Abstract
This study investigated the role of long-chain n-3 polyunsaturated fatty acids (LCn-3PUFAs) of muscle phospholipids in the regulation of neonatal metabolism. Twenty-eight piglets were weaned at 2 days of age and raised on one of two milk formulas that consisted of either a control formula supplying 0% or a formula containing 3.5% LCn-3PUFAs until 10 or 28 days of age. There was a developmental decline in the insulin sensitivity of amino acid disposal in control pigs during the first month of life, with a slope of −2.24 μmol·kg−1·h−1 (P = 0.01) per unit of insulin increment, as assessed using hyperinsulinemic-euglycemic-euaminoacidemic clamps. LCn-3PUFA feeding blunted this developmental decline, resulting in differing insulin sensitivities (P < 0.001). When protein metabolism was assessed under parenteral feeding-induced hyperinsulinemia, LCn-3PUFAs reduced by 16% whole body oxidative losses of amino acids (from 238 to 231 μmol·kg−1·h−1; P = 0.06), allowing 41% more amino acids to accrete into body proteins (from 90 to 127 μmol·kg−1·h−1; P = 0.06). The fractional synthetic rate of muscle mixed proteins remained unaltered by the LCn-3PUFA feeding. However, LCn-3PUFAs retarded a developmental increase in the essential-to-nonessential amino acid ratio of the muscle intracellular free pool (P = 0.05). Overall, alterations in metabolism were concomitant with a preferential incorporation of LCn-3PUFAs into muscle total membrane phospholipids (P < 0.001), in contrast to intramuscular triglycerides. These results underscore the potential role of LCn-3PUFAs as regulators of different aspects of protein metabolism in the neonate.
Supplementary key words: neonatal feeding, insulin sensitivity, phenylalanine kinetics, stable isotopes
Considerable attention has been devoted to the refinement of the nutritional composition of human infant formula. Recent improvements in formulation include the incorporation of long-chain n-3 polyunsaturated fatty acids (LCn-3PUFAs) in conjunction with arachidonic acid, because these play a prominent role in visual and neural development and in the biogenesis of prostaglandins and leukotrienes (1, 2). Neonatal nutrition, which typically involves feeding numerous meals of small size to the newborn, also affects certain regulatory aspects of postprandial metabolism that are of paramount importance for development. Insulin is a primary regulator of postprandial metabolism, and the high insulin sensitivity of the neonate enables a highly efficient anabolism that critically sustains neonatal growth. As development progresses, the skeletal musculature becomes less sensitive to insulin (3, 4), and this is parallel to a simultaneous reduction in muscle anabolism. The mechanisms behind this developmental regulation include a reduction in both the responsiveness and the sensitivity of muscle to insulin (3, 4). Refinements of knowledge regarding insulin action on body tissues are critically relevant to the development of new approaches targeting the prevention and treatment of growth retardation, preterm birth nutrition, and early life programming.
Insulin resistance leading to defects in glucose metabolism in certain pathological states, such as obesity, type II diabetes, and insulin resistance attributable to high-fat feeding, can be improved through dietary LCn-3PUFAs from fish oil (5–7). In such instances, enhanced glucose metabolism in response to insulin stimulation relies on the complex biophysical and biochemical effects of highly unsaturated fatty acid content in membranes. The latter includes enhanced membrane fluidity that affects the functions of membranes and transmembrane proteins and membrane-associated enzymes, which also can lead to alterations in the activation of insulin signaling intermediates (8–10). To date, most of the focus has been devoted to the improvement of glucose metabolism for different dysregulations of insulin action. LCn-3PUFAs were recently shown to be effective at upregulating protein anabolism through enhanced insulin sensitivity in a healthy growing animal model that was otherwise more insulin-resistant as a result of maturity (11). Increased insulin-mediated amino acid disposal and activation of AktmTOR-S6K1 insulin signaling machinery, combined with reduced whole body oxidative metabolism, were among the protein-anabolic benefits observed (11). The current study was undertaken to establish whether LCn-3PUFAs would similarly affect protein metabolism in the neonatal pig to explore new avenues for intervening at different stages of development.
Materials and Methods
Animals and design
Twenty-eight cross-bred male piglets [Duroc×(Yorshire×Landrace)] were purchased at 2 days of age (1.97 ± 0.3 kg; Alfred Couture Ltée, St. Anselme, Québec, Canada). Piglets were weighed and randomly assigned to a 2 × 2 factorial arrangement of treatments according to a completely randomized design. The latter consisted of two age groups: 10 or 28 days of age, and of two semipurified milk formulas: control (0% LCn-3PUFAs) or enriched to 3.5% LCn-3PUFAs on formula total dry matter. Control oil was substituted on an isoenergetic basis with menhaden oil (Table 1). Five hours after their arrival, piglets were introduced to their respective milk formula and raised on these. Piglets were housed individually and had free access to water. Twice a week, they were weighed on two consecutive days throughout the study.
TABLE 1.
Ingredients and chemical composition of milk formulas fed to neonatal piglets
| Ingredients and Composition | Control | LCn-3PUFA |
|---|---|---|
| Ingredient (% dry matter basis) | ||
| Lactosea | 31.0 | 31.0 |
| Sodium caseinatea | 16.5 | 16.5 |
| Whey proteina | 9.8 | 9.8 |
| Plasma proteinb | 3.0 | 3.0 |
| Mineral and vitamin mixa | 5.5 | 5.5 |
| Palm oilc | 20.0 | 21.6 |
| Cottonseed oild | 6.2 | 0.0 |
| Coconut oile | 4.0 | 0.0 |
| Menhaden oilf | 0.0 | 9.3 |
| Soybean lecithine | 2.7 | 2.7 |
| Soybean emulsifiera | 0.7 | 0.7 |
| Chemical composition (% dry matter basis) | ||
| Carbohydratesg | 26.4 | 26.1 |
| Crude protein | 29.8 | 29.9 |
| Fat | 38.2 | 38.4 |
| Ashes | 5.6 | 5.6 |
LCn-3PUFA, long-chain n-3 polyunsaturated fatty acids. Milk replacers had a chemical composition similar to that of the sow's milk (13).
Grober Nutrition, Inc. (Cambridge, Ontario, Canada).
Nutrapro; JEFO Nutrition (St. Hyacinthe, Quebec, Canada).
Bedessee (Toronto, Ontario, Canada).
Cedar Vale Natural Health (Cedar Vale, KS).
Bunge Canada (Oakville, Ontario, Canada).
Omega Protein, Inc. (Reedville, VA).
Estimated by difference: carbohydrates = total dry matter − [crude protein + fat + ashes].
Dietary treatments
Two semipurified milk formulas were formulated to meet piglet nutritional requirements (Table 1) (12). These were isoenergetic and isonitrogenous, and they differed only in their fatty acid composition (Table 2). The composition of the formula's fatty acids and macronutrients was similar to that of the sow's milk (13). Oils in the milk formulas were emulsified by heating at 50°C for 15 min, with soybean lecithin and a soybean emulsifier, before mixing with other constituents. Milk replacers were offered at a minimum of 6.25% body weight on a dry matter basis to ensure ad libitum consumption (12). The dry matter concentration of the formulas was initially 10% and was increased gradually to 15% until 28 days of age, taking care to avoid diarrhea. Feeding frequency of the newborn consisted of five diurnal meals with a maximum of a 12 h overnight fast; this was reduced to four diurnal meals with a maximum of a 12 h overnight fast at 14 days old onward.
TABLE 2.
Fatty acid composition of milk fat formulas fed to neonatal piglets
| Milk Formula | ||
|---|---|---|
| Fatty Acid | Control | LCn-3PUFA |
| C8:0 | 0.26 | ND |
| C10:0 | 0.72 | ND |
| C12:0 | 6.49 | 0.74 |
| C14:0 | 3.99 | 3.45 |
| C16:0 | 33.30 | 34.81 |
| C16:1 n-7 | 0.32 | 2.95 |
| C18:0 | 4.52 | 4.78 |
| C18:1 | 30.97 | 31.54 |
| C18:2 n-6 | 18.26 | 9.54 |
| C18:3 n-3 | 0.57 | 0.88 |
| C18:4 n-3 | 0.25 | 1.22 |
| C20:0 | 0.34 | 0.38 |
| C20:1 | ND | 0.55 |
| C20:4 n-6 | ND | 0.27 |
| C20:4 n-3 | ND | 0.56 |
| C20:5 n-3 | ND | 3.29 |
| C22:5 n-3 | ND | 0.69 |
| C22:6 n-3 | ND | 4.35 |
| SAT | 49.6 | 44.2 |
| PUFA | 19.1 | 20.8 |
| Total n-6 | 18.3 | 9.8 |
| Total n-3 | 0.8 | 11.0 |
| n-3/n-6 | 0.04 | 1.12 |
| LCn-n-3PUFA | ND | 8.3a |
| PUFA/SAT | 0.38 | 0.47 |
Values shown are percentage of total fatty acids. SAT, sum of saturated fatty acids; PUFA, sum of polyunsaturated fatty acids; Total n-6, sum of n-6 fatty acids; Total n-3, sum of n-3 fatty acids; n-3/n-6, ratio of total n-3 to total n-6 fatty acids; LCn-3PUFA, 20:5 n-3 + 22:5 n-3 + 22:6 n-3; PUFA/SAT, ratio of polyunsaturated to saturated fatty acids; ND, not detected.
Equivalent to 3.5% LCn-3PUFAs on a dry matter basis of total formula constituents.
Three days before the onset of measurements (at 5 and 23 days old), piglets were anesthetized for surgical procedures after an overnight fast, as described previously (14). A catheter for sampling was placed in a carotid artery, and an infusion catheter was placed in a jugular vein. Catheters were kept patent by filling with heparinized saline (200 IU·ml−1). Piglets were returned to their crate after surgery. The experimental procedures were approved by the Animal Care and Use Committee of the Université Laval and were conducted in accordance with Canadian Council on Animal Care guidelines (15).
