Abstract
Chronic fluoxetine increases neurogenesis in the dentate gyrus (DG). In view of the widespread clinical use of fluoxetine and the well-established role of the DG in memory, surprisingly few studies have examined the effects of fluoxetine on memory and hippocampal electrophysiology. Additionally, few studies have evaluated the potential for fluoxetine to promote recovery of function after DG damage. Therefore, we studied the effects of long-term administration of fluoxetine on both spatial-reference memory and working memory, recovery of function after intrahippocampal colchicine infusions, which can destroy 50-70% of DG granule cells, and electrophysiological responses in the DG to perforant path stimulation in freely moving rats. Chronic fluoxetine did not affect matching-to-place or reference-memory performance in intact rats in the Morris watermaze task. Surprisingly, in rats with DG damage, recovery of function on both tasks was adversely affected by chronic fluoxetine. Finally, unlike an earlier study that reported fluoxetine-induced increases in hippocampal population spike amplitudes and excitatory postsynaptic potential slopes in urethane-anesthetized rats, electrophysiological measures in DG of freely moving rats were not affected by chronic fluoxetine treatment.
Keywords: antidepressant, dentate gyrus, electrophysiology, fluoxetine, hippocampus, memory, rat, selective serotonin reuptake inhibitor
Introduction
Although antidepressants are prescribed for millions of individuals each year, major gaps still exist in our understanding of how they affect the brain. Researchers have recently learned that long-term exposure to anti-depressant drugs can impact plasticity mechanisms in the hippocampus. Most notably, researchers have recently reported that antidepressants can increase neuron proliferation (neurogenesis) in the dentate gyrus (DG) of the hippocampus (Malberg et al., 2000; Manev et al., 2001, 2003; Nakagawa et al., 2002; Malberg and Duman, 2003; Santarelli et al., 2003). Evidence that antidepressant-induced increases in DG neurogenesis are functionally relevant has emerged from experiments that used genetic and radiological methods for reducing neurogenesis and a rodent model of anxiety and depression (Santarelli et al., 2003). Fluoxetine, desipramine, and imipramine each block the effects of chronic mild stress on novelty-suppressed feeding, cued fear conditioning, and grooming in wild-type mice. The neurogenic and stress-related behavioral effects of antidepressants, however, are not observed in mutant mice that lack 5-HT1A receptors. Additionally, hippocampal X-irradiation, which block DG neurogenesis, also abolish the effects of antidepressants on behavior in stressed mice (Santarelli et al., 2003). Thus, the effects of antidepressants on feeding and grooming seem to require increases in neuron proliferation within the DG.
Whether antidepressants impact the memory-related functions of the hippocampus is not yet known. Numerous reports of suspected fluoxetine-induced memory impairment have, however, been reported in the clinical literature (Mirow, 1991; Bradley and Kulik, 1993; Friedman, 1994; Bangs et al., 1994; Joss et al., 2003; Huang et al., 2004). Unfortunately, the clinical studies cited above were complicated by the fact that the psychiatric disorders for which antidepressants are prescribed can disturb cognitive performance; some researchers have concluded that antidepressants, per se, might not be responsible for the cognitive impairments sometimes reported in clinically depressed populations (Gorenstein et al., 2006). Experimental analyses of the effects of antidepressant exposure on hippocampus-dependent memory tasks are needed to resolve this issue.
In rodents, the DG is essential for normal spatial memory. A well-established procedure for studying rodent spatial memory is the Morris watermaze task (MWT) (Morris, 1984). The MWT involves training rodents to swim to an underwater escape platform in a circular swimming pool. Rats' latencies to find the escape platform decrease systematically over the course of training as they come to use progressively more direct paths to swim to the platform from any starting position in the pool. DG damage impairs the ability of the rats to learn hidden platform locations (Morris et al., 1982; Sutherland et al., 1983, 2001). In this study, we investigated the effects of fluoxetine both on the performance of a well-learned place response (reference memory) and on the rapid acquisition of a new place response (matching-to-place) in the MWT.
Prolonged exposure to elevated glucocorticoids can destroy DG granule cells. Researchers have hypothesized that antidepressants, including fluoxetine, promote recovery of function after stress-induced hippocampal damage by restoring granule-cell density and plasticity in the hippocampus (Garcia, 2002). If fluoxetine does indeed promote the regeneration of the damaged DG, as others (Garcia, 2002) have hypothesized, it is important to evaluate the possibility that fluoxetine might promote recovery of function after severe DG injury. Therefore, we assessed the effects of fluoxetine treatments on reference memory and matching-to-place abilities in rats that had received bilateral DG microinjections of colchicine, a microtubule-binding agent that is particularly toxic to granule cells (Goldschmidt and Steward, 1980, 1982, 1989; Sutherland et al., 1983; Nakayama and Sawada, 2002). For this study, a colchicine dose was selected, based on pilot studies, which showed that it consistently destroyed approximately 50-70% of the DG granule cells while generally sparing the cornu ammonis (CA) fields of the hippocampus; the goal was to preserve enough hippocampal circuitry so that behavioral recovery might be observed postoperatively.
Finally, little is known about how fluoxetine affects DG electrophysiology. In urethane-anesthetized rats, fluoxetine injections increase the slope of the field excitatory postsynaptic potential (fEPSP) recorded in the rat DG in response to a fixed perforant-path stimulus (Stewart and Reid, 2000). The increased fEPSP slope indicates that fluoxetine treatments enhanced the synaptic connections between granule-cell dendrites and perforant-path axons (Erickson et al., 1993). Urethane, the anesthetic drug used in the study discussed above, is, however, known to affect significantly neurotransmitter-receptor systems that are involved in hippocampal neurotransmission, namely N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors, and γ aminobutyric acid subtype A receptors (Hara and Harris, 2002). Therefore, this study examined the effects of chronic fluoxetine administration on perforant-path-evoked extracellular field potentials in rats that had electrodes permanently placed in the DG and the perforant path (PP), the input pathway from entorhinal cortex to the DG.
