Abstract
The acceptability of different lots of commercial components which constitute our basal medium for susceptibility testing of mycobacteria was evaluated. The basal medium consisted of Middlebrook 7H10 agar supplemented with 10% oleic acid-albumin-dextrose-catalase and 0.5% glycerol. Studies were performed by using three separate microbiologic assays, and results were compared with parallel tests on previously standardized and acceptable lots of media. Components were rejected if comparison with standardized medium showed a major change in growth support or susceptibility status of any reference strain to any antimicrobial agent tested. Of the components tested in such a manner, 7 of 23 (30%) lots of 10% oleic acid-albumin-dextrose-catalase, 2 of 13 (15%) lots of Middlebrook 7H10 agar, and 0 of 5 lots of glycerol were found to be unacceptable. This study demonstrates that individual lots of components of this basal medium may vary significantly in their suitability for susceptibility testing, and failure to detect such variation may dramatically affect susceptibility profiles.
Full text
PDF

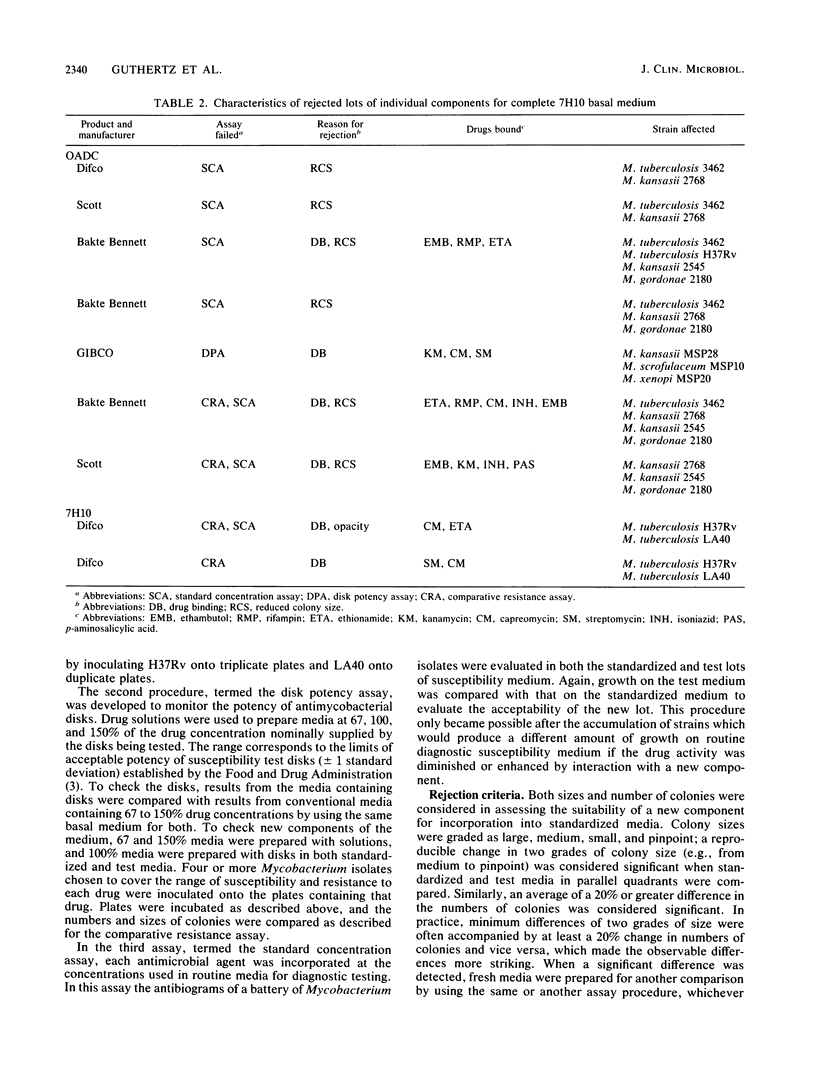
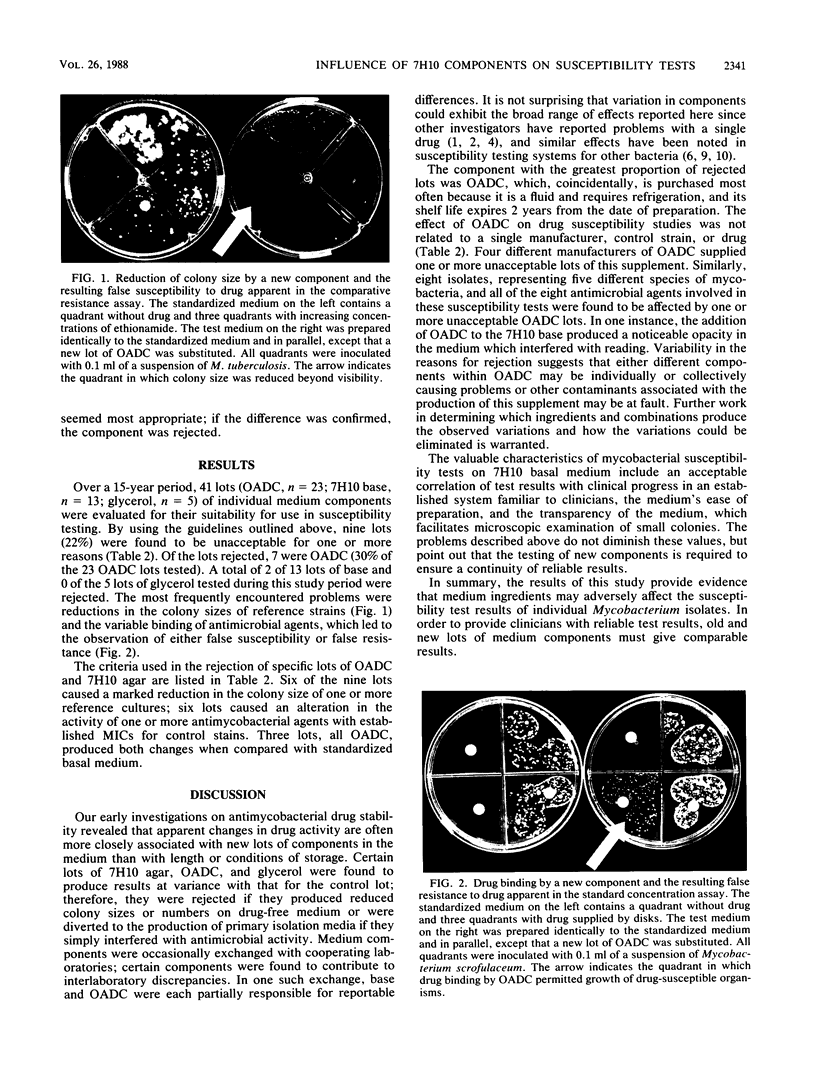