In vivo measurements and sampling procedures
Hyperinsulinemic-euglycemic-euaminoacidemic clamps
Insulin sensitivity of whole-body amino acid and glucose disposal was measured at either 7.5 ± 0.5 days of age (2.2 ± 0.3 kg body weight) or 26 ± 0.5 days of age (6.9 ± 1 kg body weight) in six piglets per milk replacer per age, for n = 12 per age group as a result of catheter patency, using the hyperinsulinemic-euglycemic-euaminoacidemic clamp procedure (3). After an overnight fast with free access to water, piglets were placed in a sling restraining system. Insulin stock solutions (1 mg·ml−1) were prepared daily by dissolving lyophilized porcine insulin (29.2 IU/mg; I-5523; Sigma Chemical, St. Louis, MO) in 0.01 N HCl followed by mixing with sterile physiological saline containing 4% filtersterilized porcine plasma. Insulin was infused into the jugular vein at 100 ng·(kg−0.66)−1·min−1. This infusion rate was specifically selected to generate fed plasma insulinemia that approximates 30 μU·ml−1 at both ages (16). A constant insulin infusion rate (12 ml·h−1) was used by individually preparing insulin syringes and by diluting the appropriate amount of stock solution with physiological saline. Fasting baseline concentrations of glucose and branched-chain amino acids, the latter used as an index of essential amino acid concentration, were established by sampling three arterial blood samples at 5 min intervals before the onset of insulin infusion. Glucose was analyzed immediately in fresh whole blood by peroxidase reaction (YSI 2300 STAT Plus analyzer; Yellow Springs Instruments, Yellow Springs, OH). A branched-chain amino acid assay was performed on plasma according to the enzymatic method of Beckett et al. (17). Remaining plasma was frozen at −20°C until further analyses of baseline concentrations of insulin and amino acids were conducted.
Once glucose and branched-chain amino acid baselines were established, the clamp was initiated with a 10 min priming insulin infusion (18). During the clamp procedure, blood samples were taken every 5 min and immediately analyzed for glucose and branched-chain amino acids. Glucose and amino acid concentrations were maintained at ±10% of baseline concentrations by adjustments of auxiliary infusions of dextrose [50% (w/v) sterile solution; Baxter Healthcare, Deerfield, IL] and a complete amino acid solution using Plum Lifecare pumps (series 1.6; Abbott Laboratories, Chicago, IL). The amino acid mixture used was adapted from Davis et al. (19) using the l-form of medical-grade amino acids as follows (mmol·l−1): alanine, 27.3; arginine, 20.1; aspartic acid, 12.0; glutamic acid, 23.9; glycine, 54.3; histidine, 17.2; isoleucine, 28.5; leucine, 34.2; lysine, 27.4; methionine, 15.1; phenylalanine, 15.5; proline, 34.8; serine, 23.8; threonine, 21.1; tryptophan, 4.4; tyrosine, 1.1; and valine, 34.1 (Ajinomoto, Sigma-Aldrich, Oakville, Ontario, Canada). Cysteine and glutamine were not added to the mixture because of solubility and stability problems. The clamp procedures lasted 150 min on average, and a 90 min period was required to reach steady utilization rates; an additional 60 min was allocated for the monitoring of glucose and amino acid disposal rates. During the last steady 45 min of the clamp procedures, four blood samples were collected at 15 min intervals. These were centrifuged and the plasma was frozen at −20°C until further analyses of insulin and individual amino acids were conducted.
Phenylalanine kinetics
Whole body protein metabolism was measured at 2 days after the clamp procedure at either 10 ± 0.5 days of age (2.7 ± 0.4 kg body weight) or 28 ± 0.8 days of age (7.5 ± 1.4 kg body weight) on seven piglets per milk replacer per age group for n = 14 piglets per age. Phenylalanine kinetics was assessed at a fed steady state after 12 h of food deprivation. The total parenteral nutrition was selected to elicit fed plasma insulin concentrations (20, 21).
Isotopes
Tracers used to conduct phenylalanine kinetics studies were as follows: l-[1-13C]phenylalanine (97.9% mole percent excess; Cambridge Isotopes Laboratories, Andover, MA), NaH13CO3 (99% atom percent excess; Cambridge Isotopes Laboratories), and NaH14CO3 (2 mCi·ml−1; MP Biochemicals, Inc., Irvine, CA). The tracer used for the quantification of plasma phenylalanine using the isotopic ratio method (22) on blood collected during the phenylalanine kinetics was l-[ring-2H5]phenylalanine (98% mole percent excess; Cambridge Stable Isotopes). Stock solutions of labeled phenylalanine were dissolved in 0.1 N HCl; stock solutions of labeled bicarbonate were dissolved in 0.05 M NaOH.
Infusions
Whole body phenylalanine kinetics were assessed over a 240 min period with piglets restrained in a sling system. The onset of the kinetic study was preceded by three arterial background samples taken every 10 min over a 30 min period to determine the natural abundance of plasma phenylalanine carbon isotope, with basal concentrations of glucose, phenylalanine, and insulin. According to a similar schedule, breath gases were sampled using a facemask equipped with a one-way valve bag for determination of CO2 isotopic ratios. Four separate lines were used for infusions: l-[1-13C]phenylalanine, NaH14CO3, fat emulsion, and the mixture of the remaining constituents of the parenteral nutrition (glucose, amino acids, vitamins, minerals). These were connected to the venous catheter using three-way valves. At time 0, body pools were primed for l-[1-13C]phenylalanine (22 μmol·kg−1), NaH13CO3 (6 μmol·kg−1), and NaH14CO3 (0.75 μCi·kg−1). Continuous infusions of l-[1-13C]phenylalanine (22 μmol·kg−1·h−1), NaH14CO3 (1 μCi·kg−1·h−1), intralipids (2.1 ml·kg−1·h−1), and the remaining parenteral nutrition (7.9 ml·kg−1·h−1) were immediately initiated and maintained throughout the 240 min period. After 195 min of infusion, four blood and breath samples were taken at 15 min intervals. Glucose and blood gas (ABL 77 series; Radiometer Medical, Copenhagen, Denmark) were immediately measured on fresh blood. A subsample of fresh blood was processed for the measurement of blood specific radioactivity (14). Remaining blood was centrifuged and kept frozen (−20°C) until analyses of plasma amino acid and insulin concentrations and of plasma phenylalanine isotopic enrichments were conducted. At 240 min, the infusions were stopped and the piglets were euthanized by an intravenous lethal injection (pentobarbital sodium, 50 mg·kg−1; CDMV, St. Hyacinthe, Quebec). Longissimus dorsi skeletal muscle was rapidly sampled and immediately frozen in liquid N2. Muscle samples were stored at −80°C for subsequent analyses of fatty acid profiling of total membrane phospholipids and intramuscular triglycerides. The concentrations of free amino acids plus the isotopic labeling of free and bound phenylalanine in muscle mixed protein homogenate were also determined.
Total parenteral nutrition
During the 240 min period of the phenylalanine kinetic studies, piglets continuously received total parenteral nutrition adapted from Burrin et al. (20) and Bertolo et al. (21) (Table 3). The amino acid composition was modified to approximate that of the sow's milk protein. Vitamins and minerals were added to the parenteral nutrition solution on the morning of their use. Emulsified lipids (20% Intralipid®, composition weight·100 ml−1: 20 g of sterile emulsion purified soybean oil, 1.2 g of purified egg phospholipids, 2.2 g of anhydrous glycerol, and water; Fresenius Kabi, Uppsala, Sweden), were administered using a separate line. The parenteral nutrition, including intralipids, provided 14.5 g amino acids·kg−1·day−1 and 1.1 MJ metabolizable energy·kg−1·day−1, for which glucose and lipid each supplied 50% of nonprotein energy intake (21).
TABLE 3.
Total parenteral nutrition continuously infused into the jugular vein of neonatal piglets during steady-fed-state phenylalanine kinetic studies
| Ingredient, Macronutrients | Amount | Ingredient, Micronutrients | Amount |
|---|---|---|---|
| Glucose, mmol·l−1 | 526.1 | Mineral | |
| Intralipids 20%, ml·l −1 | 210.9a | NaCl, mmol·l−1 | 19.2 |
| Amino acids, mmol·l−1 | 470.2 | NaOH, mmol·l−1 | 30.3 |
| Alanine | 32.7 | K acetate, mmol·l−1 | 7.7 |
| Arginine | 14.6 | KPO4, mmol·l−1 | 22.3 |
| Aspartic acid | 15.8 | Mg sulfate, mmol·l−1 | 3.2 |
| Glutamic acid | 23.1 | Ca gluconate, mmol·l−1 | 5.1 |
| Glycine | 23.0 | Zn, mg·l−1 | 6.24 |
| Histidine | 12.7 | Mn, mg·l−1 | 1.25 |
| Isoleucine | 29.9 | Cu, mg·l−1 | 1.56 |
| Leucine | 53.6 | Cr, mg·l−1 | 15.6 |
| Lysine | 32.9 | Se, μg·l−1 | 133.3 |
| Methionine | 11.9 | Vitamin | |
| Phenylalanine | 30.8 | Folic acid, mg·l−1 | 0.13 |
| Proline | 59.2 | Thiamin, mg·l−1 | 8.0 |
| Serine | 54.6 | Riboflavin, mg·l−1 | 0.4 |
| Threonine | 28.5 | Pyridoxine, mg·l−1 | 0.4 |
| Tryptophan | 5.6 | Penthotenol, mg·l−1 | 0.8 |
| Tyrosine | 1.1 | Niacin, mg·l−1 | 10.0 |
| Valine | 40.3 | Vitamin B12, μg·l−1 | 8.0 |
| Vitamin A, IU·l−1 | 916.7 | ||
| Vitamin D, IU·l−1 | 91.7 | ||
| Vitamin E, IU·l−1 | 2.75 |
Adapted from Bertolo et al. (21) and Burrin et al. (20). The parenteral nutrition composition was 48% glucose, 21% intralipids, and 31% protein on a dry matter basis; vitamin and mineral volume represented 7.6% of the nutritive solution. In total, the parenteral nutrition provided 60 g of medical-grade l-amino acids (Sigma-Aldrich) per solution liter; 75% of gross energy was provided by nonprotein nutrients such as glucose and fat at a ratio of 50% each (based on equivalence of physiological fuel values). Gross energy from nonprotein nutrients represented 2.2 MJ metabolizable energy intake·kg−1·d−1.
This amount provided 42.2 ml of intralipids in total per liter of elementary diet.
A pilot study was conducted to assess the neonatal alteration in the fatty acid composition of muscle total membrane phospholipids using 30 newborn piglets. Six of them were weaned on the control milk formula, with half raised until 10 days of age and the other half raised until 28 days of age (three piglets per age group assessing the control formula). The remaining 24 piglets were weaned on the LCn-3PUFA formula, and they were raised in groups of three until 10, 14, 21, or 28 days of age (three piglets per age group assessing the LCn-3PUFA formula). At the end of each period, piglets were euthanized by lethal injection as outlined previously. Care, feeding, and methods used in this pilot study were similar to those described for the main study.
Laboratory and analytical methods
Milk replacer analyses
Freeze-dried samples of milk were analyzed for fat and ash using standard procedures (23). Total N was analyzed by thermal conductivity (N × 6.38; model FP-428 nitrogen determinator; LECO, St. Joseph, MO). Menhaden oil was kept in N-flushed amber bottles with peroxide index determined weekly to control quality (24). The latter were kept at <10 mEq/kg throughout the study.