Methods
Housing and injections
Thirty-nine adult male Long-Evans hooded rats (Canadian Centre for Behavioural Neuroscience vivarium, Lethbridge, Alberta, Canada) initially weighing 200-250 g were used. Animal treatments and maintenance of the colony were in accordance with the United States National Institutes of Health and Canadian Research Council on Animal Care standards. Animals were individually housed (12-h light/dark cycle) with free access to food and water. Fluoxetine hydrochloride (Eli Lilly, Indianapolis, Indiana, USA), diluted in distilled water and obtained as a solution at a concentration of 4 mg/ml, was administered by intraperitoneal injection daily at a dose of 5 mg/kg (injection volume was 1.25 ml/kg). Isotonic (0.9%) sodium chloride solution was administered daily to the control animals at a volume of 1.25 ml/kg.
Spatial learning and memory
The purpose of this experiment was to determine (i) whether chronic exposure to fluoxetine affects spatial navigation in the MWT, a task for which the DG is essential; and (ii) whether fluoxetine promotes the recovery of spatial-navigation abilities in rats with DG damage. The experiment was performed in three phases: preoperative training, intrahippocampal colchicine micro-injections (versus sham surgery), and a 6-week-long regimen of daily injections (fluoxetine versus saline, intraperitoneal), during which daily (Monday to Friday) spatial-navigation testing in the watermaze task took place.
Twenty-one rats received training on the MWT. The MWT testing sessions were conducted in two circular white fiberglass pools (1.5 m diameter, 30.5 cm deep). The pools were filled with 23 cm of water and contained a small white platform (10 cm diameter, 20 cm high) that was submerged 2 cm below the surface of the water (20-22°C), which was made opaque with instant milk. The pools were located in two adjacent rooms that had large, distinctive visual stimuli, which were mounted on the walls and hung from the ceiling. A digital video tracking system (HVS Image, Twickenham, Middlesex, UK) interfaced with microcomputers recorded and stored swimming paths, speed, and trial duration.
Each rat was first trained on a reference memory problem that involved swimming to a hidden escape platform in a fixed position in one of the swimming pools. Individual trials began with a rat being placed in the pool at one of the four cardinal compass positions around the wall of the pool according to a pseudorandom sequence, such that each starting location was used once per session. The maximum duration for an individual swim trial was 90 s. If the rat found the platform within the 90-s period, it was allowed to remain on the platform for 7 s. If the rat failed to find the platform within the 90-s period, it was placed by the experimenter on the platform for 7 s and then placed back into a holding cage for approximately 5 min before starting the next trial. Daily sessions consisted of four trials and were conducted for 2 weeks (Monday to Friday).
When 10 daily sessions of preliminary training in performance setting had been completed, sessions were modified to incorporate training on the matching-to-place task, which was carried out in a second setting. During training on the matching-to-place, the submerged escape platform was moved to a new location in the pool before each session, where it remained for the entire session. The platform location was determined in a quasi-random manner, with the constraint that the new location was not permitted to be within 20 cm of the location used during the earlier session (center-to-center). Thus, each rat first received four swims each day on the reference-memory problem, and then received four swims in matching-to-place (the rooms used for the reference memory and matching-to-place were counterbalanced across the experimental conditions). Rats were trained on both problems 5 days a week (Monday to Friday) for 4 weeks, and highly stable performances on both problems were established in every rat before surgery.
To permit the rats to recover from surgery, no behavioral testing took place during the first postoperative week. During the second postoperative week, daily behavioral testing resumed. Each rat received a daily injection of fluoxetine or saline. On weekdays, all injections were given 1-2 h after the daily behavioral testing was completed; on Saturday and Sunday, they were given at approximately the same time of day as on weekdays. The injection regimen and spatial-navigation testing described above continued for 6 postoperative weeks. At the end of the behavioral study, all rats received one final session that involved swimming to an escape platform that extended 7 cm above the water surface and was covered in black terry cloth. This visible platform training session took place in the environment that was used for matching-to-place training.
Colchicine microinjections into dentate gyrus
All 21 rats that were trained on the MWT (described above) were anesthetized with isofluorane (4% with 2l/ min of oxygen and 2% after a surgical plane was established). A midline incision was made in the scalp and periosteum. Six 0.5-mm diameter holes (three overlying each hemisphere) were drilled in the skull overlying the target tissue. The stereotaxic coordinates of the holes, relative to bregma, were A-P, 3.3, 4.8, and 5.8 mm, and lateral, 1.5, 3.2, and 5.0 mm. A 30-gauge injection needle was stereotaxically inserted through each hole to the target depth (3.7, 4.2, and 7.5 mm). Microinjection of a solution containing colchicine (2 mg/ ml) and 0.1 M phosphate-buffered saline (PBS) was administered at each of three sites in the hippocampus. An infusion of 0.5 μl of solution was injected at each site at a rate of 0.125 μl/min. The injection needles remained in place for 4 min after each injection, to allow diffusion of the colchicine solution away from the needle tip. The needles were removed and the scalp closed with 9-mm autoclips. Rats were monitored until they fully recovered from anesthesia.
Six weeks postoperatively, when behavioral testing was complete, rats received an overdose of pentobarbitol (120 mg/kg) and were perfused transcardially with 200 ml of PBS, followed by an equal volume of 4% paraformalde-hyde. Brains were extracted and stored in 4% paraformaldehyde solution for 24 h. The brains were then transferred to 30% sucrose in PBS for at least 4 days before sectioning. A frozen sliding microtome was used to cut 50-μm coronal sections through the entire extent of the hippocampus. Every fifth section was mounted on a slide, stained with cresyl violet, and DG lesions were evaluated.
