Abstract
The possible role of astrocytes in the regulation of feeding has been overlooked. It is well-established that the endothelial cells constituting the blood–brain barrier transport leptin from blood to brain and that hypothalamic neurons respond to leptin to induce anorexic signaling. However, few studies have addressed the role of astrocytes in either leptin transport or cellular activation. We recently showed that the obese agouti viable yellow mouse has prominent astrocytic expression of the leptin receptor. In this study, we test the hypothesis that diet-induced obesity increases astrocytic leptin receptor expression and function in the hypothalamus. Double-labelling immunohistochemistry and confocal microscopic analysis showed that all astrocytes in the hypothalamus express leptin receptors. In adult obese mice, 2 months after being placed on a high-fat diet, there was a striking increase of leptin receptor (+) astrocytes, most prominent in the dorsomedial hypothalamus and arcuate nucleus. Agouti viable yellow mice with their adult-onset obesity showed similar changes, but the increase of leptin receptor (+) astrocytes was barely seen in ob/ob or db/db mice with their early-onset obesity and defective leptin systems. The marked leptin receptor protein expression in the astrocytes, shown with several antibodies against different receptor epitopes, was supported by RT–PCR detection of leptin receptor-a and -b mRNAs in primary hypothalamic astrocytes. Unexpectedly, the protein expression of GFAP, a marker of astrocytes, was also increased in adult-onset obesity. Real-time confocal imaging showed that leptin caused a robust increase of calcium signalling in primary astrocytes from the hypothalamus, confirming their functionality. The results indicate that metabolic changes in obese mice can rapidly alter leptin receptor expression and astrocytic activity, and that leptin receptor is responsible for leptin-induced calcium signalling in astrocytes. This novel and clinically relevant finding opens new avenues in astrocyte biology.
Keywords: leptin, obesity, astrocyte, ObR, calcium signalling
Introduction
It is increasingly recognized that astrocytes play an important role in gliotransmission and neurovascular coupling (Haydon and Carmignoto, 2006). However, the role of astrocytes in the regulation of obesity is not yet clear. Obese agouti viable yellow (Avy) mice express leptin receptors (ObR) in both astrocytes and neurons in the hypothalamus. While in lean adult B6 mice 7% of the ObR(+) cells are astrocytes (the remaining 93% being neurons), in adult Avy mice 25% of ObR is expressed in astrocytes, with a corresponding decrease of ObR (+) neurons to 75% (Pan et al., 2008a). Obese rats also have higher levels of expression of agouti-related protein (Dunbar et al., 2005). This leads to the next question: do astrocytes participate in the CNS control of obesity by upregulation of their leptin receptors?
Leptin is a 16 kD protein mainly produced by adipocytes, and its blood concentration correlates with fat mass. As a neuroendocrine hormone, one of the major targets of leptin action is the brain. Obesity is related to impaired transport of leptin across the blood–brain barrier (Kastin et al., 1999; Banks and Farrell, 2003; Banks, 2003; Banks et al., 2001, 2004), reduced neuroplasticity (Stranahan and Mattson, 2008), and decreased neurogenesis in the hippocampus (Lindqvist et al., 2006). The model of diet-induced obesity provides an additional tool to determine whether astrocytic upregulation of ObR expression is related to an intrinsic genetic predisposition in the Avy mice or is caused by epigenetic influences occurring after the onset of obesity.
The structural and biochemical changes in brain parenchyma induced by obesity appear to be rather trivial. There is no clear evidence of reactive gliosis or inflammatory cell infiltration, and there is no change in whole brain protein carbonyls (index of protein oxidation) or 4-hydroxynonenal (index of membrane lipid oxidation) in mice with a higher percent of fat (10%) in the diet as compared with the controls (5%). Yet, the triglyceride lowering agent gemfibrozil reduces protein carbonyls and 4-hydroxynonenal in these obese mice on a diet containing 10% fat for 10 months (Farr et al., 2008). But would leptin provide a metabolic switch for astrocytes?
The CNS effects of leptin are rather diverse. It is trophic to neural progenitor cells and promotes glial as well as neuronal development (Udagawa et al., 2006). It is anti-epileptic and can directly affect calcium and potassium channels (Shanley et al., 2002; Harvey, 2007; Xu et al., 2008). In the feeding circuitry, leptin facilitates the production of melanocortin in proopiomelanocortin neurons and suppresses the production of neuropeptide Y and agouti-related protein in neuropeptide Y neurons of the arcuate nucleus of the hypothalamus (Schwartz, 2001). Both proinflammatory and anti-inflammatory effects of leptin have been shown (Matarese et al., 2001; Lord, 2006; Ahmed et al., 2007; Pinteaux et al., 2007; Lin et al., 2007). The central leptin system, therefore, might be involved in neuroinflammation after the onset of the metabolic syndrome and hyperleptinaemia. Astrocytes could constitute an ideal cellular substrate for such actions. Thus, in this study we examine the important issue of astrocytic ObR regulation.
Materials and methods
Induction of diet-induced obesity and the specifics of the genetic models of obesity used in this study
C57BL/6J male mice (B6, Jackson Laboratories, Bar Harbor, ME, USA) were used to induce diet-induced obesity following a protocol approved by the Institutional Animal Care and Use Committee. Four-week-old mice were group-housed and randomly assigned to either a high fat diet (HFD, 45% kcal fat) (D12451, Research Diets, New Brunswick, NJ, USA) or regular rodent chow for 2 months or longer, as specified in the Results section. Body weight was measured weekly after induction of obesity for both the diet-induced obesity and control groups. The percentage of body fat was determined with a nuclear magnetic resonance (NMR) Bruker minispec Live Mice Analyser (model mq7.5, LF50; Bruker Optics, Inc., Billerica MA, USA), as described previously (Pan and Kastin, 2007).
The obesity phenotype of Avy mice (Jackson Laboratories) has been characterized previously (Pan and Kastin, 2007; Pan et al., 2008a). Small numbers of male ob/ob and db/db mice (Jackson Laboratories) were used in the validation of immunohistochemistry. The mutant mice were studied in parallel with age-matched male B6 controls. The ages and sample sizes are specified in the Results section.
