Abstract
Objective
The current study examined whether cancer survivors showed impairment, resilience, or growth responses relative to a sociodemographically matched sample in four domains: mental health and mood, psychological well-being, social well-being, and spirituality. The impact of aging on psychosocial adjustment was also investigated.
Design
Participants were 398 cancer survivors who were participants in the MIDUS survey (Midlife in the United States) and 796 matched respondents with no cancer history. Psychosocial assessments were completed in 1995-96 and 2004-06.
Results
Findings indicated that cancer survivors demonstrated impairment relative to the comparison group in mental health, mood, and some aspects of psychological well-being. Longitudinal analyses spanning pre- and post-diagnosis clarified that while mental health declined after a cancer diagnosis, poorer functioning in other domains existed prior to diagnosis. However, survivors exhibited resilient social well-being, spirituality, and personal growth. Moreover, age appeared to confer resiliency; older survivors were more likely than younger adults to show psychosocial functioning equivalent to their peers.
Conclusion
While younger survivors may be at risk for disturbances in mental health and mood, cancer survivors show resilience in other important domains of psychosocial adjustment.
Keywords: cancer, mental health, mood, resilience, aging
Due to advances in detection and treatment, approximately 2 in 3 adults diagnosed with cancer today can be expected to survive more than 5 years (Ries et al., 2006). This improved survival rate, combined with the aging population, has led to a substantial increase in the number of cancer survivors in the U.S., now numbering more than 10.5 million individuals (Ries et al., 2006). While the medical concerns of cancer survivors are gaining attention (Hewitt, Greenfield, & Stovall, 2005; President’s Cancer Panel, 2005-2006), there is also growing interest in understanding their psychosocial concerns.
Cancer diagnosis and treatment, and their sequelae, are typically thought of as adverse experiences. For most individuals, they represent significant stressors, and for some, the diagnosis of cancer is a traumatic event (Kangas, Henry, & Bryant, 2002; Koopman et al., 2002). O’Leary and Ickovics have proposed a model to describe three potential responses to adversity (O’Leary & Ickovics, 1995), which has been further elaborated by Carver (Carver, 1998). Following initial decline in functioning after adverse experience, Carver describes survival with impairment as continuing compromised functioning, but he distinguishes this pattern from resilience, defined as a return to normal or baseline functioning, which is then further distinguished from thriving, described as exceeding one’s original level of functioning. While most previous work has focused on identifying impairment among cancer survivors, psychological thriving or growth among cancer survivors is of growing interest (Cordova & Andrykowski, 2003; Cordova, Cunningham, Carlson, & Andrykowski, 2001; Widows, Jacobsen, Booth-Jones, & Fields, 2005).
Understanding the extent to which cancer survivors show psychological impairment, resilience, or thriving necessitates a reference for “normal” or “baseline” functioning. National survey data or health care registries, which include cancer survivors as well as population-based reference groups, partially address this issue. Most studies of this type support the survival with impairment model, with cancer survivors exhibiting greater psychological distress, poorer mental health, greater role impairment due to emotional problems, and poorer social well-being relative to those without a cancer history (Arndt, Merx, Stegmaier, Ziegler, & Brenner, 2004, 2005; Baker, Haffer, & Denniston, 2003; Hewitt, Rowland, & Yancik, 2003; Rabin et al., 2007). The disadvantage of these datasets is that most were designed to focus on broad health outcomes and thus have only brief measures assessing limited domains of psychosocial functioning. Studies designed specifically to address quality of life and psychological well-being among cancer survivors include more comprehensive and detailed psychosocial measures. These studies generally provide contrasting results to the population-based studies, with findings more consistent with the resilience model. For example, studies of breast and gynecologic cancer survivors have found no significant differences between survivors and age-matched comparison groups in multiple domains of psychosocial functioning including mental health, psychological distress, and emotional and social well-being (Bradley, Lutgendorf, Rose, Costanzo, & Anderson, 2006; Dorval, Maunsell, Deschenes, Brisson, & Masse, 1998; Ganz, Rowland, Desmond, Meyerowitz, & Wyatt, 1998; Helgeson & Tomich, 2005; Wenzel et al., 2002).
The source of these discrepant findings is unclear. Brief measures utilized in large-scale population studies may not accurately capture survivors’ psychosocial functioning. On the other hand, studies examining narrower samples of cancer survivors may be less representative of cancer survivors as a whole, and therefore, less likely to include survivors who are functioning poorly. The present study addressed these issues by drawing our sample from the National Survey of Midlife Development in the United States (MIDUS), designed to study health and well-being during midlife. Unlike most national health surveys, MIDUS respondents completed comprehensive batteries of psychosocial measures, thereby also providing the opportunity to compare those with a history of cancer to those with no such history on diverse psychological outcomes. In addition, the MIDUS dataset includes two waves of data, the second obtained approximately 9 years after the initial wave. This afforded the unique opportunity to examine psychological functioning both prior to and following diagnosis among individuals who were diagnosed with cancer between the two assessments. Determining whether the trajectory of pre-to post-diagnosis change was most consistent with impairment, resilience, or thriving models and comparing such dynamics to those without cancer were key objectives of our investigation.
Although most previous studies of cancer survivors focus on mental health symptoms and negative mood, such areas neglect ideas of thriving (i.e., positive psychological outcomes following cancer). The concept of “posttraumatic growth” suggests that individuals often experience transformations in self-perceptions, life philosophies, and interpersonal relationships following traumatic experience (Tedeschi & Calhoun, 1996). Cancer survivors have reported closer intimate relationships, positive changes in spirituality, an enhanced sense of personal strength, and an increased appreciation of life (Cordova & Andrykowski, 2003; Manne et al., 2004; Sears, Stanton, & Danoff-Burg, 2003; Widows et al., 2005). While a few studies suggest these changes exceed personal growth reported by individuals not contending with the challenge of a cancer diagnosis (Andrykowski et al., 1996; Cordova et al., 2001), most work has not included a normal or baseline reference, making it unclear whether hypothesized growth areas actually change or differ from the rest of the population following a cancer diagnosis. Therefore, in addition to traditional measures of mental health and mood, we included assessments in multiple domains where psychological growth is thought to occur.
