Abstract
Sensory hair cells of the inner ear are susceptible to death from a variety of stresses including aging, noise trauma, genetic disorders, and exposure to certain therapeutic drugs. Ototoxic drugs include the aminoglycoside antibiotics and the antineoplastic agent cisplatin. This is a short technical report describing the dissection and culture of the adult mouse utricle. This in vitro preparation allows for detailed studies of ototoxic drug-induced hair cell death in an adult mammalian system. In addition, this preparation allows for examination of the effects of specific gene products through the use of transgenic and knockout mouse models.
Introduction
Sensory hair cells of the inner ear are the mechanosensitive receptor cells of hearing and balance. These cells are sensitive to damage and death from a variety of insults including aging, noise trauma and exposure to certain therapeutic drugs that are toxic to hair cells. The two primary classes of ototoxic drugs are the aminoglycoside antibiotics and the antineoplastic agent cisplatin. Approximately 12–25% of patients receiving these drugs experience permanent hearing loss and/or balance disorders (Forge and Schacht, 2000; Simon et al., 2002). The purpose of this short technical report is to describe an in vitro preparation of the adult mouse utricle. This preparation allows for detailed biochemical and molecular analysis of hair cells from a mature mammal.
The utricle is one of the vestibular sensory organs of the inner ear, and utricular hair cells are very similar both embryologically and structurally to cochlear hair cells, especially inner hair cells. Perhaps the most notable difference between hair cells of the utricle and those of the mammalian organ of Corti is that the vestibular system has some capacity to regenerate hair cells (Forge et al., 1993; Warchol et al., 1993). Despite these differences, vestibular hair cells have proven useful in tracking the cellular mechanisms underlying ototoxicity. The in vitro utricle preparation presents several potential advantages over in vitro preparations of the cochlea. First, adult mammalian cochlear hair cells do not survive well in vitro using current culture techniques. Therefore, in vitro preparations of the cochlea usually rely on the use of neonatal tissue, which may vary significantly from mature tissue in its responses to a wide variety of stimuli, particularly ototoxic drugs (Pujol, 1986). Second, the hair cells of the mouse utricle are sensitive to death from exposure to the same therapeutic drugs that kill cochlear hair cells (Cunningham et al., 2002; Zhang et al., 2003). Third, the cellular mechanisms mediating ototoxic hair cell death appear to be similar for both utricular hair cells in vitro and cochlear hair cells in vivo (Cunningham et al., 2002; Forge and Li, 2000; Liu et al., 1998; Mangiardi et al., 2004; Matsui et al., 2003; Matsui et al., 2002; Nakagawa et al., 2003; Pirvola et al., 2000; Ylikoski et al., 2002). Finally, the use of mouse tissue allows for examination of the effects of specific gene products through the utilization of tissue from transgenic and knockout animals (Cunningham et al., 2004).
Materials and Methods
Dissection of the Mouse Utricle
Adult mice (usually 5–8 weeks of age) are euthanized by overdose of Nembutal (Ovation Pharmaceuticals, Inc. Deerfield, IL). Heads are removed using medium scissors, and the external auditory canal is snipped beneath the skin on either side using small scissors. The skin of the head is then pulled forward from back to front, and the head is bisected (also from back to front) using small scissors. The brain is removed from each half using the tip of the small scissors. The VIIIth nerve root and posterior semicircular canal can then be visualized near the back of the skull. The skull is broken (or cut) to obtain the auditory bulla and a small bit of surrounding bone. Once this gross dissection is complete, the fine dissection is completed in a tissue culture hood equipped with a stereomicroscope.
