Abstract
Human respiratory syncytial virus (HRSV), human metapneumovirus (HMPV), and human parainfluenza virus type 3 (HPIV3) are common, important respiratory pathogens, but HRSV has a substantially greater impact with regard to acute disease, long-term effects on airway function, and frequency of re-infection. It has been reported to strongly interfere with the functioning of dendritic cells (DCs). We compared HRSV to HMPV and HPIV3 with regard to their effects on human monocyte-derived immature DCs (IDC). Side-by-side analysis distinguished between common effects versus those specific to individual viruses. The use of GFP-expressing viruses yielded clear identification of robustly infected cells and provided the means to distinguish between direct effects of robust viral gene expression versus bystander effects. All three viruses infected inefficiently based on GFP expression, with considerable donor-to donor-variability. The GFP-negative cells exhibited low, abortive levels of viral RNA synthesis. The three viruses induced low-to-moderate levels of DC maturation and cytokine/chemokine responses, increasing slightly in the order HRSV, HMPV, and HPIV3. Infection at the individual cell level was relatively benign, such that in general GFP-positive cells were neither more nor less able to mature compared to GFP-negative bystanders, and cells were responsive to a secondary treatment with lipopolysaccharide, indicating that the ability to mature was not impaired. However, there was a single exception, namely that HPIV3 down-regulated CD38 expression at the RNA level. Maturation by these viruses was anti-apoptotic. Inefficient infection of IDC and sub-optimal maturation might result in reduced immune responses, but these effects would be common to all three viruses rather than specific to HRSV.
Keywords: metapneumovirus, pneumovirus, paramyxovirus, monocyte derived dendritic cells, cytokine, maturation marker
INTRODUCTION
We compared the effects of infection of human monocyte-derived immature dendritic cells (IDC) by human respiratory syncytial virus (HRSV) in a side-by-side comparison with human metapneumovirus (HMPV) and human parainfluenza virus type 3 (HPIV3) using viruses engineered to express enhanced green fluorescent protein (GFP). HRSV is recognized as the most important viral agent of serious pediatric respiratory tract disease worldwide (CDC, 2007; Collins and Crowe, 2007; Collins and Graham, 2008; Nicholson et al., 2006). Although known primarily as a pediatric pathogen, HRSV can infect and cause disease in individuals of all ages and can be particularly serious in the elderly and in severely immunosuppressed individuals such as allogeneic hematopoietic stem cell transplant recipients (Falsey and Walsh, 2000). HMPV was first described in 2001 following its isolation from infants and children who were experiencing HRSV-like disease of unknown etiology (van den Hoogen et al., 2001). HMPV has gained recognition as an important agent of the respiratory tract disease worldwide, especially in the pediatric and elderly populations, although its impact appears to be substantially less than that of HRSV (Hamelin et al., 2004; Williams et al., 2004). HPIV serotypes 1, 2, and 3 (HPIV1, 2, and 3) as a group are second only to HRSV as a cause of serious respiratory tract disease in infants and children (Hall, 2001; Welliver et al., 1986). The impact of HPIV3 is greater than that of HPIV1 and HPIV2. HRSV, HMPV and the HPIVs are non-segmented negative-strand RNA viruses of family Paramyxoviridae, which comprises two subfamilies: HRSV and HMPV belong to subfamily Pneumovirinae and are classified separately in genus Pneumovirus and Metapneumovirus, respectively, and HPIV3 belongs to subfamily Paramyxovirinae and is classified in genus Respirovirus.
Myeloid (also called conventional) DC are potent antigen presenting cells that activate CD4+ and CD8+ T lymphocytes and play a major role in initiating and modulating the adaptive immune response and also contribute to innate immunity (Grayson and Holtzman, 2007). DC reside in peripheral tissues in an immature phenotype that is specialized for the uptake and processing of antigen and exert a sentinel function for incoming antigens. In infected tissue, their numbers are augmented by the chemotactic influx of IDC precursors that appear to originate primarily from circulating monocytes (Auffray et al., 2007; Geissmann, 2007; Geissmann et al., 2008). Exposure of IDC to microbes or inflammatory cytokines such as tumor necrosis factor (TNF)-α initiates a maturation process of phenotypic and functional changes. This involves the increased surface expression of a panel of cell surface proteins that are correlates of DC maturation and T cell stimulatory capability, including Major Histocompatibility Complex (MHC) class I and II molecules; T cell co-stimulatory molecules CD80 (B7.1), CD86 (B7.2), and CD40; maturation marker CD83; the signaling receptor CD38; and CD54 (intercellular adhesion molecule-1 [ICAM-1]) (de la Fuente et al., 2005; Frasca et al., 2006; Lipscomb and Masten, 2002; Prechtel and Steinkasserer, 2007; Quezada et al., 2004; Reis e Sousa, 2006; Schuurhuis et al., 2006; Shen and Rock, 2006). Maturing DC secrete an array of chemokines, cytokines, and interferons involved in innate immunity and T cell activation. Maturation also changes the profile of chemokine receptors involved in directing DC migration (Lukacs-Kornek et al., 2008). Maturing DC traffic via the afferent lymphatics into the T cell area of the draining lymph node to interact with and activate T cells. Ex vivo, DC with a phenotype closely resembling that of IDC can be generated by culturing peripheral blood monocytes with granulocyte/macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 (Kumar and Jack, 2006). As for any in vitro model, there are caveats. In particular, the isolation process via CD14 positive selection may affect the reactogenicity in response to TLR ligands (Elkord et al., 2005). In vitro maturation conditions can not match the inflammatory environment of the lung. That said, human dendritic cells derived from GM-CSF/IL-4 treated monocytes are currently the most widely used model for detailed study of the effects of human pathogens on DC.
HRSV, HMPV, and HPIV3 are generally similar with regard to their tissue tropism in vivo and spectrum of disease. Yet, the impact of HRSV on human health is much greater both with regard to disease burden and long-term effects on airway function and reactivity (Collins and Crowe, 2007; Collins and Graham, 2008; Glezen, 1990; Lee et al., 2005). Also, while all three viruses have the capability to re-infect throughout life without significant antigenic change, re-infection by HRSV is more frequent and imposes a much greater burden of morbidity and mortality. It is commonly suspected that these attributes of HRSV may reflect an ability of the virus to manipulate and blunt the host immune response and to impart long-term deleterious effects, although the specific mechanisms involved remain poorly understood (Collins and Graham, 2008). The central role of DC in initiating and shaping the immune response, together with their presence at and recruitment to the site of infection and concomitant exposure to infectious virus, makes them obvious candidates for viral manipulation of the host immune response.
A number of studies have investigated the effects of interaction of IDC (either isolatedx directly from blood or monocyte-derived) with HRSV and, in two cases each, HMPV or HPIV3 (Bartz et al., 2002; Bartz et al., 2003; Chi et al., 2006; de Graaff et al., 2005; Guerrero-Plata et al., 2005; Horga et al., 2005; Jones et al., 2006; Plotnicky-Gilquin et al., 2001; Tan et al., 2007), but relatively few experiments had compared any of these viruses in parallel (Bartz et al., 2002; de Graaff et al., 2005; Guerrero-Plata et al., 2006; Jones et al., 2006). Previous studies have tended to highlight effects that were suggested to be specific characteristics of an individual virus, including immunosuppressive effects of HRSV observed in some studies (Bartz et al., 2003; Guerrero-Plata et al., 2006) that were not observed by others (Jones et al., 2006), differential effects on cytokine/chemokine expression by HRSV versus HMPV (Guerrero-Plata et al., 2006), impaired stimulation of DC maturation by HMPV (Tan et al., 2007), and virus-induced apoptosis by HPIV3 (Horga et al., 2005; Plotnicky-Gilquin et al., 2001). In the present study, we addressed this uncertainty by a side-by-side comparison of purified HRSV, HMPV and HPIV3, each expressing GFP. A side-by-side comparison provides for clear identification of effects that are specific for a particular virus versus effects common to all three, and the use of GFP-expressing viruses made it possible to distinguish between effects in GFP-positive and GFP-negative cells. In general, we did not observe dramatic effects specific to particular viruses. All three viruses infected IDCs inefficiently and induced low-to-moderate maturation and cytokine/chemokine expression. Infection was relatively benign, such that, for the most part, GFP-positive cells were neither more nor less able to mature compared to GFP negative bystanders. Maturation induced by each of the three viruses was anti-apoptotic. In general, we observed suboptimal maturation, rather than strong virus-specific impairment. These three common respiratory viruses may have evolved similarly to minimize their immunologic footprint.
