Abstract
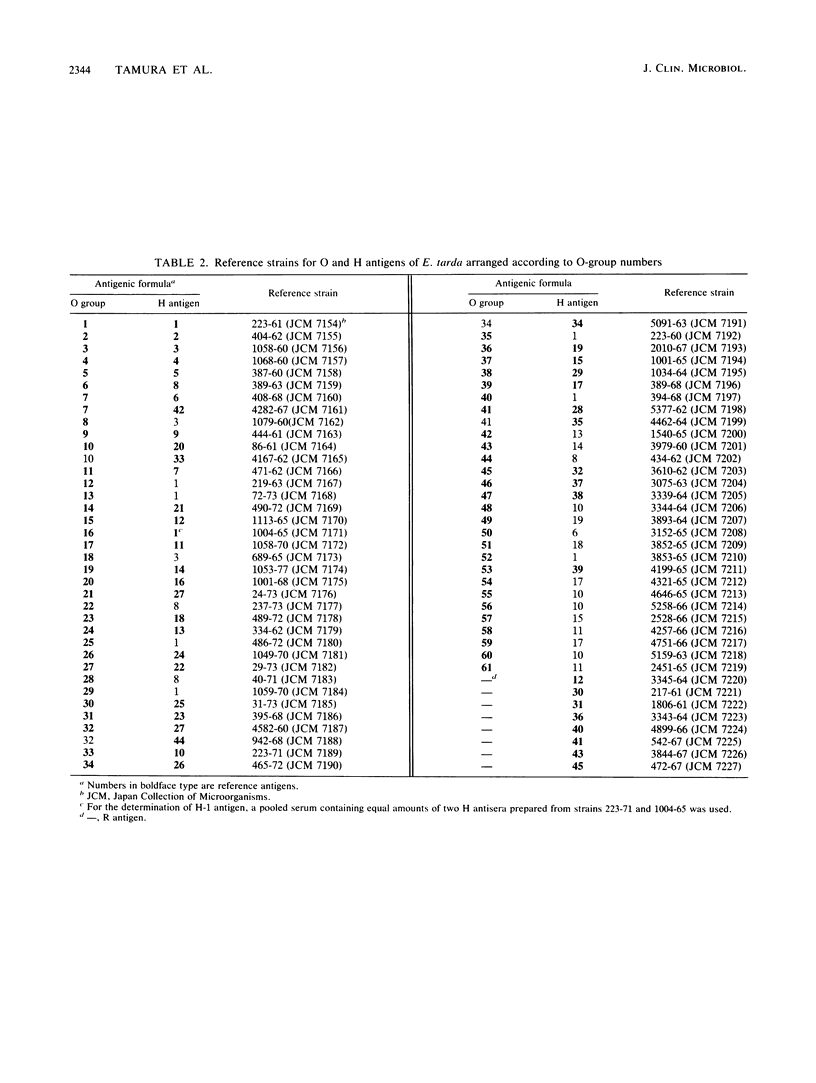
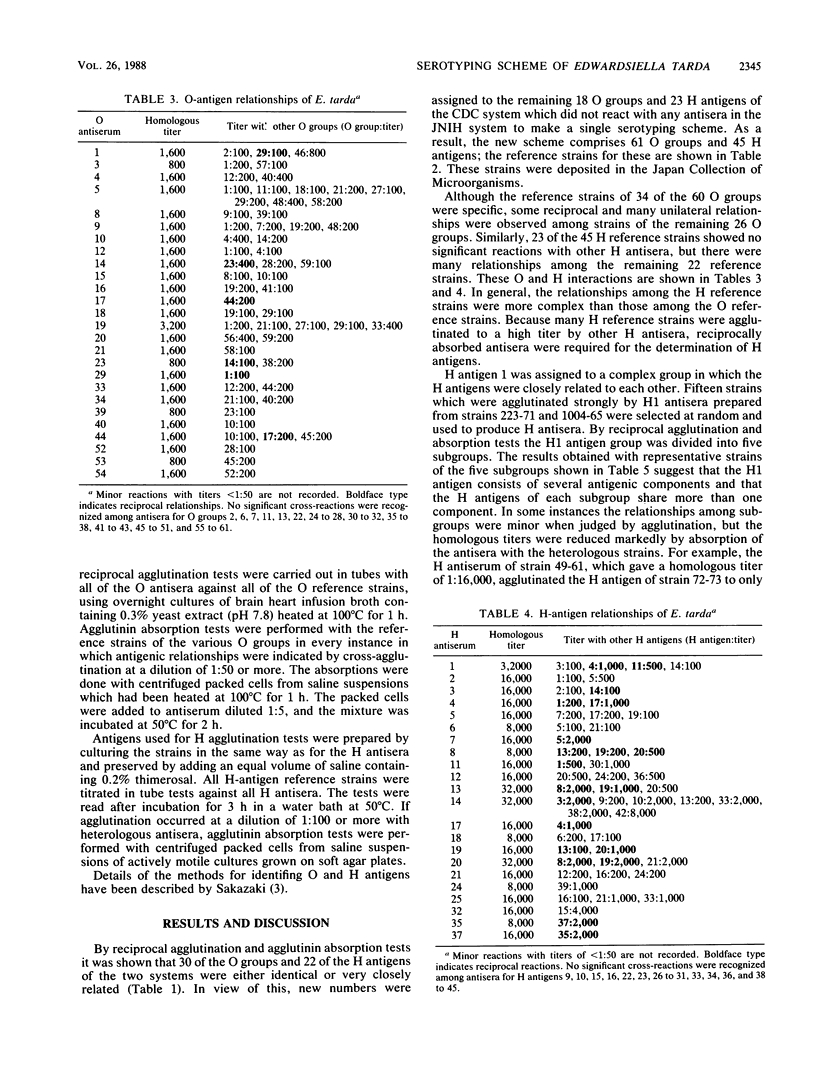
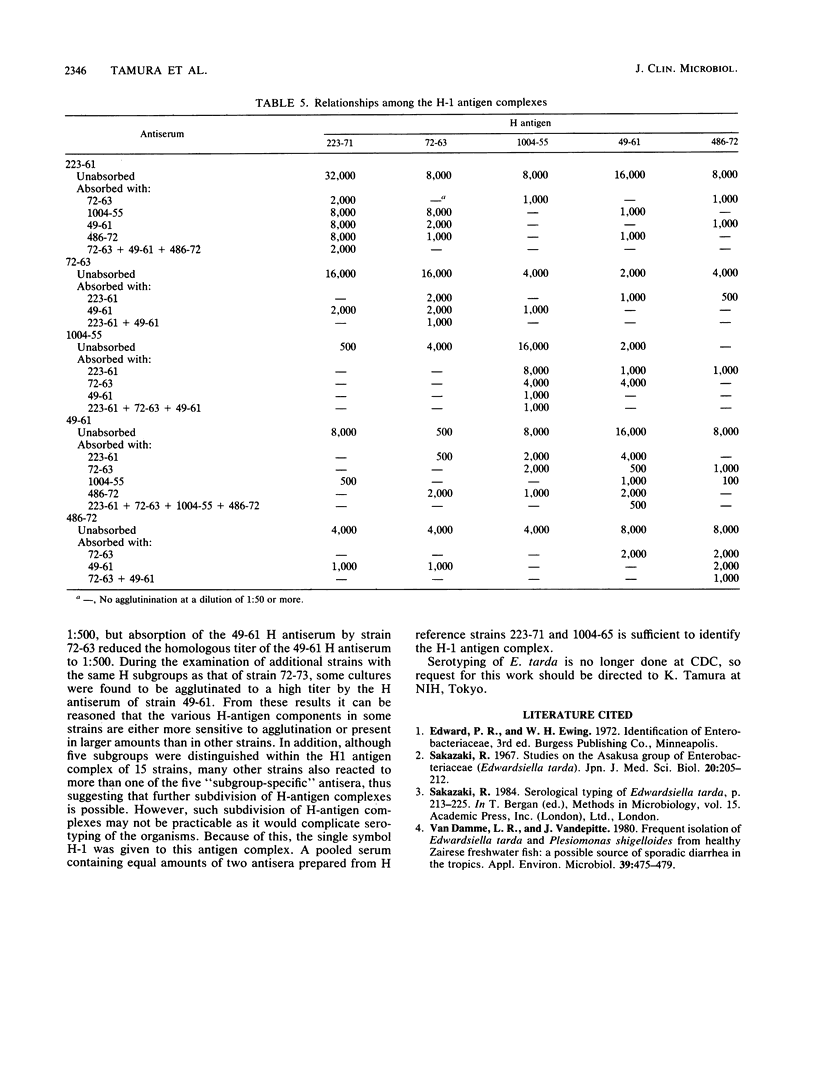
A combination of two systems for the serotyping of Edwardsiella tarda developed independently at the National Institute of Health, Tokyo, Japan, and the Centers for Disease Control, Atlanta, Ga., has enabled a single serotyping scheme comprising 61 O groups and 45 H antigens to be established for international use.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Sakazaki R. Studies on the Asakusa group of Enterobacteriaceae (Edwardsiella tarda). Jpn J Med Sci Biol. 1967 Jun;20(3):205–212. doi: 10.7883/yoken1952.20.205. [DOI] [PubMed] [Google Scholar]
- Van Damme L. R., Vandepitte J. Frequent isolation of Edwardsiella tarda and Pleisiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980 Mar;39(3):475–479. doi: 10.1128/aem.39.3.475-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


