Abstract
Opioids are widely prescribed for non-cancer pain conditions (NCPC), but there have been no large observational studies in actual clinical practice assessing patterns of opioid use over extended periods of time. The TROUP (Trends and Risks of Opioid Use for Pain) study reports on trends in opioid therapy for NCPC in two disparate populations, one national and commercially insured (HealthCore Blue Cross and Blue Shield plans) and one state-based and publicly-insured (Arkansas Medicaid) population over a six year period (2000-2005). We track enrollees with the four most common NCPC conditions: arthritis/joint pain, back pain, neck pain, headaches, as well as HIV/AIDS. Rates of NCPC diagnosis and opioid use increased linearly during this period in both groups, with the Medicaid group starting at higher rates and the HealthCore group increasing more rapidly. The proportion of enrollees receiving NCPC diagnoses increased (HealthCore 33%, Medicaid 9%), as did the proportion of enrollees with NCPC diagnoses who received opioids (HealthCore 58%, Medicaid 29%). Cumulative yearly opioid dose (in mg. morphine equivalents) received by NCPC patients treated with opioids increased (HealthCore 38%, Medicaid 37%) due to increases in number of days supplied rather than dose per day supplied. Use of short-acting Drug Enforcement Administration Schedule II opioids increased most rapidly, both in proportion of NCPC patients treated (HealthCore 54%, Medicaid 38%) and in cumulative yearly dose (HealthCore 95%, Medicaid 191%). These trends have occurred without any significant change in the underlying population prevalence of NCPC or new evidence of the efficacy of long-term opioid therapy and thus likely represent a broad-based shift in opioid treatment philosophy.
1. Introduction
The WHO Collaborative Study of Psychological Problems in General Health Care estimated that 22% of primary care patients have pain persisting at least six months that interferes with function or prompts medical care. The most commonly reported pain sites were back pain (48%), headache (45%), and joint pain (42%). While 32% of this group had one site of pain, 78% had multiple sites.[14] These prevalence data are compatible with recent single country studies in the US[28], Australia[4] and Denmark[10]. A significant percent of these patients, e.g.,13% of headache patients and 18% of back pain patients in the US, report that they have been unable to work full-time because of their pain.[27] It is estimated that 90-95% of long-term opioid therapy is prescribed for non-cancer pain conditions (NCPC). We have previously estimated that approximately 3% of the US general population without cancer uses opioids regularly for a month or more per year.[30]
There is evidence in recent years of increasing use of prescription opioids for NCPC. Between 1980 and 2000, opioid prescribing at outpatient visits for chronic musculoskeletal pain doubled from 8 to 16% of visits and the use of more potent opioids increased from 2 to 9% of visits.[6] Other studies have shown a marked increase in both use and abuse of prescribed opioids between 1997 and 2002.[13] Among Medicaid fee-for service enrollees, overall use of opioid pain medications increased 3-fold from 1996 to 2002, and varied widely from state to state.[36] Opioid use among states also varies widely among privately insured individuals.[8] This variation in practice likely results from lack of consensus about standards of care with regard to opioid therapy for NCPC.[35] While some pain specialists have seen increased opioid use as evidence of better attention to the problem of unrelieved pain[24], others are worried that we do not have enough evidence of the “safety and effectiveness of long-term opioid therapy” in NCPC.[34]
There are no large observational studies assessing patterns of opioid use in actual clinical practice over extended periods of time. The TROUP (Trends and Risks of Opioid Use for Pain) study is designed to assess trends in and risks of opioid therapy for non-cancer pain conditions in contrasting pain populations. In this first report, we describe trends in opioid use over a six year period (2000-2005) for NCPC in two disparate populations, a national commercially insured population (HealthCore Blue Cross and Blue Shield plans) and a state-based publicly-insured population (Arkansas Medicaid). We track the four most common NCPC conditions: arthritis/joint pain, back pain, neck pain, and headaches/migraines. We also track HIV/AIDS due to special concerns about pain management for this condition linked with stigma and high rates of substance abuse.[5]
2. Methods
2.1. Study populations
Two populations were chosen for this study one national commercially insured population (HealthCore Blue Cross and Blue Shield plans) and one state-based and publicly-insured (Arkansas Medicaid) population. We anticipated rates of opioid use would differ due to differences in sociodemographic characteristics and disease burden. The primary focus of these analyses is to describe the range of opioid use in different populations and note similar trends.
2.1.1. HealthCore Blue Cross/Blue Shield
The study utilized the HealthCore Integrated Research Database, which contains medical and pharmacy administrative claims and health plan eligibility data from US Blue Cross/Blue Shield (BCBS) commercial health plans. The study population is drawn from five BCBS plans representing the West, Mid West, and South East regions of the United States. The data in the study came from health plan members who were fully-insured via several commercial insurance products including health maintenance organizations (HMO), preferred provider organizations (PPO), and point of service providers (POS). The health plan members all had full medical and pharmacy coverage, with a range of co-pay and deductible benefit options. Claims submitted with partial or complete subscriber liability (due to co-pay or deductible requirements) are captured and maintained within the system. A de-identified data set as defined by the Health Insurance Portability and Accountability Act (HIPAA) was utilized for the research and HealthCore had in place all HIPAA required business associate and data use agreements with the University of Washington prior to conducting the research. The study received approval to study human subjects through the institutional review board at the University of Washington.
2.1.2.Arkansas Medicaid
Arkansas (AR) is one of the poorest states in the nation, with 26% of the AR general population qualifying for Medicaid benefits in 2005. The 2005 total expenditures for the program were $3.0 billion dollars or $4,368 per recipient which reflects payments to approximately 24,660 providers that billed AR Medicaid. Compared to national averages, AR Medicaid covers a higher percentage of the total population, pays less per recipient, and has a relatively high percentage of elderly, disabled (aged, blind, disabled), children, and poverty related eligibles. [2] The Arkansas Medicaid population is thus a very disadvantaged and vulnerable population situated in the highest opioid use and abuse region of the U.S..[7] However, in a study of opioid use in state Medicaid plans during 2002 [36], that tracked a Defined Daily Dose of opioids, Arkansas Medicaid which was in the middle of the range for all states' Medicaid use.