For the fine dissection, dissecting tools are cleaned and immersed in 70% ethanol for 15 minutes prior to being placed at a convenient location in the tissue culture hood. The necessary tools are as follows:
2 pairs #55 forceps (such as Fine Science Tools #11255-20)
1 pair #5 forceps
1 pair #3 forceps
1 scalpel with #11 blade
1 fine probe (such as half of a broken #55 forceps)
The fine dissection is carried out using sterile technique. Each ear is placed in a 35mm sterile Petri dish and covered with dissecting medium consisting of a 2:1 v/v mixture of Basal Medium Eagle and Earle’s Balanced Salt Solution (BME/EBSS). BME consists of Basal Medium Eagle (Sigma, St. Louis, MO #B-9638) supplemented with 25mM sodium bicarbonate (Sigma #S-6297) and 25mM glucose (Sigma #G5767). Earle’s Balanced Salt Solution is obtained from Sigma (#E2888).
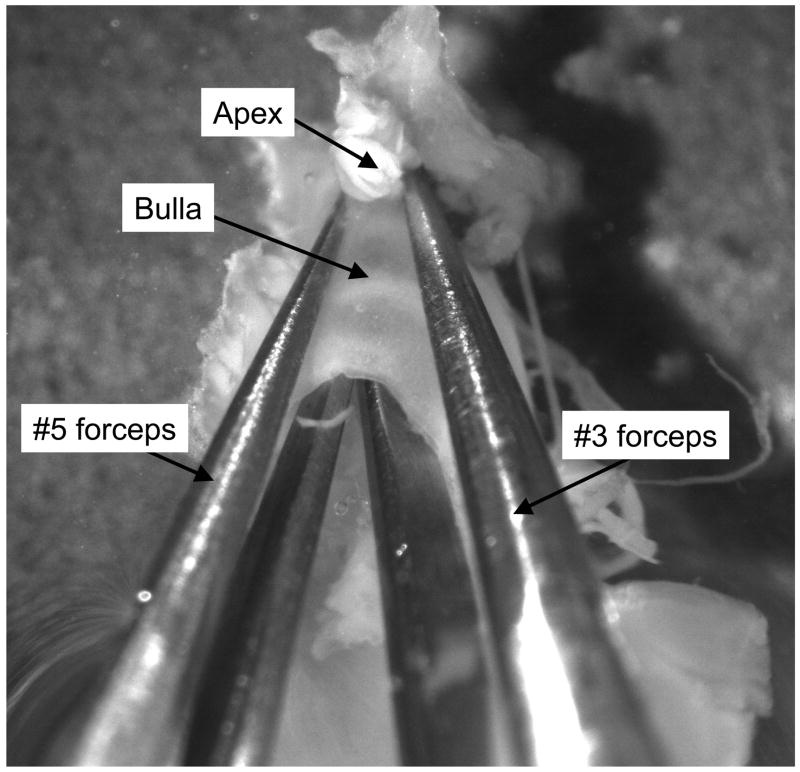
The first step in the fine dissection is to locate the external auditory canal (EAC) on one side of the preparation and the VIIIth nerve root on the other. The auditory bulla is broken open by inserting one fork of both the #3 forceps and the #5 forceps all the way into the EAC until the tips reach the bone at the apical end (Figure 1). The forceps are then pulled apart to break the bulla, exposing the bony cochlea. After the bulla is removed, the bony cochlear spiral is visible along with the ossicles and the oval and round windows. On the opposite (medial) surface of the preparation, the VIIIth nerve root and posterior semicircular canal are visible (Figure 2).
Figure 1. Opening the auditory bulla.
The first step in the fine dissection is to break open the auditory bulla using two pairs of forceps. One fork of each forceps is placed all the way down the bulla from the external auditory canal to the bony apex. The bulla is broken open by pulling the two pairs of forceps away from one another. The bony cochlea is much harder than the bulla, so this manipulation will break the bulla without damaging the bony cochlea beneath.
