Abstract
Background:
Among patients with intracerebral hemorrhage (ICH), warfarin use before onset leads to greater mortality. In a retrospective study, we sought to determine whether warfarin use is associated with larger initial hematoma volume, one determinant of mortality after ICH.
Methods:
We identified all patients hospitalized with ICH in the Greater Cincinnati region from January through December 2005. ICH volumes were measured on the first available brain scan by using the abc/2 method. Univariable analyses and a multivariable generalized linear model were used to determine whether international normalized ratio (INR) influenced initial ICH volume after adjusting for other factors, including age, race, sex, antiplatelet use, hemorrhage location, and time from stroke onset to scan.
Results:
There were 258 patients with ICH, including 51 patients taking warfarin. In univariable comparison, when INR was stratified, there was a trend toward a difference in hematoma volume by INR category (INR <1.2, 13.4 mL; INR 1.2–2.0, 9.3 mL; INR 2.1–3.0, 14.0 mL; INR >3.0, 33.2 mL; p = 0.10). In the model, compared with patients with INR <1.2, there was no difference in hematoma size for patients with INR 1.2–2.0 (p = 0.25) or INR 2.1–3.0 (p = 0.36), but patients with INR >3.0 had greater hematoma volume (p = 0.02). Other predictors of larger hematoma size were ICH location (lobar compared with deep cerebral, p = 0.02) and shorter time from stroke onset to scan (p < 0.001).
Conclusion:
Warfarin use was associated with larger initial intracerebral hemorrhage (ICH) volume, but this effect was only observed for INR values >3.0. Larger ICH volume among warfarin users likely accounts for part of the excess mortality in this group.
GLOSSARY
- AAICH
= anticoagulant-associated intracerebral hemorrhage;
- GERFHS
= Genetic and Environmental Risk Factors for Hemorrhagic Stroke;
- HR
= hazard ratio;
- ICH
= intracerebral hemorrhage;
- INR
= international normalized ratio;
- IVH
= intraventricular hemorrhage.
Warfarin use at the onset of intracerebral hemorrhage (ICH) is an independent predictor of mortality among ICH patients.1,2 Understanding the mechanism by which warfarin worsens ICH outcome is important because anticoagulant-associated intracerebral hemorrhage (AAICH) has become more common in the past two decades and now accounts for approximately 20% of all ICH.1,3
Among the factors influencing ICH outcome, hematoma volume at presentation to medical care, intraventricular extension of hemorrhage, and hematoma expansion after presentation are important variables that could be influenced by warfarin use. If hematoma expansion after presentation to medical care is a major cause of morbidity and mortality among AAICH patients, agents that promote rapid hemostasis and prompt reversal of coagulopathy could improve outcome. However, if the majority of excess mortality associated with warfarin use is caused by larger hematomas or more frequent intraventricular hemorrhage (IVH) at presentation, these therapies may be less effective because the window for intervention has passed.
Studies of the effect of warfarin on ICH volume to date have generally been small or referral based and have provided conflicting results.4–8 We sought to determine 1) the predictors of initial hematoma volume among patients with ICH in a large population-based stroke study and 2) the relationship of INR and initial hematoma volume.
METHODS
This article analyzes a group of patients with ICH ascertained as part of the Genetic and Environmental Risk Factors for Hemorrhagic Stroke (GERFHS) study. The methodology of the GERFHS study has been previously described.9 The institutional review board for each participating hospital system approved the GERFHS study.
For this report, we attempted to ascertain all persons aged ≥18 years from the five-county Greater Cincinnati/Northern Kentucky area who were hospitalized with ICH between January 1 and December 31, 2005. Cases were identified by retrospective review of primary and secondary International Classification of Diseases, 9th Revision, codes 430 through 438.9. Study nurses also maintained active surveillance (“hot pursuit”) at several hospitals that treat most ICH in the area by reviewing neurosurgery logs and patient rosters several times each week.9 All potential cases were abstracted by study nurses and reviewed in detail by study physicians. Patients living outside the five counties of interest were excluded from this analysis by zip code of residence. Other exclusion criteria were previous ICH, traumatic ICH, hemorrhagic cerebral infarction, and hemorrhage associated with brain tumor, encephalitis, recent endarterectomy, and thrombolytic treatment of ischemic stroke. Patient demographics and putative risk factors for ICH, including warfarin and antiplatelet drug use before stroke onset, were recorded by chart review. The first available international normalized ratio (INR) value upon medical presentation was recorded. For all patients, the first available CT or MRI scan was reviewed.