Electrophysiology
Eighteen rats were anesthetized with isoflurane (O2 flow rate 1.5 l/min at 1.5-2% isoflurane) for chronic electrode implantation. Electrode implantations were unilateral and placed in the right cerebral hemisphere. The stimulating and recording electrodes were made of a single strand of Teflon-coated stainless-steel wire (114 mm outer diameter). Wires having a larger diameter (178 mm), soldered to stainless-steel screws, served as the ground and indifferent electrodes, which were screwed into the frontal and occipital bones, respectively. Burr holes were made at 3.5 mm posterior to bregma and 1.8 mm lateral to the midline for the recording electrode, and 8.1 mm posterior to the bregma and 4.3 mm lateral to the midline for the stimulating electrode. The dura was punctured with a sharp needle, and the recording electrode was positioned in the hilus of the DG by monitoring multiple-unit discharges as it was lowered through the neocortical and hippocampal layers. The stimulating electrode was lowered into the PP while stimulating at a rate of 0.05 Hz. The recording electrode was further adjusted to maximize the response. Proper placements of the recording and stimulating electrodes produce an unmistakable signature waveform in the evoked response (McNaughton et al., 1986; Sutherland et al., 1997). Gold Amphenol (Wallingford, Connecticut, USA) connector pins attached to the electrodes were arranged and fixed in a stable position using dental acrylic, and anchored by jeweler's screws that were tapped into the skull. The skin incision was closed with surgical glue and the rats received injections of penicillin G (60 000 IU, intramuscular) and buprenorphine (0.10 mg/kg).
Input/output recordings
One week postoperatively, a 6-week injection regimen was initiated and electrophysiology recording sessions were carried out twice weekly. During the recording sessions, the rats were free to move about in a clear polycarbonate cage that was 35.5 cm long, 23.2 cm wide, and 19.8 cm high. For each recording session, a rat was transported from the vivarium in its home cage to the recording room, immediately plugged into the stimulating and recording apparatus, and habituated to the recording situation for 20 min. Stimuli were delivered at the rate of 0.05 Hz and monitored during the habituation period.
Constant voltage stimulus pulses (AMPI Master 8 pulse former and an Isoflex optically isolated constant current stimulator, AMPI, Jerusalem, Israel) were delivered to the PP through a stimulator that was connected to the implanted perforant-path electrode through the gold Amphenol connector pin (pulse duration = 100 μs, rate = 0.05 Hz). The output of the stimulator was controlled by a Pentium III microcomputer running DataWave (version 3.3) software (DataWave Technologies, Longmont, Colorado, USA). Granule-cell population responses were amplified by a Grass (model P15D) (Grass Technologies, West Warwick, Rhode Island, USA) differential preamplifier, filtered (1/2 amplitude low frequency - 1 Hz; 1/2 amplitude high frequency = 10 kHz), and amplified by a Neurolog filter (Digi-timer Limited, Welwyn Garden City, UK) and amplifier (total amplification was × 200). Waveforms were displayed on a Nicolet digital storage oscilloscope (Tektronix, Inc, Beaverton, Oregon, USA). The output was sampled online by the computer and stored on the hard drive for offline analysis. All recordings occurred between 13 : 00 and 18 : 00 h.
Following habituation, input/output (I/O) recordings were sampled at five different perforant-path stimulus intensities (100, 200, 300, 400, and 500 μA), always in an ascending series. For each of the stimulus-intensity series, 20 single perforant-path stimuli were delivered at a rate of 0.05 Hz. The field fEPSP component of the response was measured as the amplitude in millivolts between two points on the rising phase of the response. The amplitude, in millivolts, of the field population spike (PS) was measured from the negative peak of the waveform to a tangent fitted between the onset and offset of the spike (McNaughton et al., 1986).
A mixed-factor experimental design was used to evaluate the effects of chronic fluoxetine on evoked field potentials. The between-group factor was drug treatment (fluoxetine versus saline) and the repeated-measures factor was week (baseline through week 6 of the injection regimen). Stimulus intensity was a repeated-measures factor and was nested within week. I/O functions were obtained from each rat twice each week (i.e. Monday/ Wednesday or Tuesday/Thursday schedule), and the data from the two sessions for each individual rat were averaged before being entered into the analysis. Group assignments were based on the data from the two baseline recording sessions, such that the groups were closely matched in terms of the means and standard deviations of the groups' fEPSPs and PSs. The experimenter responsible for all electrophysiological recordings was blind with respect to the rats' experimental group assignments.
All statistical analyses reported below were conducted with the Statistical Package for the Social Sciences (SPSS 11 for Mac; SPSS, Chicago, Illinois, USA).
Results
Spatial learning and recovery from dentate gyrus damage
During the 4 weeks of preoperative training, all rats mastered both the reference-memory and matching-to-place versions of the MWT. Figure 1 shows the mean swim-path lengths based on the performances of all the rats, averaged over the final three preoperative training sessions. During the terminal sessions of preoperative training, the swimming paths on the reference-memory problem were consistently short in every trial. Therefore, the rats learned to use relatively direct routes to swim to the platform during the reference-memory problem. On the matching-to-place problem, path lengths on the first trial were relatively long; this is to be expected, given that the escape platform was moved to a new location for each session. Systematic within-session decreases in swim-path lengths were apparent and, by the fourth trial, path lengths were as short as those obtained on the fixed-platform problem. Thus, rats rapidly learned the new escape-platform location during the training on the matching-to-place problem. A repeated-measures analysis of variance (ANOVA) revealed significant main effects of task [F(1,64) = 135.55, P < 0.001] and trial [F(3,192) = 40.78, P < 0.001], and a significant task × trial interaction [F(3,192) = 6.81, P < 0.001]. Posthoc Tukey's `Honestly Significantly Different' (HSD) tests revealed that the swim-path lengths on the first trial of the repeated-acquisitions problem were significantly longer than they were on the other three trials.