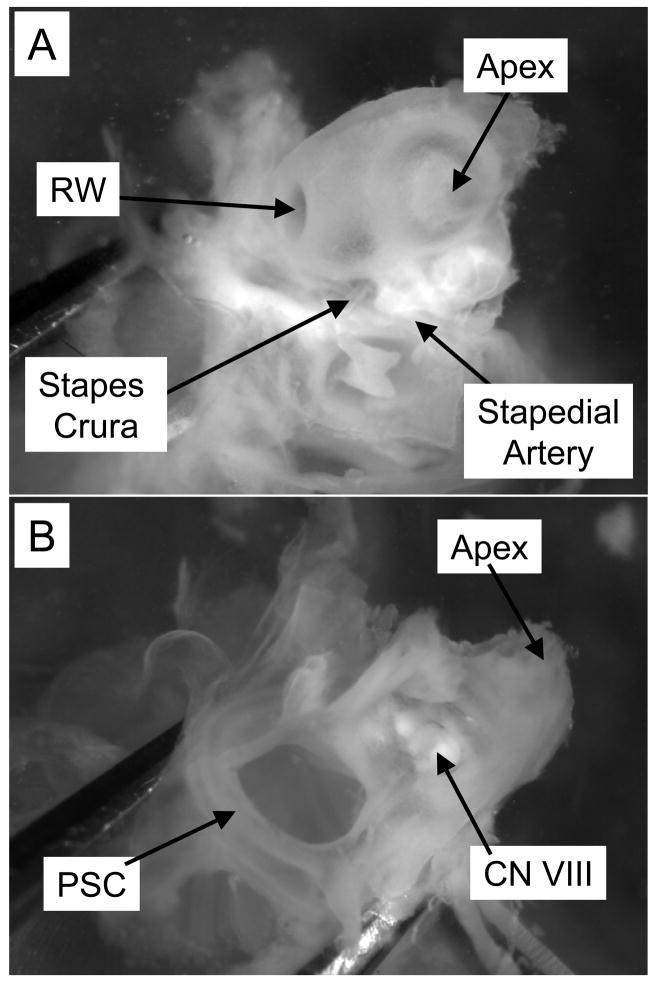
Figure 2. Views of the bony cochlea.
After the bulla is removed, the bony cochlea is visible. A: The lateral surface (bulla side) of the cochlea, with both the oval and round windows visible. B: the medial (brain side) of the cochlea, with the VIIIth nerve root visible. RW, round window; PSC, posterior semicircular canal; CN VIII, root of the VIIIth cranial nerve.
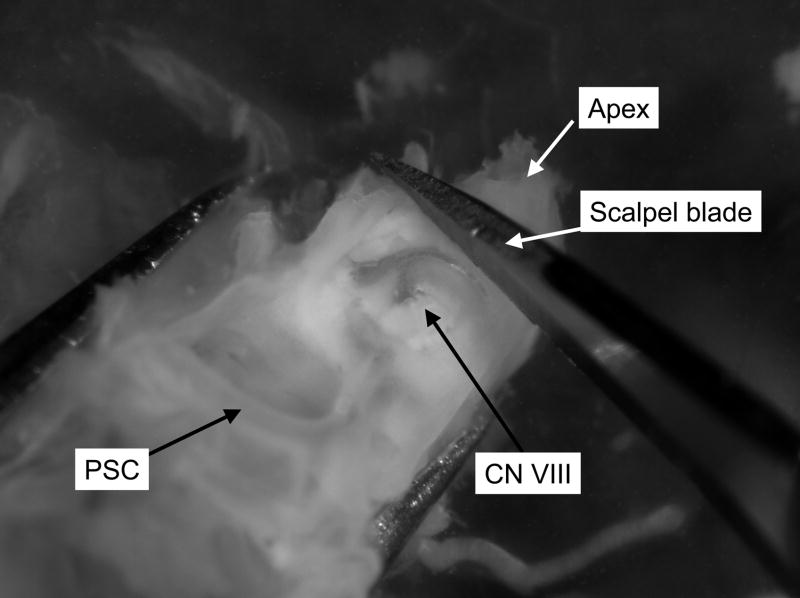
With the preparation held such that the cochlear spiral and ossicles are on the bottom of the dish and the nerve root is on top, the apex of the cochlea is removed using the scalpel. The scalpel blade is used to cut all the way through the apex of the cochlea between the VIIIth nerve root and the apex (Figure 3). The preparation is then rotated until the remaining cochlear spiral is pointing up and the preparation is resting on the semicircular canals. The posterior semicircular canal is used as a handle to hold the preparation steady by placing one fork of the #3 forceps in the canal. The remaining cochlear spiral (soft tissue) is then removed at the modiolus using #5 forceps.