Fluorescent immunohistochemistry and confocal microscopic analyses
The mice were anaesthetized by intraperitoneal injection of 40% urethane, and perfused intracardially with 4% paraformaldehyde (PFA). The brain was post-fixed in 4% paraformaldehyde for 3 days at 4°C. Afterwards, it was transferred to phosphate-buffered saline (PBS) with 15% sucrose for 8 h at 4°C, and then to 30% sucrose until it sank. HistoPrep frozen tissue embedding media (Fisher Scientific, Pittsburg, PA, USA) was used to embed the brain. The embedded tissue block was stored at −80°C for future use. Free-floating coronal sections of 20 μm thickness were obtained by a cryostat and stored in cryoprotectant (30% sucrose and 30% ethylene glycol in 0.1 M phosphate buffer) at −20°C until staining.
After being washed with PBS, the sections were blocked with 10% normal donkey serum in PBS with 0.3% Triton X-100 at room temperature for 1 h. Several ObR primary antibodies were used for immunolabelling, as shown in Table 1. Anti-glial fibrillary acidic protein (anti-GFAP, AB5804, 1:100 to 1:500; Chemicon, Temecula, CA, USA) or anti-neuronal nuclei (anti-NeuN, MAB377B, 1:100; Chemicon) primary antibodies were also used in the double-labelling process. After overnight incubation at 4°C, the sections were incubated with Alexa488 or Alexa594-labeled secondary antibodies (Invitrogen, Eugene, OR, USA), or FITC-conjugated anti-chicken secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at room temperature for 1 h. The sections were thoroughly washed with PBS between the steps. After staining, the sections were mounted with Vectashield mounting media (Vector Laboratories, Burlingame, CA, USA).
Table 1.
Features of leptin receptor (ObR) antibodies
| Epitope | Name | Source | Concentration (μg/ml) |
|---|---|---|---|
| membrane juxtapositional cytoplasmic domain (aa 877–894; greater affinity to short cytoplasmic forms of membrane-bound ObR) | M18 | SC-1834, Santa Cruz Biotechnology | 2 |
| N-terminus of ObR (all isoforms) | K20 | SC-1834, Santa Cruz Biotechnology | 2 |
| ObRb | CH14014 | CH14014, Neuromics | 10 |
The specificity of the staining was shown by the lack of signal in negative controls, including pre-adsorption of the primary antibody with a specific blocking peptide, and omission of primary antibody. For double-labelling immunofluorescence studies with a second primary antibody against GFAP, single staining experiments with each primary antibody were performed and compared to rule out the cross-reactivity of the irrelevant antibodies. Confocal images were acquired on an Olympus FV1000 microscope in our laboratory, with Argon laser excitation/filter for emission at 488/505–525 and 543/560–660 (nm) for Alexa488 and Alexa594, respectively.
Statistical analysis of the number of ObR(+), GFAP(+) or double-labelled cells in the diet-induced obesity, Avy or control groups was performed on matching sections from three different mice. The significant differences were determined by analysis of variance (ANOVA) followed by Tukey's post hoc test.
Primary culture of mouse hypothalamic astrocytes
Following approved animal protocols, the hypothalami of 7-day-old FVB/NJ (FVB) mouse pups were dissected, pooled (3 pups/ml), mechanically dissociated and cultured in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics/antimycotics in a 5% CO2 incubator at 37°C. At about 90% confluency (about 1 week in culture), the cells were agitated in an orbital shaker at 250 r.p.m. for 2 h at 37°C to detach microglial cells and remove them by change of medium. The remaining cells showed astrocytic morphology. Immunocytochemistry confirmed that >95% of the cells were GFAP (+). Two days later, the cells were used for RT–PCR analysis of ObR mRNA, or passaged to poly-d-lysine coated glass coverslips for calcium imaging.
RT–PCR analysis of ObR mRNA subtypes in hypothalamic astrocytes
The cells were lysed in RNA lysis buffer containing β-mercaptoethanol. In addition to primary mouse hypothalamic astrocytes, rat C6 astrocytoma cells were also studied. C6 cells have been shown to express ObR mRNA and protein (Morash et al., 2000) and thus were used as a positive control. Total RNA was extracted by use of an Absolutely RNA Miniprep kit (Stratagene, La Jolla, CA, USA) and reversely transcribed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The primers used for PCR amplification are listed in Table 2. Mouse primers were used for tissue studies, whereas rat primers were used for C6 astrocytoma cells. The PCR conditions were: denaturing at 95°C for 5 min, followed by 35 cycles of denaturing at 95°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 30 s, with a final extension at 72°C for 10 min. A negative control without template (water only) was included. The PCR products were electrophoresed on 1.5% agarose gel containing ethidium bromide, and imaged under a UV lamp with a Kodak EDAS 290 imaging system.
Table 2.
PCR primers for ObR subtypes
| ObR subtype | Forward primer | Reverse primer | Species | Product size (bp) |
|---|---|---|---|---|
| ObRa | GAAGTCTCTCATGACCACTACAGATGA | TTGTTTCCCTCCATCAAAATGTAA | Mouse/rat | 98 |
| ObRb | GCATGCAGAATCAGTGATATTTGG | CAAGCTGTATCGACACTGATTTCTTC | Mouse/rat | 81 |
| ObRc | AGGGCTTTATGTTGTTGTGTTC | TTTCTCTGATCAAATCCCAAAC | Mouse | 88 |
| ObRc′ | TTA ATT TCC AAA AGG TCA CT | TTT TTG CCT TTT AAA GAT GT | Rat | 105 |
| ObRd | CACACCAGAGAATGAAAA | TCTGAAAATAAAAACTTCATGT | Mouse | 119 |
| ObRe | TAATGAAGATGATGGAATGAAG | ATTGCCAGTCTACAGTGTCA | Mouse | 117 |
| ObRe′ | GTA AAT TGG CAA TCC TGA TAA TAA AG | ATT CAG TTG GAA GGT TGA CAT C | Rat | 72 |
Real-time imaging of leptin-induced calcium response
Primary hypothalamic astrocytes on glass coverslips were loaded with calcium green-1AM (4 μM, Invitrogen) for 30 min at 37°C. The coverslip was placed on a temperature-controlled perfusion chamber on the stage of an Olympus FV1000 inverted microscope, and perfused with oxygenated Kreb's solution at 1.0 ml/min. Real-time imaging was performed with a 40× objective at a frequency of 2 frames/s, and 600 frames were captured. Calcium green was excited by argon laser, and the resulting emission at 488 nm was captured. After application of leptin (6.2 nM; R&D Systems, Minneapolis, MN) or Kreb's solution for 2 min, the cells were allowed to recover for 5 min, at which time the baseline had returned to normal. The cells were then challenged with ATP (50 μM; Sigma, St Louis, MO, USA) for 30 s. This served as a positive control with calcium oscillation being elicited consistently, as has been described (Rogers and Hermann, 2008). Only one field of each coverslip was recorded, since desensitization of the leptin response had been observed that was not explained by bleaching. The percentage of responsive cells and the amplitude of increase from baseline fluorescent intensity were calculated for each coverslip. The cells treated with Kreb's solution had no change in calcium signal and thus served as negative controls. All results were replicated at least four times, and representative images are shown.