Fig. 1.
Mean baseline escape latencies in the matching-to-place (white) and reference-memory (black) settings during the final three presurgery, baseline training sessions. Bars are standard error of the mean (SEM). Where bars are not apparent, they are smaller than the symbol that represents the group mean.
Figure 2 shows representative cresyl-violet-stained coronal sections from a sham-surgery control rat and from a colchicine-lesion rat. The colchicine-induced damage encompassed virtually all the granule cells in the dorsal hippocampus [anteroposterior (AP) - 3.3 mm]. At AP - 4.3 mm, in the colchicine-lesion rats, only about 10% of the granule cells remained. In the ventral hippocampus (AP - 5.8 mm), although the granule-cell layer was visibly thinner than in sham rats, for all the rats in the colchicine group it was clear that many granule cells had been spared. The CA fields had generally been spared and appeared intact.
Fig. 2.
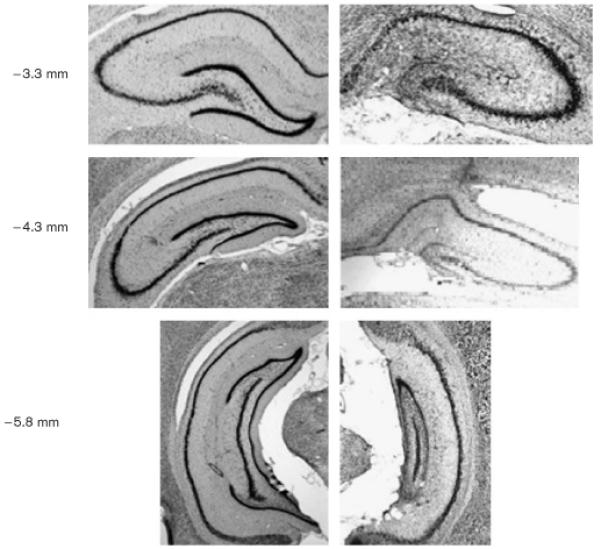
Representative coronal sections showing intact hippocampus from a rat in the sham-surgery group (left) and one that received colchicine (right) at three different anterior-posterior coordinates relative to bregma. Damage typically included extensive bilateral loss of dentate gyrus granule cells and relatively little damage to the CA3 and CA1 subfields.
Figure 3 shows the swim-path lengths during testing on the reference-memory task as a function of surgery (sham controls versus hippocampal), injection condition (saline versus fluoxetine), and week. The datapoints that represent preoperative baseline performances (time 0 in Figs 3 and 4) are each based on data from the final 3 test days before surgery. The datapoints shown for weeks 1-6 represent data averaged across five daily test sessions (Monday to Friday). Sham surgery had no effect on performance in either task. Moreover, fluoxetine did not affect the performance on either task by the sham-surgery rats (Figs 3 and 4).
Fig. 3.
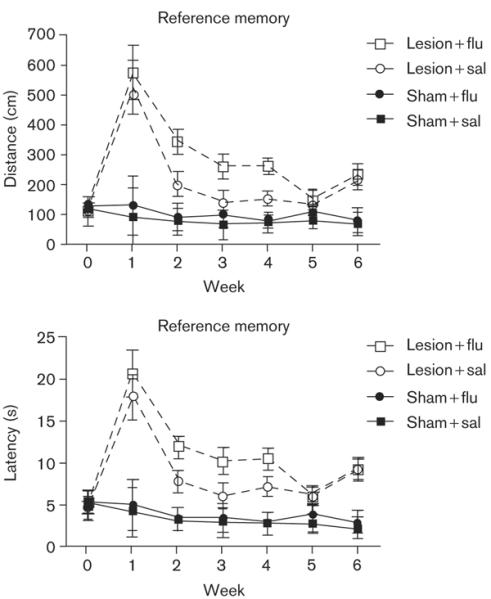
Reference-memory Morris watermaze task. Mean swimming distance (top) and escape latency (bottom) as a function of week of postoperative training (horizontal axis) and group. The datapoints shown for week 0 represent means from the final three preoperative sessions. Each datapoint for weeks 1-6 of postoperative testing represents the mean based on five daily test sessions. Bars are SEM.
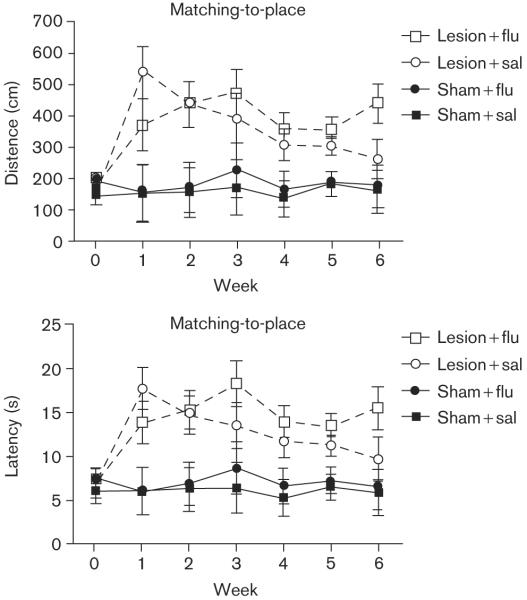
Fig. 4.
Matching-to-place Morris watermaze task. Mean swimming distance (top) and escape latency (bottom) as a function of week of postoperative training (horizontal axis) and group. The datapoints shown for week 0 represent the means from the final three preoperative sessions. Each datapoint for weeks 1-6 of postoperative testing represents the mean based on five daily test sessions. Bars are SEM.