An additional focus pertained to the question of age. Prior studies have revealed extensive age differences in psychosocial profiles (Ryff, in press), and among cancer survivors, previous work has suggested that younger adults may be more vulnerable to impairment than older survivors, with younger patients consistently reporting poorer emotional well-being, greater depressive symptomatology, and greater anxiety after treatment ends (Arndt, Merx, Sturmer et al., 2004; Mor, Allen, & Malin, 1994; Wenzel et al., 1999). Developmental theorists propose that “off-time” life events occurring outside of typical age ranges are more likely to be distressing or traumatic (Neugarten & Hagestad, 1976), and this may be true for cancer as well. Because studies of age-related differences in cancer survivors have typically not included matched comparison groups, it is, however, unknown whether the prior age effects are specific to cancer survivors or reflect similar age trends found in the general population. While there are an almost unlimited number of demographic and psychosocial factors that may potentially explain variability in psychosocial adjustment among cancer survivors, we chose to focus our investigation on age given the well-documented relationships between age and well-being in the literature, and because the broad age spectrum and developmental focus of MIDUS was particularly well-suited to investigate this issue.
In summary, the primary objectives of the present study were to examine psychosocial impairment, resilience or thriving among cancer survivors in the general population by comparing them to individuals without a cancer history, with both evaluated longitudinally. We focused on four psychosocial domains: a) distress, as defined by mental health symptoms and mood, b) psychological well-being, c) social well-being, and d) spirituality. Domains in which cancer survivors reported poorer functioning and decline in functioning following diagnosis relative to the comparison group were classified as areas of impairment. Areas showing equivalent functioning (both pre-post comparisons and cancer-noncancer comparisons) were designated as evidence of resilience. Finally, domains in which cancer survivors reported superior functioning (both pre-post and relative to the comparison group) were classified as areas of thriving. Regarding age, we hypothesized that younger adults who had been diagnosed with cancer would show the most impairment and least resilience or thriving relative to their peers with no cancer history.
Method
MIDUS
Data are drawn from MIDUS, a national survey of 7,108 adults ages 25 to 74 years completed in 1995-96 (Wave 1). MIDUS is comprised of four subsamples: a national random digit dialing (RDD) sample (n = 3,487); oversamples from five metropolitan areas (n = 757); siblings of individuals from the RDD sample (n = 950); and a national RDD sample of twin pairs (n = 1,914). The main RDD sample was selected from working telephone banks. For each household contacted, a random respondent between 25 and 74 years of age was selected. Respondents were invited to participate in a telephone interview and to complete self-administered questionnaires. Of those contacted, 70% agreed to participate in the telephone interview, and 89% of those completing the telephone interview also completed questionnaires. A longitudinal follow-up was conducted in 2004-06 (Wave 2). Of those who participated in Wave 1, 4,963 completed another telephone interview (70% response rate; 75%, when adjusted for mortality), and 81% of individuals who completed the telephone interview completed self-administered questionnaires.
Sample
Cancer survivors
Individuals who responded affirmatively to the question: “Have you ever had cancer?” at the second wave of data collection were selected for the present analysis. Participants were queried about the type of cancer and their age at diagnosis. Those who reported a diagnosis of skin cancer only were excluded. Overall, 398 individuals met eligibility criteria. These cancer survivors were a median of 10 years post-diagnosis (range 0-59 years), with the majority (n = 207) diagnosed with cancer after the baseline assessments. This subgroup of survivors was a mean of 4 years post-diagnosis (range 0-10 years). Cancer sites for the full sample included breast (26.9%), prostate (20.9%), cervical (9.8%), colon (9.3%), uterine (6.5%), leukemia or lymphoma (5.8%), ovarian (3.8%), lung (3.3%), other (19.8%), and unknown (0.5%). The percentages were similar for the subgroup of those diagnosed after the baseline assessments.
Comparison group
A computerized algorithm was used to select a comparison group matched on age (within 5 years), gender, and education level. Two individuals were randomly selected for each cancer survivor (n = 796) from the pool of all individuals with no cancer history meeting matching criteria. Participants’ ages ranged from 34 to 84 years at Wave 2 (25 to 74 years at study entry) with a mean age of 63.0 years for both groups. Full demographic data are provided in Table 1. Chi-square analyses indicated that the comparison group did not differ significantly from the cancer survivors on any demographic variables, including ethnicity, region of residence, and employment status (all p values exceeded .10).
Table 1.
Demographic Characteristics of the Full Sample of Cancer Survivors and the Comparison Group
| Cancer Survivors n = 398 % |
Comparison Group n = 796 % |
|
|---|---|---|
| Sex | ||
| Female | 62.6 | 62.6 |
| Male | 37.4 | 37.4 |
| Ethnicity | ||
| Caucasian | 92.2 | 91.7 |
| African American | 2.5 | 3.0 |
| Native American | 1.8 | 1.0 |
| Asian | 0.3 | 0.8 |
| Other | 2.8 | 2.8 |
| Relationship status | ||
| Married | 65.6 | 70.2 |
| Divorced or separated | 16.6 | 12.1 |
| Widowed | 11.3 | 12.2 |
| Never married | 6.3 | 5.5 |
| Education | ||
| Less than 12 years | 10.6 | 10.6 |
| High school graduate | 28.4 | 28.4 |
| Some college or trade school | 27.4 | 27.4 |
| College graduate/advanced degree | 33.7 | 33.7 |
| Employment status | ||
| Employed | 35.4 | 39.2 |
| Retired | 48.7 | 45.5 |
| Homemaker | 9.3 | 9.4 |
| Disabled | 2.0 | 1.0 |
| Other | 4.6 | 4.9 |
Measures
Distress
The World Mental Health Organization Composite International Diagnostic Interview Short Form (WHO CIDI-SF) (Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998) was administered by telephone to assess past-year symptoms of a major depressive episode and two anxiety disorders, generalized anxiety disorder and panic disorder, based on diagnostic criteria from the DSM-III-R (American Psychiatric Association, 1987). Participants who endorsed screening items were queried on symptoms of each disorder, and a continuous scale was constructed for each disorder based on total number of symptoms endorsed. Due to the very low number of cancer survivors who endorsed any symptoms of generalized anxiety disorder scale (n = 7), it was not used in the present study.