RESULTS
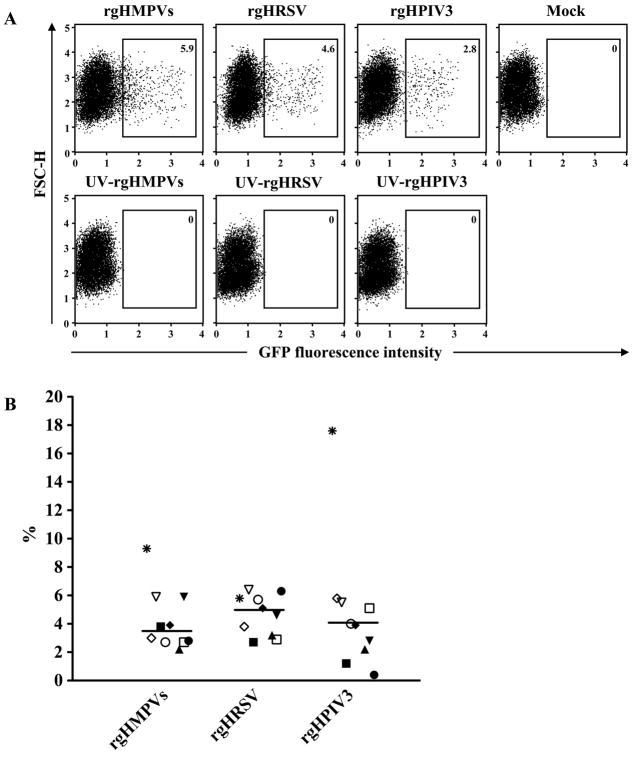
rgHMPVs, rgHRSV and rgHPIV3 have similar, low efficiencies of infection for human IDC
Human monocyte-derived IDC were infected at an input MOI of 3 PFU/cell with purified recombinant GFP-expressing HMPV, HRSV, or HPIV3 (rgHMPVs, rgHRSV, or rgHPIV3). The rgHMPVs virus was a version containing an SH gene that had been modified to silently remove tracts of A or T residues that were sites of spontaneous mutations during passage in vitro (Biacchesi et al., 2007). GFP expression was detectable by 12 to 15 h post-infection, comparable to the kinetics of expression in epithelial-type cell lines (not shown). Forty hours after infection, GFP expression was assessed by flow cytometry. Figure 1A shows dot plots of an experiment with cells from a single donor, and Figure 1B summarizes data from a total of 10 donors. As expected, no GFP-positive cells were detected in DC inoculated with UV-inactivated virus. The percentage of GFP-positive cells among the three viruses ranged from 2.2% to 9.3% for rgHMPVs (median of 3.4%), from 2.7% to 6.4% for rgHRSV (median of 4.9%), and from 0.4% to 17.6% for rgHPIV3 (median of 4.0%). DC from certain donors exhibited apparent differences in permissiveness to the three human viruses. For example, cells from one donor (indicated with asterisks in Fig. 1B) were nearly 3-fold more permissive for rgHPIV3 compared to rgHRSV. Conversely, cells from a second donor (indicated with filled circles in Fig. 1B) were 16-fold less permissive for HPIV3 than for HRSV. The possible donor-to-donor variability in permissiveness will need further studies for substantiation. Overall, the median percentage of GFP-positive cells (n=10 donors) was not significantly different among the three respiratory viruses (P>0.05), indicating a general similarity in permissiveness of IDC for infection by the three different viruses. In contrast, infection with recombinant Newcastle disease virus expressing GFP (generously provided by Dr. Siba K. Samal, University of Maryland) at the same input MOI resulted in approximately 80% GFP-positive DC (not shown), indicating the low infectivity was specific to these human viruses rather than our experimental conditions. In addition, infection of the human airway epithelium A549 cell line with these three human viruses at the same input MOI resulted in approximately 60–85% GFP-positive cells (not shown), indicating that there was at least a 15-fold difference in permissiveness between human IDC and A549 cells.
Fig. 1. Infectivity of rgHMPVs, rgHRSV, or rgHPIV3 for human IDC.
(A) Primary data from a representative donor. IDC were mock-treated or inoculated at an input MOI of 3 PFU/cell with the indicated virus or with an equivalent amount of the corresponding UV-inactivated virus. Forty hours later, the number of GFP positive cells was determined by flow cytometry. The percentages of GFP-positive cells are shown. (B) Summary of data from 10 different donors. Immature DC were inoculated with rgHMPVs, rgHRSV or rgHPIV3 and analyzed by flow cytometry as above. Each different symbol indicates the percentage of GFP positive cells for an individual donor, and the median value for each virus is indicated. This did not differ significantly between the three viruses (p<0.05).
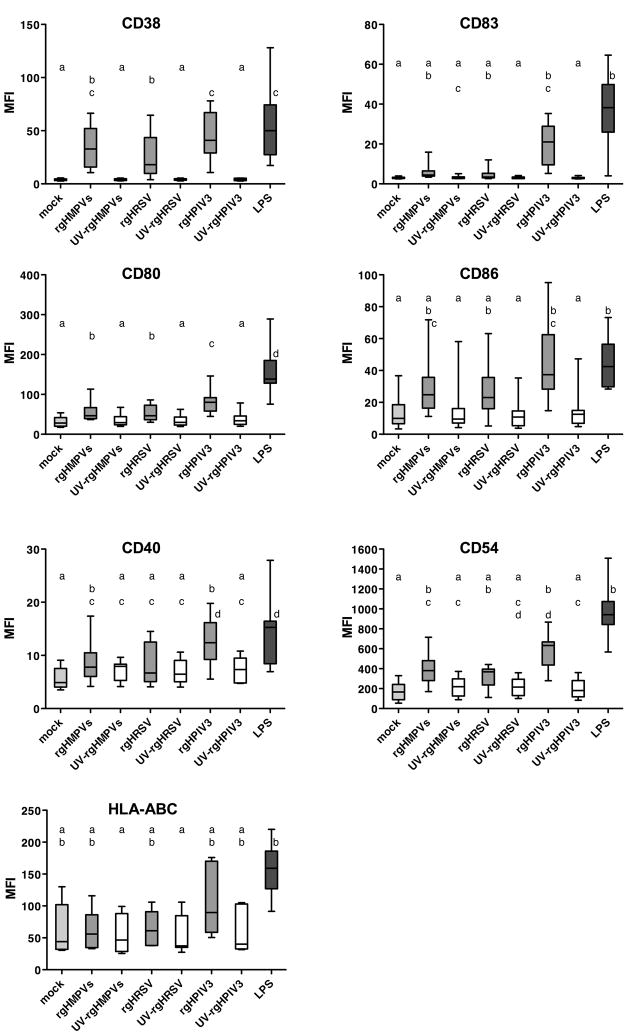
The ability of the three viruses to induce up-regulation of surface markers increased in the order: rgHRSV, rgHMPVs, rgHPIV3
IDC were mock-treated, inoculated with LPS (1 μg/ml) as a positive control, or inoculated with rgHRSV, rgHMPVs, or rgHPIV3 (each at an input MOI of 3 PFU/cell), or with UV-inactivated counterparts. Forty hours later, cells were analyzed by flow cytometry for cell surface expression of seven maturation markers: CD38, CD54, CD80, CD86, CD40, CD83, and HLA-ABC. Briefly, CD38 is a receptor and ectoenzyme involved in chemotaxis, transendothelial migration, inhibition of apoptosis, and Th1 polarization (Frasca et al., 2006); CD54 (ICAM-1) plays a key role in contact with T lymphocytes and enhances the density of MHCII and CD86 at the contact area (de la Fuente et al., 2005); CD80 and CD86 are co-stimulatory molecules that interact with CD28 on naïve T cells (Lipscomb and Masten, 2002); CD83 is involved in T cell activation (Prechtel et al., 2007); CD40 is a co-stimulatory molecule that interacts with CD40 ligand (CD154) on T cells (Quezada et al., 2004); and HLA-ABC represent the three major MHC class I genes, A, B, and C, detected by an antibody to a common epitope. Fig. S1 shows primary data from a representative donor for six of these markers, and Fig. 2 summarizes data for all markers and donors. As would be expected, mock-infected DC exhibited a low level of cell surface expression of the maturation markers (Figs. S1 and 2), with some donor-to-donor variability. Compared to mock-infected cells, the positive control LPS induced a strong, significant increase in surface expression of each of the seven markers. In general, the DC response to LPS tended to be greater than the responses to the three viruses, although this was statistically significant only versus rgHRSV for CD38, CD80, and CD40, versus rgHMPVs for CD80 and CD40, and versus rgHPIV3 for CD80. Among the viruses, the response to rgHPIV3 was greater than to rgHRSV for CD38, CD80, and CD40, and versus rgHMPVs for CD80. For all of the markers, the response to each UV-inactivated virus was very similar to that for mock-treatment. Expression was greater with live virus than its corresponding UV-inactivated control (although the increase was not always statistically significant) except in the case of CD83 and CD40 for rgHRSV and rgHMPVs and in the case of HLA-ABC. This indicated that intracellular viral RNA replication, which is the viral activity that is most sensitive to UV irradiation, was necessary to induce maturation. Taken together, these data indicate that the three viruses were broadly similar in their effect on up-regulation of DC maturation markers, but the magnitude of up-regulation increased in the order: rgHRSV, rgHMPVs, rgHPIV3.
Fig. 2. Cell surface expression of maturation markers on DC following inoculation with rgHMPVs, rgHRSV, or rgHPIV3.
IDC from individual donors [n=10, expept for CD40 (n=8) and HLA-ABC (n=7)] were mock inoculated, inoculated with an input MOI of 3 PFU/cell with the indicated live or UV-inactivated virus, or treated with 1 μg/ml of LPS. Forty hours after inoculation, the cells were harvested and analyzed by flow cytometry for cell surface expression of the indicated maturation markers. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. Treatments sharing the same lower case letters do not differ significantly at the p<0.05 confidence level for the global experiment (Materials and Methods).
Viral RNA synthesis in GFP-negative versus GFP-positive DC
GFP expression in DC, as well as in highly permissive epithelial-type cell lines, was evident only beginning at 12–15 h post-infection, and thus is a marker for robust viral RNA replication and gene expression. Therefore, it was unclear whether the GFP-negative population of virus-inoculated DC was completely resistant to infection or supported a low-level infection. To investigate this, aliquots of IDC prepared from a single donor were inoculated with each of the three viruses as well as with UV-inactivated controls. Twenty-four h post-inoculation, the cells were sorted on the basis of GFP expression and quantitative RT-PCR was used to measure the amount of cell-associated GFP RNA in the GFP-negative versus the GFP-positive population. The cells that had been inoculated with UV-inactivated virus (and had been passed through the cell sorter in parallel) served as a control for the background of preformed RNA resulting from phagocytosis or cell-surface binding of the input viral inoculum. Quantitative RT-PCR was performed on RNA from each set of GFP-positive and GFP-negative cells and the results were normalized against the respective UV-inactivated control (Table 1). As expected, in the GFP-positive population of DC inoculated with rgHRSV, rgHMPVs, or rgHPIV3, the level of GFP RNA was strongly increased compared to the UV-inactivated controls, reflecting robust viral RNA synthesis. However, in GFP-negative population, the GFP RNA levels also were substantially increased: compared to the corresponding UV-inactivated controls, the level of GFP RNA in the sorted GFP-negative cells was increased 124-fold for rgHMPVs, 182-fold for rgHRSV, and 2,600-fold for rgHPIV3. This strongly suggests that infection and viral RNA synthesis occurred in at least some of the GFP-negative cells for each of the three viruses. However, the amount of GFP RNA was 21- to 198-fold lower in the GFP-negative cells than in GFP-positive cells of the same samples. This suggests that infection in the GFP-negative cells was highly restricted and/or abortive.