Radiographs were reviewed by one of two authors (H.T. or M.L.F.). Hemorrhage volumes were measured by using the abc/2 method.10 IVH was not included in volume calculations. The intraclass correlation for hemorrhage volume was tested for 31 scans and was found to be 0.97 for the difference between the two reviewers. The degree of IVH was documented by using an ordinal scale described by Graeb, where the amount of blood in each ventricle is determined and the scores from each ventricle are summed.11–13 The intraclass correlation for IVH score was again tested for 31 scans and was found to be 0.99 for the difference between the two reviewers.
Hematoma volumes were log transformed to approximate normality. To identify potential predictors of initial hematoma volume, univariable analyses for categorical variables were performed by using t tests or analysis of variance as appropriate. The continuous variables of age and time from stroke onset to first scan were tested with a general linear model. Tested categorical variables are presented in table 1. Patients were both dichotomized as using or not using warfarin and were stratified by INR level (INR <1.2, 1.2–2.0, 2.1–3.0, >3.0).
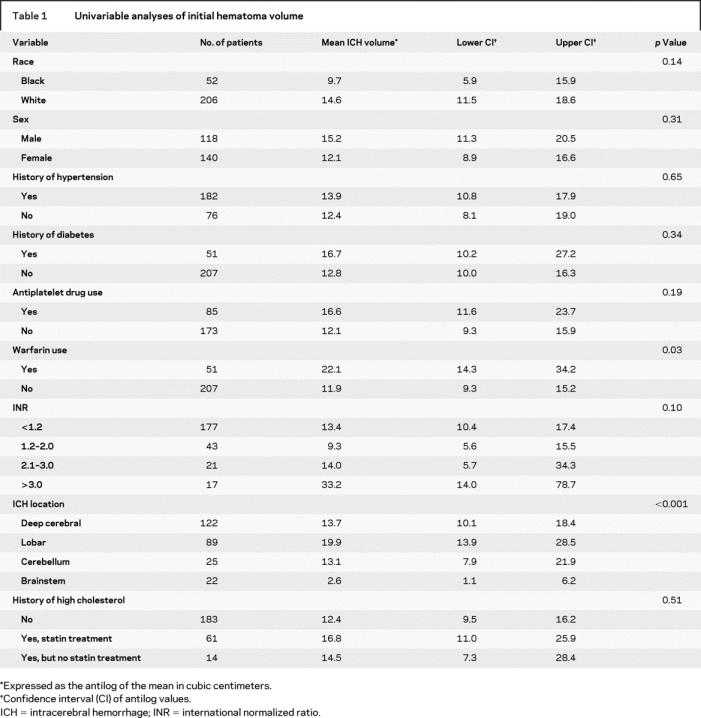
Table 1 Univariable analyses of initial hematoma volume
A multivariable generalized linear model was constructed to determine whether INR influenced ICH volume after adjusting for other factors. Variables from table 1 with p < 0.20 were included in the multivariable model and backward eliminated to a significance level of p <0.10. Age (p = 0.013) and stroke onset to scan time (p = 0.001) were also significant in univariable analysis and were included in the multivariable model. Warfarin use was not included in the multivariable model because of colinearity between the stratified INR values and the dichotomized warfarin variable. The referent values in the multivariable model were deep cerebral location, INR <1.2, and initial scan 2 hours after stroke onset.
The relationship between warfarin use and IVH was tested by using warfarin as a dichotomized variable and also by stratifying INR (<1.2, 1.2–2.0, 2.1–3.0, >3.0). This association was tested by using both IVH as a dichotomized variable (“yes” or “no”) and using the full spectrum of IVH scores via ordinal logistic regression.