The AR Medicaid program covers all federally mandated services and nearly all of the federal optional services, including, prescription drug, ambulatory surgical center services, and 44 other optional services. Most of the AR Medicaid eligible recipients participate in the primary care physician program (PCP) where recipients utilize a primary care provider to coordinate care. The AR Medicaid program also imposes some benefit limitations: 12 physician, clinic, and / or outpatient visits allowed per year, 3 prescriptions per month with possible extensions to 6 prescriptions per month, maximum of 24 inpatient days per year, and there are some co-pays and co-insurance required for prescription drugs and other services depending on eligibility type. These are relatively strict cost-containment strategies compared to other states.[26] Descriptive analyses of Medicaid claims analyses indicate that Medicaid data are generally valid and suitable for epidemiologic uses.[16] When mental health Medicaid prescription claims data were compared to chart abstracts, high levels agreement (> 85%) were attained indicating that Medicaid prescription records are reliable, however the claims may not completely capture all prescription for high utilizing subjects that are affected by maximum monthly prescription caps.[21; 22] Pain-related Medicaid claims have been used by other investigators and are likely to be at least as valid as mental health claims because they are less stigmatized.[15] However, we are unaware of studies that have validated Medicaid pain claims.
2.2. Study sample
The study sample consisted of enrollees in the two insurance types during the years from 2000 to 2005 who met the following criteria. Inclusion Criteria: 1) One or more NCPC diagnoses recorded: back pain (ICD-9-CM codes 721.3x - 721.9x, 722.2x, 722.30, 722.70, 722.80, 722.90, 722.32, 722.72, 722.82, 722.92, 722.33, 722.73, 722.83, 722.93, 724.xx, 737.1, 737.3, 738.4, 738.5, 739.2, 739.3, 739.4, 756.10, 756.11, 756.12, 756. 13, 756.19, 805.4, 805.8, 839.2, 839.42, 846, 846.0, 847.1, 847.3, 847.2, 847.9), neck pain (ICD-9CM codes 721.0X, 721.1X, 722.0X, 722.31, 722.71, 722.81, 722.91, 723.XX, 839.0, 839.1, 847. 0), arthritis/joint pain (ICD-9-CM codes >=710 and <720 or >=725 and <740), headache/migraine (ICD-9-CM codes >=346 and <347, or 307.81), and HIV/AIDS (ICD-9-CM codes 042.xx, 079.53, 279.10, 279.19, 795.71, 795.8x). We could not verify that these conditions were chronic or that these were the conditions for which opioids were prescribed, but chronic forms of these conditions are the most likely reason for long-term opioid use in a general medical population. We also identified all subjects in these insurance types who received any opioids but did not receive one of the above NCPC diagnoses. 2) Age: subjects age 18 or older. 3) Enrollment: subjects must be enrolled and eligible for benefits for at least nine continuous months in at least one calendar year during 2000-2005 to be eligible. Exclusion Criteria: 1) Cancer diagnosis at any time 2000-2005 other than nonmelanoma skin cancer (ICD-9-CM code 173), 2) resident of nursing home, 3) receiving hospice benefits. These criteria allow us to focus on enrollees likely receiving opioids for the treatment of NCPC. Our requirement that enrollees receive their NCPC diagnosis in the same year as their opioid prescription may result in an underestimation of the proportion of those receiving opioids who also have an NCPC.
2.3. Study data
Data supplied by the insurers to the research team consisted of HIPAA-compliant claims data files including: age and gender of subject, region (for HealthCore), and all opioid prescriptions (including date, dose, quantity and type of opioid dispensed) included regardless of indication for opioid use. It is not legal to treat opioid dependence in general health care settings, so methadone used for this purpose is not included. Buprenorphine was excluded.
2.3.1. Prevalence of opioid use
For each year from 2000-2005, we formed an analytic data set including all individuals with one of our tracer NCPC diagnoses (back pain, neck pain, arthritis/joint pain, headache/migraine, HIV/AIDS). We categorized the number of days of opioids supplied in the calendar year into 0, 1-30, 31-90, 91-180, and 181+ days. Days supplied is calculated and entered by the dispensing pharmacist assuming that the patient takes the medication at the maximum rate allowed by the prescribing provider. Days supplied is therefore the minimum of the actual days of use and the dose per days supplied is the maximum dose allowed per day. We do not know how many days patients actually took opioids or what dose they took on those days. We also recorded the total number of prescription fills for each patient within each calendar year.
2.3.2. Categorization of opioids
Opioids were categorized into three major groups: short-acting Drug Enforcement Administration (DEA) Schedule II opioids; long-acting DEA Schedule II opioids; and short-acting DEA Schedule III-IV opioids. Total morphine equivalents for each prescription were calculated by multiplying the quantity of each prescription by the strength of the prescription (milligrams of opioid per unit dispensed). The quantity-strength product was then multiplied by conversion factors derived from published sources to estimate the milligrams of morphine equivalent to the opioids dispensed in the prescription.[1; 11; 32]
Total morphine equivalents per patient per calendar year were calculated by adding the morphine equivalents for each prescription filled during the year for each patient. We report the following measures of opioid use: days supply of opioids dispensed per calendar year, cumulative yearly opioid dose (in milligrams morphine equivalents), opioid dose per day supplied, number of opioid prescriptions received in a calendar year, opioid dose per prescription, and cumulative opioid dose per calendar year per enrollee. We provide a table with the generic names of commonly prescribed opioids, the ratio used to calculate morphine equivalents, and their DEA Schedule (Table 1). [1; 3; 11; 23; 32; 33]. After reviewing published conversion factors, consensus was reached among two physicians with clinical experience in pain management (MS and JM), and a pharmacist pharmacoepidemiologist (DB).