Table 1.
Viral RNA in GFP-positive and GFP-negative DC 24 h after inoculation with rgHMPVs, rgHRSV, or rgHPIV3a
| GFP-negativeb | GFP-positiveb | GFP positive vs. GFP negativec | |
|---|---|---|---|
| rgHMPVs | 124 | 24558b | 198 |
| rgHRSV | 182 | 3801 | 21 |
| rgHPIV3 | 2600 | 291251 | 112 |
Monocyte-derived IDC were inoculated with rgHMPVs, or rgHRSV, or rgHPIV3 at an input MOI of 3 PFU/cell or with an equivalent amount of UV-inactivated virus, and 24 h later the cells were sorted for GFP expression. The purity of the GFP-negative cell populations was 92–99% live GFP-negative cells, with all of the remaining cells corresponding to dead GFP-negative cells. Post-sort analysis indicated that the maximum level of contaminating GFP positive cells in the GFP negative population was below 0.1 %. The purity of the GFP-positive populations was 88–91% live GFP-positive cells, with the remaining cells corresponding to live or dead GFP-negative cells and some dead GFP-positive cells. Total cell-associated RNA was prepared, and qRT-PCR was performed using primers specific for the virus-expressed GFP sequences (see Materials and Methods). Human β-actin was used as housekeeping gene for internal normalization.
Fold increase versus DC inoculated with the corresponding UV-inactivated control.
Fold difference between GFP-positive versus GFP-negative cells.
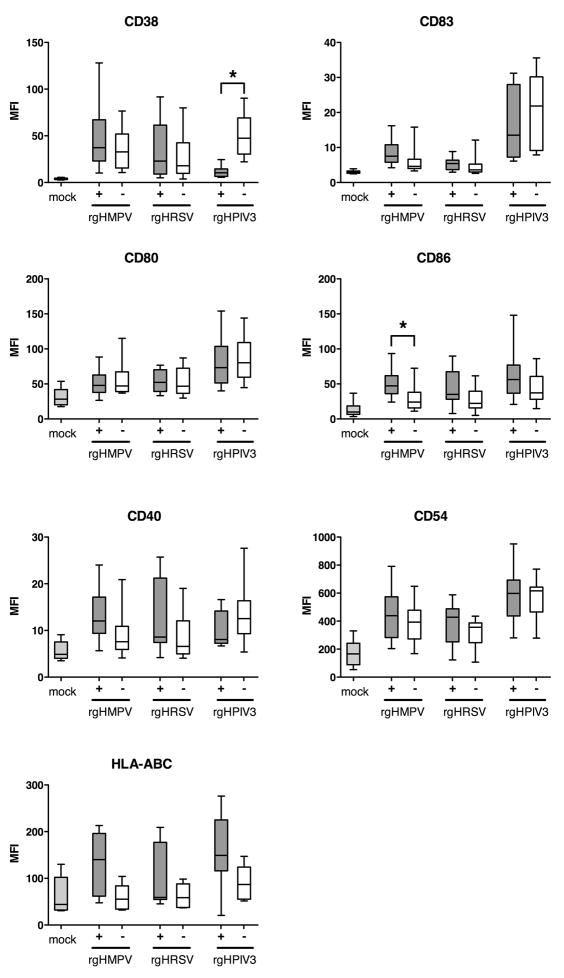
Maturation of GFP-positive versus GFP-negative DC: CD38 expression alone is down-regulated by rgHPIV3
We next investigated possible direct effects of robust viral gene expression and genome replication on maturation. This was done by analyzing the level of expression of the maturation markers in the GFP-positive versus the GFP-negative populations of IDC inoculated with each of the three viruses (Fig. 3). Remarkably, in most cases there was little difference in the cell surface expression of the above-mentioned seven maturation markers in GFP-positive versus GFP-negative cells. This suggested that, at the individual cell level, robust viral genome replication and gene expression were not required to drive maturation and, conversely, did not impair the ability of IDC to mature. There was a single instance in which there was significantly greater expression of a maturation marker in the GFP-positive versus GFP-negative population, namely CD86 for rgHMPVs. In several other instances, expression also appeared to be greater in the GFP-positive versus GFP-negative population, but the differences were not statistically significant (e.g., CD40 for rgHMPVs and rgHRSV, HLA-ABC for rgHMPVs).
Fig. 3. Cell surface expression of maturation markers on GFP-positive versus GFP-negative DC following inoculation with rgHMPVs, rgHRSV and rgHPIV3.
IDC were mock inoculated or infected at an input MOI of 3 PFU/cell with the indicated virus. Forty hours after inoculation, GFP-positive and GFP-negative cells were analyzed by flow cytometry for cell surface expression of the indicated maturation markers. n>10 different donors, exept for CD40 (n=8) and HLA-ABC (n=7). (+), GFP positive population, (−), GFP negative population. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. Significant differences at the p<0.05 confidence level for the global experiment between the GFP positive and negative populations are marked by asterisks.
There also was a single instance in which there was significantly reduced expression of a maturation marker in the GFP-positive versus GFP-negative population, namely CD38 for rgHPIV3 (Fig. 3; representative results with cells from a single donor are shown in Fig. S2). These data suggest that the more robust level of rgHPIV3 infection in the GFP-positive cells interfered with the maturation-driven up-regulation in the surface expression of CD38.
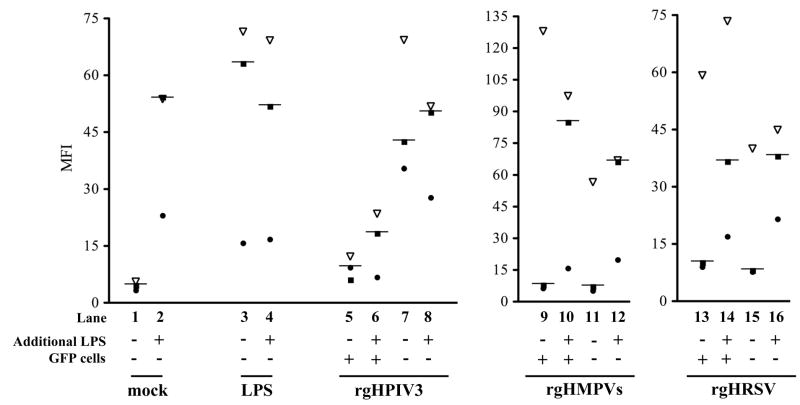
We asked whether viral infection in the rgHPIV3-GFP-positive cells actively impaired expression of CD38 associated with DC maturation, or whether rgHPIV3 simply was an inadequate stimulus. Duplicate wells of IDC from three different donors were inoculated with rgHPIV3 and incubated for 24 h, at which time LPS (1 ug/ml) was added to one of the two wells. In mock-treated control cells, stimulation with LPS for the last 24 of the 48 h culture period increased cell surface expression of CD38, as expected (Fig. 4, lane 2). Since CD38 expression was equally high in the DC treated with LPS for 48 h versus 24 h (Fig. 4, lane 3 versus lane 2), we concluded that stimulation with LPS for 24 h provided maximal stimulation for comparison to the respiratory viruses. Of the cells inoculated with rgHPIV3, the GFP-negative fraction strongly expressed CD38, and this level was increased only minimally by stimulation with LPS at 24 h (Fig. 4, lane 8 versus 7). Notably, the poor expression of CD38 in the GFP-positive fraction of rgHPIV3-inoculated cells was only marginally increased by LPS (Fig. 4, lane 6 versus 5). Finally, in contrast to rgHPIV3, LPS strongly increased the low level of CD38 expression in DC that had been inoculated with rgHMPVs (Fig. 4, lanes 12 versus 11) and rgHRSV (lanes 16 versus 15). Therefore, while the low CD38 expression in rgHMPVs- and rgHRSV-treated cells was due to insufficient stimulation, the low expression of CD38 in the rgHPIV3/GFP-positive cells was uniquely due to active impairment of CD38 expression by rgHPIV3.
Fig. 4. Reduced expression of CD38 on GFP-positive DC inoculated with rgHPIV3 is not augmented by subsequent stimulation with LPS.
Duplicate cultures of IDC were mock inoculated (lanes 1 and 2), or inoculated with 1 μg/ml of LPS (lanes 3 and 4), or inoculated with rgHPIV3 (lanes 5–8), rHMPVs (lanes 9–12), or rgHRSV (lanes 13–16) at an MOI of 3 PFU/cell. Twenty-four hours later, one culture of each pair was stimulated (or restimulated if the culture had previously received LPS) with 1 μg/ml of LPS (even numbered lanes), while the other was not stimulated (odd numbered lanes). Twenty-four hours later, the paired cultures were harvested and analyzed by flow cytometry for the cell surface expression of CD38. In the case of each pair of virus-inoculated cultures, the GFP-positive and GFP-negative fractions were analyzed separately. The different symbols indicate the three different donors. The differences are not statistically significant.