Positive and negative affect were assessed by self-administered questionnaires in which participants rated the amount of time they experienced negative and positive affective states over the past 30 days on a 5-point scale from “all of the time” to “none of the time.” The two 6-item scales were comprised of items from several well-validated measures of affect including the Affect Balance Scale (Bradburn, 1969), Composite International Diagnostic Interview (Kessler et al., 1994), Manifest Anxiety Scale (Taylor, 1953), Health Opinion Survey (MacMillan, 1957), General Well-Being Schedule (Fazio, 1977), and Center for Epidemiological Studies Depression Scale (Radloff, 1977). Both scales demonstrated excellent reliability in the MIDUS sample (Cronbach’s α = .85-.87 for negative affect and .90-.91 for positive affect).
Psychological well-being
Positive psychological functioning was assessed with four of six domains of well-being (Ryff, 1989; Ryff & Keyes, 1995) thought to be particularly relevant to cancer: Environmental Mastery, Personal Growth, Positive Relations with Others, and Self-Acceptance. Participants rated their agreement with statements regarding their well-being on a 7-point scale. An 18-item version of the scale was administered at Wave 1, and a 42-item version was administered at Wave 2. The four subscales examined in the present study showed adequate internal consistency in the Wave 1 sample (Cronbach’s α ranged from .54-.66) and good internal consistency at Wave 2 (Cronbach’s α ranged from .75-.84).
Social well-being
This 14-item scale assessed five domains of social well-being: Meaningfulness of Society, Social Integration, Acceptance of Others, Social Contribution, and Social Actualization (Keyes, 1998). Participants were asked to rate their agreement with statements regarding their social well-being using a 7-point scale. Internal consistency was low for the Acceptance of Others subscale (α= .41), which was therefore not used. Reliability was adequate for other subscales (Cronbach’s α ranged from .64-.75).
Spirituality
Four scales assessed domains of religiosity and spirituality. The 6-item Religious Identification scale asked about the extent to which a specific religion was important in participants’ lives (Garfield, Ryff, & Singer, 2001; Rossi, 2001), and the 2-item Spirituality scale similarly asked how important spirituality was in respondents’ lives (Garfield et al., 2001; Rossi, 2001) on 4-point scales from “very” to “not at all.” The 3-item Private Religious Practices scale assessed frequency of religious practices such as prayer and reading religious books on a 6-point scale from “once a day or more” to “never” (Fetzer Institute/National Institute on Aging Working Group, 1999; Koenig, Parkerson, & Meador, 1997). Finally, the 5-item Daily Spiritual Experiences scale assessed the frequency with which participants experienced spiritual states in day-to-day life on a 4-point scale from “often” to “never” (Fetzer Institute/National Institute on Aging Working Group, 1999). The Religious Identification and Spirituality scales were administered at both waves while the Private Religious Practices and Daily Spiritual Experiences scales were administered only at Wave 2. Internal consistency was very good for all measures: Religious Identification (α = .89-.90), Spirituality (α = .82-.92), Private Religious Practices (α = .71), and Daily Spiritual Experiences (α = .89).
Analyses
All variables were examined for outliers. Preliminary analyses examined whether the disease-related variables available were associated with psychosocial outcomes among cancer survivors at Wave 2. Specifically, we determined correlations between number of years since diagnosis and psychosocial outcomes and examined whether outcomes differed among the four most common disease sites: breast, prostate, gynecologic, and colon cancers. All cross-sectional analyses included the full set of 398 cancer survivors and the matched comparison sample (n = 796). Longitudinal analyses examining changes from Wave 1 to Wave 2 focused on the subset of 207 survivors who developed cancer between the two waves of data collection and their matched 414 comparison respondents.
In the initial set of cross-sectional analyses, cancer survivors were compared to the matched comparison group on mental health, mood, psychological well-being, social well-being, and spirituality measures at Wave 2 using two-tailed t-tests. Repeated-measures analysis of variance (ANOVA) was then employed to examine changes in psychosocial variables between Waves 1 and 2 in the subset of survivors who developed cancer between the two waves of data collection and their matched respondents. In ANOVA models, group was entered as the between-subjects variable, wave was specified as the repeated measure, and the cross-product of these variables was entered as an interaction term. When the interaction term was significant, mean values for psychosocial outcome variables at each wave were examined for cancer survivors and the comparison group to determine the nature of the interaction.
Hierarchical multiple regression analyses were used to determine whether group (cancer survivors versus comparison group) interacted with age in predicting psychosocial outcomes at Wave 2. Group and age were entered in the first step of each model, and their cross-product was entered in the second step. If the interaction term was statistically significant, the minimum and maximum ages (ages 34 and 84) were substituted into the regression equation for each group and regression lines were plotted to determine the nature of the interaction (Aiken & West, 1991). Interactions were then examined in a longitudinal context; we determined whether group interacted with age to predict changes in psychosocial variables between Waves 1 and 2 in the subset of survivors who were diagnosed with cancer between the two waves of data collection and comparison respondents. In these analyses, Wave 1 psychosocial variables were entered in the initial step of the model.
Results
Disease Factors and Psychosocial Status in Cancer Survivors
Preliminary analyses showed that disease variables did not significantly influence psychosocial outcomes. Among survivors, there were no significant associations between time since diagnosis and mental health, mood, or psychological well-being variables. Depression was the only outcome that differed by cancer type, F(3, 265) = 2.86, p = .04, η2 = .031. Follow-up contrasts indicated that gynecologic cancer survivors (M = 1.38, SD = 2.45) reported greater depressive symptomatology than prostate cancer survivors (M = 0.41, SD = 1.53), but cancer type was no longer significant when age was included in the model, indicating that differences between these two cancer types may be better accounted for by the older age of prostate cancer survivors and younger age of gynecologic cancer survivors.
Psychosocial Profiles of Cancer Survivors Compared to Respondents Without a Cancer History
As shown in Table 2, cancer survivors reported poorer mental health and mood than the comparison group. Specifically, cancer survivors reported greater anxiety, t(1192) = 2.28, p = .03, η2 = .004, and depressive symptomatology, t(1192) = 3.08, p = .002,η2 = .008. They also reported significantly greater negative affect, t(1006) = 3.46, p > .001,η2 = .012, and less positive affect, t(1006) = -2.90, p = .004, η2 = .008. With respect to psychological well-being, cancer survivors reported less environmental mastery, t(1006) = -2.30, p = .02, η2 = .005, less positive relations with others, t(1006) = -2.44, p = .01, η2 = .006, and less self-acceptance, t(1006) = -3.52, p < .001, η2 = .012. However, cancer survivors did not differ significantly from the comparison sample on personal growth, t(1006) = -1.59, p = .11. Moreover, there were no significant differences between cancer survivors and the comparison sample on any social well-being or spirituality measures (all p values > .10). We repeated comparisons for only the subset of survivors who were diagnosed with cancer after the initial wave of data collection. The effect sizes were similar with an identical pattern of significant results, with the exception that group differences in anxiety were no longer statistically significant.
