Abstract
In both normally hydrated and volume-expanded rats, there was a biphasic effect of corticotropin-releasing hormone (CRH) (1–10 μg, i.v.) on renal function. Within the first hour, CRH caused antidiuresis, antinatriuresis, and antikaliuresis together with reduction in urinary cGMP output that, in the fourth hour, were replaced by diuresis, natriuresis, and kaliuresis accompanied by increased cGMP output. Plasma arginine vasopressin (AVP) concentrations increased significantly within 5 min, reached a peak at 15 min, and declined by 30 min to still-elevated values maintained for 180 min. Changes in plasma atrial natriuretic peptide (ANP) were the mirror image of those of AVP. Plasma ANP levels were correlated with decreased ANP in the left ventricle at 30 min and increased ANP mRNA in the right atrium at 180 min. All urinary changes were reversed by a potent AVP type 2 receptor (V2R) antagonist. Control 0.9% NaCl injections evoked an immediate increase in blood pressure and heart rate measured by telemetry within 3–5 min. This elevation of blood pressure was markedly inhibited by CRH (5 μg). We hypothesize that the effects are mediated by rapid, direct vasodilation induced by CRH that decreases baroreceptor input to the brain stem, leading to a rapid release of AVP that induces the antidiuresis by direct action on the V2Rs in the kidney. Simultaneously, acting on V2Rs in the heart, AVP inhibits ANP release and synthesis, resulting in a decrease in renal cGMP output that is responsible for the antinatriuretic and antikaliuretic effects.
Previous studies from our laboratory and those of others have indicated that a complex hormonal interplay involving vasopressin and atrial natriuretic peptide (ANP) controls urinary excretion of water and sodium (Na+). Vasopressin has long been known to have an antidiuretic effect, mediated primarily by its action on the vasopressin type 2 receptors (V2R) in the kidney. Stimulation of these receptors activates adenylyl cyclase that converts ATP into cAMP. cAMP opens water channels in the distal nephron and promotes water reabsorption (1).
On the other hand, ANP acts on its receptor, which is an integral part of guanylyl cyclase (GC) located in renal tubules to elicit the production of cGMP, which closes Na+ channels, leading to natriuresis (2). Oxytocin, the other principal neurohypophyseal hormone, also plays an important role in the control of natriuresis by binding to its receptors in kidney tubules to activate neural nitric oxide (NO) synthase by increasing intracellular free calcium (Ca2+) that combines with calmodulin to activate the enzyme (3). The NO produced activates GC, leading to the formation of cGMP that acts to close Na+ channels, promoting natriuresis.
ANP belongs to a family of three natriuretic peptides (NP)—ANP, brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP)—that have structural homology and some biological actions in common, such as induction of diuresis, natriuresis, and vasorelaxation. Under normal physiological conditions, ANP is synthesized mainly in heart atria (4) but also is widespread in the central nervous system and several peripheral organs (5). In addition to regulating water and electrolyte output from the kidney, ANP, vasopressin, and oxytocin also interact in the brain to control water and salt intake as well as the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland (6). In the latter case, vasopressin is a potent stimulus of ACTH from the pituitary gland, whereas ANP acts as a corticotropin release-inhibiting factor (6, 7). The other peptide that is particularly important in controlling ACTH secretion is corticotropin-releasing hormone (CRH), which acts either alone or together with vasopressin to promote ACTH release (8, 9). Both have a synergistic effect to stimulate ACTH release when they are released together. Therefore, it occurred to us that the close interrelationships between these peptides in the brain also might exist in the kidney, and we evaluated the possible renal actions of CRH. The results of our study indicate that CRH indeed has potent antidiuretic and antinatriuretic influence on the kidney, effects that are mediated, at least in part, by stimulation of vasopressin and inhibition of ANP release.
Materials and Methods
Animals.
All experiments were approved by the Centre hospitalier de l'Université de Montréal Animal Care Committee. They were performed in conscious male Sprague–Dawley rats weighing 220–250 g. The animals were housed two per cage at 22°C, maintained on a 12-hr light/12-hr dark cycle (lights on from 6:00 a.m. to 6:00 p.m.), and fed Purina Rat Chow (Ralston Purina) and tap water ad libitum before the experiments.
Effect of CRH on Urinary cGMP and Electrolyte Output in Normally Hydrated Rats.
Conscious, normally hydrated rats were used to determine the dose–response relationship of systemically administered CRH on urine volume and electrolyte and cGMP excretion. All experiments began at 9:00 a.m. Previously trained rats were placed delicately in cages for 1–2 min. After washing the tail with lukewarm water, various doses of CRH (1–10 μg; Peninsula Laboratories) dissolved in saline (0.9% NaCl, 300 μl) were injected into the tail vein. Doses of 1–10 μg were chosen for these experiments because they have been shown to induce regional hemodynamic effects in conscious Long–Evans and Brattleboro rats (10). The animals in the control group were injected with an equal volume of saline.