We then asked whether the impaired expression of CD38 in the rgHPIV3-inoculated GFP-positive cells was at the level of intracellular mRNA abundance. Cells from two donors were inoculated with rgHPIV3 at an MOI of 10 PFU/cell. This resulted in 7% and 11% GFP-positive cells for donor 1 and 2, respectively, at 24 h. After 24 h, cells were sorted into GFP-positive and GFP-negative fractions. The purity of the GFP-positive population was 93% (donor 1) and 81% (donor 2). The remaining 7% and 19% of cells were live or dead GFP-negative cells, and few dead GFP-positive cells. The purity of the GFP-negative population was 97 % (donor 1) and 89 % (donor 2); the 3% and 11% of the remaining cells were identified as dead GFP-negative cells. RT-qPCR of the sorted fractions showed that the level of intracellular CD38 mRNA in the GFP-positive cells was 4 and 5-fold lower (donor 1 and 2, respectively) than that in GFP-negative cells, which could account for the magnitude of the difference in cell surface expression. Therefore, expression of CD38 is inhibited at the level of intracellular mRNA abundance.
rgHPIV3, rgHMPVs and rgHRSV induce a similar array of cytokines
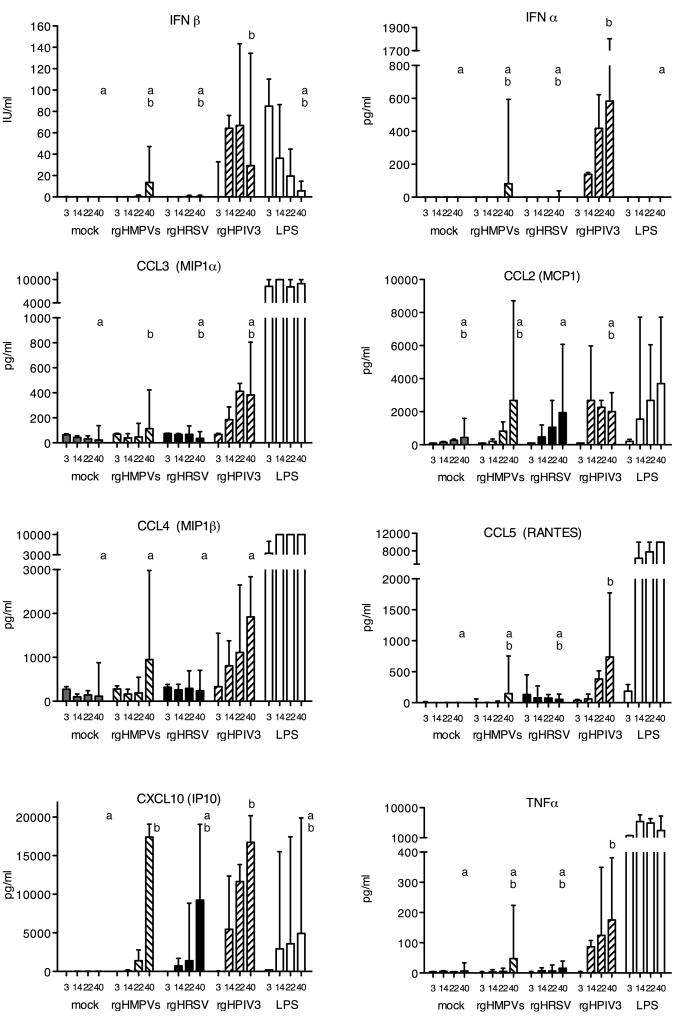
IDC were mock inoculated, or stimulated with 1 μg/ml of LPS, or inoculated at an MOI of 3 PFU/cell with rgHMPVs, rgHRSV, rgHPIV3 or with their UV-inactivated counterparts. At 3, 12, 24 and 40 h.p.i., supernatants were harvested and assayed for the presence of 30 cytokines and factors (see materials and methods for the complete list): the time course of production of selected cytokines is shown in Fig. 5, and the peak concentrations of all of the virus-induced cytokines are shown in Table 2. Out of 30 cytokines tested, 24 were detected following stimulation with LPS, whereas only 12 were detected following virus infection (Table 2). The virus-induced species included type I interferons (IFN), C-C chemokines (four out of five tested), C-X-C chemokines (two out of three tested), interleukins (three out of 13 tested), and TNFα. Growth factors (TGFα, G-CSF, GM-CSF, VEGF, EGF) were not detected. These increases were not observed for DC infected with UV-inactivated viruses (data not shown), indicating that viral genome replication was required.
Fig. 5. Cytokine production by DC.
IDC were mock inoculated, or stimulated with 1 μg/ml of LPS, or inoculated with an MOI of 3 PFU/cell with rgHMPVs, rgHRSV, rgHPIV3, or with a corresponding amount of UV-inactivated virus. At 3, 14, 22 and 40 h p.i., medium supernatants were collected from duplicate wells for each treatment and subsequently tested for cytokine quantity using a multiplex bead assay or, for IFNα, IFNβ and CXCL10, by ELISA. n= 3 different donors for the 3, 14 and 22 h time points and 3 additional donors (total of 6) for the 40 h time point. For each given cytokine, and only for the 40 h time point, treatments sharing the same lowercase letters do not differ significantly at the p<0.05 confidence level for the global experiment (Materials and Methods). LPS was not included in the statistical analysis in cases where it induced a much stronger response than the viruses, namely with CCL2, CCL3, CCL4, CCL5 and TNFα. This is part of the experiment shown in Table 2.
Table 2.
Peak concentrations of cytokines produced by DC inoculated with rgHMPVs, rgHRSV, or rgHPIV3a
| Cytokine | Mockb | rgHMPVsb | rgHRSVb | rgHPIV3b | LPSb | |
|---|---|---|---|---|---|---|
| Interferons | IFNα | 0 | 81 | 0 | 492 | 0 |
| IFNβ | 0 | 14 | 0 | 67 | 85 | |
| Chemokines | CCL2 (MCP1) | 44 | 2,681 (6) | 1,951 (4) | 2,681 (6) | 3,703 (8) |
| CCL3 (MIP1α) | 65 | 113 (2) | 75 (1) | 412 (6) | ≥10,000 (≥154) | |
| CCL4 (MIP1β) | 274 | 948 (3) | 320 (1) | 1,921 (7) | ≥10,000 (≥36) | |
| CCL5 (Rantes) | 0 | 147 | 131 | 738 | ≥10,000 | |
| CXCL8 (IL-8) | 138 | 133 (1) | 131 (1) | 352 (3) | 6,270 (45) | |
| CXCL10 (IP10) | 23 | 17,395 (756) | 9,230 (401) | 16,733 (728) | 4,921 (214) | |
| Interleukins | IL-1ra | 1156 | 4590 (4) | 3,945 (3) | 3,307 (3) | 4,233 (4) |
| IL-1α | 6 | 208 (35) | 209 (35) | 212 (35) | 356 (59) | |
| IL-6 | 29 | 343 (12) | 163 (6) | 646 (22) | ≥10,000 (≥345) | |
| TNF family | TNFα | 7 | 48 (7) | 16 (2) | 175 (25) | 5,842 (835) |
Monocyte-derived IDC were inoculated with 3 PFU/cell of the indicated virus or with 1 μg/ml LPS. Duplicate samples from six donors were taken at various times post-inoculation (see the legend to Fig. 7) and cytokine concentrations were assayed by a luminex bead assay (or by ELISA for IFNα, IFNβ and CXCL10)(parts of this experiment also are depicted in Fig. 7). Shown here are maximum median values irrespective of time. All of the cytokines from the assay set that were increased in at least one of the virus-infected cells compared to mock-inoculated cells are shown. Cytokines that were induced by LPS only (not shown) were: CX3CL1 (fractalkine), IL-7, IL-10, IL-12p70, IL-15, IFNγ, G-GSF, GM-CSF, TGFα, and VEGF. Cytokines that were not significantly induced by any inoculation were: CCL11 (Eotaxin), IL-1β, IL-2, IL-4, IL-5, IL-13, IL-17, and EGF.
Cytokine concentration in clarified supernatants, expressed as pg/ml (or IU/ml for IFNβ) and, in parentheses, as fold-difference compared to mock-inoculated DC, unless the value for mock was “0”.
The production of IFNβ by DC after virus infection was delayed as compared to the rapid production following LPS stimulation (Fig. 5). Essentially no IFNβ was induced by rgHRSV, whereas rgHPIV3 was a more efficient inducer than rgHMPVs (Table 2). The profile of IFNα production was similar to that of IFNβ, such that little or none was produced in response to rgHRSV, whereas rgHPIV3 was the most efficient inducer. Among the interleukins, treatment with LPS, rgHMPVs, rgHRSV, or rgHPIV3 up-regulated expression of the pro-inflammatory cytokines IL-1α and IL-6 as well as the IL-1 receptor antagonist (IL-1ra, also called the IL-1 inhibitor) (Table 2). IL-1ra is noteworthy because it competes with IL-1α/β for binding to the IL-1 receptor and down-regulates IL-1-mediated effects, and thus is a natural anti-inflammatory factor. It was suggested previously that production of this antagonist by HRSV might result in reduced T cell activation, as a possible mechanism of reduced immunogenicity (Salkind et al., 1991). Since all three viruses in the present study induced a high level of expression of IL-1ra, any immunosuppressive effect would not be unique to HRSV, although it remains possible that this antagonist plays a similar role for all three viruses in reducing immunogenicity. All three viruses also induced detectable levels of TNFα, although induction by rgHRSV was very weak.
Of the panel of chemokines tested, rgHPIV3 up-regulated production of four C-C chemokines, namely CCL2 (MCP1), CCL3 (MIP1α), CCL4 (MIP1β), and CCL5 (RANTES) which are chemotactic for monocytes, as well as of two C-X-C chemokines CXCL8 (IL-8, which strongly attracts neutrophils and would augment innate immunity (Piqueras et al., 2006)) and CXCL10 (IP10) (Fig. 7, Table 2). The response to rgHMPVs and rgHRSV was somewhat more circumscribed: rgHMPVs and rgHRSV did not induce increased production of CXCL8; in addition rgHRSV did not up-regulate CCL3 and CCL4. However, rgHMPVs and rgHRSV, like rgHPIV3, induced strong responses of CCL2 and CXCL10. Even though CXCL10 is regulated by type I and type II IFN, it was strongly up-regulated by all three viruses despite the lack of detectable IFNγ for any of the viruses and the lack of detectable type I IFN for HRSV. Thus, among the three viruses, the induction of IFN and pro-inflammatory cytokines was strongest for rgHPIV3 and weakest for rgHRSV, and the three viruses were comparable in the production of anti-inflammatory IL-1ra.