Table 1.
Classification of opioid medications and morphine equivalent conversion factors per milligram of opioid.1
| Major Group | Type of Opioid | Morphine equivalent conversion factor per mg of opioid |
|---|---|---|
| Short-acting Non-Schedule II | Propoxyphene (with or without aspirin/acetaminophen/ibuprofen) | 0.23 |
| Codeine + (acetaminophen, ibuprofen or aspirin) | 0.15 | |
| Hydrocodone + (acetaminophen, ibuprofen, or aspirin) Hydrocodone and homatropine | 1.0 | |
| Tramadol with or without aspirin | 0.10 | |
| Butalbital and codeine (with or without aspirin, ibuprofen, acetaminophen) | 0.15 | |
| Dihydrocodeine (with or without aspirin, ibuprofen, acetaminophen) | 0.25 | |
| Pentazocine (with or without aspirin, ibuprofen, acetaminophen) | 0.37 | |
| Short-acting, Schedule II | Morphine sulfate | 1.0 |
| Codeine sulfate | 0.15 | |
| Oxycodone (with or without aspirin, acetaminophen, ibuprofen) | 1.5 | |
| Hydromorphone | 4.0 | |
| Meperidine hydrochloride | 0.1 | |
| Fentanyl citrate transmucosal2 | 0.125 | |
| Oxymorphone | 3.0 | |
| Long-acting (Schedule II) | Morphine sulfate sustained release | 1.0 |
| Fentanyl transdermal3 | 2.4 | |
| Levorphanol tartrate | 11.0 | |
| Oxycodone HCL controlled release | 1.5 | |
| Methadone | 3.0 |
Opioids delivered by pill, capsule, liquid, transdermal patch, and transmucosal administration were included in CONSORT data. Opioids formulated for administration by injection or suppository were not included.
Transmucosal fentanyl conversion to morphine equivalents assumes 50% bioavailability of transmucosal fentanyl and 100 micrograms transmucosal fentanyl is equivalent to 12.5 to 15 mg of oral morphine..
Transdermal fentanyl conversion to morphine equivalents is based on the assumption that one patch delivers the dispensed micrograms per hour over a 24 hour day and remains in place for 3 days.
From Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter C, Silverberg M, Banta-Green C, Weisner C, De Facto Long-term Opioid Therapy for Non-Cancer Pain, Clinical Journal of Pain, In Press, used with permission
2.3.3. Data quality
To protect against data entry errors, we treated as an outlier any value for days supply or for quantity that was greater than two times the 99th percentile value for the particular type of opioid. Outliers were then handled as if they were missing data. If either quantity or days supply was missing for a particular prescription, then morphine equivalents were not calculated for that prescription. The estimate of total morphine equivalents was inflated by the total number of prescriptions in the year (including those with missing data) divided by the number of prescriptions with valid data (i.e. not counting the ones with missing/outlier data). This approach conservatively estimates the morphine equivalents for prescriptions with missing/outlier data as being equal to the average prescription in the year. In both samples, total missing was less than 1.5% and outliers were less than 0.5%
3. Analysis
The descriptive statistics (e.g. means, standard deviations, percentages) were provided by year for each insurer. We also computed the percent change between 2000 and 2005 data for each variable by dividing the difference by the 2000 result. For continuous variables, the percent change is the ratio of the difference between 2000 and 2005 means over the 2000 mean. For binary or categorical variables, the percent change is the ratio of the difference in the 2000 and 2005 percentages over the 2000 percentage. Because all the numbers were rounded for succinct presentation after the calculation was completed, minor rounding errors might be found between the means and percent change, especially for the means with smaller values. Due to the very large sample sizes, statistical tests for differences of means and linear trend are all highly significant (tests not shown). All the analyses were performed using SAS 9.1 (SAS Institute Inc, Cary, NC).
4. Results
Figure 1 displays the relative proportions of enrollees receiving one or more of the NCPC tracer diagnoses and/or opioids in 2000 and 2005 for the HealthCore and Medicaid samples. Based on whether the enrollee had received a NCPC diagnosis in the past year (yes, no), and whether the enrollee had received any opioid prescriptions in the past year (yes, no), we constructed four mutually exclusive categories. Increases in the rates of diagnosis and opioid use were linear between these years in both samples. Since 90% of the non-NCPC associated opioid use in all years was for 30 days supply or less (data not shown), we did not explore this group further.
Fig. 1.
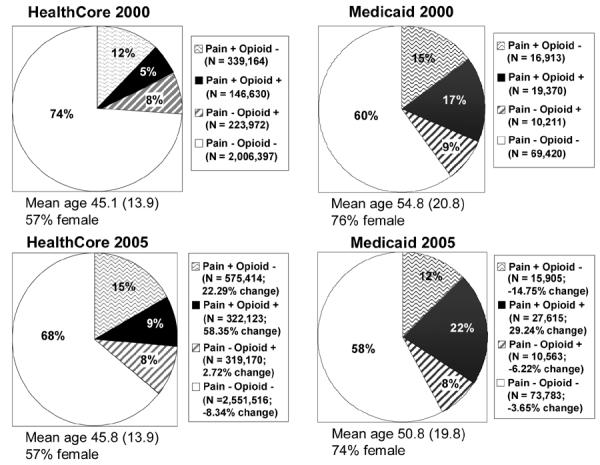
Proportions of enrollees with CNCP and/or opioid use in HealthCore and Medicaid enrollees
The HealthCore sample consisted of 2,716,163 individuals in 2000 and 3,768,223 individuals in 2005. Among these eligible subjects, 485,794 (17.9%) received at least one of our five NCPC tracer diagnoses in 2000. This increased to 897,537 (23.8%) by 2005, a 33.2% increase. This NCPC sample was 57%-58% female throughout the study period. Mean age was 45.1 years in 2000 and 45.8 years in 2005. The proportion of enrollees receiving at least one of our NCPC diagnoses and receiving opioids in the same calendar year increased 58.4% between 2000 and 2005. The proportion of enrollees receiving one of the tracer NCPC diagnoses but not prescribed any opioids increased 22.3% from 2000 to 2005. The proportion of enrollees with a NCPC diagnosis who received any opioids in the same calendar year increased from 30.1% in 2000 to 35.8% in 2005, an 19.1% increase.