Plasma substrates, hormone, and blood gas
Plasma insulin concentrations were measured using a porcine insulin radioimmunoassay kit (Linco, St. Louis, MO) that used porcine insulin antibody and human insulin standards as described previously (14) (intra-assay coefficient of variation, 8.3%). Plasma amino acid concentration on blood samples taken during the clamp procedures was determined by high-performance liquid chromatography using an ultraviolet light detector set at 254 nm (Alliance system; Waters) with precolumn derivatization using the Pico-Tag procedure (14). Amino acid concentration was corrected for an internal standard (methionine sulfone; Sigma-Aldrich) added to the sample on a weight basis. Plasma phenylalanine concentration of blood sampled during the phenylalanine kinetic studies was measured by isotopic ratio according to the method of Calder et al. (22).
Isotopic enrichments
Plasma Amino Acids
Phenylalanine in plasma was separated using a cation-exchange resin column (AG-W50 resin; Bio-Rad, Hercules, CA) and converted to the n-propyl ester heptafluorobutyramide derivative as described previously (14). The recovery of the tracers was verified by linear regression. Gas chromatographymass spectrometry was carried out on a gas chromatograph (model HP 6890; Agilent Technologies Canada, Inc., Mississauga, Canada; HP-5MS column; length, 30 m; 0.25 mm inner diameter; film thickness, 0.25 μm; Agilent Technologies, Inc.) coupled to a quadrupole mass spectrometer mass selective detector (model 5973; Agilent Technologies, Inc.) operating in the negative chemical ionization mode. Selective ion monitoring was carried out at m/z 383, 384, and 388.
Breath CO2 and Muscle Isotopic Ratios
Breath CO2 isotopic ratios were determined in triplicate on an isotope ratio mass spectrometer (IsoPrime™; GV Instruments, Ltd., Manchester, UK) monitoring for masses 44, 45, and 46 in continuous flow mode. The IsoPrime™ instrument was interfaced to a multifunctional head space analyzer (Multiflow™Bio; GV Instruments, Ltd.) configured for breath sample analysis and equipped with a Gilson autosampler (model 222XL). Duplicates of frozen longissimus dorsi muscle (50 mg) were homogenized in 2 ml of ice-cold trichloroacetic acid (10%, w/v). Subsamples of muscle homogenates were kept for additional analyses of isotopic enrichments of free phenylalanine and concentrations of free phenylalanine in homogenates. In this regard, 500 μl of homogenate was filtered through 10,000 cutoff filters at 15,000 rpm for 15 min; 50 μl of the filtrate was mixed with 50 μl of an internal standard of methionine sulfone on a weight basis and then processed according to the Pico-Tag method for HPLC analysis as outlined above. The remaining homogenate free pool was processed similarly to plasma samples for the determination of phenylalanine isotopic enrichments by GC-MS using n-propyl ester heptafluorobutyramide derivative. The trichloroacetic acidinsoluble precipitate of homogenates was further hydrolyzed in hydrochloric acid (6 N solution) at 110°C for 24 h (14). Phenylalanine released from muscle mixed proteins was converted to its n-acetyl-propyl derivative (14), separated by gas chromatography (HP 6890), and combusted. Carbon isotopic ratios were measured on a isotopic ratio mass spectrometer (IsoPrime™) by monitoring ion beams at m/z 44, 45, and 46.
Blood specific radioactivity
Blood samples withdrawn during phenylalanine kinetics studies were processed to determine the specific radioactivity of total blood CO2 (14). Briefly, 0.5 ml of fresh whole blood was added to 0.1 ml of sodium bicarbonate (1 mol·l−1) in a sterile Vacutainer. Both products were injected through the cap to maintain vacuum and kept on ice with weights recorded. Immediately after the kinetic measurement period, these processed subsamples were then injected with 5 ml of methanol, mixed gently, and centrifuged at 1,500 g for 20 min at 4°C. The supernatant was quickly poured off into a 30 ml scintillation vial, and 10 ml of scintillation cocktail (scintillation cocktail 3a70; RPI Research Products International, Mount Prospect, IL) was added to the scintillation vial. The samples were counted on a scintillation counter (KB-Wallac 1277 Gamma Master; Perkin-Elmer, Woodbridge, Ontario, Canada).
Muscle total membrane phospholipids and intramuscular triglycerides
The fatty acid composition of total phospholipids, which include plasma and organelle membranes, was assessed because it relates to insulin sensitivity (5, 25). This analysis was conducted on five piglets per milk formula per age for 20 observations, because the effect of menhaden oil on membrane composition is highly significant (11) and a large number of observations per treatment is not required to detect treatment effects. Fatty acid profiling was conducted by gas chromatography (26), as was the enteral oil profiling (27). Approximately 75 mg of frozen longissimus dorsi samples was used for this study. Lipids were extracted along with internal standards (C:15; Avanti Polar Lipids, Alabaster, AL) in a chloroform-methanol mixture (2:1, v/v). Extracted lipids were then weighed and dissolved in a chloroform-methanol mixture (3:1, v/v). Polar lipids (phospholipids) were separated by TLC (Silica Gel H, 250 μm; Analtech, Inc., Newark, DE) using an isopropyl ether-acetic acid mixture (96:4, v/v). They were then recovered in individual glass tubes, and direct transesterification was performed by adding acetyl chloride (28). Fatty acid methyl esters of milk formula were prepared by base-catalyzed transmethylation (27). Fatty acid methyl esters of phospholipids were analyzed by gas chromatography using a Hewlett-Packard 5890, series II (Hewlett-Packard, Toronto, Canada), equipped with a fused silica column (DB23; 30 m, 0.25 mm internal diameter, 0.25 μm film; Agilent Technologies), helium as the carrier gas, a split ratio of 1:72, a flow of 0.72 ml·min−1, and a coupled flame ionization detector. The fatty acid methyl esters were identified by comparison with the retention times of Supelco 37 Component FAME mix (Supelco, Inc., Bellefonte, PA) using an internal standard (C:15; Avanti Polar Lipids) and are expressed in milligrams of fatty acids per 100 g of wet tissue or in percentage of total fatty acids. Fatty acid methyl esters of formulas were similarly analyzed by gas chromatography without using an internal standard.
Calculations
Whole body irreversible loss rate of phenylalanine
This parameter was calculated by the isotopic dilution of the tracer by the tracee corrected for the tracer infusion rate:
where IEpp represents phenylalanine isotopic enrichment in the arterial plasma precursor pool. Isotopic enrichments (IE) are presented as mole percent excess and were calculated according to Campbell (29).
where RA 14CO2-infusate is the radioactivity (dpm·ml−1) of the infusate and SA 14CO2-blood is the specific activity (dpm·μmol−1) in blood. CO2 production was corrected for carbon sequestration using a recovery factor of 0.92 as measured for piglets (30).
SA of blood CO2
This parameter was calculated as follows:
where Iphe−diet is the entry rate of unlabeled phenylalanine in the central plasma compartment via the parenteral nutrition in μmol·kg−1·h−1. Whole body protein synthesis and breakdown were derived from the ILR equation as follows:
Fractional synthetic rate of muscle mixed protein homogenate
This parameter was calculated according to the precursor/product technique (31).
where IEbound is the isotopic enrichment of the bound phenylalanine in muscle mixed protein homogenate in atom percent excess at time t, IEfree is the isotopic enrichment of the free phenylalanine in muscle homogenate in atom percent excess at time t, and t is the time of labeling in minutes. The isotopic enrichment of the bound phenylalanine to protein homogenate at baseline was assumed to be similar to baseline plasma phenylalanine (31). The isotopic enrichment of free phenylalanine in muscle homogenate reliably represents that of aminoacyl-tRNA (32).
Insulin clearance rate
This parameter was calculated as follows:
Statistical analyses
Completely randomized design
The mean effects of the milk replacers on muscle total phospholipids, intramuscular triglycerides, insulin sensitivity, and protein metabolism were submitted to a variance analysis according to the mixed procedures of SAS (33) (see Tables 4–7, Fig. 3 below). Treatments were allocated according to a 2 × 2 factorial treatment distribution in a completely randomized design that compared a control with an enriched milk replacer in LCn-3PUFAs randomized to piglets raised until either 10 or 28 days of age. The statistical model included age, formula and age × formula interaction as fixed effects. Probabilities were interpreted using type 1 error. Treatment differences were considered significant at P ≤ 0.05 and tendency at 0.05 < P < 0.10.
TABLE 4.
Fatty acid composition of total membrane phospholipids of longissimus dorsi muscle in neonatal piglets
| 10 Days Old | 28 Days Old | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid | Control | LCn-3PUFA | Control | LCn-3PUFA | SEM | Age | Milk | Age × Milk |
| 14:0 | 0.34 | 0.29 | 0.27 | 0.25 | 0.02 | 0.02 | 0.11 | 0.53 |
| 16:0 | 19.2 | 20.8 | 20.6 | 22.9 | 0.46 | 0.001 | 0.0006 | 0.39 |
| 16:1 n-7 | 0.43 | 0.67 | 0.23 | 0.50 | 0.02 | <0.0001 | <0.0001 | 0.41 |
| 18:0 | 18.5 | 18.1 | 17.1 | 16.2 | 0.26 | <0.0001 | 0.01 | 0.31 |
| 18:1 n-9 | 12.8 | 11.1 | 9.5 | 8.5 | 0.35 | <0.0001 | 0.001 | 0.34 |
| 18:1 n-7 | 4.0 | 4.8 | 2.9 | 3.9 | 0.17 | <0.0001 | <0.0001 | 0.62 |
| 18:2 n-6 | 27.1 | 19.3 | 32.9 | 19.6 | 0.54 | <0.0001 | <0.0001 | <0.0001 |
| 18:3 n-3 | 0.36 | 0.35 | 0.26 | 0.40 | 0.03 | 0.41 | 0.05 | <0.05 |
| 18:4 n-3 | 0.17 | 0.18 | 0.09 | 0.20 | 0.02 | 0.20 | 0.007 | 0.02 |
| 20:0 | 0.52 | 0.46 | 0.32 | 0.40 | 0.04 | 0.008 | 0.88 | 0.10 |
| 20:1 n-9 | 0.22 | 0.25 | 0.14 | 0.20 | 0.04 | 0.16 | 0.30 | 0.77 |
| 20:3 n-6 | 0.85 | 0.82 | 0.69 | 0.64 | 0.03 | <0.0001 | 0.21 | 0.69 |
| 20:3 n-3 | 0.05 | 0.10 | 0.02 | 0.14 | 0.04 | 0.88 | 0.07 | 0.36 |
| 20:4 n-6 | 11.0 | 9.7 | 10.6 | 4.9 | 0.28 | <0.0001 | <0.0001 | <0.0001 |
| 20:4 n-3 | 0.08 | 0.24 | 0.03 | 0.39 | 0.06 | 0.42 | 0.0005 | 0.11 |
| 20:5 n-3 | 0.35 | 4.9 | 0.35 | 10.6 | 0.29 | <0.0001 | <0.0001 | <0.0001 |
| 22:4 n-6 | 1.36 | 0.84 | 1.27 | 0.23 | 0.04 | <0.0001 | <0.0001 | <0.0001 |
| 22:5 n-6 | 1.22 | 1.01 | 1.20 | 0.40 | 0.07 | 0.0003 | <0.0001 | 0.0005 |
| 22:5 n-3 | 0.77 | 1.54 | 0.60 | 2.0 | 0.07 | 0.03 | <0.0001 | 0.0001 |
| 22:6 n-3 | 0.64 | 4.6 | 0.79 | 7.5 | 0.25 | <0.0001 | <0.0001 | <0.0001 |
| SAT | 38.6 | 39.6 | 38.3 | 39.7 | 0.60 | 0.91 | 0.06 | 0.71 |
| PUFA | 44.0 | 43.6 | 48.9 | 47.1 | 0.52 | <0.0001 | 0.06 | 0.20 |
| Total n-6 | 41.5 | 31.7 | 46.7 | 25.8 | 0.70 | 0.60 | <0.0001 | <0.0001 |
| Total n-3 | 2.4 | 11.9 | 2.1 | 21.2 | 0.57 | <0.0001 | <0.0001 | <0.0001 |
| Total n-3/n-6 | 0.06 | 0.38 | 0.05 | 0.82 | 0.03 | <0.0001 | <0.0001 | <0.0001 |
| LCn-3PUFA | 1.8 | 11.0 | 1.7 | 20.1 | 0.55 | <0.0001 | <0.0001 | <0.001 |
| PUFA/SAT | 1.1 | 1.1 | 1.3 | 1.2 | 0.03 | 0.002 | 0.05 | 0.38 |
Values shown are percentage of total by weight. SAT, sum of saturated fatty acids; PUFA, sum of polyunsaturated fatty acids; Total n-6, sum of n-6 fatty acids; Total n-3, sum of n-3 fatty acids; n-3/n-6, ratio of total n-3 to total n-6 fatty acids; LCn-3PUFA, 20:5 n-3 + 22:5 n-3 + 22:6 n-3; PUFA/SAT, ratio of polyunsaturated fatty acids to saturated fatty acids. n = 5 per milk formula per age group for 20 observations; see Materials and Methods for details.
