Abstract
Cells sense their physical environment through mechanotransduction–that is, by translating mechanical forces and deformations into biochemical signals such as changes in intracellular calcium concentration or activation of diverse signalling pathways. In turn, these signals can adjust cellular and extracellular structure. This mechanosensitive feedback modulates cellular functions as diverse as migration, proliferation, differentiation, and apoptosis and is critical for organ development and homeostasis. Consequently, defects in mechanotransduction–often caused by mutations or misregulation of proteins that disturb cellular or extracellular mechanics–are implicated in the development of a wide array of diseases, ranging from muscular dystrophies and cardiomyopathies to cancer progression and metastasis.
Mechanotransduction describes the cellular processes that translate mechanical stimuli into biochemical signals, thus allowing cells to adapt to their physical environment. Extensive research over the last decades has identified several molecular players involved in cellular mechanotransduction (Box 1); however, many components, and especially the identity of the primary mechanosensor(s), remain incompletely defined.
Box 1. Cellular mechanotransduction. Several biological components, not mutually exclusive, have been proposed to act as cellular mechanosensors and are schematically depicted in a representative cell (see figure). Note that most of these features can be found in many cell types, whereas others (e.g., changes in intercellular space) might only be relevant in a subset of cells. (A) Stretch-activated ion channels in the plasma membrane open in response to membrane strain and allow influx of calcium and other ions. (B) In endothelial cells, the glycocalix, a layer of carbohydrate-rich proteins on the cell surface, can mediate mechanotransduction signalling in response to fluid shear stress. (C) Cell-cell junctional receptors or (D) cell-matrix focal adhesions allow the cells to probe its environment. (E) Force-induced unfolding of extracellular matrix proteins such as fibronectin can initiate mechanotransduction signalling outside the cell. (F) Intracellular strain can induce conformational changes in cytoskeletal elements such as filaments, crosslinkers or motor proteins, thereby changing binding affinities to specific molecules and activating signalling pathways. (G) The nucleus itself has been proposed to act as a mechanosensor. Intracellular deformations could alter chromatin conformation and modulate access to transcription factors or machinery. However, direct evidence for this mechanism is still lacking. (H) Compression of the intercellular space can alter the effective concentration of autocrine and paracrine signalling molecules. Additionally, changes in G-protein coupled receptors, lipid fluidity, and even mitochondrial activity have been proposed as mechanosensors. Generally, almost all cells respond to mechanical stimulation with adaptive changes in cell function. These changes include short term responses such as increases (or decreases) in intracellular tension, adhesion, spreading or migration, as well as long term effects (e.g., protein synthesis/secretion, structural reorganization, proliferation, viability) often mediated through multiple, overlapping and crosstalking signalling pathways.
Research in mechanotransduction has often focused on sensory cells, such as hair cells in the inner ear. These specialized cells often have evolved specific cellular structures (Fig. 1) that are tailored to transduce mechanical inputs into biochemical signals (for example, by opening ion channels in response to applied forces) and thus provide a good model to study cellular mechanosensing. Subsequently, it has become apparent that mechanotransduction signalling has a critical role in the maintenance of many mechanically stressed tissues such as muscle, bone, cartilage, and blood vessels; consequentially, research has expanded to diverse celltypes such as myocytes, endothelial cells, and vascular smooth muscle cells. Mechanotransduction is now emerging to be involved in a much broader range of cellular functions, not just in a subset of specialized cells and tissues. For example, stem-cell differentiation can be steered towards specific fates based on the geometry and stiffness of the substrate on which the cells are grown on1, and intercellular physical interactions such as tension and adhesion might be as important in embryonic development as concentration gradients of morphogenic factors (see the Review by Wozniak and Chen in this issue.)