Results
Immunohistochemistryof ObR and cellular phenotypic characterization
We have observed co-staining of ObR and GFAP in mouse hypothalamus with the M-18 antibody (sc-1834; Santa Cruz Biotechnology, Santa Cruz, CA, USA) that recognizes the membrane juxtapositional cytoplasmic domain (aa 877–894) of ObR in B6 and Avy mice in the original study (Pan et al., 2008a). To determine whether this is a general phenomenon and further identify ObR subtypes, we tested additional rodent models and ObR antibodies against different epitopes of the receptor protein. For all three antibodies used, sections incubated with secondary antibody only or with primary antibody after pre-adsorption with the respective blocking peptide overnight did not show specific signals. The negative controls thus assured the specificity of the staining.
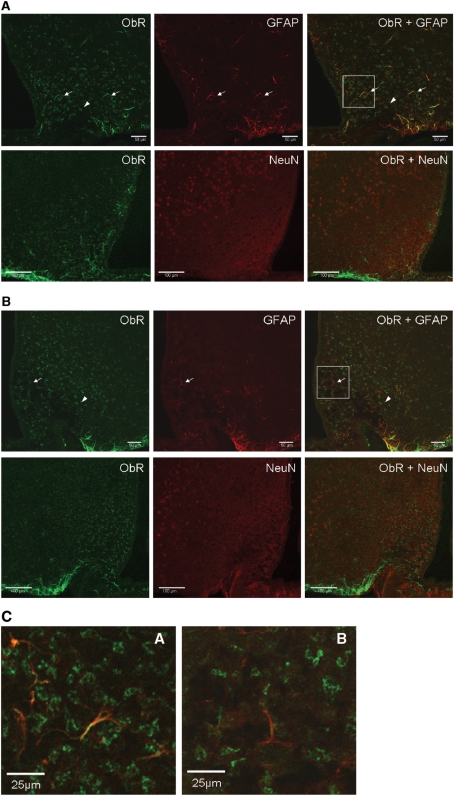
In hypothalamic sections of obese ob/ob mice (Fig. 1A) that do not produce leptin, and in obese db/db mice (Fig. 1B) that have no ObRb receptor because of an insertional mutation although other ObR isoforms are present (Chua et al., 1996), the ObR staining pattern with the M18 antibody was mainly neuronal rather than astrocytic. Figure 1C shows higher magnification images of GFAP and ObR co-localization in these strains.
Figure 1.
Expression of ObR in arcuate nucleus of different mouse models shown by immunostaining with M18 antibody. Astrocytes were immunostained with anti-GFAP (red, arrows), whereas neurons were immunostained with anti-NeuN (red, arrow heads). Yellow colouration indicates co-localization. (A) ob/ob mouse; (B) db/db mouse (confocal image, scale bar = 50 µm for GFAP; scale bar = 100 µm for NeuN). (C) Higher magnification of the co-localization of ObR and GFAP from the demarcated areas in A and B. Scale bar: 25 μm.
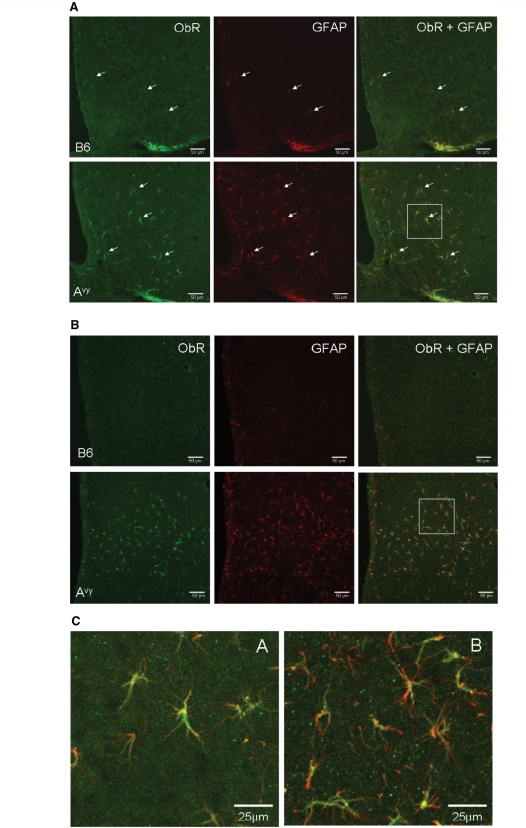
In hypothalamic sections from B6 and Avy mice, the K-20 polyclonal antibody against the N-terminus of the mouse ObR (sc-1835) also showed immunoreactivity (Fig. 2). The staining patterns were similar to those seen with the M-18 antibody. In contrast to the weak immunoreactivy in neurons, the astrocytes in the Avy mice showed strong reactivity to the K-20 antibody in both the arcuate nucleus (Fig. 2A) and dorsomedial hypothalamus (Fig. 2B). Figure 2C shows higher magnification images of the co-localization of GFAP and ObR.
Figure 2.
Expression of ObR in the arcuate nucleus (A) and dorsomedial hypothalamus (B) shown by immunostaining with the K20 antibody. In both regions, there were more ObR (+) astrocytes (arrows) in Avy than in age-matched B6 mice (confocal image, scale bar = 50 µm). (C) Higher magnification of the co-localization of ObR and GFAP from the demarcated areas in (A and B).
