Abstract
Aims
In this study, we investigated whether short-term exercise, known to promote hippocampal BDNF expression, would also enhance activity in the Porsolt forced swim test (FST), a model for assessing antidepressant efficacy. We also wished to determine whether exercise combined with antidepressants would be more effective at modifying behavior in the FST than either intervention alone. In parallel with this, we also expected that these interventions would preserve post-stress levels of BDNF, and that antidepressants designed to selectively enhance noradrenergic or serotonergic neurotransmission (reboxetine or citalopram, respectively) would have differential effects on behavior and BDNF expression.
Main methods
Male Sprague-Dawley rats were treated with exercise (voluntary wheel running), reboxetine, citalopram, or the combination of exercise and each antidepressant, for 1 week. At the end of this period, a subset of animals from each treatment group underwent the FST. Post-stress levels of hippocampal BDNF mRNA were then quantified via in situ hybridization.
Key findings
Our results indicate that while both exercise and antidepressant treatment preserved post-stress levels of hippocampal BDNF mRNA, each intervention led to a unique behavioral profile in the FST. We found that antidepressant treatment increased swimming time in the FST, but that exercise decreased swimming time. While the combination of reboxetine-plus-exercise led to an increase in climbing and diving, citalopram-plus-exercise reduced these behaviors.
Significance
It is possible that active behaviors during the FST, though specific to antidepressant medications, may not reflect increased hippocampal BDNF expression or other survival- associated benefits.
Keywords: Antidepressant, Norephinephrine, Serotonin, Growth factor, Physical activity, In situ hybridization, Depression model, Brain-derived neurotrophic factor (BDNF), norepinephrine and serotonin reuptake inhibitor (NSRI), selective serotonin reuptake inhibitor (SSRI)
Introduction
Social and physical stressors have been shown to result in decreased expression of brain-derived neurotrophic factor (BDNF) within the hippocampus (Pizarro et al. 2004; Murakami et al. 2005), and have led to functional losses (Thome et al. 2001; Kim and Diamond 2002). Furthermore, decreased BDNF expression is thought to play a role in the neurodegenerative and behavioral changes associated with chronic stress and depression (Bergström et al. 2008). Restoring these levels may underlie the therapeutic responses to antidepressant medications (Duman and Monteggia 2006).
Exercise increases the expression of hippocampal BDNF (Garza et al. 2004; Adlard et al. 2005) and reverses the harmful effects of chronic unpredictable stress on mood and spatial memory performance (Zheng et al. 2006). The combination of voluntary exercise and antidepressant treatment enhances the expression of hippocampal BDNF and that of its transcript variants in an additive manner (Russo-Neustadt et al. 1999), and evidence exists that noradrenergic activation is an essential part of this BDNF-enhancing mechanism (Garcia et al. 2003; Ivy et al. 2003; Van Hoomissen et al. 2004).
Both norepinephrine (NE) and serotonin (5-HT) selective reuptake inhibitors enhance hippocampal BDNF expression after chronic treatment, and exercise often accelerates and/or increases this effect (Russo-Neustadt et al. 2000, 2004). Reboxetine, a highly NE-selective antidepressant (Montgomery 1999), led to particularly rapid increases in hippocampal BDNF mRNA that were potentiated with concurrent exercise (Russo-Neustadt et al. 2004). Citalopram, a highly 5-HT-selective monoamine reuptake inhibitor (Sanchez and Hyttel 1999), also enhanced hippocampal BDNF transcription when combined with voluntary physical activity (Russo-Neustadt et al. 2004). Evidence suggests that 5-HT-selective agents may not be as rapidly effective as NE-selective agents for inducing changes in the expression of neurotrophins (Duman 1998), or altering behavior (Lucki 1998).