TABLE 7.
Arterial concentrations of insulin, glucose, and phenylalanine and whole body irreversible loss rate of phenylalanine kinetics measured in neonatal piglets continuously fed through total parenteral nutrition
| 10 Days Old | 28 Days Old | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Control | LCn-3PUFA | Control | LCn-3PUFA | SEM | Age | Milk | Age × Milk |
| Insulin, μU·ml−1 | 40.8 | 43.5 | 73.0 | 55.2 | 13.1 | 0.09 | 0.55 | 0.42 |
| Glucose, mg·dl−1 | 146.2 | 135.9 | 122.8 | 127.2 | 9.4 | 0.08 | 0.74 | 0.42 |
| Phenylalanine, μmol·l-1 | 358.8 | 407.7 | 491.8 | 553.4 | 39.2 | 0.001 | 0.15 | 0.86 |
| Parenteral phenylalanine entry, μmol·kg−1·h−1 | 327.5 | 327.5 | 327.5 | 327.5 | — | — | — | — |
| Initial weight,a kg | 2.01 | 2.09 | 2.06 | 1.90 | 0.13 | 0.52 | 0.78 | 0.33 |
| Final weight,b kg | 2.73 | 2.64 | 7.96 | 7.06 | 0.44 | <0.001 | 0.25 | 0.34 |
| Phenylalanine kinetics, μmol·kg−1 ·h−1 | ||||||||
| Whole body irreversible loss rate | 572.6 | 552.9 | 535.7 | 532.7 | 17.0 | 0.09 | 0.49 | 0.61 |
| Oxidation | 269.6 | 210.6 | 205.8 | 190.5 | 19.7 | 0.03 | 0.06 | 0.25 |
| Protein synthesis | 302.9 | 342.2 | 329.9 | 342.2 | 26.4 | 0.60 | 0.31 | 0.59 |
| Protein breakdown | 245.1 | 225.4 | 208.3 | 205.2 | 17.0 | 0.09 | 0.49 | 0.61 |
| Accretion | 57.9 | 116.8 | 121.7 | 136.9 | 19.7 | 0.03 | 0.06 | 0.25 |
| Fractional oxidation | 0.47 | 0.38 | 0.38 | 0.36 | 0.04 | 0.14 | 0.14 | 0.39 |
| LD FSR,c %·d−1 | 14.2 | 12.4 | 8.7 | 7.5 | 0.94 | <0.001 | 0.11 | 0.78 |
Initial body weight at 2 days old.
Final body weight at 10 ± 0.5 and 28 ± 0.8 days of age.
Fractional synthetic rate of longissimus dorsi mixed protein homogenate. n = 7 per milk formula per age group for 28 observations, see Materials and Methods for details.
Fig. 3.
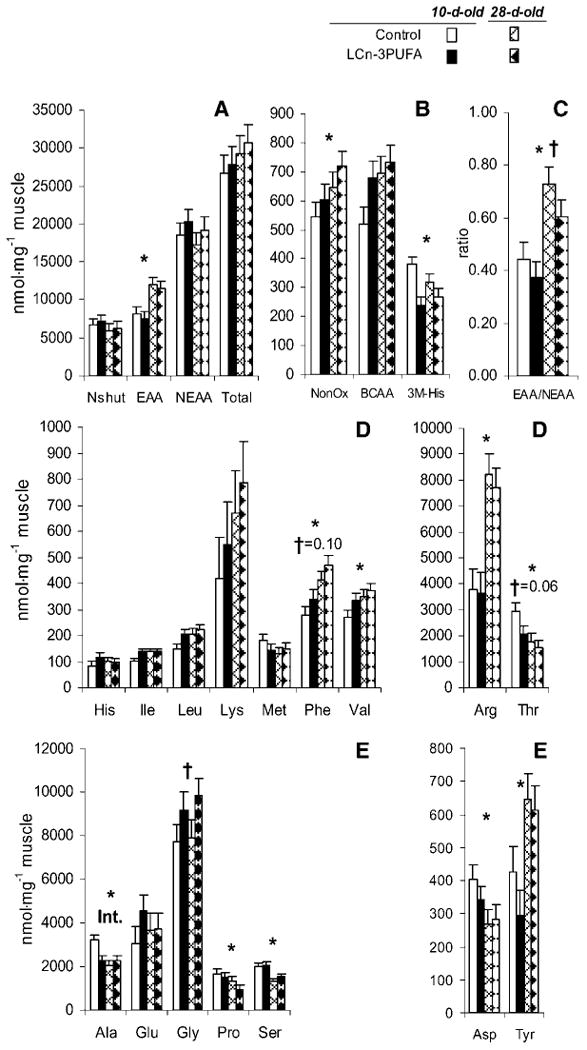
Concentrations of free amino acids in longissimus dorsi homogenate sampled at the end of the phenylalanine kinetic study. A: Sum of N shuttles (Nshut = alanine, aspartate, glutamate), essential amino acids (EAA), nonessential amino acids (NEAA), and total amino acids (Total). B: Amino acids not oxidized within the muscle (NonOx = phenylalanine, histidine, methionine), branched-chain amino acids (BCAA = valine, isoleucine, leucine), and 3-methyl-histidine (3M-His). C: Ratio of essential to nonessential amino acids (EAA/NEAA). D: Individual essential amino acids. E: Individual nonessential amino acids. * Age effect with P < 0.05; † milk formula effect with P < 0.05 unless noted otherwise. Int represents interaction between age and milk formula (P < 0.05); n = 7 piglets per milk formula per age group for 28 observations.
Regression analyses and modeling
The linear regressions between amino acid disposal, designated as the dependent variable, and hyperinsulinemic clamp-induced plasma insulin, designated as the independent variable (see Fig. 2A, B below), were performed using the regression procedure of SAS (33). The modeling of the LCn-3PUFA enrichment of muscle total membrane phospholipids and their alteration in n-6 fatty acid content during the neonatal period were assessed using the nonlinear procedure of SAS (see Fig. 4A, B below). A similar procedure was used to model insulin-stimulated amino acid disposal along with the membrane LCn-3PUFA content (see Fig. 5 below).
Fig. 2.
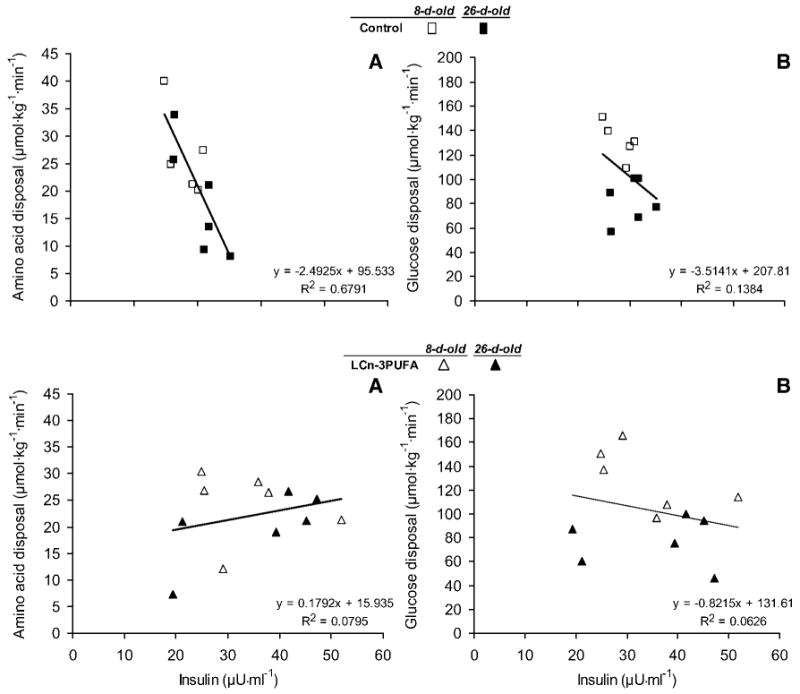
Relationship between whole body net mixed amino acid (A) or glucose (B) disposal rates and plasma insulin concentrations elicited during a 100 ng·(kg−0.66)−1·min−1 insulin clamp procedure in neonatal piglets. The piglets were fed during the first 8 or 26 days of age either a control milk formula with a similar composition to the sow's milk or a formula enriched in LCn-3PUFA. Each symbol represents the average disposal during the steady-state hour of the clamp procedure of a pig (n = 6 piglets per milk formula per age). The slopes of the amino acid disposals (A) are different (P < 0.001) between control and LCn-3PUFA piglets; the slopes of the glucose disposals (B) are not different (P > 0.05). An outlier datum was eliminated for the control milk formula at 8 days of age.