In a separate group of rats, CRH (5 μg) was injected i.v. 10 min after bolus administration of the most potent antagonist of the antidiuretic activity of vasopressin (2 or 10 μg), [d(CH2)51,d-Phe2,Ile4,Ala9-NH2]AVP (Peninsula Laboratories; no. 8115).
After the injection, the animals were placed individually in metabolic cages without food or water. Spontaneously voided urine was funneled into graduated cylinders for 4 hr. The animals were returned to their cages at the end of the experiment. Each rat took part, in random order, in two to four experiments, each performed at least 4 days apart. Urine volume was measured each hour and was analyzed for sodium and potassium with a flame photometer (Instrumentation Laboratory, Lexington, MA).
To examine the possible involvement of arginine vasopressin (AVP) and ANP in the mechanism of CRH-induced renal effects, blood samples were obtained for AVP and ANP determination in a separate group of animals 5, 15, 30 min, or 3 hr after administration of 5 μg of CRH.
Urinary cGMP was determined by a specific RIA developed in our laboratory (11).
For ANP measurements, 2 ml of blood was collected in chilled test tubes containing protease inhibitors, 2 mg of EDTA, 20 μl of 1 μM PMSF (Sigma; P-7626), and 20 μl of 0.5 μM pepstatin A (Sigma; P-4265). After centrifugation for 20 min at 4°C at 4,000 rpm, the separated plasma was immediately extracted or stored at −80°C until assayed. Plasma ANP was measured by RIA after extraction on C18 Sep Pak (Waters) cartridges, as described previously (12).
For the determination of tissue ANP concentration, the heart was removed quickly from decapitated rats, frozen in liquid nitrogen, and kept at −80°C until processing. The heart chambers were homogenized in 0.1 M acetic acid containing protease inhibitors to a final concentration of 1 mg of EDTA per ml, 5 μM pepstatin A, and 10 μM PMSF at 4°C in a Polytron homogenizer (setting: 10×3 for 20 s). After 20-min centrifugation at 30,000 × g, the pellet was washed and rehomogenized in 0.1 M acetic acid with protease inhibitors. Both supernatants were combined, aliquoted, and stored at −80°C. ANP concentration was determined by direct RIA in serial dilutions (1:2,000; 1:4,000; 1:8,000) of the homogenates (12). The values were normalized to protein concentration and reported as μg/mg protein.
Plasma AVP was determined by RIA after prior extraction with acetone, as described earlier (13).
Determination of ANP Gene Expression in the Heart.
RNA from cardiac tissues was isolated according to the guanidinium thiocyanate/phenol/chloroform method (14). The concentration of each RNA sample was determined by UV absorbance at 260 nm. Ventricular (10 μg) or atrial (2 μg) RNAs were displayed by electrophoresis through gels of 1.5% agarose containing 0.22 M formaldehyde. The gel electrophoresis buffer contained 0.02 mM Mops [3-(N-morpholino)propanesulfonic acid] (Sigma), 5 mM sodium acetate, and 0.1 mM EDTA. The accuracy of relative RNA quantitation was checked by staining the gel with ethidium bromide before capillary blotting onto nylon membranes (Hybond N+; Amersham Pharmacia). Immobilized RNA samples were hybridized with random-primed α-32P-labeled cDNA probes corresponding to ANF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA sequences. A random priming kit (GIBCO/BRL) and [α-32P]dCTP (3,000 Ci/mmol) (Amersham Pharmacia) were used for labeling the probes. The PstI-digested 660-bp fragment from the plasmid clone served as the ANP probe. A 470-bp fragment of a GAPDH cDNA probe was generated by reverse transcription of rat atrial RNA and amplification of the resulting cDNA by PCR (15). Blots were prehybridized in 120 mM Tris⋅HCl buffer (pH 7.4) containing 8 mM EDTA, 0.6 M NaCl, 0.01% sodium pyrophosphate, 0.02% SDS, and 0.01% heparin (Sigma; H-7005). Hybridization was performed for 16 hr in the same buffer solution with increased heparin content (0.03%) supplemented by 10% dextran sulfate and a 106 dpm/ml radioactive probe. After hybridization, the membranes were washed in 2× SSC/1% SDS for 10 min at room temperature, in 0.1× SSC/0.1% SDS for 15–30 min at 65°C, and in 0.1× SSC for 10 min at room temperature. The membranes were exposed on phosphor-sensitive cassettes and then scanned on the PhosphorImager. Radioactive bands were measured with image-quant software (Molecular Dynamics). Percent (or fold) increases in ANP mRNA were normalized to GAPDH and compared with values in saline-injected control rats.