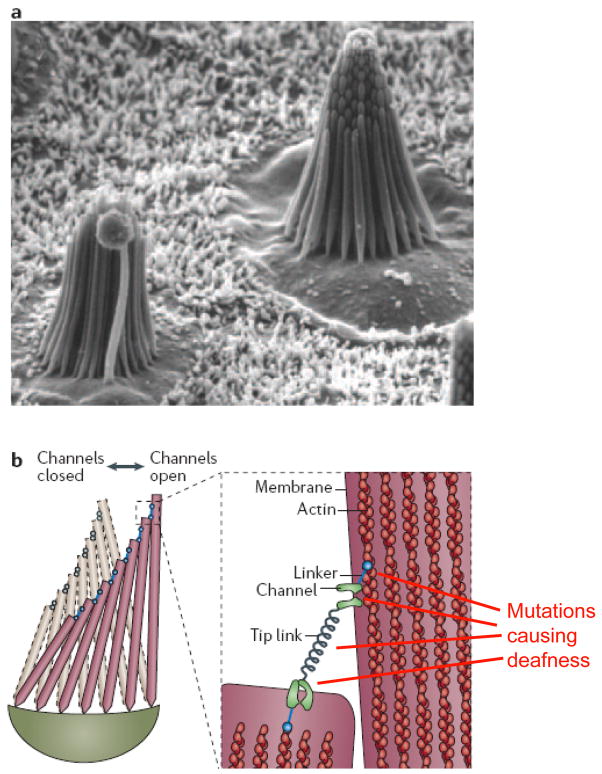
Figure 1. Mechanotransduction in hair cells.
(a). Scanning electron micrograph of two hair bundles in the sensory macula of the bull frog saccule, showing the arrangement of stereocilia with increasing heights. These bundles are ~8μm tall and contain 50–60 stereocilia. (b) Schematic drawing of a hair bundle in resting (gray) and deflected (color) configuration. Deflection, i.e., shearing of the stereocilia relative to each other, causes the ~150–200nm long tip links to pull directly on K+ channels in the stereocilia, causing the channels to open. Myosin motors that link the channels to the actin core of the sterocilia can adjust the position to restore resting tension in the tip link, allowing adaptation to persistent stimulation. Mutations in the K+ channel, the linker proteins, or the unconventional myosins (UCM), which keep the tip links under tension, can result in deafness. Figure is modified from Ref (82). Part a of the Figure is reproduced and part b of the Figure is modified with permission from Ref. (83)
In this Review, we discuss how mutations and modifications that interfere with normal mechanotransduction and cellular sensitivity to mechanical stress could be implicated in a wide spectrum of diseases that range from loss of hearing to muscular dystrophies and cancer(Table 1). Many of these diseases share few similarities at first sight. How could muscular dystrophies be related to atherosclerosis or kidney failure? In the following, we will highlight some of these disorders and discuss how they could be traced back, at least in part, to defects in mechanotransduction, revealing some unexpected similarities. Identifying the molecular details that are involved in normal and defective mechanotransduction will ultimately lead to a better understanding of the underlying disease mechanisms and normal cellular function and could provide new avenues in the therapeutic approaches to these diseases.
Table 1.
Diseases associated with defects in mechanotransduction
| Diseases | Primary cells/tissues affected | Select References |
|---|---|---|
| Deafness | Hair cells in inner ear | 6 |
| Arteriosclerosis | Endothelial and smooth muscle cells | 10–13, 19 |
| Muscular dystrophies and cardiomyopathies | Myocytes, endothelial cells and fibroblasts | 35, 36, 41, 85 |
| Osteoporosis | Osteoblasts | 20 |
| Axial myopia and glaucoma | Optic neurons and fibroblasts | 43–45 |
| Polycystic kidney disease | Epithelial cells | 51, 52 |
| Asthma and lung dysfunction | Endothelial cells and alveolar tissue | 15, 21–23 |
| Premature ageing (HGPS) | Multiple cell types and tissues | 55, 57 |
| Developmental disorders | Multiple cell types and tissues | 46–50, 86 |
| Cancer | Multiple cell types and tissues | 2, 58–60, 68, 71, 73, 87 |
| Potential immune system disorders | Leukocytes | 24–26 |
| Potential central nervous system disorders | Neurons | 27, 28 |
A literature search for “mechanotransduction AND disease” reveals more than 175 references, covering diverse areas such as loss of hearing, cardiac hypertrophy, atherosclerosis, and cancer. Despite their varied phenotypes and clinical manifestations, are there any shared characteristics between these mechanotransduction diseases? A common denominator of many mechanobiology diseases is a disruption in the intricate force transmission between the extracellular matrix (ECM), the cytoskeleton, and the nuclear interior (Fig. 2). Cellular mechanosensing is based on force induced conformational changes in mechanosensitive proteins subjected to molecular forces that result in opening of membrane channels or altered affinities to binding partners, thereby activating signalling pathways. Hence, any changes in the normal intracellular force transmission through changes in cellular (or extracellular) structure and organization can lead to altered molecular forces acting on these proteins, resulting in attenuated or increased mechanosensitive signals. In addition to the defects that affect cellular structure and organization and thus cellular mechanosensing, mutations in proteins that are involved in the downstream signalling pathways could also cause impaired mechanotransduction. Examples include mutations in proteins involved in intracellular calcium signalling or members of the Rho or mitogen-activated protein kinase (MAPK) pathways2. Generally, any changes in cellular or extracellular structure, the cellular mechanosensing process itself, or the involved downstream signalling pathways can result in altered and abnormal mechanotransduction and ultimately lead to disease (Fig. 3).