Data from the Arkansas Medicaid sample consisted of 115,914 individuals in 2000 and 127,866 individuals in 2005. Among these eligible subjects, 36,283 (31.3%) received one or more of our five NCPC tracer diagnoses in 2000. This increased to 43,520 (34.0%) by 2005, a 8.7% increase. This NCPC sample was 74%-76% female, as is typical for Medicaid cohorts. Mean age was 54.8 years in 2000, decreasing to 50.8 years by 2005, a decline of 7.4%. The proportion of enrollees that received neither a tracer NCPC diagnosis nor any opioids declined 3.7% from 2000-2005. The proportion of enrollees with a NCPC diagnosis who received any opioids in the same calendar year increased from 53.4% in 2000 to 63.5% in 2005, a 18.9% increase.
Rates of NCPC diagnosis and opioid use were initially higher in the Medicaid sample but increased more rapidly in the HealthCore sample between 2000 and 2005. The percent of enrollees with at least one NCPC diagnosis who received opioids increased from 5 to 9% in HealthCore and from 17 to 22% in AR Medicaid. In contrast, the proportion of enrollees who did not receive one of our tracer diagnoses but did receive opioids remained fairly constant in both samples. There was little change in either sample from 2000 to 2005 in the proportion of enrollees without a tracer NCPC diagnosis who received any opioids.
Tables 2 and 3 display data about opioid use among HealthCore and Arkansas Medicaid enrollees with the NCPC tracer diagnoses. We grouped enrollees into five categories according to the number of days of opioids supplied to them in the calendar year: 0 days, 1-30 days, 31-90 days, 91-180 days, 181+ days. The proportion of enrollees that received a NCPC diagnosis but did not receive opioids in a given year was greater for the HealthCore sample than for AR Medicaid (2000-46.6%; 2005-36.6%). Across both study samples, most opioid use involves less than 30 days supply. However, opioid use increased most rapidly in NCPC patients receiving > 180 days supply. The proportion of NCPC enrollees with 181+ days supply grew from 9.5% of the NCPC group to 16.0% of the NCPC group in AR Medicaid and grew from 2.1% to 3.2% of HealthCore subjects. These trends and differences between samples are reflected in the mean days supply among those receiving opioids, which increased from 86.6 to 116.1 days (34% increase) in AR Medicaid and increased from 42.7 to 52.8 days (24% increase) in the Health Core sample.
Table 2.
HealthCore: opioid use among subjects with non-cancer pain diagnoses
| Variables | Category | 2000 (N=146,630) | 2001 (N=197,581) | 2002 (N=241,482) | 2003 (N=290,060) | 2004 (N=314,354) | 2005 (N=322,123) | % change from 00~05 |
|---|---|---|---|---|---|---|---|---|
| Days Supply of Opioids-Groups | 1-30 days | 23.6% | 27.3% | 26.6% | 27.4% | 27.5% | 27.3% | 15.6% |
| 31-90 days | 3.1% | 3.5% | 3.5% | 3.6% | 3.6% | 3.8% | 23.4% | |
| 91-180 days | 1.3% | 1.5% | 1.6% | 1.6% | 1.6% | 1.6% | 21.8% | |
| 181+days | 2.1% | 2.5% | 2.7% | 2.9% | 3.0% | 3.2% | 49.9% | |
| Among Those Receiving Opioids. | ||||||||
| Days Supply of Opioids-Per Year | Mean (SD) | 42.7 (100.3) | 43.9 (104.9) | 46.8 (110.2) | 48.7 (115.7) | 50.4 (120.1) | 52.8 (124.6) | 23.5% |
| [Median] | [8] | [8] | [8] | [8] | [8] | [9] | ||
| Cumulative Yearly Opioid Dose [mg morphine equiv] | Mean (SD) | 2,473.5 (13,136.7) | 2,612.7 (15,255.9) | 2,951.9 (18,466.2) | 3,160.4 (19,718.5) | 3,235.3 (19,901.6) | 3,406.9 (20,558.5) | 37.7% |
| [Median] | [350] | [353] | [368] | [368] | [375] | [375] | ||
| Opioid-Dose per Day Supplied [in mg morph/days supplied] | Mean(SD) | 53.5 (49.0) | 53.0 (48.9) | 53.8 (52.5) | 54.1 (52.7) | 53.8 (51.3) | 53.1 (50.6) | -0.7% |
| [Median] | [38] | [38] | [38] | [38] | [38] | [38] | ||
| Number of Prescriptions Received | Mean(SD) | 3.8 (6.2) | 3.9 (6.3) | 3.9 (6.2) | 3.9 (6.1) | 3.9 (6.1) | 4.0 (6.1) | 4.3% |
| [Median] | [2] | [2] | [2] | [2] | [2] | [2] | ||
| Opioid Dose per Prescription [in mg morph/prescription] | Mean(SD) | 366.4 (663.7) | 358.2 (700.8) | 375.3 (797.1) | 384.0 (832.2) | 386.7 (844.4) | 396.8 (856.9) | 8.3% |
| [Median] | [200] | [200] | [200] | [200] | [200] | [206] | ||
| All Enrollees | ||||||||
| N=2,716,163 | N=2,968,321 | N=3,567,284 | N=3,743,373 | N=3,780,378 | N=3,768,223 | |||
| Cumulative Yearly Opioid Dose Per Year Per Enrollee | 204.3 | 247.0 | 276.2 | 318.4 | 344.6 | 371.4 | 81.8% | |
Table 3.