The viruses block apoptosis
We evaluated the extent of apoptosis 40 h following inoculation of IDC with each of the three viruses, a time span that in vivo would approximately encompass the time from antigen contact to maturation, migration, and activation of T cells in lymph nodes (Grayson and Holtzman, 2007; Piqueras et al., 2006). The extent of apoptosis was evaluated by two flow cytometry-based assays. In one assay, labeled annexin-V was used to detect cell surface phospholipid phosphatidylserine, which is translocated from the inner to the outer surface of the plasma membrane early in apoptosis. In the second assay, apoptotic cells were identified by two parameters: (i) side scatter, which measures intracellular granularity, which is reduced in apoptotic cells, and (ii) immunostaining with an antibody specific for activated caspase 3, which is one of the major executioner caspases and is generated from an inactive precursor by proteolysis.
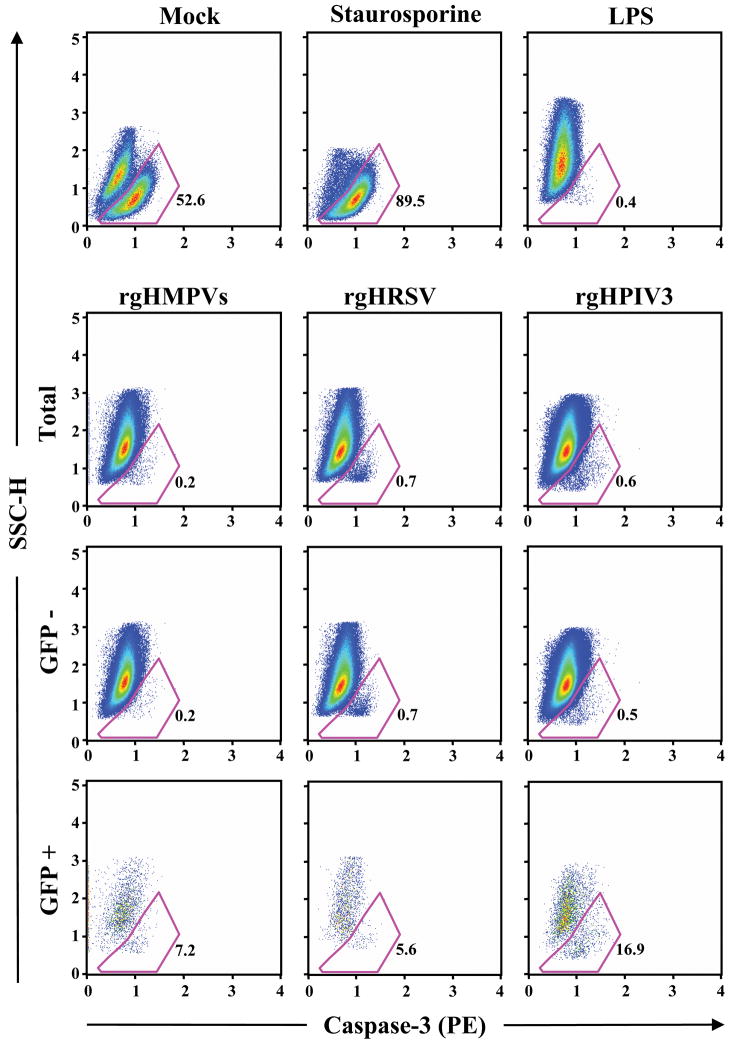
Representative data for a single donor using the side scatter/caspase 3 assay are shown in Figure 8. In this example, 52.6% of the mock-inoculated cells were apoptotic at the 40 h time point (Figure 6, top row). With the apoptosis inducer staurosporine, the percentage of apoptotic cells increased to 89.5%. Remarkably, treatment with LPS reduced the level of apoptotic cells to 0.4%, which presumably reflects an anti-apoptotic effect of DC maturation as suggested previously (Arimilli et al., 2006; Lundqvist et al., 2002). The level of apoptotic cells with the three viruses (Figure 6, second row) also was low: rgHMPV, 0.2%; rgHRSV, 0.7%, and rgHPIV3, 0.6%. Analysis of cells that were gated on the basis of GFP expression (Figure 6, bottom two rows) showed the GFP-positive fractions had the following percentages of apoptotic cells: 7.2, 5.6, and 16.9 for rgHMPVs, rgHRSV, and rgHPIV3, respectively. These values were higher than the respective values from the GFP-negative fractions: 0.2, 0.7, and 0.5, but nonetheless were low compared to the mock-inoculated control. Similar results were obtained with this assay using cells from an additional donor (Table 3, donor A) and for these two donors (donors A and B) plus a third donor (donor C) using the annexin-V assay (Table 3). This indicated that the three viruses were similar in the property of sparing DC from apoptosis, presumably through the anti-apoptotic effects of DC maturation, although this protective effect was partly negated in the small fraction of cells that was GFP-positive and thus robustly infected.
Fig. 6. Apoptosis in DC inoculated with rgHMPVs, rgHRSV, or rgHPIV3: representative data from a single donor.
IDC were inoculated individually with the indicated virus at an MOI of 3 PFU/cell, or mock inoculated, or inoculated with 1 μg/ml of LPS. Forty hours later, the cells were harvested and analyzed by flow cytometry to identify apoptotic cells by two parameters: side scatter, which characterizes intracellular granularity, and immunostaining with an antibody for activated caspase 3. Additional cells were treated for 4 h with 5 μM staurosporine as a positive control for apoptosis and analyzed in parallel. The top six panels show dot plots of the total cell population for each indicated treatment/inoculation, and the bottom six panels show GFP-negative and GFP-positive fractions of the virus-inoculated populations. Cells defined as apoptotic by the two parameters are outlined in red and the percentage is indicated. These data correspond to donor B at the bottom of Table 3.
Table 3.
Apoptosis markers in DC inoculated with rgHMPVs, rgHRSV, or rgHPIV3a
| Marker | Donor | Mock | Stauro-sporine | LPS | rgHMPVs | rgHRSV | rgHPIV3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | GFP+ | GFP − | Total | GFP+ | GFP − | Total | GFP+ | GFP − | |||||
| Annexin 5 | Donor A | 12.9 | 37.4 | 2.3 | 3.0 | 14.0 | 2.8 | 2.6 | 9.5 | 2.5 | 2.7 | 6.7 | 2.7 |
| Donor B | 48.6 | 74.5 | 11.4 | 1.7 | 5.5 | 1.7 | 1.5 | 3.5 | 1.5 | 1.5 | 7.1 | 1.4 | |
| Donor C | 19.0 | n.d.b | 1.5 | 1.2 | 3.3 | 1.1 | 1.6 | 4.1 | 1.6 | 2.9 | 5.5 | 2.7 | |
| Activated caspase 3 | Donor A | 16.3 | 66.3 | 5.6 | 0.5 | 27.7 | 0.4 | 1.2 | 16.5 | 1.1 | 4.6 | 7.8 | 4.4 |
| Donor B | 52.6 | 89.5 | 0.4 | 0.2 | 7.2 | 0.2 | 0.7 | 5.6 | 0.7 | 0.6 | 16.9 | 0.5 | |
Monocyte-derived IDC from three donors (A, B, and C) were mock-treated, or treated with 1 μg/ml LPS, or treated with 5 μM of the apoptosis inducer staurosporine, or inoculated with the indicated virus at an input MOI of 3 PFU/cell. Forty h later (or, in the case of staurosporine, 4 h later), the cells were stained with annexin V or with antibody specific for the activated form of caspase 3 and analyzed by flow cytometry. The percentage of cells positive for the indicated apoptosis marker is shown: for the virus-inoculated cultures, values are shown for the percentage apoptotic cells in the total culture as well as the percentage of GFP-positive and –negative cells that were apoptotic.
nd, not done
DISCUSSION
We made a side-by-side comparison of the ability of GFP-expressing HMPV, HRSV, and HPIV3 to infect and induce maturation in human monocyte-derived IDC. Comparison of the three viruses did not confirm dramatic differences observed in some previous studies (see Introduction and below). We found that all three viruses infected IDC poorly, with only a few percent of cells being GFP-positive and the remainder having low, abortive levels of viral RNA synthesis. The three viruses induced low-to-moderate maturation of DC and moderate cytokine/chemokine responses, with responses to HPIV3 being somewhat greater. Infection at the individual cell level tended to be relatively benign, such that GFP-positive cells were neither more nor less able to mature compared to GFP-negative bystanders. The only exceptions were that robust (GFP+) HMPV infection modestly increased expression of CD86, and robust (GFP+) HPIV3 infection strongly down-regulated CD38 expression at the RNA level. Finally, in each case, maturation was anti-apoptotic.
Each of the three viruses was able to infect monocyte-derived IDC sufficiently to mediate strong expression of GFP in approximately 4% of the cells. This low level of infectivity contrasts, for example, with influenza A virus, Sendai virus, and simian virus type 5, which have been reported to infect nearly 100% of human monocyte-derived DC (Arimilli et al., 2006; Osterlund et al., 2005). Infection by Newcastle disease virus also was efficient in our own laboratory (not shown), whereas infection by measles virus appeared to be intermediate in efficiency (Servet-Delprat et al., 2000). The permissiveness of IDC to infection by HPIV3 had not been reported previously. With HRSV and HMPV, previous studies reported similar rates of infection as well as instances of higher rates occurring at higher MOIs in a dose-dependent fashion. Since the particle to PFU ratio of preparations of HRSV has been reported to be very high, 3,000–30,000:1 (Buynak et al., 1978), we avoided using higher doses. The use of GFP expression as a marker of robust viral infection likely was more reliable and discriminating than the use of immunofluorescence detection of viral antigen, since the latter method does not distinguish between antigen in the inoculum taken up by these actively phagocytic cells versus that synthesized de novo. The three viruses in the present study were at least 15-fold less efficient in infecting human monocyte-derived IDCs compared to A549 epithelium-derived cells (data not shown).