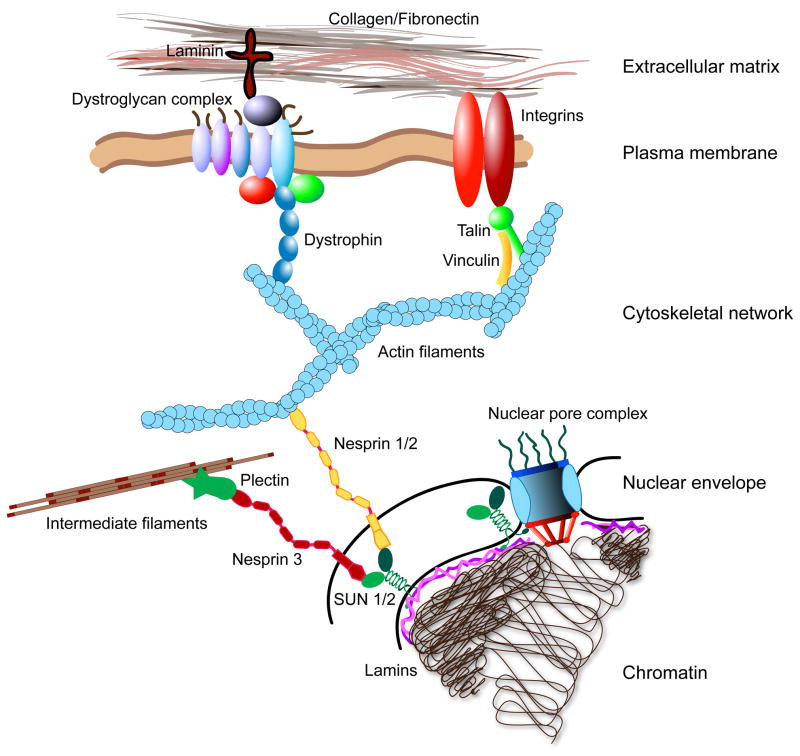
Figure 2. Force transmission between the extracellular matrix (ECM) and the nucleus.
Extracellular forces are transmitted through the ECM, consisting of tissue-specific proteins such as collagen, laminin, and fibronectin. Adhesion complexes at the cell surface physically link the ECM to the cytoskeleton. For example, focal adhesions, comprised of integrins, talin, vinculin, and other proteins, connect the ECM to actin filaments. In skeletal muscle, the dystrophin-associated protein complex links the ECM to actin filaments. The configuration and binding affinity of these complexes can be modulated through intra- and extracellular signalling. Intracellular forces are then transmitted through the cytoskeletal network (i.e., actin filaments, microtubules, and intermediate filaments). The cytoskeleton is coupled to the nucleus through nesprins and possibly other proteins on the outer nuclear membrane. The giant isoforms of nesprins-1 and -2 bind to actin filaments, whereas nesprin-3 can associate with intermediate filaments through plectin. Nesprins interact across the luminal space with inner nuclear membrane proteins (e.g., SUN1 and SUN2), that are retained there by interaction with other nuclear envelope proteins such as lamins and emerin84. Nuclear lamins and SUN proteins also bind to nuclear pore complexes, which could contribute to nuclear cytoskeletal coupling. Finally, lamins form stable nuclear structures and can bind DNA, thus completing the force transmission between the ECM and the nuclear interior. Mutations in any of these components, as well as changes in cellular structure and organization, or changes in the cellular environment, could disturb mechanotransduction signalling and result in altered cellular function; however, this has only been conclusively demonstrated for a subset of these molecules, motivating further research.