Rats that received colchicine microinjections into DG demonstrated severe behavioral impairments postoperatively on the reference-memory task (Fig. 3). During postoperative weeks 2-6, performance by the rats with DG damage showed significant improvement relative to postoperative week 1. Interestingly, during weeks 2-4, DG damaged rats that received daily saline injections performed better on the reference-memory task than those that received fluoxetine. A mixed-factor ANOVA yielded a significant main effect of surgery condition (DG damage versus sham), [F(1,18) = 52.15, P < 0.001], and a marginally significant surgery × drug (saline versus fluoxetine) interaction, [F(1,18) = 3.44, P < 0.08]. Posthoc Tukey's HSD tests revealed that the sham-surgery group had significantly shorter paths than the DG-damage group (P < 0.05), at each postoperative point shown in Fig. 3. Additionally, planned comparisons revealed that on the reference-memory problem, the DG-lesion saline group located the platform using significantly shorter swimming paths than the DG-lesion fluoxetine group did, during weeks 2-4 [P < 0.05]. Virtually identical results were observed for escape latency as for path length (bottom panel of Fig. 3).
Figure 4 shows the results from the matching-to-place task. As mentioned earlier, in the matching-to-place task, long escape paths were typical during the first trial, because the rats had not yet had an opportunity to learn the new escape-platform location. Thus, Fig. 4 does not include data from the first trial of the matching-to-place task. As was the case in the reference-memory task, the rats in the sham-surgery group traveled consistently shorter distances to find the escape platform. DG damage severely disrupted the abilities of the rats to remember new platform locations. Additionally, it is apparent from the figure that the saline-treated, DG-damage group steadily improved on the repeated-acquisitions task over the course of 6 postoperative weeks; although they remained impaired, relative to controls with intact DG. Fluoxetine-treated, DG-damaged rats, however, showed no evidence of improvement on the matching-to-place task during the postoperative phase of the experiment. A mixed-factor ANOVA revealed significant main effects of surgery [F(6,18) = 41.87, P < .001] and week [F(6,108) = 5.37, P < .001], and week × surgery interaction [F(6,108)=4.40, P < .001]. Posthoc Tukey's HSD tests confirmed that rats in the sham-surgery group had significantly shorter path lengths than rats in the DG-damage group at every postoperative time point. Again, escape latencies (bottom panel) closely paralleled the pattern observed with path lengths.
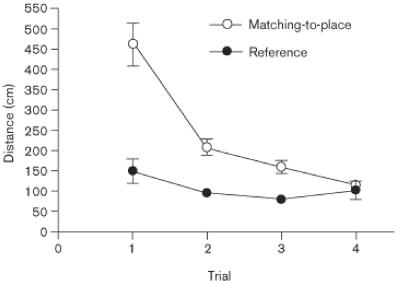
A detailed examination of the data from the matching-to-place task during week 6 is informative. Figure 5 shows average path lengths as a function of trial. First, rats in the sham-surgery group learned the new location more rapidly than DG-damaged rats, swimming minimal distances to reach the hidden escape platform by the second trial. Second, DG-damaged rats that received daily saline injections performed equally well as the sham-surgery control rats by the third trial. Finally, rats with DG damage that received fluoxetine daily failed to learn new platform locations. A mixed-factor ANOVA revealed a significant main effect of trial [F(3,300) = 21.20, P < .001], and a significant trial × drug interaction [F(3,300) = 2.69, P < .05]. Posthoc Tukey's HSD analysis confirmed that the DG-damaged rats that received saline differed from the sham-surgery groups only on trial 2, whereas the DG-damaged rats that received fluoxetine had significantly longer swimming paths than all the other groups in trials 2-4 [P < 0.05].
Fig. 5.
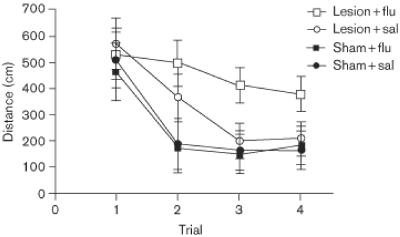
Mean escape latencies from the sixth postoperative week in a trial-by-trial format. Each datapoint represents a mean based on five sessions. Bars are SEM. Where bars are not apparent, they are smaller than the symbol that represents the group mean.
Figure 6 shows the average swimming speed for each group. It is clear that there were no group differences in the swimming speeds [all P values not significant (NS)]. Figure 7 shows average escape latencies for the rats that were tested on the visible-platform task. Rats in all the groups quickly reached the escape platform when the platform was visible, and no group differences were apparent (all P values NS).
Fig. 6.
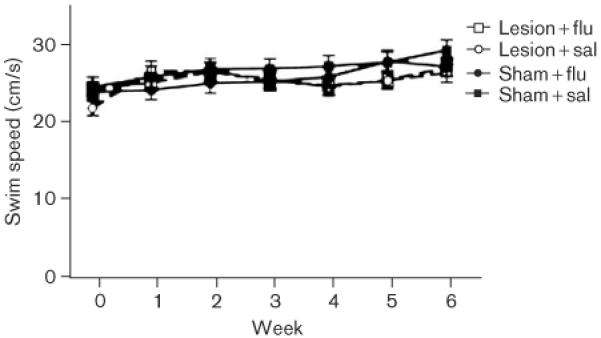
Swimming speed: mean swimming speed as a function of week of postoperative training (horizontal axis) and group. The datapoints shown for week 0 represent means from the final three preoperative sessions. Each datapoint for weeks 1-6 of postoperative testing represents the mean based on five daily test sessions. Bars are SEM.
Fig. 7.
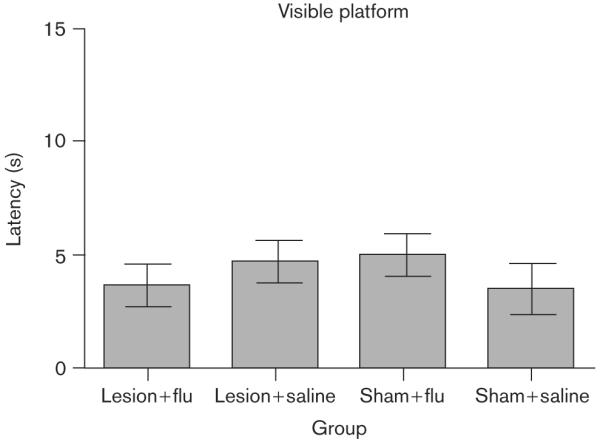
Mean escape latencies during training on the visible-platform task as a function of group (horizontal axis). Bars are SEM.