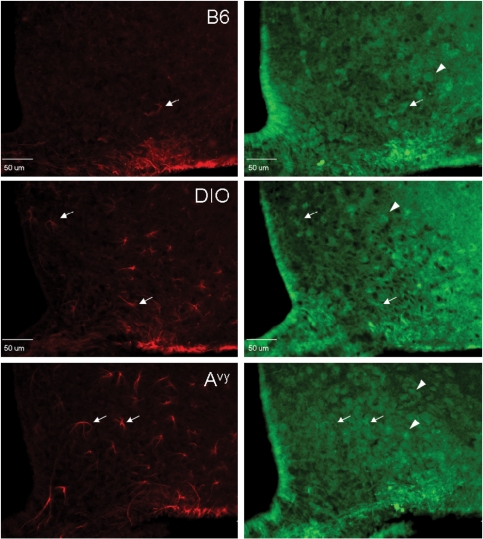
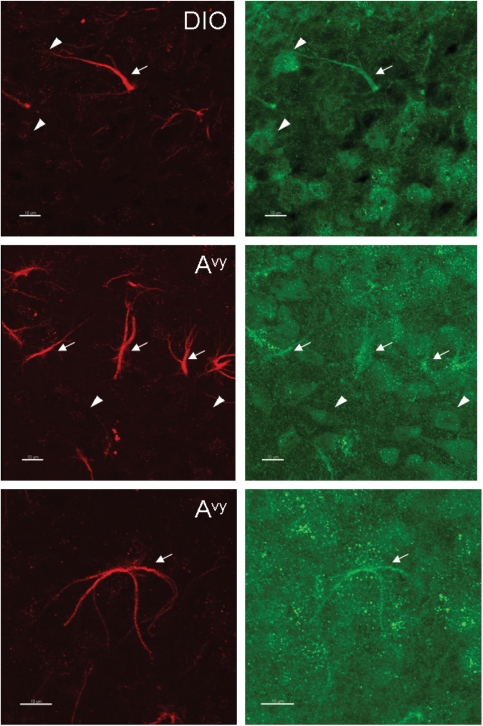
A third antibody used was targeted to the unique sequence of ObRb at its cytoplasmic tail (CH14104, Neuromics, Edina, MN, USA). This antibody was raised against rat ObRb but cross-reacted with mouse tissue. Figure 3A shows that the ObRb staining had a rather diffuse background with both neuronal and astrocytic distribution. The cells double-labelled with both the M-18 antibody (against the membrane juxtapositional cytoplasmic domain) and the CH14104 antibody (against the membrane distal cytoplasmic domain of ObRb) were mainly astrocytes. The partial overlap of the staining in cells showing typical astrocyte morphology, probably representing the co-expression of ObRb with short transmembrane forms of ObR, was seen not only in the B6 mice but also in the diet-induced obesityand Avy mice (Fig. 3B).
Figure 3.
Expression of ObR in the arcuate nucleus of B6 (top), diet-induced obesity (DIO) (middle) and Avy (bottom) mice shown by double immunostaining with M-18 (red) and CH14104 (green) antibodies. (A) M-18 immunoreactivity was mainly seen in astrocytes (arrows). CH14104 antibody stained mostly neurons (arrow heads). In comparison with the lean B6 control, the diet-induced obesityB6 mice and the Avy mice on a B6 background showed a greater number of ObR(+) astrocytes seen with the M18 antibody (epifluorescence image, scale bar = 50 µm). (B) Higher magnification images showing that astrocytes can be stained with both M-18 and CH14104 antibodies, the former providing a stronger signal with a crisper background (confocal image, scale bar = 10 µm).
Time course of the development of diet-induced obesity
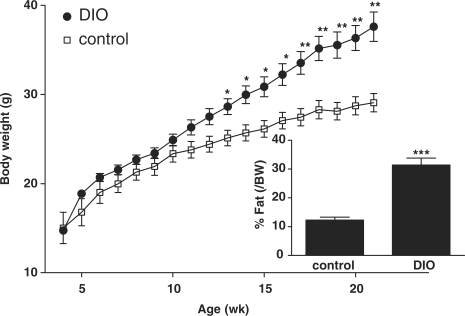
Male B6 mice were fed a 45% HFD (the diet-induced obesity group, n = 9) or rodent chow (lean control group, n = 7) between 1 and 5 months of age. Repeated measures ANOVA showed that the diet-induced obesity group had significantly higher body weight than the lean controls (P < 0.005). The post hoc test showed that the difference was apparent by 13 weeks of age (P < 0.05 on week 13–17, and P < 0.01 afterwards). Concurrent with increased body weight, there was also a significant (P < 0.005) increase of fat composition (% fat/body weight) shown by serial measurement with NMR, at 8.5 week after being placed on HFD, or at 12.5 weeks of age (Fig. 4). Based on the results, we used 3-month-old mice for immunohistological studies, immediately before the full development of the obesity phenotype.
Figure 4.
Obesity phenotype of the diet-induced obesity mice (n = 9), shown by more rapid increase of body weight over time and higher percentage of body fat at 8.5 weeks in comparison with their former littermates fed with regular rodent chow (n = 7). *P < 0.05; **P < 0.01; ***P < 0.005.
Diet-induced obesity mice show increased astrocytic ObR immunoreactivity in the hypothalamus
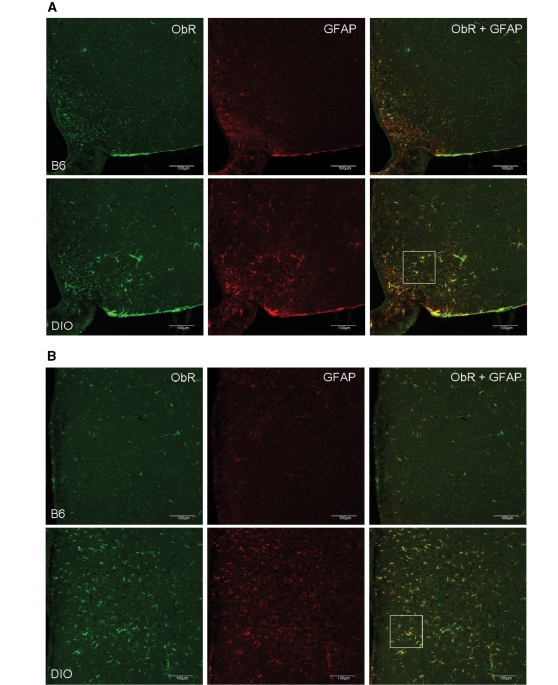
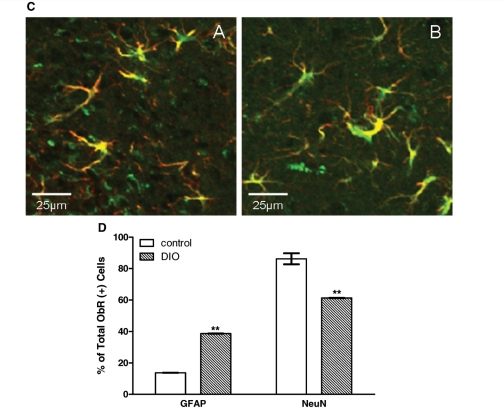
The diet-induced obesity mice, which share the body phenotype of adult-onset obese Avy mice, also showed an increase of ObR (+) cells. Besides an increase in the amount of ObR (+) cells, there also was an increase of GFAP immunoreactivity. Greater changes for both were seen in the dorsomedial hypothalamus than in the arcuate nucleus. Double-labelling with GFAP further confirmed that the newly emerged ObR (+) cells were astrocytes (Fig. 5A–C). In both control and diet-induced obesity mice, all GFAP (+) cells were also ObR (+). In contrast to the control mice where 13.7% of ObR (+) cells in the arcuate nucleus were astrocytes, in the diet-induced obesity mice 38.7% of ObR (+) cells were astrocytes. In the control mice, there were significantly (P < 0.001) fewer ObR (+) astrocytes than neurons. This neuronal predominance of ObR expression was no longer present in the diet-induced obesity mice. The diet-induced obesity group showed a greater (P < 0.01) increase of ObR (+) astrocytes than the control group, with a corresponding reduction of ObR (+) neurons (Fig. 5D). Thus, although both astrocytes and neurons express ObR, the obese diet-induced obesity mice had a higher absolute number and percentage of ObR (+) astrocytes than the lean controls. In parallel, diet-induced obesity also induced an increase of GFAP immunoreactivity.