The Porsolt forced swim test (FST), involving exposure of a rodent to swimming in a narrow glass cylinder, has served as both an acute stress paradigm and a means to assess “antidepressant-like” efficacy. A variety of clinically efficacious antidepressants reverse the reduction in swimming time that occurs the day following the initial (15 minute) exposure to this inescapable stress (Cryan et al. 2005a). Reboxetine has been shown to increase the most active behaviors observed during the FST, such as climbing the walls of the cylinder (Page et al. 2003). Serotonin-selective reuptake inhibitors, on the other hand, have tended to enhance horizontal swimming movements in the cylinder (Cryan et al. 2005b). Antidepressant treatments have been applied both acutely (between days 1 and 2 of the FST) and more chronically (up to several weeks before day 1). The latter paradigm has allowed for the evaluation of more long-term effects of interventions such as antidepressant medications and exercise, and is thought by some investigators to be more clinically relevant (Detke et al., 1997).
In this current study, our first aim was to test the hypothesis that exercise and antidepressant treatments would protect against decreased hippocampal BDNF mRNA brought about by the FST. Our second aim was to test the hypothesis that swimming time, considered a correlate of antidepressant efficacy, would be increased by exercise, and that the different antidepressant agents in combination with exercise would have unique effects on animal behavior in this test.
Materials and methods
Subjects and experimental design
All animal use procedures described below were conducted in strict accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996). All efforts were made to minimize the number of animals and any pain/distress they might incur. Male Sprague-Dawley rats (n = 85, 350g; Charles River) were housed singly in polyethylene cages (48 × 27 × 20 cm) with food and water ad libitum, and a 12:12 hr (06:00 to 18:00) light/dark cycle.
Voluntary physical activity entailed free access to running wheels for one week prior to the FST. After a week of initial acclimation to the vivarium, rats were placed in polyethylene cages equipped with running wheels (34.5 cm diameter; Nalgene, Oregon). Distance traveled on the running wheel per 24-h period was recorded by computer using Ratrun software (C. Hage Associates, CA). Control animals remained in cages of comparable size without wheels.
Rats were surgically implanted (09:00 hr) in the mid-scapular region with osmotic mini-pumps (Alza, Palo Alto, CA), which continuously infused drug (saline, reboxetine, or citalopram) subcutaneously. Citalopram (10 mg × kg−1 × day−1) or reboxetine (40 mg × kg−1 × day−1) was administered over 7 days, during which, these rats were allowed free access to their running wheels. These dosage regimens have been used in previous studies using these reboxetine or citalopram (Russo-Neustadt et al., 2004; Hesketh et al., 2008). Control rats were implanted with pumps that infused saline and were handled, but were not subjected to the FST.
Rats were then subjected to forced swim stress for 15 and 5 minutes on 2 consecutive days at 19:00 using the Porsolt method (Porsolt et al. 1977). Animals were sacrificed by rapid decapitation immediately after the last forced swim test.
Forced swim test
Rats were forced to swim by being individually placed into a cylindrical container (22.5 cm diameter, 45.5 cm height) containing a water (25°C) depth of 15 cm. Following a 15-min induction period in the water, rats were removed and dried off with a towel before being returned to their home cages. Twenty-four h later, they were placed in the cylinder for a second test of 5 minutes duration. The total time spent immobile vs. total swimming activity was measured during this 5-minute test. In addition, the proportion of active time spent climbing the walls of the cylinder and/or diving during the test period was assessed using a four-point scale from 1 to 4, based on the number of episodes of each behavior observed (see Fig. 1). Swimming behavior entailed active movements of the forepaws with directed horizontal actions such as crossing between quadrants of the cylinder and turning. Climbing was defined as upward-directed movements of the forepaws along the side of the cylindrical container. Diving involved the subject’s entire head and body being submerged beneath the water. Immobility was characterized as floating with minimal movements required to keep the head above water. The rater was blind to each animal’s treatment group. Rats were decapitated immediately following their last forced swim period and their brains excised and frozen in an isopentane/dry ice bath. Brains were stored at −80°C until processed for in situ hybridization. A total of 7 animal groups (n = 7 rats per group unless otherwise indicated) were used: saline, sedentary, no forced swim (NFS) (n = 9); saline, sedentary, forced swim (FS); saline, activity, FS; reboxetine, sedentary, FS; reboxetine, activity, FS; citalopram, sedentary, FS; and citalopram, activity, FS.