Fig. 4.
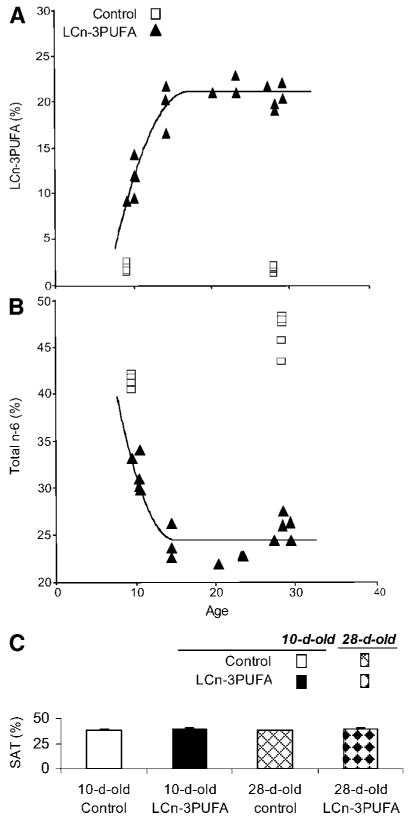
A, B: Temporal enrichment of longissimus dorsi total membrane phospholipids (percentage total by weight) in LCn-3PUFAs (A) and total n-6 PUFAs (B) in neonatal piglets fed either a control milk formula with a similar composition to the sow's milk or one enriched in LCn-3PUFAs from 2 days old until 10, 14, 21, or 28 days of age. The control milk formula was compared for the ages 10 and 28 days; the LCn-3PUFA formula was compared for the ages 10, 14, 21, and 28 days. These data originate from a pilot study (n = 3 per age group per milk formula). C: Percentage of total saturated fatty acids (SAT) in piglets of the main study; error bars indicate SEM of the complete randomized design (n = 5 per milk formula per age group).
Fig. 5.
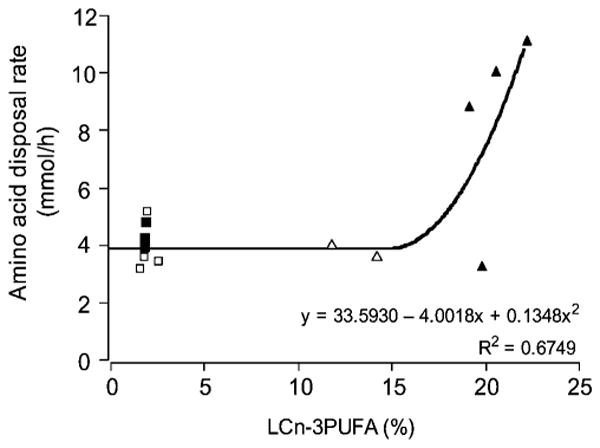
Amino acid disposal rates (mmol·h−1) against LCn-3PUFA content in longissimus dorsi total membrane phospholipids (percentage total by weight) in piglets fed either a control milk formula with a similar composition to that of the sow's milk or one enriched in LCn-3PUFAs during the first 8 or 26 days of age. Open triangles, n = 3; closed triangles, n = 4; open squares, n = 4; closed squares, n = 3. Unequal numbers of observations occurred because membrane phospholipids were analyzed for five piglets per milk formula per age group and the clamp procedure was not performed on every animal.
Other statistical tests
The baseline and the clamp-induced concentrations of glucose and branched-chain amino acids (see Fig. 1 below) were analyzed for the difference from zero using Student's paired t-test; error bars represent the 10% variation allocated to maintain the clamps. Parenteral feeding-induced and clamp-induced insulin concentrations were compared according to a factorial 2 × 2 × 2 accounting for the effects of age, formula, and the measurements (i.e., the parenteral feeding-induced and the clamp-induced insulin) using the GLM procedure of SAS and type III error (see Fig. 6 below).
Fig. 1.
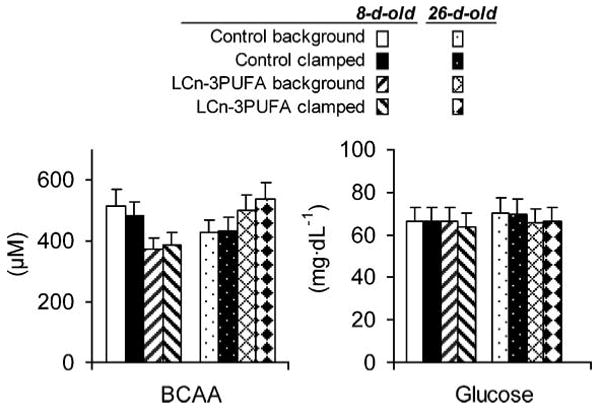
Plasma concentrations of branched-chain amino acids and glucose during the steady hour of the 100 ng·(kg−0.66)−1·min−1 hyperinsulinemic-euglycemic-euaminoacidemic clamp procedure in neonatal piglets. The concentrations were similar, with P = 0.05 according to a paired t-test. Error bars represent the 10% error allocated to maintain the clamps. LCn-3PUFA, long-chain n-3 polyunsaturated fatty acids. BCAA, branched-chain amino acids
Fig. 6.
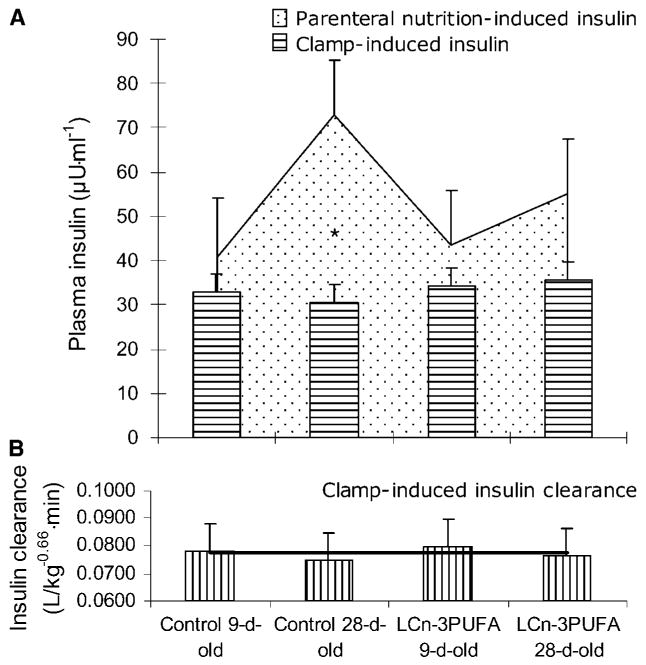
A: Plasma insulin concentrations resulting from either an exogenous insulin infusion during a clamp procedure performed at 100 ng·(kg−0.66)−1·min−1 or naturally elicited during continuous total parenteral nutrition. B: Insulin clearance rate calculated from the clamp trial. Neonatal piglets were raised on either a control milk formula with a similar composition to that of the sow's milk or one enriched in LCn-3PUFAs. Clamp procedures were conducted at 8 or 26 days of age (n = 6); kinetic studies were conducted at 10 or 28 days of age (n = 7). * Insulin is different between parenteral nutrition and the clamp procedure, with P = 0.009.
Results
Muscle membranes of the newborn are sensitive to dietary fatty acids (Table 4), and the dynamism of fatty acid acylation and deacylation enables changes in composition, which are further affected by time. Total membrane phospholipids of longissimus dorsi were enriched in LCn-3PUFAs by 776% on average, and the increment was more notable in 28 day old piglets. According to a similar pattern, the total n-3 fatty acid series was increased by 643%. This increase in n-3 fractions was attributable to augmentations in C20:5 n-3 (>800%), C22:5 n-3 (+169%), and C22:6 n-3 (+737%). As n-3 fatty acids exhibit a great affinity for phospholipids, the total n-6 fatty acid series was concomitantly decreased by 34% on average by menhaden oil feeding. This reduction in total n-6 fatty acids was mostly accounted for by decreases in C18:2 n-6 (−41%) and C20:4 n-6 (−33%) contents. Higher n-3/n-6 ratios, increasing from 0.055 to 0.605 in oil-fed piglet membranes (+10-fold), were observed in total phospholipids, and time effectiveness partially defined this response as well. Phospholipid total saturation remained unaltered, independently of dietary changes and time of weaning. The resulting saturation degree of membranes, defined as the ratio of PUFAs to saturated fatty acids, was decreased by menhaden oil feeding, showing that this index is n-6 series-driven.
The intramuscular triglycerides of the newborn are also sensitive to dietary changes, and time is an important contributor to the modeling of their composition (Table 5). However, the dynamic of fatty acid acylation and deacylation, leading to a contrasting composition to that of the phospholipids, reflects the different biochemical characteristics of these two lipids. The LCn-3PUFA content of intramuscular triglycerides increased to a lesser extent than in the phospholipids when they were expressed either in percentage (Table 5) or quantitatively in mg fatty acids·100 g−1 wet tissue (Table 6). Their contents in 10 and 28 day old piglets fed the control milk formula was 0.5% and 0.3%, respectively. When menhaden oil substituted control oil, this amount increased to 4.8% and 6.6%, respectively. The LCn-3PUFA content in triglycerides reached 36% of that of membrane phospholipids. Total n-6 fatty acid content was time-dependently reduced with menhaden oil feeding (−20%), and this was mainly accounted for by a decline in C18:2 n-6. Ratios of total n-3/n-6 fatty acids were augmented with dietary menhaden oil in a time-dependent manner.
TABLE 5.