Figure 5.
Diet-induced obesity increased ObR (+) astrocytes in the arcuate nucleus (A) and to an even greater extent in the dorsomedial hypothalamic nucleus (B) (confocal image, scale bar = 100 µm). (C) Higher magnification of the co-localization of ObR and GFAP from the demarcated areas above in diet-induced obesity. Scale bar: 25 μm. (D) Percent of ObR (+) cells that were also GFAP or NeuN (+); the diet-induced obesity group had significantly more ObR (+) astrocytes in the arcuate nucleus than the controls, with corresponding reduction of ObR (+) neurons. **P < 0.01.
ObR expression profile of primary hypothalamic astrocytes in culture
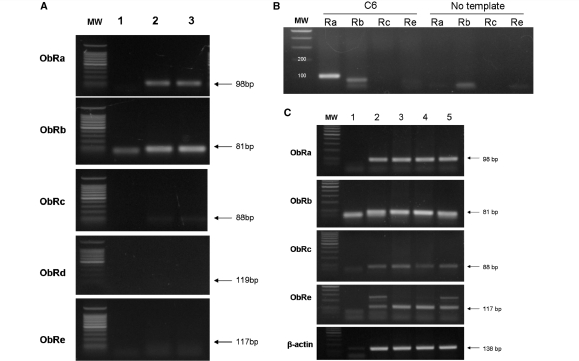
Amplification in primary astrocytes was mainly seen for ObRa and ObRb (Fig. 6A). The negative controls (no template) did not show the specific PCR product, although a lower molecular weight band was present in the ObRb group, probably representing primer dimers. C6 astrocytoma cells, which have been reported to express ObR mRNA (Morash et al., 2000), also had similar amplification of ObRa and ObRb (Fig. 6B). The signals for ObRc and ObRe were barely detectable, in contrast to the presence of these two isoforms in mouse hippocampus (Fig. 6C) as well as in hypothalamus (Pan et al., 2008a).
Figure 6.
RT–PCR analysis shows that astrocytes expressed mainly ObRa (98 bp) and ObRb (81 bp) mRNA. (A) Primary astrocytes from 1-week-old mouse pups. Lane 1: no template control; lane 2: primary astrocytes from mouse hypothalamus; lane 3: primary astrocytes from mouse striatum. (B) C6 astrocytoma cells with rat-specific primers. (C) In contrast, mouse hippocampus had not only ObRa and ObRb, but also ObRc and ObRe. Lane 1: negative control; lanes 2–5: hippocampal tissue from different mice.
Primary hypothalamic astrocytes respond to leptin by increased calcium signals
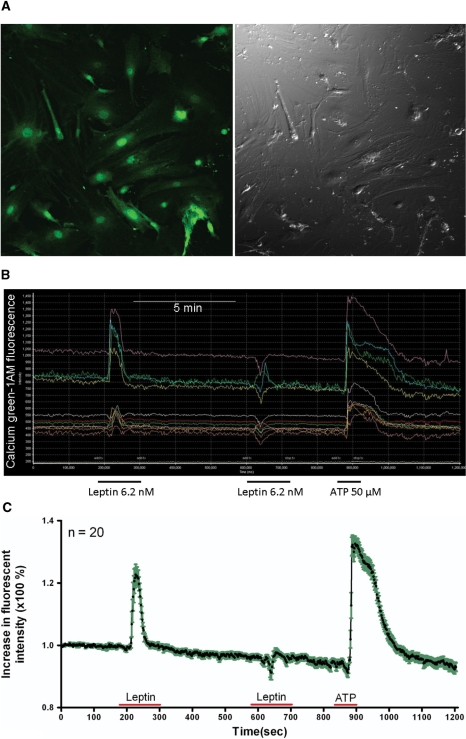
Leptin (6.2 nM) induced a robust increase of intracellular calcium green fluorescence in almost 100% of astrocytes. The response occurred immediately after initiation of the 2 min perfusion, had a rapid return to baseline, and showed similar amplitude among the cells though their baseline calcium green fluorescent intensity differed. In the cells within the microscopic field (40× objective) shown in Fig. 7, the mean increase of calcium signal was 22.7 ± 3.3% (n = 20). This contrasts with the lack of calcium response in the cells on the control coverslips perfused with Kreb's solution only. Calcium influx was not seen in cells perfused with heat-inactivated leptin or chemical inhibitors of mitogen-activated protein kinases (MAPK), indicating specificity. Repetitive application of leptin on the same coverslip 5 min later elicited a much smaller amplitude of response (4.5 ± 1.1%), suggesting desensitization (tachyphylaxis). Nonetheless, ATP (50 μM for 30 s) treatment of the same cells induced a robust and persistent increase of calcium response at the end of the study in each coverslip (32.6 ± 2.4%) (Fig. 7).