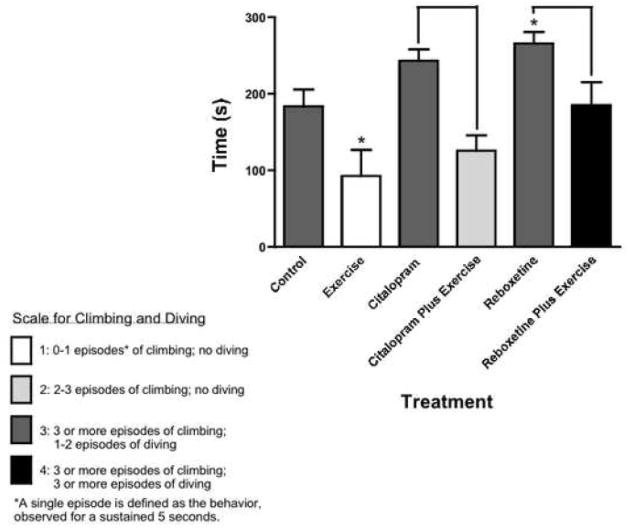
Fig. 1.
Exercise and antidepressant interventions exerted distinct effects on total swimming time (in seconds) and the degree of climbing/diving (on a scale from 1 to 4) during the 5-minute Porsolt Swim Test period. Data are the mean ± S.E.M., n = 7 per treatment group. Time data were analyzed using a one-way ANOVA with p < 0.05 level of significance, followed by Fisher’s post-hoc LSD test. * indicates a significant difference from control; brackets indicate significant difference between indicated values. The effect sizes (Cohen’s d) are as follows: 1.36 (exercise), 0.91 (citalopram), 0.892 (citalopram plus exercise), 1.21 (reboxetine) and 0.01 (reboxetine plus exercise).
cRNA probes, in situ hybridization, and data analyses
Construction of cRNA probes, in situ hybridization, and data analyses for hippocampal BDNF mRNA levels were performed as previously described (Russo-Neustadt et al. 2000).
Results
As expected for the Porsolt Forced Swim Test, antidepressant treatment increased swimming time during the five-minute test period (Figure 1). Reboxetine significantly increased swimming time [F5,36 = 6.877, p = 0.03], and citalopram led to a non-significant trend for an increase [F5,36 = 6.877, p = 0.11]. These treatments did not lead to a notable change in climbing or diving behavior. Exercise, on the other hand, significantly decreased swimming time [F5,36 = 6.877, p = 0.01], and also led to a decrease in climbing and diving behavior. Each antidepressant produced distinct behavioral responses when combined with exercise for one week prior to the test: Citalopram-plus-exercise significantly decreased swimming time, and also decreased climbing and diving behaviors. Reboxetine-plus-exercise did not increase total swimming time, but led to a vigorous level of climbing and diving. Also of note: Citalopram led to significantly greater swimming time than citalopram-plus-exercise (p = 0.01), and reboxetine treatment resulted in significantly greater swimming time than reboxetine-plus-exercise (p = 0.03). Swimming times during the 15-minute induction period (day 1) reflected similar trends to those observed during the test period, but these changes did not reach statistical significance (Table 1). The rater reliability coefficient (intraclass correlation coefficient, model 2 or ICC-2) for swimming time was found to be 0.475 (F1.23 = 2.81, p = .0082).