Figure 3. Opening the apex.
The apical portion of the bony cochlea is removed using a scalpel. The scalpel blade should be lowered onto the cochlea just apical of the VIIIth nerve root. Cut the apex by pushing straight down onto the bone without sawing. PSC, posterior semicircular canal; CN VIII, root of the VIIIth cranial nerve.
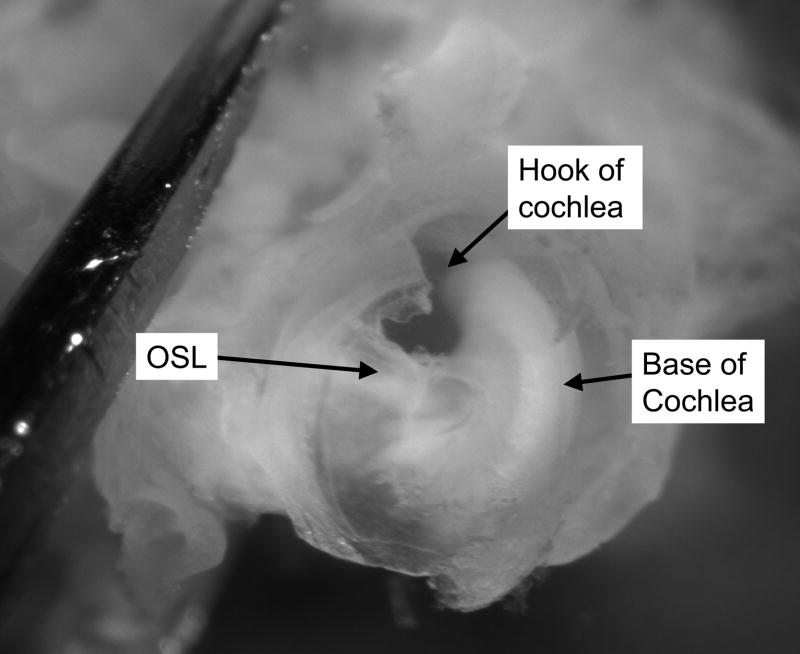
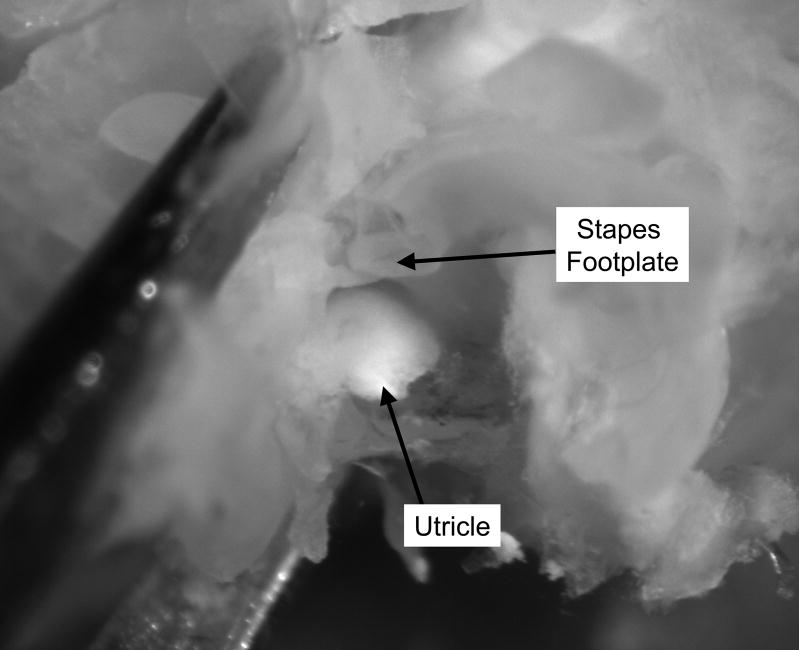
After the cochlea (except for the basal hook region) is removed, the remainder of the osseous spiral lamina is removed using the fine probe (Figure 4). The tip of the fine probe is placed beneath the remainder of the osseous spiral lamina, and the bone is carefully lifted up. Just beneath this bony shelf lies the saccule. When the saccule is removed, the utricle (surrounded by bone) is visible directly across from the stapes footplate (Figure 5). The utricle is carefully removed using #55 forceps.