Effect of CRH on Urine and Electrolyte Output in Volume-Expanded Animals.
In the initial set of experiments, rats (225–250 g) were placed in restraining cages and underwent volume expansion (VE) by injection of 0.3, 4, 6, or 8 ml of 0.9% NaCl (saline) into the tail vein in 60 s. Then, the animals were placed in metabolic cages for urine collection for 3–4 consecutive hr. To determine the effect of CRH on VE-induced water and electrolyte excretion, rats were injected with CRH (0–5 μg) 10 min before VE with 6 ml of saline. Urine was collected and measured every hour for 4 consecutive hr, and electrolyte and cGMP excretion was determined.
Effect of ACTH.
To establish whether the CRH effects were mediated by ACTH (0, 10, 100, or 5,000 ng) (Peninsula Laboratories; no. 8740), this polypeptide hormone was injected into the tail vein of normally hydrated, conscious rats.
Blood Pressure Measurement.
Systolic, diastolic, mean arterial pressure, and heart rate (HR) were measured with a telemetry system (Data Sciences International, St. Paul, MN). The monitoring system consisted of a pressure transmitter (radio frequency transducer model TL11M2-C50-PXT), receiver panel, consolidation matrix, and personal computer with program. Output from the transmitter was monitored by the receiver (RLA 2000). The signals from the receiver were consolidated by the multiplexer (BCM 100) and were stored and analyzed by microcomputer with software (a.r.t., Dataquest). The pressure signals were corrected automatically for changes in atmospheric pressure. The pressure transmitters had been calibrated by the manufacturer, but, additionally, calibration was performed before their implantation. For transmitter implantation, the animals (225- to 250-g male Sprague–Dawley rats) were anesthetized with pentobarbital, and the flexible transmitter catheter was secured surgically in the abdominal aorta below the renal arteries pointing against the flow. The transmitter was sutured to the abdominal wall. The animals recovered in individual cages for 7 days after the operation. On the day of the experiment, each cage was placed over the receiver panel and connected to the computer for collection of data. Blood pressure (BP) was measured every min for 24 hr before drug injection and for 4 hr after CRH (5 μg) injection. Systolic, diastolic, and mean arterial BP and HR were calculated with Dataquest a.r.t. software (16). Data obtained over each 5-min period from each rat were pooled.
Statistical Analysis.
The data were subjected to two-way ANOVA for repeated measures on differences between treatment vs. baseline by using the SAS computer program. P values of <0.05 were considered significant. Data are reported as mean ± SEM.
Results
Effect of CRH on Urine Output in Conscious, Normally Hydrated Rats.
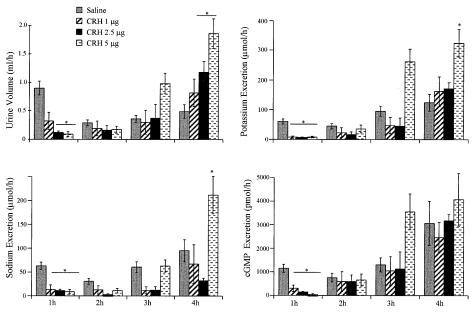
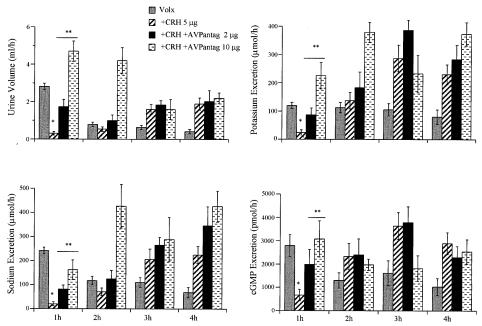
CRH administered i.v. evoked a dose-dependent effect on urine output, sodium, potassium, and cGMP excretion in normally hydrated rats (Fig. 1). cGMP was measured as an index of ANP activity. Two interesting observations are clear in this figure. First, CRH exerted a biphasic effect on urine output over a range of doses. During the first hour after peptide administration, CRH had a dose-related inhibitory action on urine output and electrolyte and cGMP excretion. Then, there was a substantial time delay until the onset of diuresis that appeared during the third and fourth hour in a dose-related manner. Electrolyte and cGMP excretion followed the same pattern. The cumulative urine output over the 4-hr period was increased in a dose-related fashion in comparison with the control group. Cumulative urine output over 4 hr in animals treated with 5 μg CRH was 3.3 ± 0.2 ml per rat (n = 14) compared with the controls that registered 2.3 ± 0.2 ml per rat (n = 40, P < 0.002). Similarly, cumulative urinary K+, Na+, and cGMP excretion increased significantly above that of the control rats.