Table 1. Swimming times during the 15-minute induction period of the Porsolt forced swim test.
| Treatment | Time Swimming (sec) |
|---|---|
| Control | 412.86 ± 39 |
| Exercise only | 305.29 ± 62 (1.04) |
| Citalopram only | 431.43 ± 55 (.18) |
| Citalopram plus Exercise | 321.14 ± 60 (.89) |
| Reboxetine only | 499.43 ± 72 (.84) |
| Reboxetine plus Exercise | 451.71 ± 88 (.38) |
| ANOVA [F(5, 36)], p | 1.38, .256 |
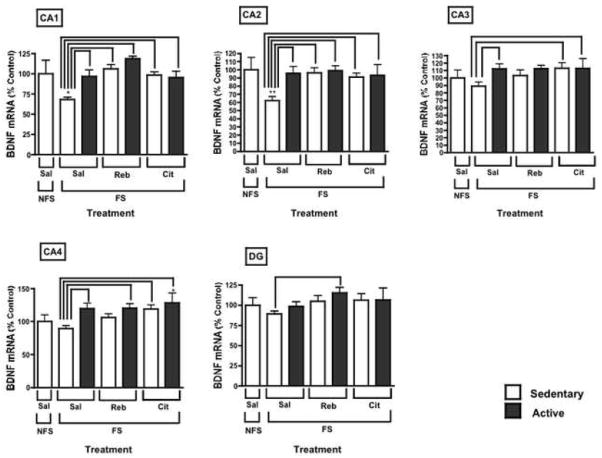
In this study, forced swimming (FS) decreased BDNF mRNA levels as compared to sedentary animals without FS in two hippocampal regions [CA1: F11,69 = 4.542, p = 0.014; CA2: F11,69 = 2.187, p = 0.0096]. All treatments prevented this decrease in BDNF mRNA due to FS in the CA1 and CA2. In addition, several treatments significantly increased BDNF mRNA relative to FS levels in the remaining hippocampal regions. Exercise alone achieved this increase in the CA3 [F11,69 = 3.836, p = 0.049] and CA4 [F11,69 = 7.162, p = 0.035]. Citalopram and citalopram-plus-exercise also both led to an increase over FS levels in the CA3 and CA4 [citalopram/stress: CA3: F11,69 = 3.836, p = 0.042; CA4: F11,69 = 7.162, p = 0.038; citalopram-plus-exercise/stress: CA3: F11,69 = 3.836, p = 0.043; CA4: F11,69 = 7.162, p = 0.0071]. The combination of reboxetine and exercise resulted in this increase in the CA4 [F11,69 = 7.162, p = 0.038] and DG [F11,69 = 3.847, p = 0.029].
Rats allowed access to their running wheels during the 1-week exercise period ran an average (mean ± S.E.M.) of 1.10 ± 0.13 (saline, physical activity), 2.06 ± 0.24 (reboxetine, physical activity), and 1.26 ± 0.18 (citalopram, physical activity) km per 24-h period. The reboxetine-plus-exercise group ran significantly more per 24-hr period than did the other two groups [F2,152 = 6.42, p = 0.0021].
Discussion
In our current study, NE- and 5-HT-selective antidepressants, exercise and the exercise-antidepressant combinations all prevented a decline in hippocampal BDNF mRNA when applied for one week prior to the Porsolt FST. As noted earlier, all of these interventions have been demonstrated to enhance hippocampal BDNF expression in unstressed animals (Russo-Neustadt et al. 2004). Exercise and antidepressants have in common the activation of monoaminergic neurotransmission, which influences central BDNF expression and confers neuroprotection (Dishman et al. 2000; Ivy et al. 2003; Duman and Monteggia 2006).
Studies in recent years have revealed a decline in hippocampal BDNF expression with acute stress (Ueyama et al. 1997), and antidepressants (Popoli et al. 2002) or antidepressant/exercise combinations (Russo-Neustadt et al. 2001) have been reported to prevent or reverse these changes. Exercise has also been reported to protect against decrements in hippocampal function and/or neurochemistry caused by stress (Zheng et al. 2006; Filipovic et al. 2007; Greenwood et al. 2007). Because an increase in BDNF expression promotes hippocampal survival (Kozisek et al. 2008), it is likely that the change evident following our interventions reflects neuroprotection. Both physical activity and antidepressants have been demonstrated to enhance hippocampal neurogenesis and provide some resistance to the damaging effects of acute stressor exposure or brain injury (Bjornebekk et al. 2005; Dranovsky and Hen 2006). In unstressed animals, the combination of exercise with a variety of antidepressant medications enhances the expression of BDNF mRNA, BDNF protein and the activation of several survival-promoting molecules in the hippocampus to a greater degree than either intervention alone (Russo-Neustadt et al. 2000, 2004; Chen and Russo-Neustadt 2005). Since the presence of the running wheel in the animal’s cage can represent a form of environmental enrichment, it is possible that enrichment could account for part of the exercise effect. It also should be noted that reboxetine significantly increased running activity during the experiment (by approximately 58%, with respect to vehicle-treated exercising animals). Therefore, part of the BDNF-enhancing effect of the reboxetine-exercise combination may have resulted from enhanced activity along with enhanced NE transmission.