To better understand the effect of INR on outcome after ICH, we analyzed the relationships between clinical and radiographic variables and 90-day mortality. Variables tested in univariable analysis were age, race, sex, history of diabetes, history of hypertension, history of heart disease, antiplatelet drug use, presence of IVH, location of hemorrhage, volume of hemorrhage, baseline Glasgow Coma Scale score, and INR stratified as <1.2 (referent), 1.2–2.0, 2.1–3.0, and >3.0. Variables with p <0.20 in univariable analysis were entered into a Cox proportional hazards model, and variables with p > 0.10 were then backward eliminated from this model.
RESULTS
There were 277 patients with ICH between January 1 and December 31, 2005, who met study criteria. After exclusion of 8 patients whose hemorrhages occurred while they were using heparin or low-molecular-weight heparin without concomitant warfarin use, 6 patients with pure IVH, and 5 patients who were not of black or white race, there were 258 patients with ICH (51 taking warfarin) remaining for analysis. Initial imaging was a CT scan for 256 of 258 patients. The mean age was 68.5 years (SD 16.4 years). The mean time from stroke onset to first scan was 14.5 hours (SD 30.6 hours). The median time from stroke onset to first scan was 4.7 hours. There were 22 patients without INR results. Because none of these patients were taking warfarin or other anticoagulants, they were assigned INR values of 1.0. The mean INR was 3.1 (SD 2.0) for warfarin users, compared with 1.1 (SD 0.2) among other patients (p < 0.001). The median INR was 2.7 for warfarin users and 1.0 for other patients. Among warfarin users, 2 (4%) had INR levels <1.2, 14 (27%) had INR values of 1.2–2.0, 18 (35%) had INR values of 2.1–3.0, and 17 (33%) had INR values >3.0. Among patients not documented to be taking warfarin, 175 (85%) had INR values <1.2, 29 (14%) had INR values of 1.2–2.0, 3 (1%) had INR values of 2.1–3.0, and 0 had INR values >3.0. Of all patients, 107 (41%) had some degree of IVH.
Univariable analyses for hematoma volume are presented in table 1. When INR was stratified, there was a trend toward a difference in hematoma volumes by INR category (p = 0.10). When patients were dichotomized according to warfarin use, patients taking warfarin had larger hematomas than those not taking warfarin (p = 0.03). Increasing systolic blood pressure (p = 0.003) and increasing glucose levels (p = 0.005) at presentation were both associated with larger hematoma size in univariable analysis, but these variables were not included in our multivariable model because we were uncertain whether their relationship to hematoma size was cause or effect.
The multivariable generalized linear model is presented in table 2. Compared with patients with INR <1.2, there was no difference in hematoma size for patients with INR 1.2–2.0 (p = 0.25) or INR 2.1–3.0 (p = 0.36), but patients with INR >3.0 had greater hematoma volume (p = 0.02). When patients with INR 1.2–3.0 who were not taking warfarin were excluded from the analysis, the mean hematoma volume in the INR 1.2–3.0 group increased, but results of the multivariable model were similar. As an example of the effect of anticoagulation, the expected hematoma volume of a deep cerebral ICH with INR <1.2 and CT scan 2 hours after stroke onset is 15.33 mL. The expected volume of a deep cerebral ICH with INR >3.0 imaged 2 hours after onset is 15.33 mL × 2.64 = 40.47 mL. The effect of INR in this instance is illustrated in the figure, which shows hemorrhages of 15 and 40 mL.