Fatty acid composition of intramuscular triglycerides of longissimus dorsi muscle in neonatal piglets
| 10 Days Old | 28 Days Old | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid | Control | LCn-3PUFA | Control | LCn-3PUFA | SEM | Age | Milk | Age × Milk |
| 14:0 | 3.5 | 2.8 | 3.9 | 2.5 | 0.07 | 0.34 | <.0001 | 0.0003 |
| 16:0 | 42.9 | 42.2 | 39.6 | 37.1 | 1.29 | 0.005 | 0.22 | 0.51 |
| 16:1 n-7 | 1.1 | 2.4 | 1.0 | 2.7 | 0.13 | 0.44 | <0.0001 | 0.22 |
| 18:0 | 5.3 | 5.3 | 7.4 | 8.3 | 0.31 | <0.0001 | 0.13 | 0.16 |
| 18:1 n-9 | 23.3 | 21.2 | 25.7 | 26.1 | 1.15 | 0.005 | 0.47 | 0.31 |
| 18:1 n-7 | 2.9 | 3.8 | 1.9 | 2.7 | 0.15 | <0.0001 | <0.0001 | 0.65 |
| 18:2 n-6 | 18.0 | 13.7 | 18.2 | 10.9 | 0.30 | 0.0004 | <0.0001 | 0.0002 |
| 18:3 n-3 | 0.65 | 0.86 | 0.59 | 0.85 | 0.02 | 0.15 | <0.0001 | 0.30 |
| 18:4 n-3 | 0.13 | 0.30 | 0.18 | 0.46 | 0.02 | <0.0001 | <0.0001 | 0.007 |
| 20:0 | 0.26 | 0.26 | 0.22 | 0.21 | 0.01 | 0.009 | 0.80 | 0.66 |
| 20:1 n-9 | 0.43 | 0.71 | 0.24 | 0.54 | 0.06 | 0.007 | 0.0001 | 0.87 |
| 20:3 n-6 | 0.20 | 0.24 | 0.11 | 0.12 | 0.02 | 0.0005 | 0.30 | 0.66 |
| 20:3 n-3 | 0.05 | 0.13 | 0.05 | 0.11 | 0.02 | 0.48 | 0.0005 | 0.40 |
| 20:4 n-6 | 0.51 | 0.48 | 0.51 | 0.48 | 0.07 | 0.99 | 0.66 | 0.96 |
| 20:4 n-3 | 0.07 | 0.40 | 0.00 | 0.52 | 0.03 | 0.41 | <0.0001 | 0.004 |
| 20:5 n-3 | 0.01 | 0.47 | 0.01 | 1.2 | 0.06 | <0.0001 | <0.0001 | <0.0001 |
| 22:4 n-6 | 0.36 | 0.58 | 0.17 | 0.26 | 0.05 | <0.0001 | 0.005 | 0.18 |
| 22:5 n-6 | 0.22 | 0.50 | 0.05 | 0.25 | 0.02 | <0.0001 | <0.0001 | 0.04 |
| 22:5 n-3 | 0.22 | 1.4 | 0.14 | 1.4 | 0.05 | 0.88 | <0.0001 | 0.12 |
| 22:6 n-3 | 0.12 | 2.4 | 0.10 | 3.3 | 0.15 | 0.02 | <0.0001 | 0.01 |
| SAT | 52.0 | 50.6 | 51.1 | 48.1 | 1.14 | 0.18 | 0.08 | 0.51 |
| PUFA | 20.5 | 21.5 | 20.1 | 19.9 | 0.32 | 0.006 | 0.30 | 0.11 |
| Total n-6 | 19.3 | 15.5 | 19.0 | 12.0 | 0.34 | <0.0001 | <0.0001 | 0.0002 |
| Total n-3 | 1.3 | 6.0 | 1.1 | 7.8 | 0.26 | 0.004 | <0.0001 | 0.001 |
| Total n-3/n-6 | 0.06 | 0.38 | 0.06 | 0.65 | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| LCn-3PUFA | 0.4 | 4.8 | 0.30 | 6.6 | 0.25 | 0.005 | <0.0001 | 0.001 |
| PUFA/SAT | 0.40 | 0.42 | 0.39 | 0.41 | 0.01 | 0.59 | 0.04 | 0.69 |
Values shown are percentage of total by weight. SAT, sum of saturated fatty acids; PUFA, sum of polyunsaturated fatty acids; Total n-6, sum of n-6 fatty acids; Total n-3, sum of n-3 fatty acids; n-3/n-6, ratio of total n-3 to total n-6 fatty acids; LCn-3PUFA, 20:5 n-3 + 22:5 n-3 + 22:6 n-3; PUFA/SAT, ratio of polyunsaturated fatty acids to saturated fatty acids. n = 5 per milk formula per age group for 20 observations; see Materials and Methods for details.
TABLE 6.
Composition between different fatty acid groups of total membrane phospholipids and intramuscular triglycerides of longissimus dorsi muscle in neonatal piglets expressed on a quantitative basis
| 10 Days Old | 28 Days Old | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid Group | Control | LCn-3PUFA | Control | LCn-3PUFA | SEM | Age | Milk | Age × Milk |
| Total membrane phospholipids, mg·100 g−1 wet tissue | ||||||||
| SAT | 169 | 174 | 131 | 136 | 5 | <0.0001 | 0.34 | 0.92 |
| PUFA | ||||||||
| Total n-6 | 182 | 139 | 160 | 89 | 8 | 0.0003 | <0.0001 | 0.10 |
| Total n-3 | 11 | 52 | 7 | 73 | 3 | 0.007 | <0.0001 | 0.0004 |
| Total n-3/n-6 | 0.058 | 0.378 | 0.043 | 0.830 | 0.028 | <0.0001 | <0.0001 | <0.0001 |
| LCn-3PUFA | 8 | 50 | 6 | 71 | 3 | 0.003 | <0.0001 | 0.0004 |
| PUFA/SAT | 1.14 | 1.10 | 1.27 | 1.19 | 0.03 | 0.004 | 0.08 | 0.51 |
| Intramuscular triglycerides, mg·100 g−1 wet tissue | ||||||||
| SAT | 473 | 328 | 324 | 310 | 42 | 0.07 | 0.08 | 0.14 |
| PUFA | ||||||||
| Total n-6 | 175 | 101 | 120 | 76 | 13 | 0.008 | 0.0003 | 0.27 |
| Total n-3 | 11 | 38 | 7 | 50 | 4 | 0.31 | <0.0001 | 0.04 |
| Total n-3/n-6 | 0.06 | 0.39 | 0.05 | 0.66 | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| LCn-3PUFA | 4 | 31 | 2 | 42 | 4 | 0.17 | <0.0001 | 0.06 |
| PUFA/SAT | 0.40 | 0.42 | 0.39 | 0.41 | 0.01 | 0.59 | 0.04 | 0.69 |
Values shown are g/100 g wet tissue. SAT, sum of saturated fatty acids; PUFA, sum of polyunsaturated fatty acids; Total n-6, sum of n-6 fatty acids; Total n-3, sum of n-3 fatty acids; n-3/n-6, ratio of total n-3 to total n-6 fatty acids; LCn-3PUFA, 20:5 n-3 + 22:5 n-3 + 22:6 n-3; PUFA/SAT, ratio of polyunsaturated fatty acids to saturated fatty acids. n = 5 per milk formula per age group for 20 observations; see Materials and Methods for details.
Branched-chain amino acids and glucose disposals were maintained during clamp procedures (Fig. 1) at a fed insulinemia [33.3 ± 4.3 μU·ml−1 (SEM)] generated by a 100 ng·(kg−0.66)−1·min−1 insulin infusion. The decline in insulin sensitivity with age was readily apparent when amino acids and glucose disposals were regressed against plasma insulin in 8 and 26 day old pigs (4) (Fig. 2A, B). Control piglets demonstrated the neonatal decline of muscle sensitivity to use amino acids in response to insulin stimulation. Within 8 and 26 days of age, amino acid utilization rates were reduced, with a sharp downward slope of −2.24 μmol·kg−1·min−1 per unit of insulin increment within a narrow range of insulin concentration (r2 = 0.63, P = 0.01), and the lower utilization rates belonged mainly to older piglets. In contrast, the 26 day old piglets fed the LCn-3PUFA-enriched milk formula elicited amino acid disposal rates that were similar to those in 8 day old piglets. This was shown with a nil slope along with increments of plasma insulin (slope = 0.3078, r2 = 0.30, P = 0.53). The slopes of amino acid disposals expressed against plasma insulin between the two formulas were different (P < 0.001). Glucose disposal in response to insulin stimulation declined with age in both groups of piglets, as lower utilization rates belonged mainly to older piglets (Fig. 2B).
In contrast, when the mean effect of milk formula was investigated regardless of plasma insulin concentration using ANOVA, age reduced the utilization rates of amino acids by 28% (from 27.1 to 19.4 μmol·kg−1·min−1, SEM = 3.6; P = 0.04) and of glucose by 42% (from 24.8 to 14.4 μmol·kg−1·min−1, SEM = 2.0; P < 0.001). Using this statistical approach, the milk formula effect per se is no longer discernible. These contrasting conclusions emphasize a conjectural inability to detect an interaction effect by ANOVA, which uses a mean error term, and potentially because of an insufficient observation number. In the context of this study, the slope comparison is a more appropriate approach to assess the formula effects with more statistical power: the error term is calculated via a deviation between the individual observations and a prediction curve. Because the data are consistent with the regression models, this offsets the effect of the observation number and increases the power of the test. The mean plasma insulin concentrations monitored during the clamp procedures were similar between age groups and milk replacers and averaged 34 and 33 μU·ml−1 (SEM = 4.1) in 8 and 26 day old piglets, respectively. Body weight was not altered by milk formula, nor was body weight gain. Feed intake was not measured, as piglets were fed in bolls and spillage compromised the measurement. A previous study with steers showed that the improvement in insulin sensitivity translated into a reduction of feed intake with similar body weight gain, and these resulted in a tendency for improved feed conversion (11).
To further investigate the effect of LCn-3PUFAs on insulin-stimulated protein metabolism, tracer kinetic studies were performed when insulinemia was naturally induced through continuous parenteral nutrition. At steady state, insulin, glucose, and amino acid concentrations tended to change with age (P ≤ 0.10) (Table 7), and LCn-3PUFA feeding did not further alter these parameters. Development tended to reduce the whole body irreversible loss rate of phenylalanine (−5%; P = 0.09), its entry into plasma from protein breakdown (−15%, tendency, P = 0.09) (14), and it decreased oxidative metabolism (−17%; P = 0.03), as reported previously (14). This study further shows that LCn-3PUFA feeding to neonates decreased the oxidative metabolism of phenylalanine by 16% compared with control feeding (P = 0.06). Concomitantly, accretion of phenylalanine into body proteins tended to increase by 41% (P = 0.06).
Amino acid metabolism in skeletal muscle was also affected by LCn-3PUFA feeding. The fractional synthetic rate of muscle mixed proteins was downregulated by development (−39%; P = 0.001) (Table 7), whereas the intracellular concentration of total amino acids remained steady (Fig. 3). The intracellular concentration of 3-methyl-histidine followed a similar pattern to that of the fractional synthetic rate of protein, mirroring protein turnover. The ratio of essential to nonessential amino acids in the muscle free pool increased (+64%) with development (Fig. 3) (P < 0.0001). The latter was responsible for increased essential amino acids such as arginine (+114%), phenylalanine (+43%), and valine (+19%); threonine decreased (−33%) with development. In contrast, the concentrations of many nonessential amino acids such as alanine (−21%), aspartate (−26%), proline (−28%), and serine (−30%) were reduced, but tyrosine increased (+74%) in a parallel manner to phenylalanine. LCn-3PUFAs did exert further regulation on free amino acids in muscle. LCn-3PUFA consumption retarded the developmental increase in the elevation of the ratio of essential to nonessential amino acids in the free pool of muscle homogenate (−17%; P = 0.03).