Figure 1.
Effect of increasing doses of CRH on urine output and electrolyte and cGMP excretion during 4 hr after peptide administration in conscious, normally hydrated rats (n = 6–10 rats per group per experiment). The data are expressed as means ± SEM of at least two experiments. *, P < 0.001 vs. control rats receiving saline.
Role of AVP and ANP in the Renal Effects of CRH.
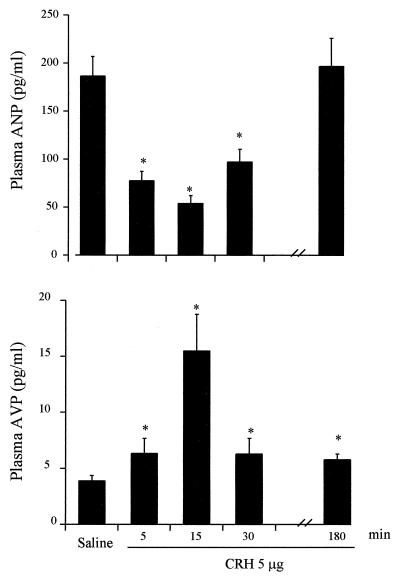
Plasma ANP concentrations were decreased significantly at 5, 15, and 30 min after CRH administration from a baseline of 186.3 ± 20.5 pg/ml (n = 23) in control rats to 77.5 ± 9.8 pg/ml (n = 10, P < 0.001), 54.1 ± 8.0 pg/ml (n = 9, P < 0.001), and 97.1 ± 13.4 pg/ml (n = 13, P < 0.001) at the respective times (Fig. 2). In contrast to the impact of CRH on plasma ANP, plasma AVP was increased significantly (P < 0.01) 5, 15, 30 min, and 3 hr after peptide administration (Fig. 2). Vasopressin concentrations rose to a peak at 15 min, declined significantly at 30 min, but remained elevated above initial values at 180 min (n = 15, P < 0.002).
Figure 2.
Effect of CRH (5 μg/300 μl saline) on plasma ANP level (Upper) and plasma AVP (Lower) at 5, 15, 30, and 180 min after drug administration. Baseline hormone levels were measured in rats injected i.v. with 300 μl of saline.
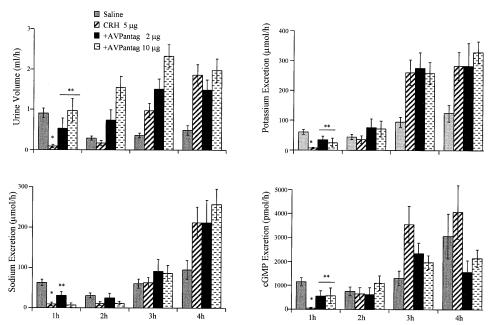
Injection of the vasopressin receptor antagonist (2 or 10 μg) 10 min before CRH significantly (P < 0.01) reversed the antidiuretic effect of CRH (5 μg) in a dose-dependent manner (Fig. 3). The higher dose (10 μg) completely reversed the inhibitory influence of CRH on urine output. The vasopressin antagonist partially reversed the antikaliuretic action of CRH at 1 hr, but had no effect at later times. The antinatriuretic effect of CRH was only partially inhibited by the lower dose of the antagonist at 1 hr. Both doses of the antagonist significantly reduced the inhibition of cGMP excretion induced by CRH during the first hour.
Figure 3.
Urine output and sodium, potassium, and cGMP excretion in CRH (5 μg)-treated rats in the absence or presence of two doses (2 and 10 μg) of the AVP receptor antagonist. Values are expressed as means ± SEM. *, P < 0.001 CRH-treated rats vs. controls (n = 14). **, P < 0.01 AVP antagonist-treated animals vs. CRH.
Effect of CRH in Blood VE Animals.
Because plasma AVP was increased and ANP was decreased after CRH administration, we determined the effect of CRH during inhibition of AVP and activation of ANP release that occurs after isotonic blood VE. In preliminary experiments, the impact of VE was studied by i.v. injection of increasing doses of saline. A dose-related rise in urine output and electrolyte excretion was observed during the first hour after VE. Urine output in control rats injected i.v. with 0.3 ml of saline was 0.91 ± 12 ml (mean ± SEM) (n = 10), whereas in animals injected with 4, 6, or 8 ml it was 1.99 ± 0.3 ml, 2.8 ± 0.2 ml, and 3.99 ± 0.23 ml, respectively, during the first hour. Sodium excretion was augmented from 62.7 ± 8 μmol during the first hour to 147.9 ± 25 μmol, 241.7 ± 14 μmol, and 371 ± 26 μmol in rats injected i.v. with 4, 6, or 8 ml saline, respectively. Urinary potassium and cGMP exhibited similar VE-induced increases.