Table 2 Multivariable generalized linear model of initial hematoma volume
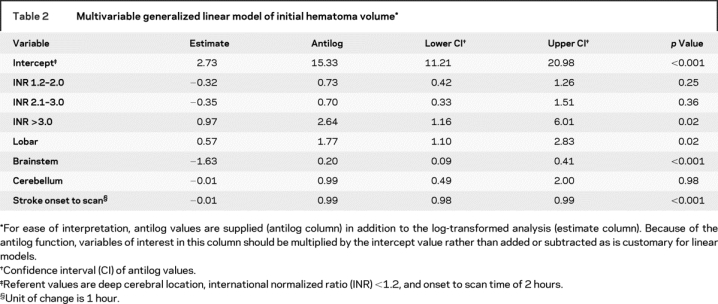
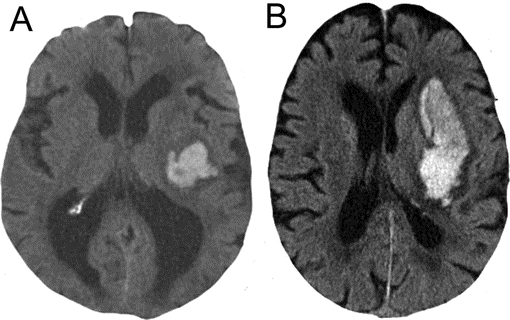
Figure Comparison of 15- and 40-mL intracerebral hematomas
CT scans from 15-mL (A) and 40-mL (B) deep cerebral intracerebral hemorrhages (ICHs). This approximates the difference between an ICH with an international normalized ratio (INR) <1.2 and an ICH with an INR >3.0 as seen in the multivariable generalized linear model.
We also modeled hematoma volume (nontransformed) against the actual INR values and a second-order term (square of INR) to examine whether the relationship between hematoma volume and INR was nonlinear. We used all the other factors that were previously included in the multivariable model and backward eliminated to include only those that had p <0.10. The p values for the linear term and the quadratic term for INR were 0.002 and 0.02. This suggests that the volume of hemorrhage was nonlinearly related to the INR.
Other factors that independently predicted larger hematoma size were lobar location (p = 0.02) and shorter time from stroke onset to scan (p <0.001). Antiplatelet drug use showed a trend toward association with larger hematoma volume in univariable analysis but fell out of multivariable analysis. When forced into the final model, the p value for antiplatelet drug use was 0.36.
In univariable analysis, warfarin users were not more likely to have IVH than other patients (45% vs 41%; p = 0.58). There was no association between warfarin use and degree of IVH as measured by the Graeb scale (p = 0.72), or between stratified INR values and the presence or degree of IVH.
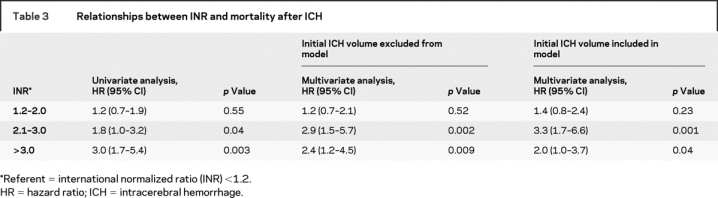
The relationships between INR strata and 90-day mortality after ICH are presented in table 3. In both univariable and multivariable analyses, compared with INR <1.2, initial INR of 1.2–2.0 was not associated with worse outcome, while INR of 2.1–3.0 and INR >3.0 were associated with worse outcome. Multivariable analyses are presented with and without initial hemorrhage volume included in the model. The INR stratum of >3.0 conferred the highest point estimate of risk in univariable analysis. This effect was attenuated in multivariable analyses, especially when volume was included in the model.
Table 3 Relationships between INR and mortality after ICH
DISCUSSION
Warfarin anticoagulation is increasingly relevant to the care of patients with ICH.3 During 2005, nearly 20% of patients with ICH in Greater Cincinnati were taking warfarin, with a much smaller number receiving heparin anticoagulation. Mortality is increased for AAICH patients. In Greater Cincinnati from 1998 to 2003, the 1-day mortality rate among AAICH patients was 33%, compared with 16% for ICH patients without coagulopathy.14 This mortality gap remained constant through 1 year.
Patients with AAICH are on average older and have more medical comorbidities than other ICH patients, factors that likely contribute to worse outcomes.14 In addition to these factors, three important influences on outcome after ICH that may be affected by warfarin use are initial hematoma volume, IVH, and hematoma growth.