Figure 4. Base of the cochlea.
After most of the soft tissue of the cochlea is removed, only the basal “hook” region remains. The Osseous Spiral Lamina will be lifted up from beneath using a fine probe. OSL, Osseous Spiral Lamina.
Figure 5. Utricle and stapes footplate.
With the Osseous Spiral Lamina removed, the utricle is visible immediately adjacent to the stapes footplate. The utricle is removed gently using fine forceps.
Culture Conditions and Ototoxic Drug Exposures
Utricles are cultured free-floating (2–8 utricles per well) in sterile 24-well tissue culture plates. Culture medium consists of dissecting medium (BME and EBSS, 2:1 v/v) supplemented with 5% fetal bovine serum (FBS). For ototoxic drug experiments, neomycin sulfate solution (Sigma) is added to culture medium at final concentrations ranging from 0.1mM to 5.0mM. Cisplatin is supplied as a 1 mg/ml stock solution (Bedford Laboratories, Bedford, OH) and diluted into culture medium at final concentrations ranging from 1.0 μg/ml to 10.0 μg/ml. No neomycin or cisplatin is added to control cultures. Utricles are incubated at 37°C in a 5% CO2/95% air environment.
Otoconia Removal
At the end of the culture period, utricles are fixed overnight at 4°C in 4% paraformaldehyde. Utricles are then washed in phosphate buffered saline (PBS). Otoconia are removed by holding the edge of the utricle with a pair of #55 forceps and directing a stream of PBS across the utricle from a syringe with a 23-gauge needle. This technique can also be used to remove otoconia from fresh utricles prior to culturing them.
Labeling Hair Cells
For hair cell counts, fixed utricles can be prepared for whole mount double-label fluorescent immunohistochemistry using a monoclonal antibody against calmodulin (Sigma C-3545) and a polyclonal antibody against calbindin (Chemicon AB1778) (Cunningham et al., 2002; Cunningham et al., 2004). The double label immunohistochemistry protocol allows for separate counts of the two types of hair cells in the mouse utricle: anti-calmodulin labels all hair cells in the utricle, and anti-calbindin labels only the hair cells in the striolar region. Striolar hair cells are more sensitive than non-striolar hair cells to aminoglycoside-induced death (Cunningham et al., 2002).
After staining, whole utricles are mounted on glass slides in Fluoromount-G (Southern Biotechnology, Birmingham, AL) and coverslipped.
Discussion
This report describes the dissection and culture of the adult mouse utricle. This in vitro preparation presents specific advantages over other in vitro preparations used to examine sensory hair cells. For example, this preparation allows for the examination of hair cells from a mature mammalian epithelium. An additional advantage of this preparation is the potential to examine the effects of specific genes by utilizing existing transgenic and knockout mouse models (Cunningham et al., 2004).