Table 2.
Psychosocial Profile of the Full Sample of Cancer Survivors at Wave 2 Relative to the Comparison Group
| Cancer Survivors n = 398 |
Comparison Group n = 796 |
|
|---|---|---|
| M and SD | M and SD | |
| Mental health | ||
| Depression** | 0.90 (2.05) | 0.56 (1.67) |
| Anxiety* | 0.40 (1.12) | 0.27 (0.91) |
| Mood | ||
| Negative affect*** | 1.60 (0.67) | 1.46 (0.54) |
| Positive affect** | 3.37 (0.76) | 3.51 (0.67) |
| Psychological well-being | ||
| Environmental mastery* | 37.8 (7.9) | 38.9 (7.3) |
| Personal growth | 37.7 (7.4) | 38.4 (6.8) |
| Positive relations with others** | 40.5 (7.2) | 41.6 (6.6) |
| Self-acceptance*** | 37.0 (8.8) | 38.9 (7.9) |
| Social well-being | ||
| Meaningfulness of society | 8.9 (3.1) | 8.7 (3.2) |
| Social integration | 14.8 (4.2) | 15.1 (4.0) |
| Social contribution | 15.2 (3.8) | 15.3 (3.9) |
| Social actualization | 12.4 (4.0) | 12.3 (4.0) |
| Spirituality/Religiosity | ||
| Religious identification | 20.6 (5.3) | 20.6 (5.1) |
| Spirituality | 6.7 (1.5) | 6.6 (1.5) |
| Private religious practices | 10.7 (4.4) | 10.4 (4.3) |
| Daily spiritual experiences | 16.4 (3.0) | 16.4 (3.0) |
Cancer survivors differ from the comparison group at p < .05.
p < .01
p < .001
Changes in Psychosocial Status from Pre- to Post-Diagnosis
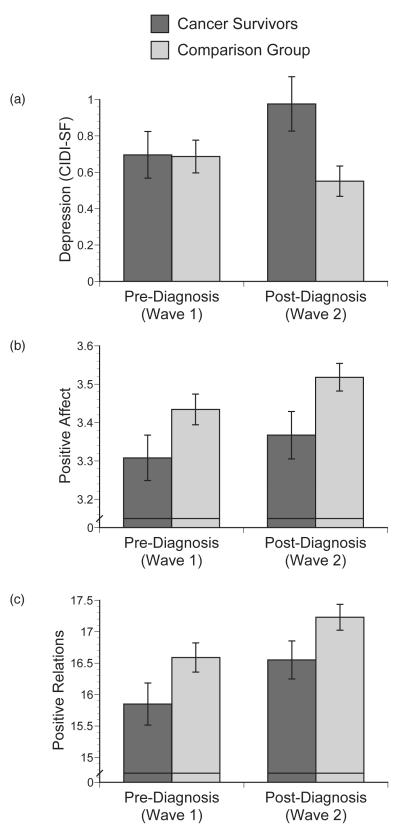
Table 3 provides mean scores for psychosocial measures prior to and following diagnosis among the subset of cancer survivors diagnosed with cancer between the two waves of data collection and their comparison sample. In repeated-measures ANOVA models, group significantly interacted with wave in predicting depressive symptomatology, F(1, 619) = 6.10, p = .01, η2 = .003, and was marginally significant for the model predicting anxiety, F(1, 619) = 3.36, p = .067, η2 = .002. Plots indicated that mental health symptoms increased from Wave 1 to Wave 2 among cancer survivors, but decreased slightly during the same period in the comparison group (see Figure 1a on depression). There were no significant main effects of group or wave on mental health.
Table 3.
Pre -to Post-Diagnosis Change in Mean Psychosocial Variables
| Wave 1: Pre-Diagnosis | Wave 2: Post-Diagnosis | ||||
|---|---|---|---|---|---|
| Cancer Survivors n = 207 |
Comparison Group n = 414 |
Cancer Survivors n = 207 |
Comparison Group n = 414 |
Significant Effects (p < .05) |
|
| Mental health | |||||
| Depression | 0.70 (1.85) |
0.69 (1.82) |
0.98 (2.14) |
0.55 (1.68) |
Group X Wave |
| Anxiety | 0.38 (1.09) |
0.45 (1.26) |
0.43 (1.17) |
0.30 (1.00) |
|
| Mood | |||||
| Negative affect | 1.61 (0.76) |
1.48 (0.62) |
1.60 (0.72) |
1.46 (0.53) |
Group |
| Positive affect | 3.31 (0.76) |
3.43 (0.73) |
3.37 (0.80) |
3.51 (0.64) |
Group Wave |
| Psychological well-being | |||||
| Environmental mastery |
16.1 (3.6) |
16.5 (3.3) |
16.7 (3.5) |
17.1 (3.0) |
Wave |
| Personal growth | 17.2 (3.5) |
17.8 (3.1) |
16.8 (3.3) |
17.1 (3.1) |
Wave |
| Positive relations with others |
15.9 (4.3) |
16.6 (4.2) |
16.6 (3.9) |
17.2 (3.7) |
Group Wave |
| Self-acceptance | 16.2 (3.8) |
16.7 (3.4) |
15.9 (4.1) |
16.6 (3.5) |
|
| Social well-being | |||||
| Meaningfulness of society |
8.8 (3.5) |
8.4 (3.5) |
9.0 (3.3) |
8.5 (3.1) |
|
| Social integration | 13.9 (5.0) |
14.9 (4.2) |
14.5 (4.1) |
14.8 (3.9) |
|
| Social contribution |
15.1 (4.0) |
15.4 (4.0) |
14.9 (3.8) |
15.2 (3.8) |
|
| Social actualization |
12.1 (4.6) |
11.9 (4.4) |
12.7 (4.1) |
12.2 (3.9) |
Wave |
| Spirituality | |||||
| Religious identification |
16.7 (4.5) |
17.6 (4.2) |
20.0 (5.6) |
20.7 (5.1) |
Wave |
| Spirituality | 6.1 (1.6) |
6.3 (1.5) |
6.5 (1.6) |
6.5 (1.5) |
Wave |
Figure 1.