The low efficiency of infection of IDC with these viruses may have consequences for the adaptive immune response. The classical pathway by which DCs activate CD8+ T cells involves degradation of de novo-synthesized, intracellular antigen and presentation of the resulting oligopeptides on MHC class I molecules. Inefficient infection and concomitant inefficient antigen expression would reduce the efficiency of this pathway. As an alternative pathway, IDC also can take up exogenous antigen and degrade and display this antigen on MHC class I molecules, a process called cross-presentation (Shen and Rock, 2006). The inefficient infection observed in the present study with HRSV, HMPV, and HPIV3 suggests that activation of CD8+ T cells against these viruses may be more dependent on cross-presentation and thus may be less robust than if both pathways were efficient. It also might be delayed in time, since cross-presentation would largely depend on the initial infection of epithelial cells. As another factor, in the absence of efficient viral infection, DC maturation might be dependent on exogenous stimuli. Indeed, we recently showed that the maturation of human IDC in response to HRSV infection is dependent in large part on secreted type I IFN and is partially suppressed by the IFN antagonists encoded by the virus (Munir et al., 2008).
We also analyzed infectivity by qRT-PCR of viral RNA present in GFP-negative and GFP-positive cells. This showed that the GFP-negative fraction of each population of virus-inoculated DC contained a substantial increase in virus-specific RNA compared to controls inoculated with UV-irradiated virus. The primary effect of UV irradiation is to block viral genome replication (Ball and White, 1976), and thus the higher level of viral RNA in the GFP-negative fractions compared to the cells inoculated with the UV-treated controls indicated that viral genome replication occurred in the GFP-negative population for all three viruses. However, the amount of viral RNA in the GFP-negative fraction was 21- to 198-fold lower than for the GFP-positive fraction. These results suggest that infection occurred in the GFP-negative cells, but aborted at a step following attachment, entry, primary transcription, and one or more initial rounds of genome replication. However, it is unknown what percentage of GFP-negative cells were abortively infected.
For all three viruses, maturation was ablated by UV-inactivation of the virus and thus was dependent on intracellular genome replication. This sensitivity to UV-inactivation had previously been noted for HRSV and HMPV (de Graaff et al., 2005; Guerrero-Plata et al., 2006). However, the present results differ from previous studies with HPIV3 in which UV-inactivated virus was shown to induce an increase of CD54 and MHCII (Plotnicky-Gilquin et al., 2001), and CD86 and CD83 (Horga et al., 2005; Plotnicky-Gilquin et al., 2001). Whether this reflects a difference in strain or methodology, such as the use of unpurified virus in previous studies, is unknown. The dependence on infectivity indicates that contact and uptake of the viral inoculum by IDC was insufficient to induce maturation. This was the case even though, as noted above, preparations of these viruses typically contain very much more viral material than is suggested by the number of PFU and typically contain RNA of both polarities. Thus, detection of endocytosed viral RNA by toll-like receptor 3 (TLR3) or, in the case of HRSV, the ligation of virion-associated F protein with TLR4 (Kurt-Jones et al., 2000), apparently was insufficient to induce maturation under these conditions. The dependence on RNA replication suggests that maturation was dependent on signaling in infected cells mediated by cytoplasmic pattern recognition receptors such as RIG-I, which recognizes 5′-triphosphorylated RNAs produced during RNA replication (Hornung et al., 2006; Pichlmair et al., 2006).
All three viruses induced a significant up-regulation of CD38 and CD80. Additionally, rgHPIV3 induced significant increases of CD83, CD86, CD40, and CD54, while the increases associated with rgHRSV and rgHMPVs were not statistically significant. Previous studies had reported varying extents of maturation (Bartz et al., 2002; Bartz et al., 2003; de Graaff et al., 2005; Guerrero-Plata et al., 2006; Horga et al., 2005; Jones et al., 2006; Plotnicky-Gilquin et al., 2001), with some studies in particular describing incomplete maturation in response to HRSV (Bartz et al., 2002; Bartz et al., 2003) and HMPV (Tan et al., 2007). To further investigate whether robust infection promoted or impaired the expression of the surface maturation markers, we evaluated the extent of maturation of GFP-positive versus GFP-negative cells. For rgHRSV and rgHMPVs, the extent of expression in GFP-positive cells was similar to (e.g. CD38, CD83, CD80, CD54) or greater than (e.g. CD86 and CD40) that of GFP-negative cells. This suggested that robust viral genome replication and gene expression was not inhibitory and, in the latter instances, was somewhat stimulatory. The results were similar for rgHPIV3 except that the expression of CD38 was significantly reduced in GFP-positive versus GFP-negative cells. Thus, robust rgHPIV3 genome replication and gene expression was inhibitory to CD38 expression. Quantitative RT-PCR provided evidence that this occurred at the level of the accumulation of CD38 mRNA. The low level of CD38 expression in rgHPIV3-inoculated cells was not boosted by secondary stimulation with LPS, consistent with inhibition of expression. In contrast, the low levels of CD38 expression in rgHPMVs- and rgHRSV-inoculated cells was boosted by LPS challenge. This also was noted for several other maturation markers (not shown), suggesting that the low-to-moderate levels of expression of maturation markers reflected insufficient stimulation rather than direct impairment of maturation, with the notable exception of CD38/rgHPIV3.
The basis for the reduced expression of CD38 in response to rgHPIV3 remains unknown. CD38 is an ectoenzyme involved in inducing calcium signaling, and also is a receptor that mediates intracellular signaling following binding to its counter-receptor CD31. Signaling induced by CD38/CD31 interaction up-regulates CD83 expression and IL-12 production and enhances DC-induced T cell activation (Fedele et al., 2004; Frasca et al., 2006). CD38 clusters at the immunologic synapse between DC and T lymphocytes (Munoz et al., 2008). The enzymatic and receptor activities of CD38 also are important for DC recruitment to inflamed tissue and subsequent migration to secondary lymphoid tissue (Frasca et al., 2006; Partida-Sanchez et al., 2007). Thus, reduced expression of CD38 has the potential for multiple effects on HPIV3 immunobiology that remain to be investigated. To the best of our knowledge, the only other instance of down-regulation of CD38 surface expression on human DC was a recent report involving the spirochete bacterium Borrelia garinii (Hartiala et al., 2007). Inhibition of the expression of other maturation markers has been noted previously with certain viruses. For example, infection of DC with herpes simplex virus type 1 (Kruse et al., 2000) or human cytomegalovirus (Senechal et al., 2004) results in degradation of CD83.
In vivo, chemokines and cytokines expressed by maturing DC play important roles in augmenting the inflammatory response in the infected tissue as well as attracting and activating lymphocytes later in the lymph node (Piqueras et al., 2006). Thus, impaired or altered expression potentially could lead to reduced or inappropriately polarized immune responses. Various previous reports have described possible deficiencies in the response by individual viruses among the trio tested here, including impaired production of chemokines and cytokines by HMPV (Guerrero-Plata et al., 2006; Tan et al., 2007), or a response to HRSV that is biased towards the expression of immunosuppressive mediators such as IL-10 (Bartz et al., 2002) or IFN types I and III (Chi et al., 2006), or IL-1ra (Salkind et al., 1991), or that HPIV3 was notably deficient in the induction of IL-12 (Horga et al., 2005). In the present side-by-side comparison, rgHRSV was notably deficient in producing IFNα/β whereas a response was noted for rgHMPVs, and rgHPIV3 induced the greatest response of all. With regard to the other assayed factors, the patterns of expression by the three viruses in this side-by-side comparison were remarkably similar, although the responses tended to be somewhat lower for rgHRSV and somewhat higher for rgHPIV3.
Measles virus and vaccinia virus are examples of pathogens that interfere with DC function by inducing apoptosis (Engelmayer et al., 1999; Fugier-Vivier et al., 1997). There are reports of increased apoptosis occurring following inoculation of IDC with HRSV (Bartz et al., 2003) or HPIV3 (Plotnicky-Gilquin et al., 2001). However, in the present study, inoculation with each of the viruses - or with LPS - resulted in a decrease rather than an increase in apoptosis at 40 h compared to mock-treated cells. This decrease, rather than increase, in apoptosis probably reflects anti-apoptotic effects of maturation, possibly mediated through the induction of anti-apoptotic proteins of the Bcl-2 family (Arimilli et al., 2006; Lundqvist et al., 2002).
In conclusion, when compared side-by-side, rgHMPVs, rgHRSV, and rgHPIV3 were poorly infectious for monocyte-derived human IDC and induced a low-to-moderate level of maturation as measured by the expression of cell surface markers and cytokines. Extrapolating to clinical infection, the low level of infectivity for DC and the low-to-moderate level of induced DC maturation might provide for reduced antigen presentation and T cell activation. This might result in suboptimal immune responses, which could impede resolution of infection and reduce protection against re-infection. This effect would be the greatest for HRSV since it was the least efficient in inducing DC maturation, and would be consistent with the greater role of HRSV in acute disease and re-infection (Collins and Crowe, 2007; Glezen, 1990; Lee et al., 2005).
MATERIALS AND METHODS
Virus stock preparation
The construction of recombinant GFP-expressing (rg) HMPV (strain CAN97-83), rgHRSV (strain A2) and rgHPIV3 (strain JS) was described previously (Biacchesi et al., 2004; Hallak et al., 2000; Zhang et al., 2005). The further modification of rgHMPV to create rgHMPVs, which involved silently removing tracts of A or T residues that were sites of spontaneous mutations during passage in vitro, was described previously (Biacchesi et al., 2007). To prepare virus stocks, confluent 225 cm2 flasks of Vero cells were infected at the low multiplicity of infection (MOI) of 0.1 PFU/cell with low-passage recombinant rgHMPVs, rgHRSV or rgHPIV3 in OptiPro SFM medium (Invitrogen, Carlsbad, CA) supplemented with 4 mM L-glutamine (Invitrogen). These conditions were used to keep the abundance of defective interfering particles low, and at comparable levels for all three viruses. Every two days, 40 μl per ml of medium of trypsin (TrypLE Select, Invitrogen) was added to the rgHMPVs-infected flasks to allow for cleavage activation of the fusion protein needed to obtain infectious particles. After 6 to 8 days, the cells were scraped into the overlying medium. The suspension was vortexed for 30 s to release cell associated viral particles. Cells and debris were removed by centrifugation at 1,000 × g for 10 min. The clarified supernatants were combined and were loaded onto 30%/60% w/v sucrose step gradients and subjected to centrifugation in a Beckman SW28 rotor at 121,000 × g for 90 min. The virus-containing band was collected from the 30%/60% sucrose interface, pooled and diluted 8-fold with Advanced RMPI 1640 (Invitrogen) supplemented with 2 mM L-glutamine. Viruses were pelleted in 40 ml polycarbonate tubes in a Beckman JA-17 rotor at 8,000 × g for 2 h. This final low speed spin removed sucrose without reducing infectivity: initial studies indicated that the presence of sucrose interfered with DC maturation (results not shown). After centrifugation, supernatants were discarded and virus pellets were resuspended in Advanced RMPI 1640 supplemented with 2 mM L-glutamine and aliquots were snap frozen and stored at −80°C until use. Virus titers were determined by plaque assay on Vero cells under methylcellulose overlay (containing trypsin for titration of rgHMPVs) as described previously (Biacchesi et al., 2004). On day 5, plaques were visualized on a Typhoon 8600 scanner (GE Healthcare, Piscataway, NJ). In all experiments, UV-inactivated viruses were included as controls and were prepared using a Stratalinker UV cross-linker (Agilent, Santa Clara, CA) at 0.5 J/cm2, with inactivation monitored by plaque assay.