Fluoxetine effects on neurotransmission in dentate gyrus
At the end of the 7-week chronic electrophysiological recording protocol, 12 of the 18 rats originally implanted for the electrophysiology study (six per group) still produced high-quality evoked field potentials. Only the data from the rats that had high-quality evoked field potentials throughout the entire protocol were included in the analysis of the electrophysiology results; however, we wish to note that the two experimental groups did not differ from one another in this respect. Individual I/O curves for each week are presented in Fig. 8 (PS) and Fig. 9 (fEPSP). The individual panels in each figure present data averaged across two of the recording sessions that were conducted each week. It is clear from the figures that PS amplitude and slope of the fEPSP increased systematically as a function of PP stimulus intensity. It is also apparent that the evoked potentials of the two groups were closely matched when the experiment began. Finally, the PS and fEPSP were not significantly (P > 0.05) affected by fluoxetine after 6 weeks of drug administration, although a nonsignificant trend toward reduced PS amplitudes and fEPSP slopes is apparent in Figs 8 and 9.
Fig. 8.
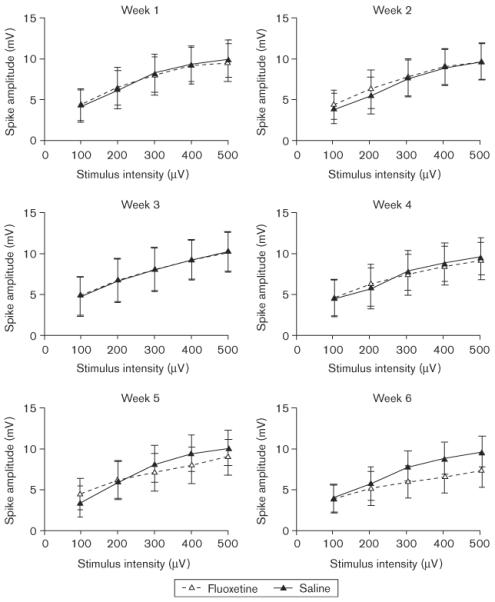
Electrophysiology input/output curves for population spike amplitudes as a function of perforant-path stimulus intensity (horizontal axis) and group. Electrophysiology recording sessions were conducted twice a week and each panel shows the population spike amplitudes averaged across both weekly sessions.
Fig. 9.
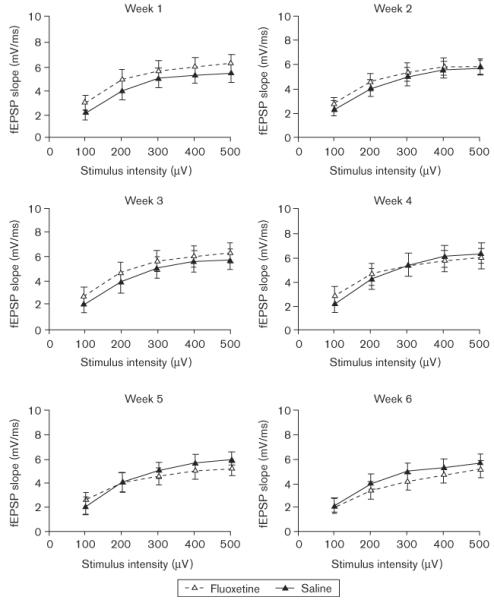
Electrophysiology input/output curves for field excitatory postsynaptic potentials as a function of perforant-path stimulus intensity (horizontal axis) and group. Electrophysiology recording sessions were conducted twice a week and each panel shows the population spike amplitudes averaged across both weekly sessions.
Discussion
The aims of the experiments presented here were to determine whether fluoxetine affected the memory processes for which the DG was essential, promoted recovery of function in animals with DG damage, and affected DG neurotransmission in nonanesthetized animals. Fluoxetine had no effect on the reference-memory or matching-to-place performances in the MWT, in rats with an intact DG. The spatial-learning procedure used for this study was one that firmly established a high degree of skill at the MWT in all the animals, before initiating the chronic fluoxetine administration regimen. Therefore, there was little scope for the observation of further improvement that could have been produced by fluoxetine. In contrast, the procedure used here was very well suited for detecting drug-induced disturbances, either in reference memory or in matching-to-place memory; earlier work by our laboratory has demonstrated that very subtle changes from steady-state baseline are more readily detected using the present procedure than by between-group procedures (Galizio et al., 2003; Keith et al., 2003; Padlubnaya et al., 2005). Numerous studies based on clinical populations have reported that fluoxetine can cause memory disturbances in depressed patients (Mirow, 1991; Bradley and Kulik, 1993; Friedman, 1994; Bangs et al., 1994; Joss et al., 2003; Huang et al., 2004). Chronic fluoxetine administration, however, disrupted neither the reference memory nor the matching-to-place memory. Our data support the conclusion that, in intact rats, chronic fluoxetine administration does not impair spatial learning or memory.