Pre- to post-diagnosis changes psychosocial measures relative to the comparison group: (a) interaction between group and wave predicts depression, F(1, 619) = 6.10, p = .01; (b) main effects of group and wave on positive affect, F(1, 493) = 4.96, p = .03 and F(1, 489) = 4.49, p = .03, respectively; (c) main effects of group and wave on positive relations with others, F(1, 493) = 3.90, p = .049 and F(1, 489) = 18.00, p < .001, respectively.
There was a group main effect on both negative affect, F(1, 493) = 6.50, p = .01, η2 = .010, and positive affect, F(1, 493) = 4.96, p = .03, η2 = .008. Somewhat surprisingly, cancer survivors reported greater negative affect and lower positive affect both prior to and following diagnosis (see Figure 1b on positive affect). There was also a main effect of wave on positive affect, F(1, 489) = 4.49, p = .03,η2 = .002, with both cancer survivors and the comparison group showing increased positive affect over time. There were no interactions between group and wave, indicating that a cancer diagnosis did not change the trajectory of affect over time.
With respect to psychological well-being, there was a group main effect on positive relations with others, F(1, 493) = 3.90, p = .049, η2 = .006, and a marginally significant effect on self-acceptance, F(1, 493) = 3.72, p = .054, η2 = .006. As was the case for affect, cancer survivors reported poorer well-being than the comparison group both prior to and following diagnosis (see Figure 1c on positive relations). There were also strong main effects of wave on three of the four psychological well-being outcomes: environmental mastery F(1, 489) = 14.13, p < .001, η2 = .007, personal growth, F(1, 489) = 18.00, p < .001, η2 = .008, and positive relations with others, F(1, 489) = 15.53, p < .001, η2 = .006. Environmental mastery and positive relations increased significantly for both groups (see Figure 1c), while personal growth decreased in both groups. This pattern did not differ between those with and without cancer.
On social well-being measures, there was a main effect of wave on social actualization, F(1, 489) = 5.38, p = .02, η2 = .003,with scores increasing from Wave 1 to Wave 2 for both cancer survivors and the comparison group. There were no significant interactions between group and wave, nor any main effects of group on social well-being measures.
Finally, there were strong effects of wave on religious identification, F(1, 489) = 486.39, p < .001, η2 = .086, and spirituality, F(1, 489) = 25.47, p < .001, η2 = .008. Both cancer survivors and the comparison group showed increased religious identification and spirituality from Wave 1 to Wave 2. There was also a marginally significant main effect of group on religious identification, F(1, 493) = 4.02, p = .056, η2 = .006, with cancer survivors reporting slightly less strong religious identification. There were no significant interactions between group and wave.
Age and Psychosocial Status
Cross-sectional models
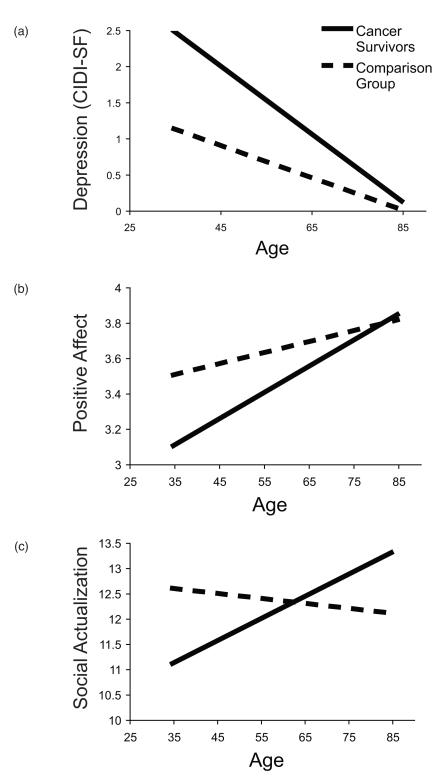
Table 4a provides an example of the hierarchical multiple regression models. The interaction between group and age was significant in models predicting depressive symptomatology, β = -.13, p < .001 (see Table 4a), and anxiety, β= -.09, p = .02. Plots of both outcomes indicated that younger cancer survivors reported higher levels of both depression and anxiety symptoms than their age-matched peers, while older cancer survivors reported low levels of symptoms consistent with others their age (see Figure 2a for depression). The interaction between group and age was also significant in predicting positive affect, β = .08, p = .03, but not negative affect, p = .14, with the pattern showing that younger cancer survivors reported much lower positive affect than those of the same age with no cancer history, while older cancer survivors were similar to their same-age peers (see Figure 2b). Similar patterns were found for positive relations with others, β = .07, p = .07, self-acceptance, β = .07, p = .06, and social integration, β = .07, p = .08, but these findings showed only statistical trends. Finally, there was s significant interaction in the model predicting social actualization, β = .09, p = .02. Younger cancer survivors reported less social actualization than their peers, while older survivors showed greater social actualization than those of the same age with no cancer history (see Figure 1c). There were no significant interactions for any of the models predicting spirituality measures. We repeated this set of analyses restricting the sample to the subset of survivors who developed cancer between the two waves and comparison respondents. While the pattern of effects was similar and effect sizes were somewhat larger for most interactions, the smaller sample size reduced statistical power and the interaction between group and age remained significant only in the model predicting depression, β = -.13, p = .02.
Table 4.
Interactions Between Group and Age in Multiple Regression Models Predicting Symptoms of Depression at Wave 2
| (a) Cross-Sectional Model (n =1194) | ||||
|---|---|---|---|---|
| Variable | R | ΔR2 | β (final model) |
p |
| Step 1 | .226 | .051 | <.001 | |
| Group | .105 | .001 | ||
| Age | -.131 | .001 | ||
| Step 2 | .248 | .011 | <.001 | |
| Group X Age | -.127 | <.001 | ||
| (b) Longitudinal Model (n = 621) | ||||
|---|---|---|---|---|
| Variable | R | ΔR2 | β (final model) |
p |
| Step 1 | .427 | .183 | <.001 | |
| Wave 1 depression | .395 | <.001 | ||
| Step 2 | .468 | .037 | <.001 | |
| Group | .134 | .001 | ||
| Age | -.093 | .06 | ||
| Step 3 | .475 | .006 | <.001 | |
| Group X Age | -.098 | .046 | ||
Figure 2.