Figure 7.
Leptin-induced calcium signalling in primary astrocytes from mouse hypothalamus. (A) A field of astrocytes on glass coverslips after calcium green-1AM loading. (B) Time-lapse image showing that all cells responded to leptin and ATP. (C) Time-lapse image showing average amplitude of the calcium response (n = 20). Green area surrounding the line shows SD of the response from individual cells.
Discussion
The notion of astrocytic expression of ObR has encountered much resistance among some obesity researchers focusing on endothelial and neuronal ObR in the CNS. Nonetheless, Avy mice, which manifest a genetic predisposition to adult-onset obesity, recently have been shown to express increased astrocytic ObR in the hypothalamus (Pan et al., 2008a). This indicates regulatory changes of astrocytic ObR. Here we further determined whether this upregulation is a general mechanism in obesity or is specific for genetic models of obesity.
The distribution of ObR mRNA in rat brain has been mapped by in situ hybridization with radioactive probes for ObRb and the entire ObR (Elmquist et al., 1998). In the rat hypothalamus, we also found co-localization of ObRb mRNA and GFAP protein, by fluorescent in situ hybridization and double labelling immunohistochemistry (Hsuchou et al., unpublished observations). ObR is a single-transmembrane receptor that interacts with Janus kinase (JAK)-2, Signal Transducer and Activator of Transcription (STAT)-3, phosphoinositide-3 kinase, and MAPK. There are at least four major forms of ObR splice variants in mice, including the soluble ObRe, the short cytoplasmic tail ObRa and ObRc, and the long cytoplasmic tail ObRb that activates STAT-3. ObRd is not detected in the mouse hypothalamus or cerebral vasculature (Pan et al., 2008b). By use of three different polyclonal antibodies against different epitopes of ObR and co-immunostaining of cellular phenotypic markers, we differentiated the relative abundance of the ObR isoforms in astrocytes and neurons.
The K20 antibody recognizes all ObR isoforms, the CH14104 antibody is specific for ObRb, whereas the relative selectivity of the M18 antibody is highest for ObRa, less for ObRc and ObRd (if present), and the least for ObRb. The M18 antibody revealed both neuronal and astrocytic patterns of ObR immunoreactivity, similar in rats and mice. The specificity of the staining was illustrated not only by the lack of staining in the presence of blocking peptide or secondary antibody only, but also by different patterns in the ob/ob mice, in which leptin is not produced, and in the db/db mice, in which an insertional mutation abolishes the production of ObRb although other ObR isoforms are present (Chua et al., 1996). In the ob/ob and db/db mice, there were few ObR (+) astrocytes, and the neuronal ObR staining was also less intense than the age-matched B6 lean controls.
Confocal analyses of the images showed that astrocytes (GFAP-positive) had strong immunoreactivity to the M18 antibody, K20 antibody, and to a lesser extent the CH14104 antibody. Neurons (NeuN-positive), in contrast, revealed a greater immunreactivity to CH14104 and weaker signals with M18 and K20. Although the fluorescent intensity is influenced by the affinity of the antibodies, comparison from the same antibody staining between the two major populations of ObR (+) cells in CNS parenchyma suggests that astrocytes showed short ObR predominance whereas the neurons had more ObRb.
It should be noted that the lack of an endothelial or microvascular pattern of staining does not detract from the sensitivity of the detection method nor indicate the absence of ObR at the blood–brain barrier. Microvessels composing the blood-brain barrier have a specific array of ObR subtypes and mediate the transport of leptin from blood to brain (Bjørbæk et al., 1998; Boado et al., 1998; Kastin et al., 1999; Maness et al., 2000; Hileman et al., 2002; Pan et al., 2008b). The blood-brain barrier is a three-dimensional structure. In human brain averaging 1200 g, capillaries span 650 km with a volume of 1 ml. These thin structures provide tremendous surface area for exchange of information between the circulation and CNS parenchyma, with no neuron >8–10 μm away from a capillary (Yoshida and Ikuta, 1984; Abbott et al., 2006; Abbott, 2005, 2008). Thus, enriched microvessels show a many-fold greater extent of expression of cytokine receptors than tissue homogenates from the same region (Pan et al., 2008a, 2008b), yet the immunostaining in individual endothelial cells may not be significant.
In general, astrocytes have a close structural association with the endothelia and play important roles in regulating blood-brain barrier functions (Nedergaard et al., 2003). Specialized astrocytic endfeet cover up to 90% of vessel walls, as shown by electron microscopy and aquaporin-4 staining (Abbott, 2008). Other investigators even estimate that >99% of the cerebrovascular surface is ensheathed by astrocyte processes (Kacem et al., 1998; Agulhon et al., 2008). In primary astrocytes from mouse hypothalamus, both ObRa and ObRb mRNAs were detected. This is consistent with findings in C6 cells shown both here and in the literature (Morash et al., 2000), and supports the immunohistochemical findings described above. The composition of ObR subtypes in astrocytes is similar to that shown in cerebral microvessels (Hileman et al., 2002; Pan et al., 2008b). Although it is yet to be determined how the endothelial ObR, astrocytic ObR, and neuronal ObR act in concert or differentially in response to blood-borne leptin, astrocytes apparently play an intricate role in the blood–brain barrier transport of leptin into the CNS.
A high fat diet increases body weight and blood leptin levels, and induces leptin resistance shown both by defective STAT3 signalling in neurons that express ObRb, and by saturation of blood–brain barrier transport (Coculescu, 1999; El Haschimi et al., 2000; Hileman et al., 2002; Banks et al., 2003; Pan and Kastin, 2003; Kastin and Pan, 2003; Levin et al., 2004; Pan et al., 2004). We also showed that different ObR isoforms all participate in leptin transport (Tu et al., 2007, 2008), that interactions of leptin with other ingestive peptides may transform feeding behaviour (Kastin et al., 2002; Pan et al., 2004), and that the ObR isoforms are differentially regulated by neonatal development as well as obesity (Pan et al., 2008a, 2008b). In addition, this study indicates that astrocytic ObR is implicated in the mechanism of leptin resistance. The elevation of astrocytic ObR in the diet-induced obesity mice is similar to that seen in obese Avy mice (Pan et al., 2008a). The percent of ObR (+) astrocytes in the control group was higher than in the previous study, probably related to differences in staining intensity, individual mice, and cell counting resulting from different demarcation of the borders of the hypothalamic nuclei. Regardless, the results indicate that metabolic signals in obesity can rapidly alter astrocytic phenotype, representing a unique aspect of CNS plasticity.