Contrary to our initial expectations, combination treatments of exercise plus antidepressant were not more effective than individual interventions in preventing the decline in hippocampal BDNF mRNA occurring as a result of the FST. Exercise combined with tranylcypromine, a monoamine oxidase inhibitor, has been evidenced to protect against BDNF mRNA deficits due to a different form of swimming stress in one previous study (Russo-Neustadt et al. 2001). Since acute stress and glucocorticoid production can suppress hippocampal BDNF expression (Smith et al. 1995; Pizarro et al. 2004; Gronli et al. 2006), it is possible that our interventions may have met with a ceiling effect not present in unstressed or mildly stressed animals.
In contrast to the uniform neurochemical effect reported above, each of our interventions had unique behavioral effects during the FST. As evident in several previous studies (Detke et al. 1997; Connor et al. 1999), both antidepressant medications tended to increase total swimming time during the 5-minute test period (though only reboxetine produced effects that reached statistical significance). In contrast to this, exercise decreased swimming time, and physical activity also decreased the amount of climbing and diving observed. Although physical activity, like antidepressant treatment, increases hippocampal BDNF and confers protection against deleterious effects of stress in the hippocampus (reviewed above), exercise in the current study produced behavioral effects in the FST that were the opposite of those associated with antidepressants. Therefore, there appears to be a lack of correlation between BDNF levels and motor activity in the FST following voluntary exercise. Previous studies have been conducted on exercise and the FST, with mixed results. Some groups have reported increased swimming time during the FST following exercise (Bjornebekk et al. 2005; Duman et al. 2008; Trejo et al. 2008), and some have found either no significant change or a decrease (Solberg et al. 1999; Yoo et al. 2000). It is possible that specific experimental conditions, like animal strain, or duration, mode or intensity of the exercise, may account for the different outcomes in these studies (Calil and Marcondes 2006). For example, it is possible that a greater duration of voluntary exercise (perhaps two weeks or more) is necessary to produce behavioral activation in the FST. It is also important to note that our FST was conducted with a water depth of 15 cm, which is significantly less than the water depth used by some groups (Calil et al., 2006 and Connor et al., 1999: 20 cm.; Cryan et al., 2002: 30 cm.). The ability of animals to feel the bottom of the cylinder with their tails can significantly affect their behavior in the FST (Cryan et al., 2002). Time of day may also be a very important contributing factor, as our animals were tested at the beginning of the dark phase, and rats have been observed to take part in significantly less escape-oriented activity during the dark phase, a result that may be associated with decreased anxiety and differential hormone levels (Kelliher et al. 2000). Importantly, exercise has been reported to produce significant anti-anxiety effects (Fulk et al. 2004; Dishman et al., 1996; Greenwood et al., 2007), and the decreased swimming, climbing and diving noted in our study may reflect decreased anxiety during the FST. It has been called to question in recent years whether decreased swimming time during the FST reflects behavioral despair, or may denote something altogether different (Nishimura et al. 1988; Holmes, 2003; Cryan et al. 2005a), such as a more relaxed mental state, or greater physical fitness for maintaining the floating posture. For example, some research groups have followed decreased swimming time in the FST as an indicator of a lessened stress response (Kelliher et al. 2000). Decreased anxiety after exercise may explain the lack of correlation between hippocampal BDNF levels and motor activity in the FST; it is possible that increased motor activity, though a specific response to antidepressants, does not correlate with hippocampal neuroprotection.