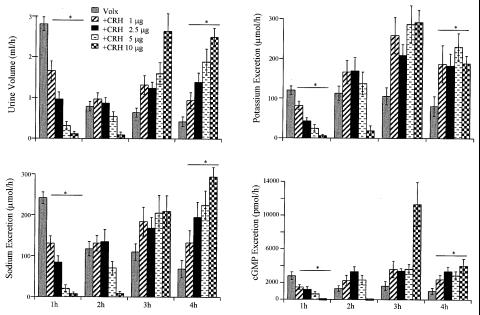
The 6-ml dose of saline was used in further experiments. CRH injection 10 min before VE inhibited VE-induced diuresis and electrolyte excretion in a dose-dependent manner (Fig. 4).
Figure 4.
Effect of increasing doses of CRH on urinary output and electrolyte (Na and K) and cGMP excretion in volume-expanded (6 ml) rats during 4 hr. Means ± SEM of three separate experiments are shown.
In an effort to identify the mechanism responsible for this biphasic renal effect of CRH, we evaluated the role of vasopressin in the inhibition of urine output and electrolyte and cGMP excretion after CRH administration by injecting the vasopressin antagonist at the two doses used before. The AVP antagonist reversed, in a dose-related fashion, all of the effects of CRH during the first hour (Fig. 5). Diuresis and K+ excretion were increased significantly above control levels with the higher dose of the antagonist, and the increased diuresis and kaliuresis was maintained for 2 hr and was accompanied by increased natriuresis in the second hour (Fig. 5).
Figure 5.
Effect of two doses (2 and 10 μg) of the vasopressin receptor antagonist [d(CH2)51,d-Phe2,Ile4,Ala9-NH2]AVP on CRH (5 μg)-induced inhibition of urinary output, electrolytes, and cGMP in volume-expanded (6 ml) rats. Means ± SEM of three separate experiments are shown. (Total number of rats, n = 8.)
Effect of CRH on ANP Synthesis.
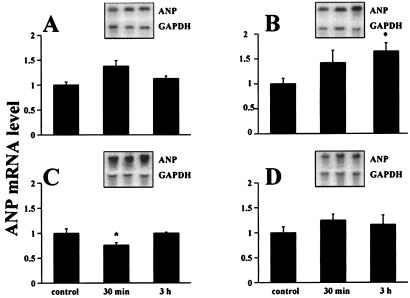
To determine the effect of CRH on ANP synthesis, ANP mRNA was analyzed in the various chambers of the heart by Northern blotting using an ANP cDNA probe. A significant decrease of ANP mRNA in the left ventricle that coincided with reduced plasma ANP concentrations was observed 30 min after CRH injection (Fig. 6). Three hours later, ANP mRNA synthesis was increased significantly in the right atrium, accompanied by a return of plasma ANP to normal values, and was coupled with significantly elevated right atrial ANP levels. Right atrial concentration was augmented from 7.0 ± 0.9 μg/mg protein (n = 5) in control animals to 11.6 ± 1.6 μg/mg protein (n = 5) in the experimental group injected with 5 μg of CRH (P < 0.03).
Figure 6.
Effect of 5-μg CRH injection on ANP mRNA in rat heart chambers analyzed by Northern blotting after 30 min and 3 hr. (A) Left atrium. (B) Right atrium. (C) Left ventricle. (D) Right ventricle. Insets show representative mRNA hybridization bands obtained with radioactive ANP cDNA probe and radioactive GAPDH cDNA probe used as an internal control. The ANP mRNA level in individual samples was calculated as a signal ratio of ANP and GAPDH bands scanned by PhosphorImager. The histogram depicts average values obtained from scans of three independent experiments (n = 10; *, P < 0.05).
Effect of ACTH on Renal Excretion.
To eliminate the possibility that the observed renal effects were due to CRH-induced secretion of ACTH, the peptide (10, 100, or 5,000 ng), in doses known to mimic the increase in plasma ACTH that follows the CRH administration and have been shown to affect blood pressure (17), was injected i.v. into conscious, normally hydrated animals, and their renal parameters were determined. ACTH (5 μg) had no significant impact on urinary volume or electrolyte or cGMP excretion. Urine volume was 1.1 ± 0.3 ml (mean ± SEM) in the first hour in ACTH-treated rats vs. 0.98 ± 0.3 ml/hr in saline-injected controls. Cumulative urine volume over 4 hr was 2.38 ± 0.3 ml in ACTH-injected rats vs. 2.85 ± 0.2 ml in control animals. No change was found in electrolyte or urinary cGMP excretion during the first hour or in cumulative values over 4 hr after CRH administration. Injection of ACTH (10 or 100 ng) had no effect on urinary excretion.