Discussion
There are several important issues highlighted by the use of a neonate animal model in this study. During the postnatal period of the piglet, comprising the first month of life, there is a dramatic decrease in the rate of protein metabolism (34). The developmental decline in protein metabolic rate is paralleled to a developmental decrease in insulin sensitivity. Previous investigations in the field have elucidated endocrine, nutritional, and molecular aspects involved in this regulation (3, 35–38). In this study, the effect of the feeding of a milk-based diet supplemented or not with LCn-3PUFAs from 2 days after birth until 28 days of age enabled the exploration of the effectiveness of LCn-3PUFAs at regulating protein metabolism in the neonatal period. This goal was achieved using two approaches: the monitoring of whole body amino acid and glucose disposal during a predefined fed hyperinsulinemia using the clamp procedure with fixed insulin entry rate, and the monitoring of phenylalanine kinetics during a naturally induced hyperinsulinemia elicited using total parenteral nutrition. The mature sow's milk composition was reproduced in the experimental formula to not denature the newborn piglet model. Natural milk is a source of different nutrients and bioactive factors that are not found in formula (39–41). However, natural milk is low in LCn-3PUFAs, and these are now frequently added to formula to meet requirements for neural and visual development (42–44). In this regard, LCn-3PUFA supply may become more relevant in many respects.
Membranes are structures that undergo continual remodeling of fatty acyls (45), and muscle membrane phospholipids of the newborn are tremendously dynamic in this respect. The current study shows that the fatty acid modeling of muscle total membranes with LCn-3PUFAs is sensitive to dietary intake, with a 776% increase in their content in response to a LCn-3PUFA intake that represented 3.5% of the formula. The period of time allocated to the enrichment of membranes with LCn-3PUFAs is a second dominant feature that was noticeable when the piglets were raised with the supplemented milk replacer until 28 days of age. Further modeling of the temporal shaping of piglet muscle membrane phospholipids (Fig. 4A) reveals a sharp enrichment in LCn-3PUFA, starting from a 3% baseline, that is typical of humans or animals fed a diet deprived of marine products (5, 6, 25), to 10–14% within 7 days of supplementation (from 2 to 10 days old). Only 12 days in total of LCn-3PUFA feeding were sufficient to reach a plateau of 18–22% in total muscle membranes, which was maintained onward along with a constant LCn-3PUFA intake. The latter suggests that a maximal LCn-3PUFA incorporation threshold in muscle phospholipids is effective. A similar plateau attaining 20–22% was noticeable for liver plasma membranes after ∼2 weeks of LCn-3PUFA supplementation (46, 47).
In this study, the partial substitution of dietary n-6 of the control oil by n-3 fatty acids with menhaden oil was almost symmetrical to the impoverishment of membranes in the n-6 series over the same period of time (Fig. 4B). Higher enzyme affinity for the acylation of absorbed LCn-3PUFAs, combined with direct LCn-3PUFA recycling between phospholipid fractions within the membrane, facilitate a dynamic favoring n-3 retention in membranes at the expense of n-6 series (5, 6, 45). These substitutions within PUFA fractions occur without alteration in membrane saturation, in accordance with other animal models (11, 46, 48). The latter observation is consistent with the findings that the LCn-3PUFA effects on metabolism are mediated through the membrane ratio of n-3 to n-6 fatty acids. In parallel, menhaden oil feeding was accompanied by a reduction of arachidonic acid content in muscle membranes. Because arachidonic acid plays crucial roles in prostaglandin biosynthesis, and membranes are seen as a biological reservoir of the latter, further investigations will be required to address this implication.
Intramuscular triglycerides are a major form of energy storage and represent a significant fuel for cellular metabolism (49). In the current study, this lipid fraction was mainly modified according to the dietary profile. In contrast to phospholipids, there was no preferential incorporation of n-3 versus n-6 fatty acids into triglycerides on a qualitative or quantitative basis. Accordingly, membranes contained 61 mg LCn-3PUFA·100 g−1 wet tissue muscle, whereas triglycerides achieved a content of 31 mg LCn-3PUFA·100 g−1 on average for the same period of time of feeding the enriched formula. The difference between structural (phospholipids) and storage (triglycerides) lipids corroborates specific requirements coupled to a greater affinity for n-3 fatty acids in the modeling of membranes that relate to their biological functions (50, 51).
Insulin is central to the growth of neonates, as they are frequently fed small meals and require the efficient use of dietary nutrients to sustain their development. In this study, LCn-3PUFAs affected certain insulin-regulated facets that are otherwise developmentally downregulated. Under a continuous exogenous entry of insulin that generated a fed insulinemia of 33 μU·ml−1, the control piglets exhibited a developmental decrease in amino acid disposal (3, 4). The LCn-3PUFA feeding blunted this developmental decline, resulting in amino acid disposal rates that were similar for both age intervals. These insulin-induced net movements of amino acids in LCn-3PUFA-fed piglets suggest that the pathways of amino acid oxidation plus accretion into proteins are maintained throughout the first 28 days of age, but their relative extent may differ. The current study also highlights the facts that the amino acid disposal rate induced by insulin was a function of the LCn-3PUFA content in total muscle membrane phospholipids and that a minimal threshold of 15–18% LCn-3PUFAs was required to elicit greater amino acid disposal (Fig. 5). These regulations occurred while insulin concentrations and hepatic clearance were similar between age and milk formula (Fig. 6B).
The amino acid metabolic pathways that underlie the net movement of amino acids measured during the clamp procedures were further investigated using tracer kinetic studies during insulinemia elicited naturally by parenteral nutrition. In this respect, the negative effect of development on insulin-mediated amino acid disposal in the control piglets was translated by a tendency for a decrease in whole body protein turnover. This was accounted for by reduced phenylalanine exit from the pool for oxidative pathways, whereas entries into the plasma were characterized by a decreased protein breakdown, as reported previously (52). As LCn-3PUFA feeding maintained high rate of insulin-induced amino acid disposal during the first 28 days of age, the pathways by which LCn-3PUFAs affect protein metabolism needed to be delineated. During parenteral nutrition-induced insulinemia, the LCn-3PUFA-enriched milk formula augmented phenylalanine accretion into body proteins by 41%. Because net accretion results from the positive balance between protein synthesis and proteolysis, the latter supports an anabolic effect of LCn-3PUFA feeding. Amino acid oxidation tended to be decreased by 16%, which reduces amino acid losses through catabolic pathways. This decline in amino acid oxidation contributed to the anabolic milieu facilitating a positive balance between synthesis and breakdown and retention into body proteins. We recently reported a similar downregulation of oxidative metabolism and improved muscle anabolism via the activation of the PKB-mTOR-S6K1 signaling pathway by LCn-3PUFA feeding in growing steers (11). The present study further supports the speculation of a more insulin-resistant environment in the control 28-day-old piglets compared with the other pigs, as the plasma insulin resulting naturally from the parenteral feeding was augmented to a greater extent compared with the controlled clamp-induced insulin (P = 0.009) (Fig. 6A). A previous study with steers showed that the improvement in insulin sensitivity translated to a tendency for a reduction in the amount of food converted to gain 1 kg of body weight (11). In the current study, body weight was unaltered by milk formula, as monitored previously, and intake was not measured; in this respect, feed conversion was not assessed as a whole body parameter of insulin sensitivity.
Further explorations of the specific effects of LCn-3PUFAs on skeletal muscle nitrogen homeostasis showed that the fractional synthetic rate of muscle mixed protein was reduced (−39%) with development. However, LCn-3PUFAs did not alter the fractional synthesis rate of muscle protein during the neonatal period. To enable a 41% higher whole body protein accretion in response to LCn-3PUFA supplementation, for which 49% was potentially partitioned toward the hindlimb skeletal musculature (14), the fractional protein breakdown must have been decreased proportionally with the LCn-3PUFA formula. The concentration of free amino acids in the muscle has been associated with sustained (53–55), increased (53, 56, 57), and even decreased (54) muscle fractional synthetic rates of proteins in the context of enhanced anabolism by feeding, exercise, and development; proteolysis is suspected of playing a codominant role in shaping intracellular pool size as a counterregulatory control of net accretion. The unaltered total concentration and increased essential amino acids in the cellular milieu do not suggest that a developmental limitation in essential amino acids restrained protein synthesis by the lack of availability for aminoacyl-tRNA formation and incorporation into proteins (53–55). Conversely, increased concentrations of many essential amino acids (cysteine, tyrosine, histidine, leucine, methionine, and threonine) in L6 myotube culture have been shown to inhibit the insulin signaling pathway (58), and as such, an eventual in vivo regulation appears developmentally plausible. Interestingly, the ratio of essential to nonessential amino acids was increased by development, and LCn-3PUFA feeding retarded this increase, thereby reducing the effect of development on this parameter. The current data do not delineate whether nonessential amino acids exert a coregulation in this process. Because many of the nonessential amino acids were reduced along with the fractional protein synthetic rate, and the sum of amino acids shuttling nitrogen was kept stable with development, we speculate that the synthesis of nonessential amino acids in the milieu is central to the cellular nitrogen homeostasis that affects certain gene expression (59, 60). Further tracer investigation studies will be required to better understand whether the alteration of this ratio is a consequence of synthesis that mirrors lower turnover and low enzymatic activity or that reflects a requirement for some nonessential amino acids to sustain nitrogen management within the cellular milieu.
Perspectives
The results of this study show that the composition of muscle membrane phospholipids in the neonate is highly sensitive to dietary intake and that membrane phospholipids, in contrast to intramuscular triglycerides, have a high affinity for LCn-3PUFAs. Importantly, LCn-3PUFA feeding maintained the sensitivity of insulin-regulated amino acid disposal during the neonatal period, whereas it was otherwise reduced by development when the control formula was consumed. Furthermore, a minimal LCn-3PUFA muscle membrane content of 18–22% elicited an improvement in the insulin sensitivity of amino acid disposal. A parenteral nutrition-elicited insulinemia consistently confirmed the more anabolic conditions conferred by the LCn-3PUFAs throughout the neonatal period, potentially by a reduction in oxidative metabolism that allocated more amino acids for accretion into whole body proteins. Although the developmental decline in the fractional synthetic rate of muscle proteins was not altered by LCn-3PUFA feeding, the intracellular composition of the essential-to-nonessential amino acid ratio was developmentally up-regulated, and LCn-3PUFA feeding retarded this increase. These findings provide important new evidence of the potential role of LCn-3PUFAs in the regulation of protein metabolism and insulin sensitivity in the neonate.