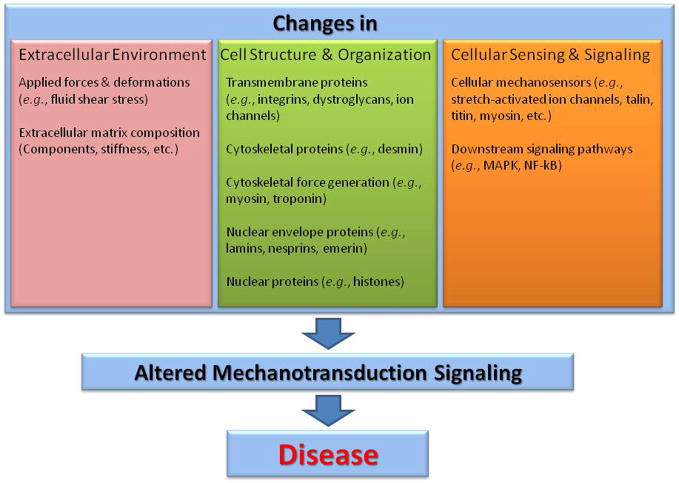
Figure 3. Unifying characteristics of mechanotransduction disorders.
Altered cellular mechanotransduction signalling can be caused by changes in the extracellular environment (e.g., variation in the mechanical forces or deformations experienced by the tissue, or changes in extracellular matrix composition that affect its stiffness and biochemical properties), the cellular structure and organization, or elements of the mechanotransduction process itself. Changes in cellular structure and organization often result from inherited or de novo mutations in proteins that are part of the force generating machinery, the cytoskeletal network, or the nuclear envelope and interior. This category also includes transmembrane proteins involved in cell-cell or cell-ECM adhesion. Abnormal function of these proteins can alter the intracellular force distribution and thus mechanotransduction signalling. In contrast, defects in the cellular mechanosensors can disturb mechanotransduction signalling even in the case of normal force distribution. Note that many proteins can fall into more than one category, as structural proteins could also have mechanosensing capabilities, and mechanotransduction signalling can in turn cause changes in cellular structure and organization and the extracellular environment. Importantly, mechanical activation often initiates multiple signalling pathways at once that can significantly overlap and cross-talk, making it more difficult to study specific pathways. Furthermore, several of the signalling pathways are often shared with “classical”, receptor-mediated pathways. For example, the mitogen-activated protein kinase (MAPK) pathway can be turned on by mechanical strain as well as by receptor-linked tyrosine kinases (e.g., epidermal growth factor receptor). Ultimately, excessive and prolonged disturbances in the normal mechanotransduction signalling can result in many disease conditions.
Cells need to feel the force
All cells and organisms across the evolutionary spectrum from the most primitive to the most complex are mechanosensitive3, 4. This ‘universal’ property allows them to relay mechanical stimuli from their physical surroundings or from within the organism itself to electrochemical or biochemical signals, which then regulate a wide repertoire of physiological responses. As such, mechanotransduction–be it involved in sensing externally applied forces (for example, in touch sensation) or in regulating forces and tension within the body (for example, in muscle tension or blood pressure regulation)–is essential to developmental pathways and to normal tissue homeostasis, primarily in the maintenance of tissues in which cellular adaptive responses are critical to counteract substantial variations from normal conditions4, 5. For example, muscle tissue responds to exercise with hypertrophic growth, that is, an increase in cell size, and the vascular system can maintain a fairly constant blood pressure despite changes in cardiac output by vasoconstriction and vasodilation (the contraction or relaxation of the smooth muscle cells that surround the blood vessels).
One often cited example of mechanotransduction is its role in hearing and balance, which result from electrochemical responses to sound waves, pressure, and gravity (see Review by Chalfie in this issue). These mechanical forces cause small displacements in the stereocilia of hair cells in the inner ear that are arranged in bundles with centrally increasing height. The deflection of the stereocilia causes tension in the tip links, small extracellular filaments that connect the stereocilia tip with adjacent sterocilia, thereby pulling open mechanically-gated ion channels (Fig. 1). The rapid influx of calcium and other ions can then initiate further downstream signalling. Motor proteins that are located at the distal end of the tip link can relax or contract to restore resting tension, thus providing a mechanism to allow the system to adjust to dynamic stimulation6. Similarly, mechanotransduction is pivotal to touch sensation and proprioception (the internal sensing of the relative position of one’s body parts), which share similar underlying mechanisms for mechanotransduction signalling as in hearing7,8.