Effects of CRH on BP and Heart Rate.
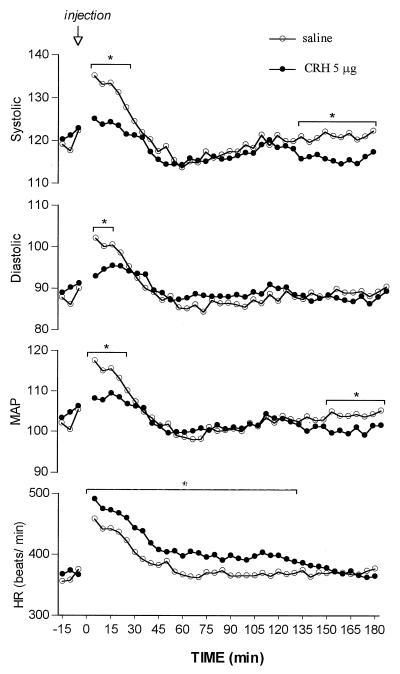
To establish whether possible hemodynamic effects of CRH could play a role in the renal actions of CRH, BP was monitored by telemetry (Fig. 7). The injection of the saline diluent induced a rapid elevation within the 3–5 min in all parameters of BP and in HR. These elevations in BP were reduced dramatically by CRH (5 μg) at 3–5 min after injection, at which time heart rate was elevated further. In the saline-injected rats, the elevated values of the parameters of BP and HR declined to minimal values by 45–60 min. Parameters of BP were no longer lower in CRH-injected than those of saline-injected rats by 30 min, but systolic and mean pressure again were significantly lower than those in saline-injected rats toward the end of the experiment (135–180 min). HR in CRH-injected rats, although declining, remained significantly higher than that in saline-injected rats for 135 min.
Figure 7.
Continuous recordings of systolic, diastolic, and mean arterial blood pressure and heart rate over 3 hr after CRH injection. The pooled values of 5-min recordings were plotted for the graph. The mean values of six animals in each group are shown. The error bars were eliminated because they were almost the same size as the symbols; *, P < 0.02.
Interestingly, this second significant decline of BP around the third hour after CRH administration coincided with significant diuresis, natriuresis, kaliuresis, and increased urinary cGMP. Indeed, plasma ANP rose significantly from 97.1 ± 13.4 pg/ml at 30 min to 196.9 ± 29.2 pg/ml at 180 min; however, there was no significant difference in comparison with the controls (Fig. 2 Upper). At 3 hr, AVP was still elevated.
Discussion
The present study demonstrates dramatic effects of systemically administered CRH on renal function. The initial effect is a rapid inhibition of urine output and Na+, K+, and cGMP excretion. This is accompanied by a decrease of plasma ANP together with a transient decline of ANP mRNA in the left ventricle during this first hour after CRH administration, with a concomitant, marked increase of circulating vasopressin. The effects on renal excretion are caused, at least in part, by this stimulation of vasopressin release, as indicated by the fact that the V2 receptor antagonist completely blocked the antidiuresis. The antinatriuretic and antikaliuretic actions of CRH were accompanied by a decrease of cGMP excretion, and this decline also was reversed by the V2 receptor antagonist. Therefore, it appears that at least part of the antinatriuretic effect was due to the suppression of ANP release because ANP acts by increasing cGMP release that closes Na+ and K+ channels in the tubules, thereby promoting natriuresis and kaliuresis (3).
What Is the Mechanism by Which CRH Induces These Profound Renal Actions?
Surprisingly, even though the rats had been trained by sham injections into the tail, there was a dramatic increase in BP and heart rate already maximal by 5 min after injection of saline, indicating an acute sympathoadrenal medullary discharge of catecholamines. Equally surprising was the rapid reduction in all parameters of BP accompanied by an increased heart rate also present at 5 min after CRH (5 μg) injection. The rapidity of these effects on BP and HR suggests that CRH has a direct effect on the vascular system. Indeed, recent experiments have shown that CRH relaxed vascular smooth muscle and that this effect may be due to its release of endothelial-derived relaxing factor, now known to be NO (18). Therefore, we hypothesize that the changes observed in our experiments are caused by the direct vasodilating properties of CRH that would lower BP almost immediately, which would decrease baroreceptor input to the brain, which induces the release of vasopressin, causing antidiuresis. Because the vasopressin antagonist reversed all of the renal actions of CRH in the first hour after its injection, the most likely explanation of the renal actions of CRH is that they are all caused by release of AVP.