Acknowledgments
A special thank you is extended to Grober Nutrition (Cambridge, Ontario, Canada), which kindly provided expertise on the effective preparation of milk replacers and provided lactose, sodium caseinate, whey protein, vitamin, and mineral mix with soybean emulsifiers. Special thanks are also extended to Omega Protein, Inc. (Reedville VA), which kindly provided the menhaden oil required in these studies. The authors gratefully thank Mr. Richard Prince and Gaston Côté for their great animal care and assistance in conducting studies. Appreciation is also extended to Mrs. Line Berthiaume, who performed the analyses of the fatty acid profiles of muscle phospholipid fractions. Mr. Gaétan Daigle is thanked for his assistance with the statistical analyses. This work was supported by grants from the Fonds Québéquois de la Recherche sur la Nature et les Technologies, the Conseil de Recherches en Pêche et Agriculture du Québec, and the Institute of Nutraceuticals and Functional Food, Faculty of Food Sciences and Agriculture, Laval University.
Abbreviations
- IE
isotopic enrichments
- LCn-3PUFA
long-chain n-3 polyunsaturated fatty acid
- PB
protein breakdown
- PS
protein synthesis
References
- 1.Makrides M, Gibson RA, Udell T, Ried K. Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr. 2005;81:1094–1101. doi: 10.1093/ajcn/81.5.1094. [DOI] [PubMed] [Google Scholar]
- 2.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 3.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acids clamps decreases with development. Am J Physiol Endocrinol Metab. 1997;273:E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr. 1998;128(Suppl):347–350. doi: 10.1093/jn/128.2.347S. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary omega-3 and polyunsaturated fatty acids modify fatty acyl composition and insulin binding in skeletal-muscle sarcolemma. Biochem J. 1994;299:831–837. doi: 10.1042/bj2990831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chishlom DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328:238–244. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- 7.Storlien LH, Pan DA, Kriketos AD, O'Connor J, Caterson ID, Cooney GJ, Jenkins AB, Baur LA. Skeletal muscle membrane lipids and insulin resistance. Lipids. 1996;31(Suppl):261–265. doi: 10.1007/BF02637087. [DOI] [PubMed] [Google Scholar]
- 8.Daveloose D, Linard A, Asfi T, Viret J, Christon R. Simultaneous changes in lipid composition, fluidity and enzyme activity in piglet intestinal brush border membrane as affected by dietary polyunsaturated fatty acid deficiency. Biochim Biophys Acta. 1993;1166:229–237. doi: 10.1016/0005-2760(93)90102-f. [DOI] [PubMed] [Google Scholar]
- 9.Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab. 2002;282:E664–E671. doi: 10.1152/ajpendo.00320.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kamada T, Yamashita T, Baba Y, Kai M, Setoyama S, Chuman Y, Otsuji S. Dietary sardine oil increases erythrocyte membrane fluidity in diabetic patients. Diabetes. 1986;35:604–611. doi: 10.2337/diab.35.5.604. [DOI] [PubMed] [Google Scholar]
- 11.Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombroski L, Couture Y, Dubreuil P, Myre A, Bergeron K, et al. Long-chain omega-3 fatty acids regulate whole-body protein metabolism by promoting muscle insulin signaling to the AktmTOR-S6K1 pathway and insulin sensitivity. J Physiol (Lond) 2007;579:269–284. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Research Council. Nutrient Requirements of Swine. 10th revised. National Academy of Sciences; Washington, DC: 1998. [Google Scholar]
- 13.Pond WG, Mersmann HJ. Biology of the Domestic Pig. Cornell University Press; Ithaca, NY: 2001. [Google Scholar]
- 14.Thivierge MC, Bush JA, Suryawan A, Nguyen HV, Orellana RA, Burrin DG, Jahoor F, Davis TA. Whole body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J Nutr. 2005;35:1430–1435. doi: 10.1093/jn/135.6.1430. [DOI] [PubMed] [Google Scholar]
- 15.Canadian Council on Animal Care. Guide to Care and Use of Experimental Animals. 2nd. Bradda Printing Services, Inc.; Ottawa, Ontario, Canada: 1993. [Google Scholar]
- 16.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- 17.Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophotometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem. 1996;240:48–53. doi: 10.1006/abio.1996.0329. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 19.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- 20.Burrin DG, Stoll B, Jiang R, Cahng X, Hartmann B, Holst HJ, Greeley GH, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–1610. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 21.Bertolo RFP, Chen CZL, Law G, Pencharz PB, Ball RO. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. J Nutr. 1998;128:1752–1759. doi: 10.1093/jn/128.10.1752. [DOI] [PubMed] [Google Scholar]
- 22.Calder AG, Garden KE, Anderson SE, Lobley GE. Quantification of blood and plasma amino acids using isotope dilution electron impact gas chromatography/mass spectrometry with U-13C amino acids as internal standards. Rapid Commun Mass Spectrom. 1999;13:2080–2083. doi: 10.1002/(SICI)1097-0231(19991115)13:21<2080::AID-RCM755>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Association of Official Analytical Chemists, editor. Official Methods of Analysis. 15th. AOAC; Arlington, VA: 1990. [Google Scholar]
- 24.Chapman RA, Mackay K. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J Am Oil Chem Soc. 1949;26:321–325. [Google Scholar]
- 25.Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julien C, Berthiaume L, Hadj-Tahar A, Rajput AH, Bedard PJ, Paolo TD, Julien P, Calon F. Postmortem brain fatty acid profile of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem Int. 2006;48:404–414. doi: 10.1016/j.neuint.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr. 1999;129:1579–1584. doi: 10.1093/jn/129.8.1579. [DOI] [PubMed] [Google Scholar]
- 28.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 29.Campbell IM. Incorporation and dilution values—their calculation in mass spectrally assayed stable isotope labeling experiments. Bioorg Chem. 1974;3:386–397. [Google Scholar]
- 30.Kyu-Il K, McMillan I, Bayley HS. Determination of amino acid requirements of young pigs using an indicator amino acid. Br J Nutr. 1983;50:369–382. doi: 10.1079/bjn19830104. [DOI] [PubMed] [Google Scholar]
- 31.Wykes LJ, Fiorotto ML, Burrin DG, Del Rosario M, Frazer ME, Pond WG, Jahoor F. Chronic low protein intake reduces tissue protein synthesis in a pig model of protein malnutrition. J Nutr. 1996;126:1481–1488. doi: 10.1093/jn/126.5.1481. [DOI] [PubMed] [Google Scholar]
- 32.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol. 1999;277:E103–E109. doi: 10.1152/ajpendo.1999.277.1.E103. [DOI] [PubMed] [Google Scholar]
- 33.Statistical Analysis System. Release 8.02. SAS Institute, Inc.; Cary, NC: 2000. [Google Scholar]
- 34.Reeds PJ, Burrin DG, Davis TA, Fiorotto ML, Stoll B, Van Goudoever JB. Protein nutrition of the neonate. Proc Nutr Soc. 2000;59:87–97. doi: 10.1017/s0029665100000112. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor PMJ, Kimball SR, Suryawan A, Bush JA, Nguyen HV. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2004;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 37.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;281:E908–E915. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- 38.Kimball SR, Farrell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol Endocrinol Metab. 2001;282:E585–E592. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- 39.Savino F, Lupica MM. Breast milk: biological constituents for health and well-being in infancy. Recent Prog Med. 2006;97:519–527. [PubMed] [Google Scholar]
- 40.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependant factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res. 1995;37:593–599. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ. Colostrum enhances the nutritional stimulation of vital organ protein synthesis in neonatal pigs. J Nutr. 1997;127:1284–1289. doi: 10.1093/jn/127.7.1284. [DOI] [PubMed] [Google Scholar]
- 42.Crawford MA. Placental delivery of arachidonic and docosahexaenoic acids: implications for the lipid nutrition of preterm infants. Am J Clin Nutr. 2000;71(Suppl):275–284. doi: 10.1093/ajcn/71.1.275S. [DOI] [PubMed] [Google Scholar]
- 43.Arbuckle LD, MacKinnon MJ, Innis SM. Formula 18:2(n-6) and 18:3(n-3) content and ratio influence long-chain polyunsaturated fatty acids in the developing piglet liver and central nervous system. J Nutr. 1994;124:289–298. doi: 10.1093/jn/124.2.289. [DOI] [PubMed] [Google Scholar]
- 44.Smit EN, Martini IA, Kemperman RFJ, Schaafsma A, Muskiet FAJ, Boersma ER. Fatty acids in formulae for term infants: compliance of present recommendations with the actual human milk fatty acid composition of geographically different populations. Acta Pediatr. 2003;92:790–796. [PubMed] [Google Scholar]
- 45.MacDonald JIS, Sprecher H. Phospholipid fatty acid remodeling in mammalian cells. Biochim Biophys Acta. 1991;1084:105–121. doi: 10.1016/0005-2760(91)90209-z. [DOI] [PubMed] [Google Scholar]
- 46.Abel S, Gelderblom WCA, Smuts CM, Kruger M. Thresholds and kinetics of fatty acid replacement in different cellular compartments in rat liver as a function of dietary n-6/n-3 fatty acid content. Prostaglandins Leukot Essent Fatty Acids. 1997;56:29–39. doi: 10.1016/s0952-3278(97)90522-6. [DOI] [PubMed] [Google Scholar]
- 47.Innis SM, Clandinin MT. Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. Biochem J. 1981;193:155–167. doi: 10.1042/bj1930155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 49.Vessby B. Dietary fat and insulin action in humans. Br J Nutr. 2000;83(Suppl):91–96. doi: 10.1017/s000711450000101x. [DOI] [PubMed] [Google Scholar]
- 50.Kuksis A. Fatty acids and glycerides. In: Hanahan DJ, editor. Handbook of Lipid Research. Plenum Press; New York: 1978. pp. 341–379. [Google Scholar]
- 51.Voelker DR. Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiol Rev. 1991;55:543–560. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab. 1996;270:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- 53.Biolo G, Williams BD, Fleming RYD, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- 54.Bolster DR, Pikosky MA, Gaine CG, Martin W, Wolfe RR, Tipton KD, Maclean D, Maresh CM, Rodriguez NR. Dietary protein intake impacts human skeletal muscle protein fractional synthetic rates after endurance exercise. Am J Physiol Endocrinol Metab. 2005;289:E678–E683. doi: 10.1152/ajpendo.00060.2005. [DOI] [PubMed] [Google Scholar]
- 55.Tipton KD, Wolfe RR. Exercise-induced changes in protein metabolism. Acta Physiol Scand. 1998;162:377–387. doi: 10.1046/j.1365-201X.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 56.Volpi E, Ferrando A, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prod'homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, Grizard J. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adults and old rats. J Physiol. 2005;563:235–248. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 59.Cardenas ME, Cutler NS, Lorenz MC, Como CJD, Heitman J. The Tor signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komeili A, Wedaman KP, O'Shea EK, Powers T. Mechanism of metabolic control: target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J Cell Biol. 2000;151:863–878. doi: 10.1083/jcb.151.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