Hematoma growth has received considerable attention as a target for therapeutic intervention using ultra-early hemostatic therapy.15,16 Although prospective data are lacking, small retrospective studies suggest that warfarin-associated ICH is more likely to expand and expand for a longer duration than ICH without coagulopathy.8,17 Ultra-early hemostatic therapy and rapid reversal of anticoagulation are therefore attractive targets for improving outcome after AAICH.
Our study found that warfarin anticoagulation with INR values >3.0 was associated with larger initial hematoma volumes. This association and the finding of a nonlinear relationship between hematoma volume and INR suggest there may be a threshold for the effect of INR on hematoma volume. However, interpretations should be tempered by the relatively small numbers of patients in our upper INR strata. Confirmation of our results in another population-based study of ICH patients would be helpful.
Although hemostasis and anticoagulant reversal remain important targets to improve outcome among AAICH patients, our findings indicate that some of the excess mortality among AAICH patients will not be remediable by these strategies. In a recently published multivariable model of ICH outcome among 218 patients without coagulopathy, for each 1.0-mL increase in baseline hematoma volume, the hazard ratio for death increased by 1%.18 When modeling hematoma size in our population, patients with INR >3.0 had initial hematoma volumes approximately 25 mL greater than those of patients without coagulopathy, enough to produce a potent effect on mortality in this group. Subjects with INR of 2.1–3.0 had greater risk of death but did not have larger initial hematomas than subject with lower INR, indicating that hematoma expansion or medical comorbidities likely contribute to mortality for subjects with INR >2.0. Again, because of relatively small numbers in the higher INR strata, replication in an independent, population-based data set would help substantiate these findings.
Intraventricular hemorrhage is an additional factor that leads to poor outcome after ICH. Both IVH at presentation and growth of IVH over time have been reported to negatively affect prognosis.19 We did not find an effect of warfarin on initial presence or severity of IVH, but we cannot exclude the possibility that warfarin produces more IVH growth with time.
Some studies have found antiplatelet drug use to be an independent predictor of death after ICH,20,21 whereas others have not.2,22,23 Our data do not show an association between antiplatelet use and initial hematoma volume. This indicates that an adverse effect of antiplatelet use on survival, if present, is due to another mechanism. The larger size of lobar hemorrhages relative to deep cerebral hemorrhages has been reported. However, deep cerebral ICH is more likely to rupture into the ventricular system than is lobar ICH.24,25 These counterbalancing influences may explain why prognoses after lobar ICH and deep cerebral ICH are similar.1 Finally, patients with larger and more devastating ICH are likely to present to the emergency department more rapidly, explaining the relationship between onset to scan time and initial hematoma volume.
Our study is limited by its retrospective nature. However, it is strengthened by our population-based case ascertainment, which includes patients receiving only one brain scan, patients not referred to tertiary centers, and patients receiving comfort care after diagnosis. We do not have data on the timing of anticoagulant reversal. INR testing was likely undertaken before the initiation of reversal in most cases. Patients who had their warfarin reversed before a blood draw for INR may have partially confounded our results. We did not review all scans subsequent to emergency department presentation for all patients. Therefore, we cannot determine whether warfarin-related hemorrhages expand or rupture into the ventricles more frequently than other hematomas do after initial presentation, nor can we estimate the potential benefit of hemostatic therapy in this setting. Ideally, a prospective study will be undertaken that includes all ICH patients in a defined population and obtains standardized, serial scans over time, allowing comparison of the natural history of ICH with and without anticoagulant use.
AUTHOR CONTRIBITIONS
Statistical analyses were performed by P.S.
Address correspondence and reprint requests to Dr. Matthew L. Flaherty, Department of Neurology, University of Cincinnati Academic Health Center, 260 Stetson St., Room 2316, PO Box 670525, Cincinnati, OH 45267-0525 matthew.flaherty@uc.edu
Supported in part by the National Institute of Neurological Disorder and Stroke (R-01-NS 36695) and a University of Cincinnati College of Medicine Medical Student Summer Research Fellowship.