The current study also addresses the issue of whether the DG is equivalently involved in reference and matching-to-place learning in the MWT. Although the adaptation of the MWT that was used in this study has also been used in earlier studies that examined the pharmacology of spatial learning, the effects of DG damage under this particular procedure had not been reported previously (Galizio et al., 2003; Keith et al., 2003; Padlubnaya et al., 2005). Colchicine microinjections into the DG profoundly impaired the reference-memory and matching-to-place performances during the early postoperative period, confirming that the DG is essential for the adaptation of the MWT procedure used here. Although improvement was evident over the 6 weeks of postoperative training, the rats with DG lesions remained impaired, relative to rats with an intact DG throughout that period. The impairments that were observed in the animals with DG damage were not due to poor swimming ability (speed of swimming was not affected by surgery), or general disruptions of sensorimotor factors, or motivation to escape from the water, as evidenced by the normal performances on the visible escape-platform task by rats with DG damage. The finding that relatively small hippocampal lesions, which were restricted largely to the DG, could cause enduring retrograde and anterograde amnesias in a spatial-learning task (characteristic of more complete hippocampal lesions) is noteworthy (Sutherland et al., 2001). Clearly, an intact DG is necessary for both retention and acquisition of new spatial information.
The hypothesis that fluoxetine promotes DG repair, however, was not supported by our results. Environmental enrichment and exercise, both factors that impact the structure and physiology of the hippocampus, have been reported to improve spatial navigation in rats after ischemic or neurotoxic brain lesions (Risedal et al., 2002; Dahlqvist et al., 2003; Will et al., 2004; Gobbo and O'Mara, 2005). Conversely, in this study, rats that received fluoxetine demonstrated poorer postoperative spatial-navigation performance both on the reference-memory and matching-to-place tasks, relative to DG-damaged rats that received saline. A limitation of this study is that we did not evaluate the effects of fluoxetine treatment on neurogenesis. Thus, our results did not permit a direct evaluation of the relationship between DG neurogenesis and recovery of function after DG damage. These findings might, nevertheless, have some relevance to the clinical use of fluoxetine in the treatment of poststroke depression. The findings suggest that further research assessing possible adverse cognitive effects of fluoxetine in patients with gross hippocampal damage is warranted.
In view of the numerous pilot studies that assessed many different colchicine injection doses, we have found that the colchicine dose used in this study creates the smallest DG lesions that reliably impair spatial learning and memory. The extent of DG damage induced by colchicine injections in this study was, nevertheless, far greater than what one would expect to observe from exposure to high glucocorticoid levels. Thus, the lesion method used in this study was not an ideal test of the hypothesis that more subtle DG damage, such as that caused by chronically elevated glucocorticoids, can be reversed by fluoxetine. As an alternative to colchicine injections, in a recent study, we used adrenalectomy (ADX) to produce selective DG granule-cell loss (Spanswick et al., 2007). Bilateral ADX reduced the average thickness of the DG granule-cell layer by approximately 62%; it significantly impaired spatial learning and memory on the same behavioral tests as were used in this study. As in this study, chronic fluoxetine treatment did not reverse the spatial-learning impairment caused by ADX-induced granule-cell loss, even though a substantial population of granule cells had been spared by the ADX.
Daily fluoxetine (5 mg/kg) injections did not affect perforant-path→DG-evoked fEPSPs or PSs, when measured in nonanesthetized, freely moving rats. Other researchers have reported that chronic exposure to fluoxetine can increase both the slope of the fEPSP and the amplitude of the PS (Stewart and Reid, 2000). The methodological details of this study differed from previously published work in three key aspects: fluoxetine dose, duration of the dosing protocol, and state of animals when the electrophysiological measurements were collected. First, in the earlier study, fluoxetine (1 mg/kg) was administered daily for 15 days; whereas, in this study, the fluoxetine (5 mg/kg) administration protocol lasted for 6 weeks. Second, earlier researches on the effects of fluoxetine on in-vivo hippocampal electrophysiology were carried out in a single recording session when the rats were still under deep urethane anesthesia and undergoing surgery at the time of data collection (Stewart and Reid, 2000). In this study, electrophysiological measurements were made twice a week for 6 weeks in nonanesthetized freely moving rats.
Given the fact that chronic fluoxetine exposure has been reported to increase DG neurogenesis, the failure to observe significant effects of long-term exposure to fluoxetine on DG electrophysiology was surprising. Evidence from in-vitro studies of newborn neurons in the subgranular zone of the DG in hippocampal slices suggests that the addition of large numbers of immature neurons can alter the overall electrophysiology of the DG. For example, whole-cell patch-clamping studies in hippocampal slices have revealed that immature DG granule cells display membrane properties that differ from those observed in mature granule cells. Compared with mature granule cells, immature granule cells have slower membrane time constants, higher input resistance, more depolarized resting membrane potentials, generate action potentials with smaller peak amplitudes, and can generate isolated Ca2+ spikes that boost fast Na+ action potentials (Overstreet et al., 2004; Schmidt-Hieber et al., 2004). These properties of immature neurons might be expected to increase the fEPSP and PS in a DG that contained significantly larger than normal numbers of new neurons; such electrophysiological effects were not observed in this study.
The neuropharmacological properties of fluoxetine are, however, complex; one can identify evidence from the literature to support a prediction that chronic exposure to fluoxetine would decrease the fEPSP and PS. For example, fluoxetine increases the 5-HT1A receptor sensitivity in the DG (Elena Castro et al., 2003). That 5-HT1A receptor activation causes neuronal hyperpolarization through G-protein coupled opening of K+ channels has been established well (Nicoll et al., 1990). Ultrastructural localization studies have reported evidence that 5-HT1A receptors are present both somatically and on the dendrites of glutamatergic hippocampal neurons (Kia et al., 1996). Collectively, fluoxetine-induced increases in extracellular 5-HT and the sensitization of 5-HT1A receptors would be expected to increase the 5-HT1A receptor-mediated tonic hyperpolarization in granule cells. Again, however, no evidence was found that such fluoxetine-induced changes detectably influence either the fEPSP or PS responses to perforant-path inputs. The multiple opposing neuropharmacological properties of chronic fluoxetine treatments, such as increases in the population of immature neurons in the DG and changes in receptor sensitivities, might, possibly, counteract one another.