Interactions between group and age at Wave 2 predict (a) depression, β = -.13, p = .001, (b) positive affect, β = .08, p = .03, and (c) social actualization, β = .09, p = .02.
Longitudinal models
After adjusting for Wave 1 scores on psychosocial outcomes, the interaction between group and age was significant in models predicting Wave 2 depressive symptomology, β= -.10, p = .046, and anxiety, β= -.12, p = .02. Table 4b provides the regression model for depression. The forms for both interactions were the same as is illustrated in Figure 2a. Interpreting the pattern in a longitudinal context suggests that the increase in psychological symptoms observed pre- to post-diagnosis (see Figure 1a) was most pronounced for the younger cancer survivors, while the older cancer survivors showed similar cross-time trajectories as their same-age peers. There were no significant interactions in models predicting psychological and social well-being or spirituality.
Discussion
Cancer survivors participating in MIDUS exhibited poorer psychological functioning in a variety of domains, including mental health, mood, environmental mastery, and self-acceptance as compared to an age-, gender-, and education-matched sample of respondents with no cancer history. The findings are consistent with an impairment model of survival following the adverse experience of a cancer diagnosis and treatment. However, survivors demonstrated resilient functioning in other domains thought to be linked to posttraumatic growth, including social well-being, spirituality, and personal growth. Longitudinal analyses spanning pre- and post-diagnosis further suggested that while mental health declined after a cancer diagnosis, poorer functioning in other domains existed prior to diagnosis. These findings are summarized in Table 5. Age analyses clarified that psychosocial differences between cancer survivors and individuals without a cancer history and pre- to post-diagnosis declines in mental health are most predominant among younger age groups.
Table 5.
Summary of Findings with Respect to Impairment, Resilience, and Growth/Thriving Models
| Group Differencesa | Pre- to Post- Diagnosis Changeb |
Change Relative to Comparison Groupc |
|
|---|---|---|---|
| Mental health | |||
| Depression | Impairment | Impairment | Impairment |
| Anxiety | Impairment | Impairment | Impairment* |
| Mood | |||
| Negative affect | Impairment | Resilience | Resilience |
| Positive affect | Impairment | Thriving | Resilience |
| Psychological well-being | |||
| Environmental mastery | Impairment | Thriving | Resilience |
| Personal growth | Resilience | Impairment | Resilience |
| Positive relations with others | Impairment | Thriving | Resilience |
| Self-acceptance | Impairment | Resilience | Resilience |
| Social well-being | |||
| Meaningfulness of society | Resilience | Resilience | Resilience |
| Social integration | Resilience | Resilience | Resilience |
| Social contribution | Resilience | Resilience | Resilience |
| Social actualization | Resilience | Thriving | Resilience |
| Spirituality/Religiosity | |||
| Religious identification | Resilience | Thriving | Resilience |
| Spirituality | Resilience | Thriving | Resilience |
| Private religious practices | Resilience | Not available | Not available |
| Daily spiritual experiences | Resilience | Not available | Not available |
Impairment indicates that cancer survivors reported poorer functioning than did the comparison group at Wave 2. Resilience indicates no significant group differences.
Impairment indicates a decline, resilience indicates no change, and thriving indicates improvement in functioning from Wave 1 (pre-diagnosis) to Wave 2 (post-diagnosis).
Group X Wave interactions: Impairment indicates cancer survivors declined in functioning relative to the comparison group from Wave 1 (pre-diagnosis) to Wave 2 (post-diagnosis). Resilience indicates groups showed equivalent decline or improvement over time (although overall group differences were present in some cases).
Group X Wave effects were marginally significant.
Areas of Impaired Functioning
Results suggest that having cancer places individuals at risk for heightened anxiety and depression. Cancer survivors reported greater anxiety and depressive symptomatology relative to a matched comparison group, with longitudinal analyses clarifying that such differences in mental health symptoms emerged after diagnosis. Prior to diagnosis, cancer survivors reported similar levels of anxiety and depression as their peers. After diagnosis, however, depression and anxiety worsened among the cancer survivors, while those without cancer showed improved mental health over the same 9-year period. Results suggest that the experience of cancer may alter typical age-related trajectories of declining anxiety and depression.
This pattern of mental health findings is consistent with a model of impaired functioning following a cancer diagnosis, thereby strengthening findings from previous studies utilizing national survey data or health care registries, albeit with less comprehensive measures of psychological functioning (Arndt, Merx, Stegmaier et al., 2004; Arndt et al., 2005; Baker et al., 2003; Hewitt et al., 2003; Rabin et al., 2007). However, findings stand in contrast to other studies designed to assess the quality of life and psychological well-being of cancer survivors, which often have not shown decrements in emotional well-being and mental health among cancer survivors (Bradley et al., 2006; Dorval et al., 1998; Ganz et al., 1998; Helgeson & Tomich, 2005; Wenzel et al., 2002). This discrepancy may be due to sampling differences, namely, that national health surveys may involve less selection bias (e.g., those with lower educational status) than studies of cancer survivorship. Moreover, participants may respond differently to a distress measure in the context of a cancer survivorship study than in a national survey. Participating in the former may evoke memories of the cancer experience, which in turn, leads participants to rate their current distress lower because they may be comparing it to a time when their distress was elevated. This type of response shift has been implicated in longitudinal assessments of mental health and quality of life among individuals with health problems (Schwartz, Sprangers, Carey, & Reed, 2004; Sprangers & Schwartz, 1999).
Areas of Resilient Functioning and Thriving
The decrements in psychosocial status observed paint only part of the picture, however. Results also indicate that cancer survivors are functioning as well as their peers in several psychosocial domains, including social well-being, spirituality, and personal growth. Moreover, none of the measures of social well-being or spirituality declined following diagnosis. In fact, patients reported greater spirituality and social actualization, a belief that society is improving for oneself and others. However, the comparison group also improved in the same areas, supporting the model of resilience rather than thriving. Previous population-based studies have not captured these areas of resilience among survivors due to the traditional focus on psychological distress. Moreover, this resiliency occurs in domains proposed to be influenced by posttraumatic growth, including measures of social relationships, spirituality, and a direct measure of personal growth.