Mechanotransduction also has a fundamental role in regulating physiological phenomena in other specialized tissues that are not directly involved in sensory functions. For example, skeletal and cardiac muscles respond to increased load, such as through intensive resistance exercise, with hypertrophic growth, whereas immobilized muscles will atrophy over time9. The role of regulatory mechanotransduction in the cardiovascular system is particularly fascinating. It is now well established that the morphology and physiology of the heart and vasculature are influenced by pressure and shear stress generated from flowing blood10–13, and low interstitial flow rates are sufficient to stimulate lymphangiogenesis14. Sophisticated in vivo studies in zebrafish embryos have revealed the presence of distinct high-shear flow patterns during critical stages in the developing heart: artificially perturbing the shear forces by occlusion results in abnormally formed hearts (for example, abnormal third chamber, reduced looping, or defective valves), with defects that are similar to those observed in some congenital heart diseases15. In the mature cardiovascular system, it is thought that laminar shear stress and circumferential vessel stretch exert an atheroprotective effect on endothelial cells. Consistent with this idea, atherosclerotic lesions are often found at specific sites of disturbed (that is, turbulent) flow patterns, characterized by low and oscillatory shear stress at the endothelium. (See the Review by Hahn and Schwartz in this issue).
Another example for the role of mechanotransduction in tissue maintenance is bone. Compact bone is comprised of concentric layers of bone matrix, in which small cavities known as lacunae are interspersed at regular intervals. These lacunae harbour osteocytes and are connected through canaliculi, a network of interconnecting canals. Gravity and compressive forces generated by muscle contractions during locomotion result in small deformations of the poro-elastic bone, resulting in pressure gradients that drive interstitial fluid flow through the lacunae-canalicular network. This load-induced fluid flow is thought to stimulate localized bone remodelling and optimize the physical performance of the bone through mechanotransduction signalling 16. Likewise, chondrocytes (the main cells that comprise cartilage) adapt to widely varying stresses by secreting the glycosaminoglycan-rich ECM that gives cartilage its dynamic mechanical properties. Moreover, lung physiology from development through maturation is influenced by the continuously changing mechanical stress and strain caused by the cyclic distension and contraction of the lungs17. Similarly, urine flow inside the kidney tubules has a central role in regulating kidney morphogenesis, as this cell sense the fluid shear stress by the bending of primary cilia18.
Mechanotransduction and disease
The ability of cells to respond to changes in their physical environment is critical in the development and maintenance of tissues that are exposed to varying mechanical stress (for example, muscle and bone), but also in physiological processes which affect the entire organism (for example, control of blood pressure and blood flow). On the cellular level, mechanotransduction can modulate diverse functions such as protein synthesis, secretion, adhesion, migration, proliferation, viability and apoptosis. Consequently, defects in cellular mechanotransduction–often through inherited or acquired mutations–can result in or can at least contribute to, various human diseases (Table 1). Alternatively, changes in the cellular physical environment could elicit pathological consequences, even when the cellular mechanotransduction processes function properly. Examples for this scenario include disturbed fluid shear stress at bifurcations that trigger vascular remodelling that can result in the development of atherosclerosis19, or the loss of bone mass in a microgravity environment20. In these cases, it is the abnormal mechanical stress at the cellular level that–through (normal) mechanotransduction signalling–modulates cellular processes that can result in break-down of normal tissue function.
So what diseases could arise from defects in mechanotransduction? As almost all cells rely on mechanotransduction signalling for normal function, many tissues can be affected by impaired biomechanics or mechanosensing. An obvious example is loss of hearing caused by mutations in the deafness genes that encode mechanosensitive proteins6 (Fig. 1). Other examples of affected tissues include bone16,20, cartilage, the lung21–23, the immune system24–26, and the central nervous system27,28. Below, we focus on mechanotransduction defects in in skeletal and cardiac muscle that result in muscular dystrophy or cardiomyopathies29–31, and also briefly discuss defects in the eye.
Cardiac mechanosensing and hypertrophy
More than 400 different mutations have been identified in patients with cardiomyopathy, affecting 9 separate sarcomeric genes, including actin, α-tropomyosin, troponin, titin, and, most commonly, β-myosin heavy chain32. To understand how mutations in these structural proteins can result in pathological hypertrophy, it is helpful to view them in the context of cardiac mechanotransduction.