Conclusion
In summary, spatial reference and matching-to-place memory were not affected by fluoxetine. Fluoxetine treatments did adversely affect recovery of function in rats with DG lesions, indicating that rather than generally promoting DG repair, at least under some circumstances, fluoxetine can exacerbate the cognitive effects of hippocampus injury. Finally, unlike the results observed using anesthetized rats, recordings of chronic DG electrophysiology over a 6-week fluoxetine regimen did not reveal an effect of fluoxetine in awake, freely moving rats.
Acknowledgements
This study was supported by NIMH MH06715601A1 (J.R.K) and NIMH MH061460 and AHFMR (R.J.S).
References
- Bangs ME, Petti TA, Janus MD. Fluoxetine-induced memory impairment in an adolescent. J Am Acad Child Adolesc Psychiatry. 1994;33:1303–1306. doi: 10.1097/00004583-199411000-00012. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Kulik L. Fluoxetine and memory impairment. J Am Acad Child Adolesc Psychiatry. 1993;32:1078–1079. doi: 10.1097/00004583-199309000-00034. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Ronnback A, Risedal A, Nergardh R, Johansson IM, Seckl JR, et al. Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats. Neuroscience. 2003;119:643–652. doi: 10.1016/s0306-4522(03)00195-7. [DOI] [PubMed] [Google Scholar]
- Elena Castro M, Diaz A, del Olmo E, Pazos A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology. 2003;44:93–101. doi: 10.1016/s0028-3908(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Erickson CA, McNaughton BL, Barnes CA. Comparison of long-term enhancement and short-term exploratory modulation of perforant path synaptic transmission. Brain Res. 1993;615:275–280. doi: 10.1016/0006-8993(93)90038-o. [DOI] [PubMed] [Google Scholar]
- Friedman EH. Fluoxetine and memory impairment. J Am Acad Child Adolesc Psychiatry. 1994;33:763. doi: 10.1097/00004583-199406000-00029. [DOI] [PubMed] [Google Scholar]
- Galizio M, Keith JR, Mansfield WJ, Pitts RC. Repeated spatial acquisition: effects of NMDA antagonists and morphine. Exp Clin Psychopharmacol. 2003;11:79–90. doi: 10.1037//1064-1297.11.1.79. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, metaplasticity, and antidepressants. Curr Mol Med. 2002;2:629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RB, Steward O. Preferential neurotoxicity of colchicine for granule cells of the dentate gyrus of the adult rat. Proc Natl Acad Sci U S A. 1980;77:3047–3051. doi: 10.1073/pnas.77.5.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt RB, Steward O. Neurotoxic effects of colchicine: differential susceptibility of CNS neuronal populations. Neuroscience. 1982;7:695–714. doi: 10.1016/0306-4522(82)90075-6. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RB, Steward O. Comparison of the neurotoxic effects of colchicine, the vinca alkaloids, and other microtubule poisons. Brain Res. 1989;486:133–140. doi: 10.1016/0006-8993(89)91285-7. [DOI] [PubMed] [Google Scholar]
- Gorenstein C, de Carvalho SC, Artes R, Moreno RA, Marcourakis T. Cognitive performance in depressed patients after chronic use of anti-depressants. Psychopharmacology (Berl) 2006;185:84–92. doi: 10.1007/s00213-005-0274-2. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- Huang SC, Tsai SJ, Chang JC. Fluoxetine-induced memory impairment in four family members. Int J Psychiatry Med. 2004;34:197–200. doi: 10.2190/DY6D-33BP-5WPB-1VDX. [DOI] [PubMed] [Google Scholar]
- Joss JD, Burton RM, Keller CA. Memory loss in a patient treated with fluoxetine. Ann Pharmacother. 2003;37:1800–1803. doi: 10.1345/aph.1D154. [DOI] [PubMed] [Google Scholar]
- Keith JR, Pitts RC, Pezzuti T, Galizio M. Effects of positive GABA(A) modulators on a multiple-component, repeated-test of spatial learning. Behav Pharmacol. 2003;14:67–75. doi: 10.1097/00008877-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Manev R. Glia as a putative target for antidepressant treatments. J Affect Disord. 2003;75:59–64. doi: 10.1016/s0165-0327(02)00044-7. [DOI] [PubMed] [Google Scholar]
- Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100beta in the rat hippocampus. Eur J Pharmacol. 2001;420:R1–2. doi: 10.1016/s0014-2999(01)00989-x. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Rao G, Baldwin J, Rasmussen M. Long-term enhancement of hippocampal synaptic transmission and the acquisition of spatial information. J Neurosci. 1986;6:563–571. doi: 10.1523/JNEUROSCI.06-02-00563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirow S. Cognitive dysfunction associated with fluoxetine. Am J Psychiatry. 1991;148:948–949. doi: 10.1176/ajp.148.7.948. [DOI] [PubMed] [Google Scholar]
- Morris RG. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Sawada T. Involvement of microtubule integrity in memory impairment caused by colchicine. Pharmacol Biochem Behav. 2002;71:119–138. doi: 10.1016/s0091-3057(01)00634-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, et al. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neuro-transmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, et al. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlubnaya D, Galizio M, Pitts RC, Keith JR. Chlordiazepoxide interactions with scopolamine and dizocilpine: novel cooperative and antagonistic effects on spatial learning. Behav Neurosci. 2005;119:1331–1338. doi: 10.1037/0735-7044.119.5.1331. [DOI] [PubMed] [Google Scholar]
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson BB. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Res Bull. 2002;58:315–321. doi: 10.1016/s0361-9230(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Spanswick SC, Epp JR, Keith JR, Sutherland RJ. Adrenalectomy-induced granule cell degeneration in the hippocampus causes spatial memory deficits that are not reversed by chronic treatment with corticosterone or fluoxetine. Hippocampus. 2007;17:137–146. doi: 10.1002/hipo.20252. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, et al. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus. 2001;11:27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]