Arkansas Medicaid: opioid use among subjects with non-cancer pain diagnoses
| Variables | Category | 2000 (N=23,155) | 2001 (N=25,261) | 2002 (N=27,896) | 2003 (N=30,079) | 2004 (N=33,028) | 2005 (N=35,697) | % Change 00~05 |
|---|---|---|---|---|---|---|---|---|
| Days Supply of Opioids-Groups | 1-30 days | 29.5% | 29.3% | 30.2% | 29.3% | 29.9% | 29.5% | 0.1% |
| 31-90 days | 9.1% | 9.5% | 9.7% | 10.6% | 10.5% | 11.0% | 21.6% | |
| 91-180 days | 5.4% | 5.9% | 6.3% | 6.4% | 6.8% | 7.0% | 29.5% | |
| 181+days | 9.5% | 10.9% | 11.5% | 12.6% | 13.9% | 16.0% | 68.5% | |
| Among Those Receiving Opioids | ||||||||
| Days Supply of Opioids Per Year | Mean (SD) | 86.6 (129.1) | 94.4 (135.4) | 96.3 (137.6) | 103.3 (145.3) | 109.2 (151.6) | 116.1 (153.5) | 34.0% |
| [Median] | [25] | [29] | [30] | [31] | [33] | [38] | ||
| Cumulative Yearly Opioid Dose [mg morphine equivalents] | Mean (SD) | 5,059.8 (13,534.4) | 5,520.2 (14,656.0) | 5,634.3 (15,961.9) | 6,129.0 (17,829.9) | 6,705.9 (19,569.4) | 6,952.4 (18,377.1) | 37.40% |
| [Median] | [1117] | [1185] | [1200] | [1301] | [1373] | [1450] | ||
| Opioid Dose per Day Supplied [in mg morph/day supplied] | Mean(SD) | 54.3 (40.3) | 53.0 (39.9) | 51.7 (40.9) | 51.1 (42.4) | 50.9 (43.4) | 50.2 (41.7) | -7.49% |
| [Median] | [41] | [39] | [38] | [38] | [38] | [38] | ||
| Number of Prescriptions Received | Mean(SD) | 5.3 (5.4) | 5.5 (5.5) | 5.5 (5.5) | 5.8 (5.7) | 6.0 (5.8) | 6.2 (5.8) | 17.13% |
| [Median] | [3] | [3] | [3] | [3] | [4] | [4] | ||
| Opioid Dose per Prescription [in mg morph/prescription] | Mean (SD) | 633.6 (824.2) | 655.5 (874.0) | 653.4 (921.5) | 667.1 (989.0) | 689.5 (1063.3) | 713.9 (1027.5) | 12.66% |
| [Median] | [398] | [395] | [380] | [378] | [383] | [406] | ||
| All Enrollees | N=115,914 | N=117,935 | N=119,338 | N=123,080 | N=128,628 | N=127,866 | ||
| Cumulative Opioid Dose Per Year Per Enrollee [mg morphine equivalents] | 1003.3 | 1137.4 | 1234.3 | 1346.4 | 1529.3 | 1662.0 | 65.66% | |
Mean cumulative opioid dose received in a calendar year by those with NCPC diagnoses increased in both samples. It rose 37.4% from 5,060 to 6,952 mg morphine equivalents in AR Medicaid and increased 37.7% from 2,474 to 3,407 in HealthCore between 2000 and 2005. This is because of the increase in the days supplied rather than the dose per day supplied, as the dose per day during this period actually declined slightly. The cumulative opioid dose per days supplied was similar between HealthCore and AR Medicaid and did not increase over time in either insurance type. There was a modest increase in the number of prescriptions received in both samples while AR Medicaid users had more prescriptions and higher opioid doses per prescription.
Table 4 displays opioid types used by enrollees with our NCPC tracer diagnoses according to Drug Enforcement Administration Schedule and duration of action. Since the rates of increase between 2000 and 2005 were quite linear (data not shown), we display the data only for the years 2000 and 2005. The two most commonly used short-acting Schedule II opioids in both samples were: oxycodone and meperidine. The two most commonly used long-acting Schedule II opioids in both samples were: sustained release oxycodone and transdermal fentanyl. The most commonly used Schedule III and IV opioids were: hydrocodone and codeine combined with either acetaminophen or aspirin.
Table 4.