Besides our observation of regulatory changes of ObR in astrocytes, several groups have shown functional leptin receptors in astrocytes. Systemically delivered hydroxystilbamidine (FluoroGold equivalent) can be taken up by astrocytes in selective regions in the brain 2–6 h later, mainly in the median eminence and the adjacent ventral part of the arcuate nucleus. Many of these GFAP (+) cells also express leptin receptors and neuropeptide Y Y1 receptors, whereas the surrounding neurons (NeuN-positive) or adjacent tanycytes (vimentin-positive) do not take up the dye (Cheunsuang and Morris, 2005). In the subcommissural organ of rabbits, ObR staining was seen in astrocytes, and fasting decreased ObR distribution in the cytoplasm but increased it in plasma membranes of these GFAP (+) cells (Cecilia et al., 2006). Leptin induces differentiation of astrocytes from neurospheres, and helps to maintain BrdU (+) cells in the embryonic neuroepithelium (Udagawa et al., 2006). C6 astrocytoma cells express the short form of ObR mRNA and cytoplasmic staining of ObR; since the leptin transcript is also found in these cells, it is suggested that leptin may be an autocrine factor acting on ObR (Morash et al., 2000). In primary rat astrocytes from the nucleus tractus solitarius, all membrane-bound ObR isoforms are present, and are differentially regulated by fasting and estradiol treatment (Dallaporta et al., 2009). Consistent with observations in Avy mice (Pan et al., 2008a), the increased astrocytic expression in diet-induced obesity mice in this study suggests its role in feeding regulation.
In the obese Avy and diet-induced obesity mice, there also was an increase of GFAP immunoreactivity. This indicates that obesity increases the level of expression of GFAP, an intermediate filament protein that plays essential roles in astrocyte function. In the Avy mice, Western blotting analysis showed that the level of GFAP protein was indeed increased in the hypothalamus (He et al., unpublished observations). Although there is astrogenesis in the adult hypothalamus, and our preliminary data showed an increase of BrdU-labeled cells, some of which co-localized with GFAP and S100β (Hsuchou et al., unpublished observations), the newly emerged astrocytes from de novo mitosis would not be sufficient to explain the abundant increase of GFAP (+) astrocytes in the obese mice. Rather, it is more likely that the expression level of both GFAP and ObR in the existing astrocytes was enhanced. Further, there seems to be an interaction between leptin and GFAP, either directly or modified by signals induced by the changed feeding status,
Calcium signaling is an important means of astrocytic activity. Our study is the first to show that leptin activates calcium signaling in astrocytes. The brief time course of the increase of calcium signal was related to the short application time by perfusion and showed desensitization to repetitive application of leptin. Desensitization to the second leptin stimulation, 5 min after recovery to the baseline, was reflected by 80% reduction in the amplitude of the response and probably was explained by leptin-mediated endocytosis of the receptors, as we have shown in cellular trafficking studies (Tu et al., 2008). Nonetheless, the cells retained their response to ATP by calcium oscillation similar to that seen in naïve cells. ATP is a P2X3 purinergic receptor agonist that increases cation channel permeability and spontaneous glutamate mediated excitatory postsynaptic currents (Jin et al., 2004). In non-astrocytes, both stimulatory and inhibitory effects of leptin on calcium influx have been reported. Leptin increases calcium influx in somatotropes (Glavaski-Joksimovic et al., 2004) and NMDA-receptor mediated calcium influx in hippocampal neurons (Shanley et al., 2001). In cerebellar granular cells, leptin acts through ObR and MAPK to facilitate N-methyl-d-aspartate (NMDA) receptor-mediated Ca2+ influx (Irving et al., 2006). Leptin directly inhibits voltage-gated calcium channels in lateral hypothalamic neurons (Jo et al., 2005), and reduces angiotensin II-induced calcium influx in muscle cells (Fortuno et al., 2002). These different results may depend on the type of preparation, CNS region, ObR subtype, and intracellular pathways.
It has been shown that activation of calcium signaling in hypothalamic astrocytes indicates the specific role of astrocytes in the regulation of synaptic transmission and neurovascular coupling (Haydon and Carmignoto, 2006). A subpopulation of astrocytes that are immunopositive for brain fatty acid binding protein is adjacent to neurons that respond to leptin by STAT3 activation (Young, 2002). Much needs to be done to further determine the mechanisms of leptin-induced calcium activation in astrocytes; however, the alteration of astrocytic calcium signaling by leptin suggests a novel role of these ObR(+) astrocytes in neuroendocrine regulation. With upregulation of astrocytic ObR by obesity, it is possible that the astrocytic calcium signaling in response to leptin is also enhanced. Thus, the ObRs in astrocytes may be critically involved in nutritional sensing and regulation of feeding circuitry.
In summary, we showed the presence and obesity-induced regulation of astrocytic ObR in the hypothalamus by immunohistochemistry with three different antibodies and blocking peptides, and by RT–PCR demonstrating ObRa and ObRb mRNA in purified astrocytes from mouse brain. The increased astrocyte ObR in obese diet-induced obesity and Avy mice was not seen in ob/ob or db/db obese mice, further supporting the specific regulatory changes in adult-onset obesity. Hypothalamic astrocytes showed increased GFAP expression, and responded to leptin by calcium influx. They probably play an important role in communication with blood-borne factors across the blood–brain barrier and with neurons. Overall, the upregulation of astrocytic ObR by a high-fat diet shows the plasticity of the brain in response to obesity, and opens a new direction for determination of the role of these astrocytes in neurovascular coupling.
Funding
National Institutes of Health (DK54880, NS45751, NS46528, NS62291).
Acknowledgements
We thank Ms. Montina J. Van Meter for helpful discussions on ObRb staining, and the Comparative Biology and Animal Metabolic and Behavior Core Services of PBRC for providing facilities.
