Abstract
Animals sense light and chemical signals through proteins called G-protein-coupled receptors. The crystal structure of one such receptor in complex with a G-protein fragment shows how these receptors are activated.
The cell membranes of animals – everything from mammals to molluscs, insects and flatworms – contain proteins called G-protein- coupled receptors. Also known as 7TM receptors because of their seven transmembrane helices, they are involved in sensing light and a multitude of chemical signals: not only hormones and other transmitter molecules, but also odours, pheromones and flavours1. Many therapeutic drugs work by regulating the behaviour of specific 7TM receptors, and these proteins constitute the largest family of targets pursued by the pharmaceutical industry. Structural information about the receptors and their activation mechanisms is therefore clearly essential, but crystal structures have been available only for 7TM receptors in inactive forms. On page 497 of this issue, Scheerer et al.2 report a long-awaited breakthrough: the first convincing structure of a 7TM receptor protein in an active conformation.
Until a year ago, the only high-resolution structures available for 7TM receptors were those for the inactive state of rhodopsin, a light receptor found in the rod cells of the retina3. Rhodopsin consists of a protein called opsin and a covalently bound ligand molecule called 11-cis retinal, which prevents the protein from signalling. Light converts the retinal to the active isomer (all-trans retinal), which stabilizes the active conformation of the receptor. As with all 7TM receptors, active rhodopsin binds and activates a subunit (the α-subunit) of an intracellular G protein (Fig. 1), and thereby translates the light signal into a cellular response. Several structures of rhodopsin that contain all-trans retinal have been obtained, but all of these essentially show the inactive conformation of the protein and offer few, if any, clues to the active state3.
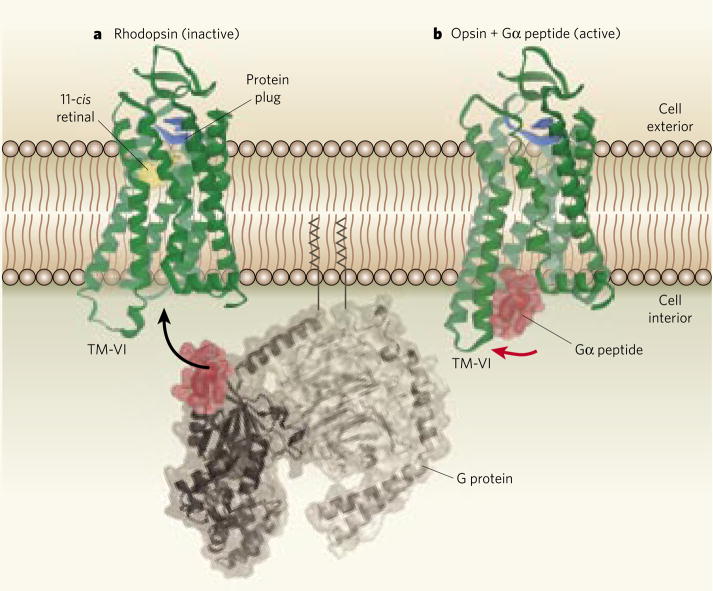
Figure 1. Activation of a G-protein-coupled receptor.
a, Rhodopsin, shown here in its inactivated conformation, is a light-sensing receptor found in cell membranes. It consists of a protein (opsin, green) and a ligand (retinal, yellow, also shown in its inactivated conformation). When activated by light, rhodopsin binds to part of an adjacent G protein (binding region in red), triggering a cascade of biological responses. The protein plug (blue) is part of the extracellular domain of opsin, and immobilizes the extracellular transmembrane segments of the receptor. b, Scheerer et al.2 have determined the activated structure of opsin in complex with the receptor-binding peptide fragment of the G protein (the Gα peptide). The most notable difference when compared with the inactivated receptor is that transmembrane helix 6 (TM-VI) has moved substantially outward (indicated by the red arrow), thereby creating the binding pocket for the G-protein peptide.
Rhodopsin has long been seen as the archetypal 7TM receptor, because particularly detailed biophysical and structural data were available for it. But last year saw the publication4–6 of the first structures of a 7TM receptor–the β2-adrenergic receptor – that is activated by a diffusible ligand, rather than light. More recently, the X-ray structure of a stabilized mutant of the β1-adrenergic receptor was also solved7. These structures differed substantially from that of rhodopsin, mainly in their extracellular domains. In each case, however, the adrenergic receptors were crystallized with antagonists (receptor blockers), and so the structures determined are thought to be inactive conformations. Nevertheless, smaller, but still noteworthy, conformational differences in the intracellular parts of the trans-membrane helices were observed compared with rhodopsin, especially for key amino-acid residues that are presumed to be involved in the activation process.
Scheerer et al.2 now report the structure of opsin in the elusive activated state. In cells, the active conformation of a 7TM receptor is normally stabilized not only by an agonist (a receptor activator) at the extracellular side, but also by the α-subunit of the G protein at the intracellular side (Fig. 1). The latter effect was exploited by the authors, who crystallized opsin in complex with the main peptide fragment of the Gα protein to which opsin binds.