The current study did not find any domains in which cancer survivors on the whole demonstrated superior functioning to their peers, however. Longitudinal analyses suggested improved positive affect, environmental mastery, and positive relationships with others following diagnosis, in addition to growth in social actualization and spirituality as noted above. However, the non-cancer comparison group showed the same trajectories of improvement, therefore suggesting that such changes may be related to normal aging or external events (e.g., the events of September 11) rather than the experience of cancer per se. The point, however, is that cancer survivors show the same age-related gains.
Pre-Cancer Vulnerability
In addition to the decrements in mental health, cancer survivors at Wave 2 reported greater negative affect, lower positive affect, and poorer psychological well-being on measures of environmental mastery, positive relations with others, and self-acceptance. While these results initially appear to support the impairment model, the longitudinal analyses clarified, rather surprisingly, that cancer survivors differed on most of these measures even before they were diagnosed with cancer. Because the impairment model requires a decline from functioning prior to the life challenge, our findings are thus not entirely consistent with this concept. Instead, results suggest a vulnerability to mood disturbance and poorer well-being among individuals who ultimately go on to develop cancer.
Such findings might suggest that poorer psychosocial functioning plays a causal role in the development of cancer. Evidence for a direct role of psychosocial factors in the initiation of cancer is, however, equivocal at best (Butow et al., 2000; Garssen, 2004; Lutgendorf, Costanzo, & Siegel, 2007). Instead, these factors may have an indirect role in cancer development. Obesity, poor diet, lack of physical activity, and tobacco use are, for example, known risk factors for cancer. Mood disturbance or poorer psychological well-being may promote such risk behaviors, and these health behaviors may also instigate mood disturbance and poorer well-being. Hormonal factors also play a role in certain cancers such as breast and gynecologic cancers and may also be associated with psychological functioning. Thus, future work needs to examine the interplay of health behaviors, biomarkers, and psychosocial morbidity in the etiology of cancer. Finally, it is possible that already existing but undetected malignancies may have adversely influenced psychosocial status at the initial wave of data collection, although this is a less plausible explanation for most respondents given that a median of 5 years had passed between initial participation in the study and cancer diagnosis.
Impact of Aging on Post-Diagnosis Adjustment
We extend previous work by including a comparison group of age-matched peers, thus clarifying whether age patterns in psychosocial functioning are unique to cancer survivors. Results indicated that differences in mental health, mood, and psychological well-being and pre- to post-diagnosis increases in mental health symptoms were most pronounced among younger cancer survivors. Such findings are consistent with the distressing or traumatic nature of “off- time” life events (Neugarten & Hagestad, 1976), presumably due to the lack of rehearsal or anticipation for such challenges as well as the diminished availability of social support from others dealing with similar problems. Younger adults may also have greater demands in the areas of work or parenting and fewer coping resources, which make contending with cancer particularly stressful. In contrast, older cancer survivors were similar to their peers on most measures and in their trajectories over time, and even reported greater social actualization, the sense that the society is becoming a better place for oneself and others.
Limitations and Conclusions
As a group, survivors in the present study were many years past their cancer diagnosis: a median of 10 years post-diagnosis (4 years post-diagnosis for survivors included in longitudinal analyses). Although we did not find links between years since diagnosis and psychosocial outcomes, results may not generalize to a population of more recently diagnosed survivors. Another limitation is the paucity of disease- and treatment-related information on survivors, such as initial disease stage, treatment history, current cancer status (in remission or living with recurrent or chronic disease), or ongoing sequelae. These factors, as well as other sociodemographic and psychosocial differences, likely play an important role in long-term psychosocial functioning, whether impaired, resilient, or thriving, and as such, will be important to examine in future work. Finally, longitudinal analyses focused on a smaller subset of survivors who developed cancer between the two waves of data collection and thus had less statistical power than did the cross-sectional analyses.
Findings of the present study clarify important areas of psychosocial impairment and resilience among cancer survivors. While data do not support ideas of posttraumatic growth following a cancer diagnosis, wherein superior levels of personal growth, social well-being, or spirituality are evident, areas of resilience among survivors are nonetheless evident. Such outcomes are all the more remarkable considering that anxiety and depressive symptoms increase following a cancer diagnosis. In other words, cancer survivors are resilient not only in spite of their cancer, but also in the face of greater mood disturbance and psychiatric symptoms. Further, although cancer survivors report poorer functioning on these distress measures, decrements are primarily found among younger survivors. Older cancer survivors function as well as, and in one instance, better than, their peers, such that older age itself appears to be an important resilience factor in contending with cancer.
A recent report from the Institute of Medicine recommends instituting comprehensive, coordinated care for survivors (Hewitt et al., 2005). Findings from the present study suggest that assessment of and interventions targeting psychological functioning should be part of this care. In addition, it will be important for future work to clarify psychosocial, behavioral, and physiological factors beyond age that confer risk or resilience for the long-term psychosocial adjustment of cancer survivors. Such data will help to determine targets for evidence-based interventions to promote optimal psychological functioning in this growing segment of our population.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01 AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation and a National Research Service Award from the National Institute of Mental Health (T32 MH18931). The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
Footnotes
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/hea/
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Newbury Park: 1991. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed., revised American Psychiatric Association Press; Washington, D.C.: 1987. [Google Scholar]
- Andrykowski MA, Curran SL, Studts JL, Cunningham L, Carpenter JS, McGrath PC, et al. Psychosical adjustment and quality of life in women with breast cancer and benign breast problems: A controlled comparison. Journal of Clinical Epidemiology. 1996;49:827–834. doi: 10.1016/0895-4356(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. Journal of Clinical Oncology. 2004;22:4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Persistence of restriction in quality of life from the first to the third year after diagnosis in women with breast cancer. Journal of Clinical Oncology. 2005;23:4945–4953. doi: 10.1200/JCO.2005.03.475. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. European Journal of Cancer. 2004;40:673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97:674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Aldine; Chicago: 1969. [Google Scholar]