References
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Cellular compositions of the blood-brain barrier (BBB) In: Kastin AJ, Pan W, editors. The Henry Stewart Talk series: the blood-brain barrier. London: Henry Stewart Talks; 2008. [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, et al. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–46. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Shaban Z, Yamaji D, Okamatsu-Ogura Y, Soliman M, Abd EM, et al. Induction of proinflammatory cytokines and caspase-1 by leptin in monocyte/macrophages from holstein cows. J Vet Med Sci. 2007;69:509–14. doi: 10.1292/jvms.69.509. [DOI] [PubMed] [Google Scholar]
- Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des. 2003;9:801–9. doi: 10.2174/1381612033455350. [DOI] [PubMed] [Google Scholar]
- Banks WA, Altmann J, Sapolsky RM, Phillips-Conroy JE, Morley JE. Serum leptin levels as a marker for a Syndrome X-like condition in wild baboons. J Clin Endocrinol Metab. 2003;88:1234–40. doi: 10.1210/jc.2002-021695. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol. 2003;285:E10–15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- Banks WA, King BM, Rossiter KN, Olson RD, Olson GA, Kastin AJ. Obesity-inducing lesion of the central rervous system alter leptin uptake by the blood-brain barrier. Life Sci. 2001;69:2765–73. doi: 10.1016/s0024-3205(01)01346-7. [DOI] [PubMed] [Google Scholar]
- Bjørbæk C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Golden PL, Levin N, Pardridge WM. Up-regulation of blood-brain barrier short-form leptin receptor gene products in rats fed a high fat diet. J Neurochem. 1998;71:1761–4. doi: 10.1046/j.1471-4159.1998.71041761.x. [DOI] [PubMed] [Google Scholar]
- Cecilia D, Ceccarelli P, Pascucci L, Brecchia G, Boiti C. Receptors for leptin and estrogen in the subcommissural organ of rabbits are differentially modulated by fasting. Brain Res. 2006;1124:62–9. doi: 10.1016/j.brainres.2006.09.091. [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia. 2005;52:228–33. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- Chua SC Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–6. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Coculescu M. Blood-brain barrier for human growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab. 1999;12:113–24. doi: 10.1515/jpem.1999.12.2.113. [DOI] [PubMed] [Google Scholar]
- Dallaporta M, Pecchi E, Pio J, Jean A, Horner KC, Troadec J. Expression of leptin receptors by glial cells of the nucleus tractus solitarius: possible involvement in energy homeostasis. J Neuroendocrinol. 2009;21:57–67. doi: 10.1111/j.1365-2826.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- Dunbar J, Lapanowski K, Barnes M, Rafols J. Hypothalamic agouti-related protein immunoreactivity in food-restricted, obese, and insulin-treated animals: evidence for glia cell localization. Exp Neurol. 2005;191:184–92. doi: 10.1016/j.expneurol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- El Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–32. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–47. [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–36. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuno A, Rodriguez A, Gomez-Ambrosi J, Muniz P, Salvador J, Diez J, et al. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology. 2002;143:3555–60. doi: 10.1210/en.2002-220075. [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A, Rowe EW, Jeftinija K, Scanes CG, Anderson LL, Jeftinija S. Effects of leptin on intracellular calcium concentrations in isolated porcine somatotropes. Neuroendocrinology. 2004;80:73–82. doi: 10.1159/000081843. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–7. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El Haschimi K, Banks WA, et al. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- Irving AJ, Wallace L, Durakoglugil D, Harvey J. Leptin enhances NR2B-mediated N-methyl-D-aspartate responses via a mitogen-activated protein kinase-dependent process in cerebellar granule cells. Neuroscience. 2006;138:1137–48. doi: 10.1016/j.neuroscience.2005.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–17. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–66. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kastin AJ, Pan W. Feeding peptides interact in several ways with the blood-brain barrier. Curr Pharm Des. 2003;9:789–94. doi: 10.2174/1381612033455378. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Akerstrom V, Hackler L, Wang CF, Kotz CM. Novel peptide-peptide cooperation may transform feeding behavior. Peptides. 2002;23:2189–96. doi: 10.1016/s0196-9781(02)00247-4. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–53. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol. 2004;286:R143–50. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- Lin J, Yan GT, Xue H, Hao XH, Zhang K, Wang LH. Leptin protects vital organ functions after sepsis through recovering tissue myeloperoxidase activity: an anti-inflammatory role resonating with indomethacin. Peptides. 2007;28:1553–60. doi: 10.1016/j.peptides.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–8. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Lord GM. Leptin as a proinflammatory cytokine. Contrib Nephrol. 2006;151:151–64. doi: 10.1159/000095326. [DOI] [PubMed] [Google Scholar]
- Maness LM, Banks WA, Kastin AJ. Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res. 2000;873:165–7. doi: 10.1016/s0006-8993(00)02520-8. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Blood SR, et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31:1324–32. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Morash B, Johnstone J, Leopold C, Li A, Murphy P, Ur E, et al. The regulation of leptin gene expression in the C6 glioblastoma cell line. Mol Cell Endocrinol. 2000;165:97–105. doi: 10.1016/s0303-7207(00)00259-8. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–30. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Pan W, Akerstrom V, Zhang J, Pejovic V, Kastin AJ. Modulation of feeding-related peptide/protein signals by the blood-brain barrier. J Neurochem. 2004;90:455–61. doi: 10.1111/j.1471-4159.2004.02502.x. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, et al. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008a;149:2798–806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008b;149:877–85. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr Pharm Des. 2003;9:827–31. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Mahogany, blood-brain barrier, and fat mass surge in A(VY) mice. Int J Obesity. 2007;31:1030–2. doi: 10.1038/sj.ijo.0803536. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Inoue W, Schmidt L, Molina-Holgado F, Rothwell NJ, Luheshi GN. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–33. doi: 10.1111/j.1471-4159.2007.04559.x. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides. 2008;29:1716–25. doi: 10.1016/j.peptides.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW. Brain pathways controlling food intake and body weight. Exp Biol Med. 2001;226:978–81. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933–44. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Med. 2008;10:209–18. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–5. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa - ObRd. J Cell Physiol. 2007;212:215–22. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, et al. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology. 2006;147:647–58. doi: 10.1210/en.2005-0791. [DOI] [PubMed] [Google Scholar]
- Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, et al. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–80. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Ikuta F. Three-dimensional architecture of cerebral microvessels with a scanning electron microscope: a cerebrovascular casting method for fetal and adult rats. J Cereb Blood Flow Metab. 1984;4:290–6. doi: 10.1038/jcbfm.1984.40. [DOI] [PubMed] [Google Scholar]
- Young JK. Anatomical relationship between specialized astrocytes and leptin-sensitive neurones. J Anat. 2002;201:85–90. doi: 10.1046/j.1469-7580.2002.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]