Also, in this study each antidepressant medication produced distinct effects when combined with exercise. Reboxetine combined with exercise increased climbing and diving, whereas citalopram combined with exercise decreased climbing/diving behaviors and also produced a trend towards decreasing swimming time. Other investigators have presented evidence that norepinephrine-selective reuptake inhibitors increase climbing during the FST, and significantly less of this specific behavior is observed in animals treated with 5-HT-selective agents (Detke et al. 1995; Cryan et al. 2005b). Since exercise is known to enhance the central release of both NE and 5-HT (Chaouloff 1997; Schmid et al. 1998; Dishman et al. 2000), it is possible that physical activity accentuated the neurotransmitter-selective behavioral effects of each antidepressant. Furthermore, the 5-HT component may be responsible for a calming effect on animal behavior. Our results indicate that interventions with a serotonergic component, such as citalopram and exercise, may have decreased the more active behaviors such as climbing and diving. Additionally, previous research has shown that not all antidepressants increase swimming time equally well in the FST. It has been reported that the SSRI antidepressants, in particular, produce a reliable response in the tail suspension test (TST), but often fail to do so in the Porsolt FST (Cryan et al. 2002). Nevertheless, SSRIs are among the most effective and widely prescribed medications today (Cryan et al. 2002). Some research groups have made modifications to the FST that enable SSRI-induced antidepressant-like behaviors, such as an increase in swimming, to be measured (Detke et al. 1995; Lucki 1997).
Conclusion
In conclusion, the results of our study suggest that, while antidepressant treatment and voluntary physical exercise produce very similar effects on BDNF levels and survival signaling in the hippocampus, each intervention produces unique behaviors during the FST. A re-examination of the meaning of active behaviors during the FST (what they may reflect in terms of emotional function and well-being of the CNS) would be warranted.
Fig. 2.
Forced swimming decreased BDNF mRNA levels in the CA1 and CA2; this effect was prevented with all interventions tested. Several interventions also significantly increased BDNF mRNA relative to FS levels in the remaining hippocampal regions. Results are displayed as the percentage of control (Saline/Sedentary/No forced swim) and represent the mean ± S.E.M. Asterisks denote statistically significant differences from control group (*p < 0.05, **p < 0.01). Bridges between bars denote statistical significance between the indicated groups (p < 0.05). All treatments were administered for an acute period of 1 week (NFS: no forced swim; FS: forced swim; Sal: saline; Reb: reboxetine; Cit: citalopram; Sedentary: no access to running wheel; Active: voluntary access to running wheel).
Acknowledgments
This study was supported by PHS grant MH59776 to ARN. Citalopram and reboxetine were kindly provided as gifts by Lundbeck and Pharmacia, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging. 2005;26(4):511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bergström A, Jayatissa MN, Mørk A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Research. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. International Journal of Neuropsychopharmacology. 2005;8(3):357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Calil CM, Marcondes FK. The comparison of immobility time in experimental rat swimming models. Life Sciences. 2006;79(18):1712–1719. doi: 10.1016/j.lfs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Medicine & Science in Sports & Exercise. 1997;29(1):58–62. doi: 10.1097/00005768-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Research. Molecular Brain Research. 2005;135(1–2):181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analyses for the Behavioral Sciences. Lawrence Erlbaum Assoc., Inc.; New Jersey: 1988. [Google Scholar]
- Connor TJ, Kelliher P, Harkin A, Kelly JP, Leonard BE. Reboxetine attenuates forced swim test-induced behavioural and neurochemical alterations in the rat. European Journal of Pharmacology. 1999;379(2–3):125–133. doi: 10.1016/s0014-2999(99)00492-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in Pharmacological Sciences. 2002;23(5):238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berlin) 2005b;182(3):335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews. 2005a;29(4–5):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Experimental and Clinical Psychopharmacology. 1997;5(2):107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berlin) 1995;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5 (2):107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav. 1996;60 (3):699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, White-Welkley JE, Burke KA, Bunnell BN. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Research Bulletin. 2000;52(5):337–342. doi: 10.1016/s0361-9230(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biological Psychiatry. 