Opioid types used by enrollees with non-cancer pain conditions (NCPC) who used opioids according to DEA schedule and duration of action for Arkansas Medicaid and HealthCore members: 2000 vs. 2005
| Opioid Types | HealthCore 2000 N= 146530 | HealthCore 2005 N= 322123 | % Change 2000-2005 | Medicaid 2000 N= 23155 | Medicaid 2005 N= 35697 | % Change 2000-2005 | |
|---|---|---|---|---|---|---|---|
| Short-Acting Schedule II Users Per Yeara | % | 12.4% | 18.8% | 52.0% | 13.3% | 17.5% | 31.2% |
| Mean Total Yearly Dose Per Short-Acting Schedule II NCPC opioid userb | Mean (SD) | 1,345.6 (10,190.0) | 2,631.2 (21,897.3) | 95.5% | 1,528.1 (5,360.6) | 4,450.3 (11,484.4) | 191.2% |
| [Median] | [300] | [300] | [300] | [465] | |||
| Short-Acting Schedule II Proportion of Cumulative yearly opioid dose among all NCPC opioid usersc | Mean (SD) | 7.2% (23.3%) | 11.0% (27.8%) | 54.0% | 5.4% (19.2%) | 7.4% (23.2%) | 38.3% |
| Long-Acting Schedule II Users Per Yeara | % | 4.0% | 4.7% | 19.5% | 6.6% | 9.3% | 40.8% |
| Mean Total Yearly Dose Per Long-Acting Schedule II NCPC opioid userb | Mean (SD) | 20,936.8 (44,365.4) | 29,155.7 (54,450.7) | 39.3% | 24,479.8 (38,031.7) | 29,722.9 (41,288.1) | 21.4% |
| [Median] | [3,600] | [8,400] | [10,035] | [14,700] | |||
| Long-Acting Schedule II Proportion of Cumulative yearly opioid dose among all NCPC opioid usersc | Mean (SD) | 2.5% (13.7%) | 2.8% (14.1%) | 11.2% | 4.4% (18.5%) | 6.1% (21.1%) | 37.2% |
| Schedule III-IV Opioid Users Per Yeara | % | 94.3% | 92.4% | -2.0% | 95.4% | 94.2% | -1.3% |
| Mean Total Yearly Dose Per Schedule III-IV NCPC opioid userb | Mean (SD) | 1,493.1 (3,738.0) | 1,503.5 (3,625.4) | 0.7% | 3,401.5 (5,892.9) | 3,630.4 (5,624.5) | 6.7% |
| [Median] | [338] | [340] | [1011] | [1,250] | |||
| Schedule III-IV Opioids Proportion of Cumulative yearly opioid dose among all NCPC opioid usersc | Mean(SD) | 90.3% (27.0%) | 86.1% (31.2%) | -4.7% | 90.2% (26.5%) | 86.5% (30.5%) | -4.1% |
Proportion of all enrollees receiving a NCPC diagnosis and opioids in a calendar year who received this type of opioid
Mean total dose (in mg morphine equivalents) of this opioid type received in a calendar year by NCPC enrollees receiving that opioid type that year
Proportion of cumulative yearly opioid dose (in mg morphine equivalents) received by enrollees with a NCPC diagnosis that is of this type of opioid
The proportion of enrollees using short-acting Schedule II opioids, the mean total yearly dose per patient, and the proportion of the cumulative yearly opoid dose among all NCPC opioid users increased markedly in both health plans between 2000 and 2005. In HealthCore, the proportion receiving short-acting Schedule II opioids increased 52%, while in Arkansas Medicaid, the proportion increased 31%. Most striking is that cumulative yearly dose per enrollee with a NCPC diagnosis increased by 95.5% in HealthCore and by 191.2% in Arkansas Medicaid. The proportion of cumulative yearly opioid dose received by all enrollees with a NCPC diagnosis that was short-acting Schedule II opioids also increased significantly, by 54% in HealthCore and 38% in Medicaid.
The proportion of enrollees using long-acting Schedule II opioids, the mean total yearly dose per patient, and the proportion of the cumulative yearly opoid dose among all NCPC opioid users increased significantly in both populations between 2000 and 2005, though not as much as for short-acting schedule II opioids. In HealthCore, the proportion receiving long-acting Schedule II opioids increased 19%, while in Arkansas Medicaid, the proportion increased 41%. Cumulative yearly dose per enrollee with a NCPC diagnosis increased by 39% in HealthCore and by 21% in Arkansas Medicaid. Though these rates of increase are lower than those for the short-acting Schedule II opioids, it is important to note that the mean doses are nearly ten times greater. The proportion of cumulative yearly opioid dose received by all enrollees with a NCPC diagnosis that was accounted for by long-acting Schedule II opioids also increased significantly, by 11% in HealthCore and 37% in Medicaid.
Over 90% of all NCPC opioid users received schedule III and IV opioids and nearly 90% of all the opioids received by this population were schedule III and IV opioids. However, there was little change between 2000 and 2005 in the proportion of enrollees using Schedule III and IV opioids or the mean total dose of Schedule III and IV opioids received in either insurance type. The proportion of the cumulative yearly opioid dose among all NCPC opioid users that was Schedule III and IV opioids declined slightly in both health plans. This is expected given the large dose increases in Schedule II opioids among the NCPC population described above.
5. Discussion
Our study demonstrates a very broad-based increase in the use of potent opioids in publicly and privately insured enrollees between 2000 and 2005. This appears to be due to multiple factors. First, the proportion of enrollees receiving NCPC diagnoses increased in both health plans. Second, among enrollees with NCPC, the proportion that received opioids increased in both populations. Throughout the study period, rates of opioid use were markedly higher in the AR Medicaid population than the commercial population. The proportion of NCPC subjects receiving opioids, total days supply, cumulative yearly opioid dose, and number of opioid prescriptions were all higher in Arkansas Medicaid enrollees than in HealthCore enrollees. This likely reflects the much greater disability and disease burden in a Medicaid population. Despite this greater burden in the Medicaid sample, the proportion of NCPC subjects using opioids, the cumulative yearly opioid dose, and the cumulative opioid dose per enrollee all grew faster in the HealthCore than the Medicaid sample. For example the cumulative opioid dose per enrollee grew 66% in the Arkansas Medicaid population, but grew 82% in the HealthCore population. These findings suggest a very broad-based liberalization in opioid use for non-cancer pain that is not limited to one geographic region or socioeconomic group.
