated axons also arose late during evolution and are a vertebrate-specific feature (Hartline and Colman, 2007). In paranodal SJs, a Caspr/contactin complex at the neuronal side binds NF155 at the glial side and links SJs to the axonal cytoskeleton (Sousa and Bhat, 2007). Whereas NF155 is believed to be the counterpart of the Caspr/contactin complex, we still do not know what is the counterpart of Caspr at the calyceal junctions. It would be interesting to identify the additional molecular components and how they are organized to establish the septate-like junctional structures at the calyceal synapses.
Footnotes
This work was supported by National Institute on Deafness and Other Communication Disorders, Division of Intramural Research, National Institutes of Health (NIH; B.K.), and NIH Grant GM063074 (M.A.B.). We thank Cole Graydon and Drs. Mark Schneider, Stephan Brenowitz, and Ronald Petralia for helpful discussions, and Uri Manor for assistance on statistical analysis of measurements.
References
- Bagger-Sjöbäck and Gulley, 1979.Bagger-Sjöbäck D, Gulley RL. Synaptic structures in the type II hair cell in the vestibular system of the guinea pig. A freeze-fracture and TEM study. Acta Otolaryngol. 1979;88:401–411. doi: 10.3109/00016487909137185. [DOI] [PubMed] [Google Scholar]
- Banerjee et al., 2006.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Beisel et al., 2005.Beisel KW, Rocha-Sanchez SM, Morris KA, Nie L, Feng F, Kachar B, Yamoah EN, Fritzsch B. Differential expression of KCNQ4 in inner hair cells and sensory neurons is the basis of progressive high-frequency hearing loss. J Neurosci. 2005;25:9285–9293. doi: 10.1523/JNEUROSCI.2110-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat et al., 2001.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Dumont et al., 2001.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21:5066–5078. doi: 10.1523/JNEUROSCI.21-14-05066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock et al., 1998.Eatock RA, Rüsch A, Lysakowski A, Saeki M. Hair cells in mammalian utricles. Otolaryngol Head Neck Surg. 1998;119:172–181. doi: 10.1016/S0194-5998(98)70052-X. [DOI] [PubMed] [Google Scholar]
- Eatock et al., 2008.Eatock RA, Xue J, Kalluri R. Ion channels in mammalian vestibular afferents may set regularity of firing. J Exp Biol. 2008;211:1764–1774. doi: 10.1242/jeb.017350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse and Tsukita, 2006.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco et al., 2006.Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault et al., 2003.Girault JA, Oguievetskaia K, Carnaud M, Denisenko-Nehrbass N, Goutebroze L. Transmembrane scaffolding proteins in the formation and stability of nodes of Ranvier. Biol Cell. 2003;95:447–452. doi: 10.1016/s0248-4900(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Goldberg, 1996.Goldberg JM. Theoretical analysis of intercellular communication between the vestibular type I hair cell and its calyx ending. J Neurophysiol. 1996;76:1942–1957. doi: 10.1152/jn.1996.76.3.1942. [DOI] [PubMed] [Google Scholar]
- Gulley and Bagger-Sjöbäck, 1979.Gulley RL, Bagger-Sjöbäck D. Freeze-fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea-pig vestibular system. J Neurocytol. 1979;8:591–603. doi: 10.1007/BF01208511. [DOI] [PubMed] [Google Scholar]
- Hartline and Colman, 2007.Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Holt et al., 2007a.Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol. 2007a;98:1083–1101. doi: 10.1152/jn.00332.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt et al., 2007b.Holt JR, Stauffer EA, Abraham D, Géléoc GS. Dominant-negative inhibition of M-like potassium conductances in hair cells of the mouse inner ear. J Neurosci. 2007b;27:8940–8951. doi: 10.1523/JNEUROSCI.2085-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley et al., 2006.Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci. 2006;26:10253–10269. doi: 10.1523/JNEUROSCI.2596-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar et al., 1986.Kachar B, Christakis NA, Reese TS, Lane NJ. The intramembrane structure of septate junctions based on direct freezing. J Cell Sci. 1986;80:13–28. doi: 10.1242/jcs.80.1.13. [DOI] [PubMed] [Google Scholar]
- Kharkovets et al., 2000.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval et al., 2008.Laval M, Bel C, Faivre-Sarrailh C. The lateral mobility of cell adhesion molecules is highly restricted at septate junctions in Drosophila. BMC Cell Biol. 2008;9:38. doi: 10.1186/1471-2121-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband and Trimmer, 2001.Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Salzer, 2003.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Shapiro et al., 2007.Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- Skaer and Maddrell, 1987.Skaer HB, Maddrell SHP. How are invertebrate epithelia made tight? J Cell Sci. 1987;88:139. [Google Scholar]
- Sousa and Bhat, 2007.Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles' heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto et al., 2002.Soto E, Vega R, Budelli R. The receptor potential in type I and type II vestibular system hair cells: a model analysis. Hear Res. 2002;165:35–47. doi: 10.1016/s0378-5955(01)00418-x. [DOI] [PubMed] [Google Scholar]
- Yamashita and Ohmori, 1990.Yamashita M, Ohmori H. Synaptic responses to mechanical stimulation in calyceal and bouton type vestibular afferents studied in an isolated preparation of semicircular canal ampullae of chicken. Exp Brain Res. 1990;80:475–488. doi: 10.1007/BF00227989. [DOI] [PubMed] [Google Scholar]