- Bradley SL, Lutgendorf SK, Rose S, Costanzo ES, Anderson B. Quality of life and mental health in cervical and endometrial cancer survivors. Gynecologic Oncology. 2006;100:479–486. doi: 10.1016/j.ygyno.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Butow PN, Hiller JE, Price MA, Thackway SV, Kricker A, Tennant CC. Epidemiological evidence for a relationship between life events, coping style, and personality factors in the development of breast cancer. Journal of Psychosomatic Research. 2000;49:169–181. doi: 10.1016/s0022-3999(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Carver CS. Resilience and thriving: Issues, models, and linkages. Journal of Social Issues. 1998;54:245–266. [Google Scholar]
- Cordova MJ, Andrykowski MA. Responses to cancer diagnosis and treatment: posttraumatic stress and posttraumatic growth. Seminars in Clinical Neuropsychiatry. 2003;8:286–296. [PubMed] [Google Scholar]
- Cordova MJ, Cunningham LLC, Carlson CR, Andrykowski MA. Posttraumatic growth following breast cancer: a controlled comparison study. Health Psychology. 2001;20:176–185. [PubMed] [Google Scholar]
- Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. Journal of Clinical Oncology. 1998;16:487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- Fazio A. Vital and health statistics publication. Government Printing Office; Washington, D.C.: 1977. A concurrent validational study of the NCHS General Well-Being Schedule. (Series 2, No. 73). [PubMed] [Google Scholar]
- Fetzer Institute/National Institute on Aging Working Group . Multidimensional measurement of Religiousness/Spirituality for Use in Health Research: A Report of the Fetzer Institute/National Institute on Aging Working Group. Fetzer Institute; Kalamazoo, MI: 1999. [Google Scholar]
- Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. Journal of Clinical Oncology. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- Garfield AM, Ryff CD, Singer B. Religion and health: Probing the connections. Paper presented at the Poster presented at the 13th Annual Conference of the American Psychological Society.2001. [Google Scholar]
- Garssen B. Psychological factors and cancer development: evidence after 30 years of research. Clinical Psychology Review. 2004;24:315–338. doi: 10.1016/j.cpr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psycho-Oncology. 2005;14:307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Institute of Medicine and National Research Council; Washington, D.C.: 2005. [Google Scholar]
- Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry JL, Bryant RA. Posttraumatic stress disorder following cancer: A conceptual and empirical review. Clinical Psychology Review. 2002;22:499–524. doi: 10.1016/s0272-7358(01)00118-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H-U. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. [Google Scholar]
- Kessler RC, MacGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-II-R psychiatric disorders in the United States. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Keyes CLM. Social well-being. Social Psychology Quarterly. 1998;61:121–140. [Google Scholar]
- Koenig H, Parkerson GR, Meador KG. Religion index for psychiatric research. American Journal of Psychiatry. 1997;154:885–886. doi: 10.1176/ajp.154.6.885b. [DOI] [PubMed] [Google Scholar]
- Koopman C, Butler LD, Classen C, Giese-Davis J, Morrow GR, Westendorf J, et al. Traumatic stress symptoms among women with recently diagnosed primary breast cancer. Journal of Traumatic Stress. 2002;15:277–287. doi: 10.1023/A:1016295610660. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Costanzo ES, Siegel S. Psychosocial influences in oncology: An expanded model of biobehavioral mechanisms. In: Ader R, editor. Psychoneuroimmunology. 4th Ed Elseiver Academic Press; San Diego: 2007. pp. 869–896. [Google Scholar]
- MacMillan AM. The Health Opinion Survey: Techniques for estimating the prevalance of psychoneurotic and related types of disorder in communities. Psychological Reports. 1957;3:325–339. [Google Scholar]
- Manne S, Ostroff J, Winkel G, Goldstein L, Fox K, Grana G. Posttraumatic growth after breast cancer: Patient, partner, and couple perspectives. Psychosomatic Medicine. 2004;66:442–454. doi: 10.1097/01.psy.0000127689.38525.7d. [DOI] [PubMed] [Google Scholar]
- Mor V, Allen S, Malin M. The psychosocial impact of cancer on older versus younger patients and their families. Cancer. 1994;74:2118–2127. doi: 10.1002/1097-0142(19941001)74:7+<2118::aid-cncr2820741720>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Neugarten BL, Hagestad GO. Age and the life course. In: Binstock RE, Shanas E, editors. Handbook of Aging and Social Sciences. Van Nostrand Reinhold; New York: 1976. [Google Scholar]
- O’Leary VE, Ickovics JR. Resilience and thriving in response to challenge: An opportunity for a paradigm shift in womens health. Women’s Health: Research on Gender, Behavior, and Policy. 1995;1:121–142. [PubMed] [Google Scholar]
- President’s Cancer Panel . Assessing Progress, Advancing Change. U.S. Department of Health and Human Services; Washington, D.C.: 20052006. [Google Scholar]
- Rabin C, Rogers ML, Pinto BM, Nash JM, Frierson GM, Trask PC. Effect of personal cancer history and family cancer history on levels of psychological distress. Social Science and Medicine. 2007;64:411–416. doi: 10.1016/j.socscimed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. SEER Cancer Statistics Review, 1975-2003. National Cancer Institute; Bethesda, MD: 2006. [Google Scholar]
- Rossi AS. Caring and doing for others: Social responsibility in the domains of family, work, and community. University of Chicago Press; Chicago: 2001. Developmental Roots of Adult Social Responsibility. [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology. 1989;57:1069–1081. [Google Scholar]
- Ryff CD. Challenges and opportunities at the interface of aging, personality, and well-being. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and Research. 3rd Edition. Guilford Press; New York: (in press) [Google Scholar]
- Ryff CD, Keyes CLM. The structure of psychological well-being revisited. Journal of Personality and Social Psychology. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Sprangers MAG, Carey A, Reed G. Exploring response shift in longitudinal data. Psychology and Health. 2004;19:51–69. [Google Scholar]
- Sears SR, Stanton AL, Danoff-Burg S. The yellow brick road and the emerald city: Benefit finding, postive reappraisal coping, and posttraumatic growth in women with early-stage breast cancer. Health Psychology. 2003;22:487–497. doi: 10.1037/0278-6133.22.5.487. [DOI] [PubMed] [Google Scholar]
- Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Social Science and Medicine. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Taylor JA. A personality scale of manifest anxiety. Journal of Abnormal Psychology. 1953;48:285–290. doi: 10.1037/h0056264. [DOI] [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Donnelly JP, Fowler JM, Habbal R, Taylor TH, Aziz N, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psycho-Oncology. 2002;11:142–153. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Fairclough DL, Brady MJ, Cella D, Garrett KM, Klushman BC, et al. Age-related differences in quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–1774. [PubMed] [Google Scholar]
- Widows MR, Jacobsen PB, Booth-Jones M, Fields KK. Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychology. 2005;24:266–273. doi: 10.1037/0278-6133.24.3.266. [DOI] [PubMed] [Google Scholar]