This increase in opioid use is largely due to increasing days supplied of DEA Schedule II opioids in enrollees with NCPC diagnoses. There was no significant increase in the proportion of enrollees without our tracer NCPC diagnoses using opioids. Furthermore, 90% of this use among enrollees without NCPC diagnoses is for 30 days supply or less. There was no significant increase in use of the most commonly used Schedule III and IV opioids in either health plan. More NCPC enrollees used short-acting Schedule II opioids and the rates of increase from 2000 to 2005 were greater than seen with long-acting Schedule II opioids. However, for enrollees receiving Schedule II long-acting opioids, mean yearly cumulative dose was ten times larger for long-acting than for short-acting opioids, reflecting the greater days supplied for patients using long-acting opioids.
Our study suggests that the trend of increasing opioid use for NCPC from 1980 to 2000 noted by Caudill-Slossberg et al is continuing. It confirms their finding that increases are concentrated in the more potent Schedule II opioids. This increase is seen in both privately-insured (BCBS plans) groups and in publicly-insured (Medicaid) groups. Future TROUP analyses will examine whether this increase in concentrated in specific age, gender, pain or mental health groups.
The large increases in proportion of enrollees receiving opioids and in the dose received likely reflect changes in attitudes and policies concerning opioid use rather than changes in the prevalence or severity of the NCPC conditions being treated. The proportion of enrollees receiving NCPC diagnoses increased 9% in Arkansas Medicaid and 33% in the commercially insured population. This is unlikely to be due to an increase in the underlying prevalence of NCPC, but could reflect the increasing attention being paid attention to pain by health systems and regulatory bodies, i.e. “pain as the fifth vital sign.”[17; 31]
The rate of NCPC diagnosis increased faster than the rate of opioid use among those with NCPC diagnoses in the HealthCore population (33% vs 19%), but the reverse was true of the Arkansas Medicaid population (9% vs 19%). This could reflect wider recognition of NCPC as serious problems that deserve medical treatment.[29] Mean dose levels generally increased more than median dose levels, suggesting that the largest increases in opioid use are concentrated in those receiving the largest doses. The overall effect is a marked increase (approximately 66% in Medicaid and 82% in the commercially insured population) in the cumulative yearly dose of opioids received per enrollee.
This large increase in the use of opioids is not accompanied by new evidence of the effectiveness of long-term opioid therapy for NCPC. A recent meta-analysis of 41 randomized trials documented that opioids were more effective for pain relief in NCPC than placebo, but the mean duration of treatment was only 5 weeks (range 1-16 weeks) and 33% of opioid-treated patients dropped out.[12] Recent systematic reviews question the efficacy of long-term opioid therapy for back pain.[9; 20] There is also concern about opioid treatment of headaches.[19] Due to health risks associated with NSAIDs and Cox-2 inhibitors, however, there is increasing advocacy for the use of opioids as a second-line treatment for osteoarthritis.[25] Overall, the broad-based increase in proportion of NCPC patients treated with opioids, in the number of days supplied, and in the potency of the opioids used can likely be attributed to both increased attention to the problem of untreated pain and to the liberalization of clinical standards and attitudes concerning the use of opioids for NCPC.
It is not possible to determine from our data whether this increasing use of opioids for NCPC provides net benefit or net harm to patients. Many aspects of benefit (pain reduction, functional improvement, distress reduction) need to be carefully weighed against many aspects of harm (opioid-induced hyperalgesia, reduced function, increased depression, iatrogenic addiction). Future studies are needed to provide the necessary information for making clinical decisions that result in net benefit to patients.
There are important limitations to our study. First, the only data available on study subjects is administrative claims data submitted to the health plan. No independent clinical assessment of study subjects to confirm diagnoses was done. Primary pain diagnoses are likely accurate, though there may be under-coding of comorbid conditions as secondary diagnoses.[18] Data concerning opioid prescriptions is likely accurate since this is a covered benefit for all enrollees and other studies suggest that over 90% of prescriptions are filled within the health plan. We did not link the NCPC diagnosis with the opioid prescription more directly than determining that they were made in the same calendar year in order to maintain consistency with the year-based analyses in the rest of our paper, and because we have no specific information concerning the indication for the opioid prescriptions. Previous studies by ourselves and others[30] suggest that opioid recipients have multiple painful and non-painful physical health and mental health conditions, and therefore linking the opioid prescription with any one diagnosis is difficult and likely invalid. Second, we analyzed each year's data separately and did not track individual subjects' status from year to year. Hence, our results should be interpreted as population trends and not the trends of individual enrollees. Third, we present data from two specific insurance types. It is not known how well these findings generalize to other private and public health plans. The Medicaid data was from a single state and differences we report between the plans may represent regional prescribing variations or inherent differences in the level of disease and pain burden between a Medicaid and commercially insured population. Furthermore, our findings may also be influenced by Medicaid and commercial specific policies that may not be found in the Medicaid or commercial plans of other states.
In summary, we found large increases in the proportion of enrollees receiving opioid therapy for non-cancer pain conditions in both privately and publicly insured populations. Among those receiving opioids, doses increased significantly due to increased in the days supplied rather than dose per day supplied. Dose increases were most marked among those receiving long-term opioid therapy and were concentrated in more potent DEA Schedule II opioids. These trends have likely occurred without any significant change in the underlying prevalence or severity of chronic non-cancer pain or evidence base concerning the efficacy of long-term opioid therapy and thus represent significant shifts in diagnosing and prescribing patterns and in opioid treatment philosophy. Further research is necessary to determine if these shifts have resulted in net benefit or net harm to patients.
Acknowlegements
This research was supported by a grant from the National Institute on Drug Abuse DA022560 to Mark D. Sullivan. None of the authors have any financial conflicts of interest. We are grateful to Gary Moore for building the Arkansas Medicaid analytic dataset and Tosmai Puenpatom for building the HealthCore analytic dataset.