The decreased baroreceptor input induced by CRH also would induce activation of the sympathoadrenal medullary system, which would attempt to compensate by increasing pulse rate and sympathetic vasoconstrictor tone. This vasoconstrictor effect was antagonized by the vasorelaxant action of CRH, but CRH did not antagonize the sympathoadrenal-induced increase in heart rate. Consequently, although CRH decreased blood pressure, heart rate was accelerated further. The antidiuretic effect of CRH can be attributed to the antidiuretic action of AVP via activation of V2 receptors in the kidney with resultant generation of cAMP and opening of tubular water channels (aquaphorins), leading to increased water reabsorption and consequent antidiuresis. Vasopressin, by these same receptors, acts directly on the heart to increase cAMP in the right atrium and other chambers to decrease ANP release and synthesis as determined by decreased levels of ANP mRNA. The result is decreased activation of ANP receptors in the renal tubules, decreased cGMP formation, and opening of Na+ and K+ channels, resulting in antinatriuresis and antikaliuresis.
It is interesting that in our experiments we detected different changes in ANP gene expression in particular compartments of the heart. This is not an isolated observation. Before ANP was discovered, Marie et al. (19) established that the degree of granularity varied in both atria, depending on sodium and water homeostasis. Because atrial granules harbor ANP, their granularity usually parallels atrial ANP concentration. Marie et al. (19) reported that the right atrium in normal animals contains a greater number of granules, and we have shown higher ANP concentrations per gram of tissue (20). Total water restriction for 5 days increased the number of granules in the right atrium only. On the contrary, total sodium restriction for 3 weeks augmented granularity in the left atrium without changes in the right atrium. Furthermore, morphine treatment increased only right atrial ANP mRNA 4 hr after administration (21, 22) whereas left ventricular ANP concentration rose with dexamethasone treatment by day 2 (23). These observations indicate that changes in the ANP cardiac system are induced differentially because of functional differences in the heart chambers.
Beginning around 3 hr, there was a reversal of the antidiuretic/antinatriuretic action of CRH. Urinary output and Na+ excretion together with cGMP excretion increased above corresponding values in saline-injected controls, accompanied by an elevation of plasma ANP and enhancement of right atrial ANP gene expression. One possibility is that this rebound simply may be the result of fluid and Na+ retention that occurs in the initial period. However, the increase in Na+ and water excretion was significantly greater than the amount retained during the initial period, suggesting the possibility of a separate action of CRH that develops more slowly, accounting for this overshoot of renal water and electrolyte excretion, which may be mediated also by ANP, as indicated by increased plasma ANP concentration and the augmentation of ANP mRNA in the right atrium. This delayed stimulation of ANP release and synthesis may be caused by a possible direct effect of CRH and/or vasopressin on the heart or could be initiated by many of the other changes that occur in the initial period after CRH injection. As indicated above, right atrial ANP mRNA increased 4 hr after morphine (100 mg/kg) administration to male rats. Although ACTH that is released by CRH had no effect on renal function at the doses employed here, CRH also releases equimolar concentrations of β-endorphin from the anterior pituitary. Like morphine, β-endorphin primarily activates the μ opiate receptors. Therefore, the β-endorphin released by CRH is a likely candidate to cause the delayed increase in plasma ANP and right atrial ANP mRNA accompanied by increased renal cGMP, natriuresis, kaliuresis, and diuresis that occurred in the fourth hour after CRH. Further research is necessary to elucidate the mechanism of this delayed diuretic/natriuretic action.
Does This Dramatic Effect of CRH on Renal Function Have Any Physiological Significance?
Stress is known to elicit the secretion of not only vasopressin but also CRH into hypophyseal portal vessels, which evokes increased ACTH secretion, resulting in elevated corticoid levels in the peripheral circulation. It has been thought that CRH concentrations in peripheral blood, in contrast to those of vasopressin, can be too low to have any physiological effect. However, Gibbs and Vale (24) have found that CRH levels in peripheral circulation are only four times less than in hypophyseal portal blood. There is also ample evidence of CRH secretion by peripheral organs (25). Therefore, it is possible that the CRH doses given i.v. in this study could approximate levels of CRH that occur in stress situations. If that is the case, then CRH could mediate stress-induced antidiuresis and antinatriuresis by the mechanisms described here. Further research will determine whether CRH plays a physiologically significant role in stress-induced antinatriuresis and antidiuresis.
Acknowledgments
We express our gratitude to Céline Coderre and Nathalie Charron for their technical contributions, Marie-Claude Guertin for her statistical analysis of the BP and HR data, and Ovid da Silva for editing this. We also thank Catherine Boily, who performed her part of this work during her summer training, and Dominique Poutrieux for her skillful secretarial assistance. This work is supported by the Medical Research Council of Canada and the Heart and Stroke Foundation of Canada (J.G.) and the National Institutes of Health (National Institute of Mental Health to S.M.M.).