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs W. H., Andrews F. A. Nonspecific ionic inhibition of ethambutol binding by Mycobacterium smegmatis. Antimicrob Agents Chemother. 1973 Aug;4(2):115–119. doi: 10.1128/aac.4.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Gonzales E. R. Influence of the medium on the in vitro susceptibility of Mycobacterium tuberculosis to ethambutol. Am Rev Respir Dis. 1970 Oct;102(4):653–655. doi: 10.1164/arrd.1970.102.4.653. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Sands J. G., Bennett E. O. Antibiotic activity in the presence of agar. Appl Microbiol. 1967 Jan;15(1):31–34. doi: 10.1128/am.15.1.31-34.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Burchall J. J. Reversal of the antimicrobial activity of trimethoprim by thymidine in commercially prepared media. Appl Microbiol. 1971 Nov;22(5):812–817. doi: 10.1128/am.22.5.812-817.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Edmondson W. P. Inhibitor of antibiotics in bacteriologic agar. Proc Soc Exp Biol Med. 1968 Oct;129(1):118–122. doi: 10.3181/00379727-129-33264. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G., COHN M. L. Bacteriology of tuberculosis: laboratory methods. Am J Public Health Nations Health. 1958 Jul;48(7):844–853. doi: 10.2105/ajph.48.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Kelly G. D., Cauthen G. M., Thompson N. J., Kilburn J. O. Infection and disease among contacts of tuberculosis cases with drug-resistant and drug-susceptible bacilli. Am Rev Respir Dis. 1985 Jul;132(1):125–132. doi: 10.1164/arrd.1985.132.1.125. [DOI] [PubMed] [Google Scholar]