Compared with inactive receptors, the hallmarks of the activated structure are a relatively large tilt of transmembrane helix 6 (TM-VI) and a smaller motion of helix 7 (TM-VII) at the inner surface of the cell membrane. These movements open a cleft that presents the ligand-binding site to the G protein. Scheerer and colleagues’ results broadly match those of earlier biophysical studies8, and agree quantitatively with those of work9 that used pairs of ‘spin labels’ – which contain unpaired electrons that can be detected spectroscopically – to map out helix movement in activated rhodopsin. On the other hand, the authors’ structure contrasts with a previously reported crystal structure10 of activated rhodopsin (in the absence of a G protein) that differed little from the inactive state. Given the extensive biochemical and biophysical data in support of Scheerer and colleagues’ structure for the activated state, it now seems likely that the previously reported structure10 was modified by the conditions used to stabilize the crystals.
It might seem strange that the activating ligand, all-trans retinal, is not present in Scheerer and colleagues’ opsin complex. But it is well established that active conformations of unliganded receptors, including opsin11, exist in equilibrium with the inactive state. Indeed, 7TM receptors are often observed signalling with high activity through G proteins in the absence of agonist ligands. The crucial point in Scheerer and colleagues’ structure2 is that the observed active form is stabilized by a large excess of the G-protein fragment (the Gα peptide). It is the presence of this peptide that identifies the structure as a true active conformation. What is more surprising is that, in the absence of both all-trans retinal and the Gα peptide, opsin crystallizes in the same conformation as the authors report here (as reported earlier this year12 by the same group).
The X-ray structure and orientation of the bound Gα peptide2 are almost identical to those previously determined by nuclear magnetic resonance (NMR) studies13 on solutions of a similar peptide interacting with activated rhodopsin. But Scheerer and colleagues’ structure now shows how the peptide is stabilized by interactions with specific amino-acid residues that are exposed by the movement of TM-VI. Of particular note is that the peptide makes direct contact with the most evolutionarily conserved residue in 7TM receptors, an arginine residue at the intracellular end of TM-III.
The mechanism underlying the molecular recognition between opsin and the Gα peptide will probably be a good model for the interactions between 7TM receptors and the α-subunits of G proteins in general. The selectivity of these interactions is largely determined by the five carboxy-terminal residues of the α-subunits14. But it has been unexpectedly difficult to identify the corresponding selectivity-determining footprint on the receptors. The structure of Scheerer and colleagues’ complex will therefore undoubtedly spur a wave of studies aimed at understanding this recognition process in detail. Moreover, interactions of 7TM receptors with G proteins, and their subsequent activation processes, are known to involve the recognition of additional structural elements on both the receptor and the G protein (Fig. 1). These processes can now be investigated in structure-based studies, as suggested by Scheerer and co-workers2.
The observed conformation of activated opsin on the intracellular side of the cell membrane probably represents the activated state for 7TM receptors in general. But the relatively minor conformational changes that occur in the ligand-binding domain – which lies between the extracellular segments of the transmembrane helices – is likely to be unique to opsin. In rhodopsin, part of the extracellular region of opsin forms a protein ‘plug’ that fills the entrance to the main ligand-binding pocket, preventing movement of the transmembrane helices (Fig. 1). In contrast, in the previously determined structures of adrenergic receptors5–7, the main ligand-binding crevice forms an open funnel towards the extracellular space. This allows ligands to diffuse in and out of the binding site, and could also allow inward movement of the extracellular segments of the transmembrane helices so that they surround the ligand. Such movement might aid ligand binding, and form part of a global ‘toggle-switch’ mechanism of receptor activation8. To test this possibility, we will need structures of 7TM receptors in complex not only with the G protein, but also with small-molecule agonists.
Footnotes
Competing financial interests: declared (see online article for details).
Contributor Information
Thue W. Schwartz, Email: tws@sund.ku.dk, Laboratory for Molecular Pharmacology, University of Copenhagen, Blegdamsvej 3, 2200 Copenhagen, Denmark.
Wayne L. Hubbell, Email: hubbellw@jsei.ucla.edu, Jules Stein Eye Institute and the Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, California 90095, USA.
References
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Nature Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Scheerer P, et al. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 3.Schertler GF. Curr Opin Struct Biol. 2005;15:408–415. doi: 10.1016/j.sbi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SGF, et al. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum DM, et al. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 6.Cherezov V, et al. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warne T, et al. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 9.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. Proc Natl Acad Sci USA. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salom D, et al. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff ML, et al. Nature Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 13.Koenig BW, et al. J Mol Biol. 2002;322:441–461. doi: 10.1016/s0022-2836(02)00745-3. [DOI] [PubMed] [Google Scholar]
- 14.Conklin BR, Farzel Z, Lustig KD, Julius D, Bourne HR. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]