Abbreviations
- NP
natriuretic peptide
- ANP
atrial NP
- ACTH
adrenocorticotropic hormone
- CRH
corticotropin-releasing hormone
- AVP
arginine vasopressin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- VE
volume expansion
- BP
blood pressure
- HR
heart rate
References
- 1.Bailly C. Kidney Int Suppl. 1998;65:S29–S35. [PubMed] [Google Scholar]
- 2.Ballermann B J, Brenner B M. J Clin Invest. 1985;76:2041–2048. doi: 10.1172/JCI112206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares T J, Coimbra T M, Martins A R, Pereira A G, Carnio E C, Branco L G, Albuquerque-Araujo W I, De Nucci G, Favaretto A L, Gutkowska J, et al. Proc Natl Acad Sci USA. 1999;96:278–283. doi: 10.1073/pnas.96.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bold A J. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 5.Gutkowska J, Nemer M. Endocr Rev. 1989;10:519–536. doi: 10.1210/edrv-10-4-519. [DOI] [PubMed] [Google Scholar]
- 6.King M S, Baertschi A J. Endocrinology. 1989;124:286–292. doi: 10.1210/endo-124-1-286. [DOI] [PubMed] [Google Scholar]
- 7.Gutkowska J, Antunes-Rodrigues J, McCann S M. Physiol Rev. 1997;77:465–515. doi: 10.1152/physrev.1997.77.2.465. [DOI] [PubMed] [Google Scholar]
- 8.Vale W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 9.McCann S M. In: Elsevier's Encyclopedia of Neuroscience. Adelman G, Smith B H, editors. Amsterdam: Elsevier; 1999. pp. 927–930. [Google Scholar]
- 10.Gardiner S M, Compton A M, Bennett T. Br J Pharmacol. 1988;95:197–208. doi: 10.1111/j.1476-5381.1988.tb16565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutkowska J, Mukaddam-Daher S, Tremblay J. J Pharmacol Exp Ther. 1997;281:670–676. [PubMed] [Google Scholar]
- 12.Gutkowska J. Nucleic Med Biol. 1987;14:323–331. doi: 10.1016/0883-2897(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 13.Baranowska B, Tremblay J, Gutkowska J. Peptides. 1988;9:189–192. doi: 10.1016/0196-9781(88)90243-4. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Jankowski M, Petrone C, Tremblay J, Gutkowska J. Regul Pept. 1996;62:53–61. doi: 10.1016/0167-0115(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 16.Davidson A O, Schork N, Jaques B C, Kelman A W, Sutcliffe R G, Reid J L, Dominiczak A F. Hypertension. 1995;26:452–459. doi: 10.1161/01.hyp.26.3.452. [DOI] [PubMed] [Google Scholar]
- 17.Van Bergen P, Vleeming W, Van Heijst B G, Versteeg D H, De Wildt D J. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:220–229. doi: 10.1007/pl00005246. [DOI] [PubMed] [Google Scholar]
- 18.Grunt M, Glaser J, Schmidhuber H, Pauschinger P, Born J. Am J Physiol. 1993;264:H1124–H1129. doi: 10.1152/ajpheart.1993.264.4.H1124. [DOI] [PubMed] [Google Scholar]
- 19.Marie J P, Guillemot H, Hatt P Y. Pathol Biol. 1976;24:549–554. [PubMed] [Google Scholar]
- 20.Gutkowska J, Horky K, Thibault G, Januszewicz P, Cantin M, Genest J. Biochem Biophys Res Commun. 1984;125:315–323. doi: 10.1016/s0006-291x(84)80370-8. [DOI] [PubMed] [Google Scholar]
- 21.Fukui K, Iwao H, Nakamura A, Tamaki T, Abe Y. Jpn J Pharmacol. 1991;57:45–50. doi: 10.1254/jjp.57.45. [DOI] [PubMed] [Google Scholar]
- 22.Gutkowska J, Strick D M, Pan L, McCann S M. Eur J Pharmacol. 1993;242:7–13. doi: 10.1016/0014-2999(93)90003-z. [DOI] [PubMed] [Google Scholar]
- 23.Fullerton M J, Krozowski Z S, Funder J W. Mol Cell Endocrinol. 1991;82:33–40. doi: 10.1016/0303-7207(91)90006-e. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs D M, Vale W. Endocrinology. 1982;111:1418–1420. doi: 10.1210/endo-111-4-1418. [DOI] [PubMed] [Google Scholar]
- 25.Suda T, Tomori N, Tozawa F, Mouri T, Demura H, Shizume K. J Clin Endocrinol Metab. 1984;59:861–866. doi: 10.1210/jcem-59-5-861. [DOI] [PubMed] [Google Scholar]