2006;59(12):1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biological Psychiatry. 1998;44(5):324–335. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. The effect of repeated physical exercise on hippocampus and brain cortex in stressed rats. Annals of the New York Academy of Sciences. 2007;1096:207–219. doi: 10.1196/annals.1397.087. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. International Journal of Sports Medicine. 2004;25(1):78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119(3):721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacology Biochemistry and Behavior. 2004;77(2):209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proceedings of National Academy of Sciences of the United States of America. 2006;103(35):13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral Neuroscience. 2007;121(5):992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacology Biochemistry and Behavior. 2006;85(4):842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Hesketh SA, Brennan AK, Jessop DS, Finn DP. Effects of chronic treatment with citalopram on cannabinoid and opioid receptor-mediated G-protein coupling in discrete rat brain regions. Psychopharmacology (Berl) 2008;198 (4):29–36. doi: 10.1007/s00213-007-1033-3. [DOI] [PubMed] [Google Scholar]
- Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic interference. Critical Reviews in Neurobiology. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacology Biochemistry and Behavior. 2003;75(1):81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Kelliher P, Connor TJ, Harkin A, Sanchez C, Kelly JP, Leonard BE. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiology & Behavior. 2000;69(4–5):531–539. doi: 10.1016/s0031-9384(00)00213-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacology & Therapeutics. 2008;117(1):30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioral Pharmacology. 1997;8(6–7):523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Montgomery SA. Predicting response: noradrenaline reuptake inhibition. International Clinical Psychopharmacology. 1999;14(Suppl 1):S21–26. doi: 10.1097/00004850-199905001-00005. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neuroscience Research. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M. Is immobility of rats in the forced swim test “behavioral despair”? Physiology & Behavior. 1988;42(1):93–95. doi: 10.1016/0031-9384(88)90266-1. [DOI] [PubMed] [Google Scholar]
- Page ME, Brown K, Lucki I. Simultaneous analyses of the neurochemical and behavioral effects of the norepinephrine reuptake inhibitor reboxetine in a rat model of antidepressant action. Psychopharmacology (Berlin) 2003;165(2):194–201. doi: 10.1007/s00213-002-1269-x. [DOI] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Research. 2004;1025(1–2):10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disorder. 2002;4(3):166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Lepichon M, Jaifre M. Depression-new animal-model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21(5):679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain- derived neurotrophic factor and behavior in an animal model. Behavioral Brain Research. 2001;120(1):87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29(12):2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cellular and Molecular Neurobiology. 1999;19(4):467–489. doi: 10.1023/a:1006986824213. [DOI] [PubMed] [Google Scholar]
- Schmid A, Huonker M, Stahl F, Barturen JM, Konig D, Heim M, Lehmann M, Keul J. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. Journal of the Autonomic Nervous System. 1998;68(1–2):96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. Journal of Neuroscience. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. American Journal of Physiology. 1999;276(1 Pt 2):R152–161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- Strohle A, Feller C, Onken M, Godemann F, Heinz A, Dimeo F. The acute antipanic activity of aerobic exercise. The America Journal of Psychiatry. 2005;162(12):2376–2378. doi: 10.1176/appi.ajp.162.12.2376. [DOI] [PubMed] [Google Scholar]
- Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA. Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biological Psychiatry. 2001;50(10):809–812. doi: 10.1016/s0006-3223(01)01229-x. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Molecular and Cellular Neuroscience. 2008;37(2):402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neuroscience Research. 1997;28(2):103–110. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behavioral Neuroscience. 2004;118(6):1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Bunnell BN, Crabbe JB, Kalish LR, Dishman RK. Failure of neonatal clomipramine treatment to alter forced swim immobility: chronic treadmill or activity-wheel running and imipramine. Physiology & Behavior. 2000;70(3–4):407–411. doi: 10.1016/s0031-9384(00)00261-4. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behavioural Brain Research. 2006;168(1):47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]