The molecular mechanisms underlying cell death and survival are complex, and multiple regulatory systems exist. Rigorous biochemical examination of these mechanisms requires the use of in vitro model systems, which allow for more control and easier manipulation of experimental conditions relative to in vivo models. In addition, in vitro model systems allow for more precise examination of the timing of specific cellular events. In recent years, our understanding of the mechanisms underlying ototoxic drug-induced cell death has been advanced by the use of in vitro model systems, including neonatal cochlear explants and immortalized cell lines (Devarajan et al., 2002; Ding et al., 2002; Kalinec et al., 2003; Liu et al., 1998; Pirvola et al., 2000; Wang et al., 2003b; Zhang et al., 2003). The primary disadvantage of in vitro model systems is that they may not accurately predict the in vivo condition. However, in vivo experiments in both birds (Cheng et al., 2003; Matsui et al., 2004; Matsui et al., 2003) and mammals (Okuda et al., 2005; Pirvola et al., 2000; Wang et al., 2003a; Ylikoski et al., 2002) indicate that the mechanism(s) underlying aminoglycoside-induced hair cell death are similar to those that have been reported for the mouse utricle preparation in vitro. Still, there may be differences in the mechanisms underlying ototoxicity in vivo and in vitro (Jiang et al., 2005). An in vitro model system utilizing mouse tissue allows for follow-up in vivo studies in the same animal, since an in vivo model of drug-induced ototoxicity has been characterized in mouse (Wu et al., 2001). The long-term goal of studies of sensory hair cell survival and death is to improve the lives of humans by inhibiting the hearing loss caused by loss of sensory cells. In vitro model systems represent important tools in all areas of experimental biology, and those findings that represent potential advances in human medicine must be followed up with in vivo testing. The mouse utricle preparation represents one model system in which sensory hair cell death can be precisely examined and manipulated with the goal of improving our understanding of its underlying molecular mechanisms. This mechanistic information then can be tested in vivo in order to develop therapies aimed at preventing or reversing hearing loss.
Supplementary Material
Acknowledgments
The author is grateful to Dr. Jonathan I. Matsui and Dr. Remy Pujol for helpful comments on an earlier version of this manuscript. Many thanks to Jim Nicholson for video production.
Footnotes
Supplementary Material: A movie of the fine dissection of the adult mouse utricle is available at http://www.musc.edu/pathology/cvs/cunningham.htm
References
- Cheng AG, Cunningham LL, Rubel EW. Hair Cell Death in the Avian Basilar Papilla: Characterization of the in vitro Model and Caspase Activation. J Assoc Res Otolaryngol. 2003;4:91–105. doi: 10.1007/s10162-002-3016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase Activation in Hair Cells of the Mouse Utricle Exposed to Neomycin. Journal of Neuroscience. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164:115–26. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8:177–89. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Liu W, Staecker H, Stupak H, Malgrange B, Lefebvre P, Van De Water TR. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport. 1998;9:2609–14. doi: 10.1097/00001756-199808030-00034. [DOI] [PubMed] [Google Scholar]
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Gale JE, Warchol ME. Critical signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J Neurobiol. 2004;61:250–66. doi: 10.1002/neu.20054. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Haque A, Huss D, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Dickman JD, Warchol ME. Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo. J Neurosci. 2003;23:6111–22. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–27. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kim TS, Murai N, Endo T, Iguchi F, Tateya I, Yamamoto N, Naito Y, Ito J. A novel technique for inducing local inner ear damage. Hear Res. 2003;176:122–7. doi: 10.1016/s0378-5955(02)00768-2. [DOI] [PubMed] [Google Scholar]
- Okuda T, Sugahara K, Takemoto T, Shimogori H, Yamashita H. Inhibition of caspases alleviates gentamicin-induced cochlear damage in guinea pigs. Auris Nasus Larynx. 2005;32:33–7. doi: 10.1016/j.anl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R. Periods of sensitivity to antibiotic treatment. Acta Otolaryngol Suppl. 1986;429:29–33. doi: 10.3109/00016488609122727. [DOI] [PubMed] [Google Scholar]
- Simon T, Hero B, Dupuis W, Selle B, Berthold F. The incidence of hearing impairment after successful treatment of neuroblastoma. Klin Padiatr. 2002;214:149–52. doi: 10.1055/s-2002-33179. [DOI] [PubMed] [Google Scholar]
- Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae Extract) Preparations Attenuate Aminoglycoside-Induced Free Radical Formation In Vitro and Ototoxicity In Vivo. Antimicrob Agents Chemother. 2003a;47:1836–41. doi: 10.1128/AAC.47.6.1836-1841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003b;23:8596–607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans [see comments] Science. 1993;259:1619–22. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–78. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.