Generation of monocyte-derived IDC
Elutriated monocytes obtained from healthy adult donors at the National Institutes of Health Clinical Center Blood Bank (clinical protocol number 99-CC-0168) were subjected to CD14+ positive sorting on an Automacs separator (Miltenyi Biotec, Auburn, CA) using magnetic microbeads coated with a CD14 specific monoclonal antibody (Miltenyi Biotec). The purity of the monocyte preparation was confirmed by flow cytometry to be > 98%. The CD14+ monocytes were seeded in 12-well plates at 6×105 cells per well in Advanced RMPI 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen), 200 U/ml penicillin, 200 μg/ml streptomycin (Invitrogen), 0.05 mM 2-mercaptoethanol (Sigma, St. Louis, MO), 16 ng/ml recombinant human IL-4 (R&D Systems, Minneapolis, MN) and 50 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukine®, Bayer Healthcare, Wayne, NJ) at 37°C in 5% CO2. On day 7, the immature DC were carefully harvested, washed and resuspended in Advanced RMPI 1640 supplemented with 2 mM L-glutamine before infection. The cell surface marker profiles were evaluated by flow cytometry, and found to be typical for IDC (CD1a+, CD14 low, CD38 low, CD11c high).
Inoculation or stimulation of DC
IDC were seeded in 12-well plates at 6×105 cells per well and were mock infected or infected with live virus at an input MOI of 3 PFU/cell or with an equivalent amount of UV-inactivated virus. Parallel cells were incubated with 1 μg/ml of lipopolysaccharide (LPS) from Escherichia Coli O55:B5 (Sigma) as a positive control for cell maturation. All experiments were performed in Advanced RMPI 1640 supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 200 U/ml penicillin and 200 μg/ml streptomycin at 37°C in 5% CO2.
Flow cytometry analysis
The percentage of GFP-positive cells and the level of surface expression of maturation markers were assessed by flow cytometry. Forty hours after inoculation, the DC were carefully harvested and pelleted by centrifugation at 300 × g for 10 min. After centrifugation, DC were resuspended in cold washing solution (PBS [Invitrogen] supplemented with 2% heat-inactivated FBS and 2 mM EDTA [Quality Biological, Inc., Gaithersburg, MD]). To determine the level of expression of the maturation markers, cells were stained with phycoerythrin (PE)- or allophycocyanin (APC)-conjugated anti-human mAbs from the following panel: PE-conjugated anti-CD1a (HI149), anti-CD11c (S-HCL-3), anti-CD14 (M5E2), anti-CD54 (HA58), anti-CD80 (L307.4) (all from BD Biosciences, San Jose, CA), and anti-HLA-ABC (W6/32) (AbD Serotec, Raleigh, NC); and APC-conjugated anti-CD38 (HIT2), anti-CD40 (5C3), anti-CD83 (HB15e), anti-CD86 (2331-FUN-1) (all from BD Biosciences). Isotype-matched mAbs as negative controls were included in all experiments. The cells were incubated with antibodies on ice for 20 min in the dark. After incubation, cells were washed 3 times with cold washing solution and resuspended in 200 μl of cold washing solution. Before flow cytometry analysis, 10 μl of propidium iodide (PI) solution (200 μg/ml, Sigma) was added to discriminate between live (PI negative) and dead (PI positive) cells. Compensation was performed manually using hardware compensation with single color control samples for GFP, PE and APC. Spectral overlap of PE into the FL3, the PI detector for dead cell exclusion, was also corrected using hardware compensation. Data was acquired using a FACSCalibur flow cytometer (BD Biosciences) on 20,000 events, excluding cell debris and dead cells. Forward scatter, side scatter, live/dead staining, GFP expression, and cell surface marker expression were analyzed using FlowJo version 8.5.2 software (©Tree Star, Inc., Ashland, OR). Maturation marker expression was analyzed individually (CD38, CD83, CD40, CD54) or in combination (CD80, CD86). The use of multiple staining tubes per sample allowed to control for absence of sample-to-sample variation of GFP expression.
Measurement of cytokine production by matured DC
Culture supernatants were collected from duplicate wells of DC at various time points (3, 14, 22 and/or 40 h) post treatment or infection and clarified by centrifugation at 300 × g for 10 min. Clarified supernatants were stored at −80°C in the presence of a protease inhibitor cocktail (Roche Applied Science, Indianapolis, MN) until analysis. ELISA kits were used to assess the concentration of IFN-α (Human Interferon alpha multi subtype ELISA Kit, PBL Biomedical Laboratories, Piscataway, NJ), IFN-β (Invitrogen), and CXCL10 (IP10) (R&D Systems) according to the manufacturer’s instructions. All other cytokines (IL-1β, IL-2, IL-1ra, IL-4, IL-5, EGF, IL-6, IL-7, TGFα, CX3CL1 (Fractalkine,) CXCL8 (IL8), IL-10, IL-12(p70), IL-13, IL-15, IL-17, IL-1α, IFN-γ, G-CSF, GM-CSF, TNFα, CCL11 (Eotaxin), CCL2 (MCP1), CCL3 (MIP1α), CCL4 (MIP1β), CCL5 (RANTES) and VGEF were detected with a Luminex multiplex bead assay (Linco Research, St Charles, MO) according to the manufacturer’s instructions.
Reverse transcription and quantitative PCR (qRT-PCR)
Cell-associated RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA) as recommended by the manufacturer and treated with DNAse to remove residual genomic DNA. One to two hundred ng of isolated RNA was reverse transcribed using SuperScript II (Invitrogen) in a 25 μl mix using random primers. Two μl of the cDNA mix were used in each quantitative TaqMan PCR (Applied Biosystems, Foster City, CA) for quantification of the targets of interest, namely CD38 or virus-expressed GFP; β–actin or 18S rRNA were used for normalization. The three viruses contained different versions of the GFP gene and were amplified with different primer sets: for rgHMPVs, the forward primer was 5′-GAGCGCACCATCTTCTTCAAG-3′, the TaqMan probe was 5′-ACGACGGCAACTACA-3′, and the reverse primer was 5′-TGTCGCCCTCGAACTTCAC-3′; for rgHRSV, the forward primer was 5′-AGACCATATGAAGCAGCATGACTTTT-3′, the TaqMan probe was 5′-TCCTGCACATAGCCC-3′ and the reverse primer was 5′-GTCTTGTAGTTCCCGTCATCTTTGA-3′; and for rgHPIV3, the forward primer was 5′-GCGGGATTTGTATACGAAAGAACGT-3′, the TaqMan probe was 5′-CCAGTCCACCATCTTC-3′, and the reverse primer was 5′-CTCTGTAGACAAACATCTCCTCGATTAAAT-3′. qPCR results were analyzed using the comparative threshold cycle (ΔΔCT) method, normalized to β-actin or 18S rRNA. Mock infected cells or cells inoculated with UV-inactivated virus were used as calibrator. Results were expressed as a fold-difference relative to uninfected cells (in the case of CD38) or relative to cells inoculated with UV-inactivated virus (in the case of GFP).
Apoptosis assays
IDC that were mock-inoculated or inoculated with the three viruses as described above were evaluated for apoptosis 40 h post-inoculation by flow cytometry using (i) APC-labeled annexin-V (Invitrogen) to detect cell membrane phospholipid phosphatidylserine or (ii) a PE-labeled antibody to detect the activated form of caspase-3 (BD Biosciences). Dead cells and cell debris were excluded from analysis. As a positive control, additional cells were treated for 4 h with 5 μM staurosporine, a known inducer of apoptosis.
Statistical analysis
Data sets were assessed for significance using parametric one-way repeated measures ANOVA with the Tukey post hoc tests for normally distributed data sets or the non-parametric Friedman test with Dunns post hoc test for non-normal data sets. A log10 transformation was applied to data sets when necessary to obtain equal standard deviations among groups, a necessary requirement of both tests. A final Bonferroni correction was applied to the whole family of the ANOVA/Tukey and Friedman/Dunns tests for each “global” experiment to maintain a total significance level of 0.05. These “global” experiments were (i) the analysis of the maturation marker expression of the global populations of DC (Figure 3; 10 different donors, 8 different treatments and 7 maturation markers), (ii) the analysis of the maturation markers expression of the GFP positive versus GFP negative cells (Figure 4; 10 different donors, 7 different treatments, 7 different markers), and (iii) the analysis of the cytokine concentrations at 40 h in the supernatant of mock- or virus-inoculated DC (Figure 7; 6 different donors at the 40 h p. i time point, 5 different treatments, 8 different cytokines). Statistics were performed on the Prism 5 version (© 1992–2008 GraphPad Software, Inc, San Diego, CA). Data were only considered significant at (family-wise error rate) P<0.05.