References
- [1].American Pain Society . Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 5th edition American Pain Society; Glenview, IL: 2003. [Google Scholar]
- [2].Arkansas Department of Health and Human Services . Program Overview. Little Rock, AR: 2005. Division of Medical Services. [Google Scholar]
- [3].Back I. Palliative Medicine Handbook [Google Scholar]
- [4].Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(23):127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- [5].Breitbart W, Rosenfeld B, Passik S, Kaim M, Funesti-Esch J, Stein K. A comparison of pain report and adequacy of analgesic therapy in ambulatory AIDS patients with and without a history of substance abuse. Pain. 1997;72(12):235–43. doi: 10.1016/s0304-3959(97)00039-0. [DOI] [PubMed] [Google Scholar]
- [6].Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [7].Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. J Pain. 2005;6(10):662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [8].Curtis LH, Stoddard J, Radeva JI, Hutchison S, Dans PE, Wright A, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41(3 Pt 1):837–55. doi: 10.1111/j.1475-6773.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deshpande A, Furlan A, Mailis-Gagnon A, Atlas S, Turk D. Opioids for chronic low-back pain. Cochrane Database of Systematic Reviews. 2007;(Issue 3):CD004959. doi: 10.1002/14651858.CD004959.pub3. [DOI] [PubMed] [Google Scholar]
- [10].Eriksen J, Jensen MK, Sjogren P, Ekholm O, Rasmussen NK. Epidemiology of chronic non-malignant pain in Denmark. Pain. 2003;106(3):221–8. doi: 10.1016/S0304-3959(03)00225-2. [DOI] [PubMed] [Google Scholar]
- [11].Fine P, Portenoy RK. A Clinical Guide to Opioid Analgesia. McGraw-Hill Healthcare Information; Minneapolis: 2004. [Google Scholar]
- [12].Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002. J Pain Symptom Manage. 2004;28(2):176–88. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [14].Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280(2):147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- [15].Hartung DM, Middleton L, Haxby DG, Koder M, Ketchum KL, Chou R. Rates of adverse events of long-acting opioids in a state Medicaid program. Ann Pharmacother. 2007;41(6):921–8. doi: 10.1345/aph.1K066. [DOI] [PubMed] [Google Scholar]
- [16].Hennessy S, Bilker WB, Weber A, Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol Drug Saf. 2003;12(2):103–11. doi: 10.1002/pds.765. [DOI] [PubMed] [Google Scholar]
- [17].Joint Commission on Accreditation of Healthcare Organizations . Pain: current understanding of assessment, management and treatments. National Pharmaceutical Council; 2001. pp. 1–101. [Google Scholar]
- [18].Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40(9):1594–600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- [19].Lipton RB, Bigal ME. Opioid therapy and headache: a cause and a cure. Neurology. 2004;62(10):1662–3. doi: 10.1212/wnl.62.10.1662. [DOI] [PubMed] [Google Scholar]
- [20].Martell BA, O'Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- [21].Martin BC, McMillan JA. Bias associated with missing out-of-plan prescription data for Medicaid recipients. J Res Pharmaceutical Econ. 1996;7(3):65–78. [Google Scholar]
- [22].McKenzie DA, Semradek J, McFarland BH, Mullooly JP, McCamant LE. The validity of medicaid pharmacy claims for estimating drug use among elderly nursing home residents: The Oregon experience. J Clin Epidemiol. 2000;53(12):1248–57. doi: 10.1016/s0895-4356(00)00259-6. [DOI] [PubMed] [Google Scholar]
- [23].Oregon Health Sciences University Opioids and Chronic Non-malignant Pain: A Clinicians' Handbook [Google Scholar]
- [24].Portenoy RK. Appropriate use of opioids for persistent non-cancer pain. Lancet. 2004;364(9436):739–40. doi: 10.1016/S0140-6736(04)16951-1. [DOI] [PubMed] [Google Scholar]
- [25].Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol. 2006;25(Suppl 1):S22–9. doi: 10.1007/s10067-006-0203-8. [DOI] [PubMed] [Google Scholar]
- [26].Soumerai SB, Avorn J, Ross-Degnan D, Gortmaker S. Payment restrictions for prescription drugs under Medicaid. Effects on therapy,cost, and equity. N Engl J Med. 1987 Aug 27;317(9):550–6. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- [27].Stang P, Von Korff M, Galer BS. Reduced labor force participation among primary care patients with headache. J Gen Intern Med. 1998;13(5):296–302. doi: 10.1046/j.1525-1497.1998.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Strine TW, Hootman JM, Chapman DP, Okoro CA, Balluz L. Health-related quality of life, health risk behaviors, and disability among adults with pain-related activity difficulty. Am J Public Health. 2005;95(11):2042–8. doi: 10.2105/AJPH.2005.066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sullivan M, Ferrell B. Ethical challenges in the management of chronic nonmalignant pain: Negotiating through the cloud of doubt. J Pain. 2005;6(1):2–9. doi: 10.1016/j.jpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- [30].Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain. 2005;119(13):95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- [31].Veterans Health Administration . Clinical Practice Guideline for the Management of Opioid Therapy for Chronic Pain (Version 1.0) Department of Defense; Washington, D.C.: 2003. [Google Scholar]
- [32].Vieweg WV, Lipps WF, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry. 2005;7(3):86–8. doi: 10.4088/pcc.v07n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Von Korff M. De facto long-term opioid therapy for non-cancer pain. Clin J Pain. doi: 10.1097/AJP.0b013e318169d03b. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109(3):207–9. doi: 10.1016/j.pain.2004.02.019. [DOI] [PubMed] [Google Scholar]
- [35].Wennberg JE. Understanding geographic variations in health care delivery. N Engl J Med. 1999;340(1):52–3. doi: 10.1056/NEJM199901073400111. [DOI] [PubMed] [Google Scholar]
- [36].Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44(11):1005–10. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]