Disclosure: M.L.F. has received compensation for activities with Novo Nordisk and provided a grand rounds presentation sponsored by an unrestricted educational grant from PhotoThera, Inc. J.P.B. has received compensation for activities with Ono Pharmaceuticals, Novo Nordisk, and Boehringer-Ingelheim. He was a member of the steering committee for trials of activated recombinant factor VII for treatment of acute intracerebral hemorrhage. He has received financial support/grant support from EKOS Corporation, AstraZeneca, and Genentech. B.K. has received honoraria from Boehringer-Ingelheim and Sanofi-Bristol Myers Squibb. D.K. has received honoraria from Boehringer-Ingelheim. The remaining authors have no disclosures.
Received May 13, 2007. Accepted in final form May 23, 2008.
REFERENCES
- 1.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66:1182–1186. [DOI] [PubMed] [Google Scholar]
- 2.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology 2007;68:116–121. [DOI] [PubMed] [Google Scholar]
- 4.Franke CL, de Jonge J, van Swieten JC, Op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke 1990;21:726–730. [DOI] [PubMed] [Google Scholar]
- 5.Berwaerts J, Dijkhuizen RS, Robb OJ, Webster J. Prediction of functional outcome and in-hospital mortality after admission with oral anticoagulant-related intracerebral hemorrhage. Stroke 2000;31:2558–2562. [DOI] [PubMed] [Google Scholar]
- 6.Radberg JA, Olsson JE, Radberg CT. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke 1991;22:571–576. [DOI] [PubMed] [Google Scholar]
- 7.Fogelholm R, Eskola K, Kiminkinen T, Kunnamo I. Anticoagulant treatment as a risk factor for primary intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1992;55:1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty ML, Woo D, Haverbusch M, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke 2005;36:934–937. [DOI] [PubMed] [Google Scholar]
- 10.Kothari RU, Brott TG, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volume. Stroke 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 11.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computer tomographic diagnosis of intraventricular hemorrhage. Neuroradiology 1982;143:91–96. [DOI] [PubMed] [Google Scholar]
- 12.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 1998;29:1352–1357. [DOI] [PubMed] [Google Scholar]
- 13.Zahuranec DB, Gonzales NR, Brown DL, et al. Presentation of intracerebral haemorrhage in a community. J Neurol Neurosurg Psychiatry 2006;77:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty ML, Haverbusch M, Sekar P, et al. Location and outcome of anticoagulant-associated intracerebral hemorrhage. Neurocrit Care 2006;5:197–201. [DOI] [PubMed] [Google Scholar]
- 15.Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke 2003;34:224–229. [DOI] [PubMed] [Google Scholar]
- 16.Mayer SA, Brun NC, Begtrup K, et al., for the Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–785. [DOI] [PubMed] [Google Scholar]
- 17.Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007;38:1257–1262. [DOI] [PubMed] [Google Scholar]
- 18.Davis SM, Broderick J, Hennerici M, et al., for the Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 19.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 2006;59:767–773. [DOI] [PubMed] [Google Scholar]
- 20.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen E-R, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:123–133. [DOI] [PubMed] [Google Scholar]
- 21.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005;252:412–416. [DOI] [PubMed] [Google Scholar]
- 22.Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T, for the Arbeitsgruppe Schlaganfall Hessen. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke 2006;37:2165–2167. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson OG, Lindgren A, Brandt L, Saveland H. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg 2002;97:531–536. [DOI] [PubMed] [Google Scholar]
- 24.Massaro AR, Sacco RL, Mohr JP, et al. Clinical discriminators of lobar and deep hemorrhages: the Stroke Data Bank. Neurology 1991;41:1881–1885. [DOI] [PubMed] [Google Scholar]
- 25.Inagawa T, Ohbayashi N, Takechi A, Shibukawa M, Yahara K. Primary intracerebral hemorrhage in Izumo City, Japan: incidence rates and outcome in relation to the site of hemorrhage. Neurosurgery 2003;53:1283–1298. [DOI] [PubMed] [Google Scholar]