Supplementary Material
Fig. S1. Cell surface expression of maturation markers on DC following inoculation with rgHMPVs, rgHRSV and rgHPIV3: primary data from a representative donor. IDC were mock-treated, or inoculated with an input MOI of 3 PFU/cell with the indicated virus, or treated with 1 μg/ml of LPS. Forty hours after inoculation, the cells were harvested, stained for surface expression of the indicated marker or incubated with an isotype control, and analyzed by flow cytometry. Median Fluorescent Intensity (MFI) values of mock (blue) and LPS or virus treated sample (red) are indicated. Data for the complete panel of makers involving multiple donors is summarized in Fig. 3.
Fig. S2. Reduced expression of CD38 on GFP-positive DC inoculated with rgHPIV3: representative data from a single donor. IDC were mock inoculated, or inoculated with 1 μg/ml of LPS, or inoculated at an MOI of 3 PFU/cell with rgHMPVs, rgHRSV or rgHPIV3 or their UV-inactivated counterparts. Forty hours later, GFP positive cells were analyzed by flow cytometry for the expression of CD38. The percentage of cells in each of the four different populations is indicated.
Acknowledgments
We thank Dr. Brian Murphy for reviewing the manuscript and for useful suggestions and Jeff Skinner for help with the statistical analyses. The rgHRSV virus was made in collaboration with Dr. Mark E. Peeples (Ohio State University). This research was supported by the Intramural Research Program of the NIAID, NIH. Disclaimer: The views expressed in this report are the personal opinions of the authors and are not the official opinion of the U.S. Food and Drug Administration, the National Institutes of Health, or the Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimilli S, Alexander-Miller MA, Parks GD. A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function. J Virol. 2006;80(7):3416–27. doi: 10.1128/JVI.80.7.3416-3427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Ball LA, White CN. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976;73(2):442–6. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129(3):438–45. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology. 2003;109(1):49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S, Murphy BR, Collins PL, Buchholz UJ. Frequent frameshift and point mutations in the SH gene of human metapneumovirus passaged in vitro. J Virol. 2007;81(11):6057–67. doi: 10.1128/JVI.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S, Skiadopoulos MH, Tran KC, Murphy BR, Collins PL, Buchholz UJ. Recovery of human metapneumovirus from cDNA: optimization of growth in vitro and expression of additional genes. Virology. 2004;321(2):247–59. doi: 10.1016/j.virol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Buynak EB, Weibel RE, McLean AA, Hilleman MR. Live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1978;157(4):636–42. doi: 10.3181/00379727-157-40112. [DOI] [PubMed] [Google Scholar]
- CDC. Brief report: respiratory syncytial virus activity--United States, July 2006–November 2007. MMWR Morb Mortal Wkly Rep. 2007;56(48):1263–5. [PubMed] [Google Scholar]
- Chi B, Dickensheets HL, Spann KM, Alston MA, Luongo C, Dumoutier L, Huang J, Renauld JC, Kotenko SV, Roederer M, Beeler JA, Donnelly RP, Collins PL, Rabin RL. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80(10):5032–40. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Crowe JE. Respiratory syncytial virus and Metapneumovirus. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Vol. 2. Lippincott, Williams and Wilkins; Philadelphia, Pa: 2007. pp. 1601–1646. [Google Scholar]
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaff PM, de Jong EC, van Capel TM, van Dijk ME, Roholl PJ, Boes J, Luytjes W, Kimpen JL, van Bleek GM. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol. 2005;175(9):5904–11. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- de la Fuente H, Mittelbrunn M, Sanchez-Martin L, Vicente-Manzanares M, Lamana A, Pardi R, Cabanas C, Sanchez-Madrid F. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol Biol Cell. 2005;16(7):3314–22. doi: 10.1091/mbc.E05-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114(2):204–12. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163(12):6762–8. [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–84. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele G, Frasca L, Palazzo R, Ferrero E, Malavasi F, Ausiello CM. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur J Immunol. 2004;34(5):1342–50. doi: 10.1002/eji.200324728. [DOI] [PubMed] [Google Scholar]
- Frasca L, Fedele G, Deaglio S, Capuano C, Palazzo R, Vaisitti T, Malavasi F, Ausiello CM. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006;107(6):2392–9. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186(6):813–23. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8(6):558–60. doi: 10.1038/ni0607-558. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008 doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Glezen WP. Morbidity associated with the major respiratory viruses. Pediatr Ann. 1990;19(9):535–6. 538–540. doi: 10.3928/0090-4481-19900901-09. passim. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Holtzman MJ. Emerging role of dendritic cells in respiratory viral infection. J Mol Med. 2007;85(10):1057–68. doi: 10.1007/s00109-007-0212-3. [DOI] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005;79(23):14992–7. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34(3):320–9. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74(22):10508–13. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38(7):983–90. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartiala P, Hytonen J, Pelkonen J, Kimppa K, West A, Penttinen MA, Suhonen J, Lahesmaa R, Viljanen MK. Transcriptional response of human dendritic cells to Borrelia garinii--defective CD38 and CCR7 expression detected. J Leukoc Biol. 2007;82(1):33–43. doi: 10.1189/jlb.1106709. [DOI] [PubMed] [Google Scholar]
- Horga MA, Macip S, Tuyama AC, Tan MC, Gusella GL. Human parainfluenza virus 3 neuraminidase activity contributes to dendritic cell maturation. Viral Immunol. 2005;18(3):523–33. doi: 10.1089/vim.2005.18.523. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Jones A, Morton I, Hobson L, Evans GS, Everard ML. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin Exp Immunol. 2006;143(3):513–22. doi: 10.1111/j.1365-2249.2005.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74(15):7127–36. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Jack R. Origin of monocytes and their differentiation to macrophages and dendritic cells. J Endotoxin Res. 2006;12(5):278–84. doi: 10.1179/096805106X118861. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Lee MS, Walker RE, Mendelman PM. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum Vaccin. 2005;1(1):6–11. doi: 10.4161/hv.1.1.1424. [DOI] [PubMed] [Google Scholar]
- Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82(1):97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Engel D, Tacke F, Kurts C. The role of chemokines and their receptors in dendritic cell biology. Front Biosci. 2008;13:2238–52. doi: 10.2741/2838. [DOI] [PubMed] [Google Scholar]
- Lundqvist A, Nagata T, Kiessling R, Pisa P. Mature dendritic cells are protected from Fas/CD95-mediated apoptosis by upregulation of Bcl-X(L) Cancer Immunol Immunother. 2002;51(3):139–44. doi: 10.1007/s00262-002-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, Le Nouen C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol. 2008;82(17):8780–96. doi: 10.1128/JVI.00630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P, Mittelbrunn M, de la Fuente H, Perez-Martinez M, Garcia-Perez A, Ariza-Veguillas A, Malavasi F, Zubiaur M, Sanchez-Madrid F, Sancho J. Antigen-induced clustering of surface CD38 and recruitment of intracellular CD38 to the immunologic synapse. Blood. 2008;111(7):3653–64. doi: 10.1182/blood-2007-07-101600. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, McNally T, Silverman M, Simons P, Stockton JD, Zambon MC. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24(1):102–8. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79(15):9608–17. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–83. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107(7):2613–8. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnicky-Gilquin H, Cyblat D, Aubry JP, Delneste Y, Blaecke A, Bonnefoy JY, Corvaia N, Jeannin P. Differential effects of parainfluenza virus type 3 on human monocytes and dendritic cells. Virology. 2001;285(1):82–90. doi: 10.1006/viro.2001.0933. [DOI] [PubMed] [Google Scholar]
- Prechtel AT, Steinkasserer A. CD83: an update on functions and prospects of the maturation marker of dendritic cells. Arch Dermatol Res. 2007;299(2):59–69. doi: 10.1007/s00403-007-0743-z. [DOI] [PubMed] [Google Scholar]
- Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol. 2007;178(9):5454–64. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Salkind AR, Nichols JE, Roberts NJ., Jr Suppressed expression of ICAM-1 and LFA-1 and abrogation of leukocyte collaboration after exposure of human mononuclear leukocytes to respiratory syncytial virus in vitro. Comparison with exposure to influenza virus. J Clin Invest. 1991;88(2):505–11. doi: 10.1172/JCI115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhuis DH, Fu N, Ossendorp F, Melief CJ. Ins and outs of dendritic cells. Int Arch Allergy Immunol. 2006;140(1):53–72. doi: 10.1159/000092002. [DOI] [PubMed] [Google Scholar]
- Senechal B, Boruchov AM, Reagan JL, Hart DN, Young JW. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103(11):4207–15. doi: 10.1182/blood-2003-12-4350. [DOI] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol. 2000;164(4):1753–60. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18(1):85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tan MC, Battini L, Tuyama AC, Macip S, Melendi GA, Horga MA, Gusella GL. Characterization of human metapneumovirus infection of myeloid dendritic cells. Virology. 2007;357(1):1–9. doi: 10.1016/j.virol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver RC, Wong DT, Sun M, McCarthy N. Parainfluenza virus bronchiolitis. Epidemiology and pathogenesis. Am J Dis Child. 1986;140(1):34–40. doi: 10.1001/archpedi.1986.02140150036029. [DOI] [PubMed] [Google Scholar]
- Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE., Jr Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79(2):1113–24. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cell surface expression of maturation markers on DC following inoculation with rgHMPVs, rgHRSV and rgHPIV3: primary data from a representative donor. IDC were mock-treated, or inoculated with an input MOI of 3 PFU/cell with the indicated virus, or treated with 1 μg/ml of LPS. Forty hours after inoculation, the cells were harvested, stained for surface expression of the indicated marker or incubated with an isotype control, and analyzed by flow cytometry. Median Fluorescent Intensity (MFI) values of mock (blue) and LPS or virus treated sample (red) are indicated. Data for the complete panel of makers involving multiple donors is summarized in Fig. 3.
Fig. S2. Reduced expression of CD38 on GFP-positive DC inoculated with rgHPIV3: representative data from a single donor. IDC were mock inoculated, or inoculated with 1 μg/ml of LPS, or inoculated at an MOI of 3 PFU/cell with rgHMPVs, rgHRSV or rgHPIV3 or their UV-inactivated counterparts. Forty hours later, GFP positive cells were analyzed by flow cytometry for the expression of CD38. The percentage of cells in each of the four different populations is indicated.