Cardiac myocytes can directly respond to mechanical deformation or stretch through several internal mechanosensors, although the precise mechanosensing mechanisms often remain incompletely understood. The presumptive mechanosensors include stretch-sensitive ion channels at the cell membrane, integrins and integrin-associated proteins (such as melusin or integrin linked kinase (ILK)), sarcomeric proteins such as titin, myosin, or the small LIM-domain protein MLP, and cell surface receptors (such as G-protein-coupled receptors or angiotensin II type 1 receptors) that can be activated by stretch even in the absence of ligands. These mechanosensors activate multiple and overlapping cellular signalling pathways that include Ras/Rho and mitogen activated protein kinase (MAPK) signalling, phospholipase C activation, calcium/Calcineurin-mediated signalling, and microRNAs (Fig. 4)32. These pathways trigger the expression of hypertrophic genes and cause an increase in myocyte length and/or width (reviewed in Refs 32,33). These mechanotransduction pathways, along with often overlapping neurohormonal mechanisms (for example, G-protein coupled receptor signalling activated by angiotensin or catecholamines) allow the heart to adapt to prolonged changes in the mechanical workload with an increase in cardiac myocyte size (hypertrophy) and modification of the surrounding ECM, referred to as cardiac remodelling.
Figure 4. Cardiac mechanotransduction signalling.
Cardiac myocytes respond to altered haemodynamics by activating multiple intracellular signalling pathways that are implicated in the maintenance and regulation of cardiac myocyte fucntion. Mechanical loading can be sensed by cardiomyocytes through a diverse group of membrane anchored mechanosensors including stretch activated ion channels, cell membrane - spanning G-protein coupled receptors, growth factor receptors, and integrins. This mechanical sensation is then converted to biochemical signals by triggering the multi-step activation of downstream partners in an array of signalling cascades in the cytoplasm. The highlights of such cascades include the three modules of the MAPK family underscored by the activation of Ras, the JAK-STAT pathway, Rac activation, calcium and NO signalling. The convergence of these pathways results in the activation of select transcription factors including NF-AT and NF-kB which then translocate to the nucleus and modulate the expression of a panel of mechanosenstive genes including egr-1 and iex-1. Ultimately, the net sum of gene expression reprogramming in cardiomyocytes dictates the functional response of the cell to mechanical stress.
The cardiac hypertrophic response is often categorized into physiological or pathological hypertrophy. Physiological hypertrophy, which arises as the consequence of aerobic exercise or pregnancy, is characterized by the addition of sarcomeres in series (to lengthen the cell) and in parallel (to increase cell width), resulting in increased cardiac wall thickness and chamber dimensions to adapt to the elevated hemodynamic load. By contrast, pathological hypertrophy is caused by abnormal changes in the cardiac workload, e.g., through hypertension, aortic stenosis, myocardial infarction, or by congenital defects due to mutations in genes that encode sarcomeric proteins. As in the physiological hypertrophy, cardiac myocytes sense the increased ventricular wall stress and response with an increase in cell size. However, in pathological hypertrophy, myocytes often show a disproportional increase in either width, resulting in increased ventricular wall thickness, or in length, leading to a dilated ventricle. The hypertrophic response is initially beneficial as it strives to normalize ventricular wall stress and is thus often referred to as compensatory hypertrophy 9, 34. However, elevated stress levels that persist over extended time periods often lead to maladaptive remodelling of the myocytes and ECM which is accompanied by myocyte apoptosis and necrosis and eventually result in cardiac failure32.
The precise molecular mechanisms that govern the transition from compensatory hypertrophy to pathological remodelling remain incompletely understood. Several lines of evidence suggest that hypertrophy can further destabilize cardiac mechanics, as hypertrophic tissue is often characterized by impaired contractility and relaxation dynamics. In addition, the cellular programme that is responsible for the pathological hypertrophy results in the re-expression of genes that are normally associated with the embryonic myocardium. This causes disorganized cellular structure, impaired calcium dynamics, and increased interstitial fibrosis, worsening the mechanical imbalance between cardiac function and hemodynamic load35. The maladaptive remodelling of the ECM can also result in slippage of cardiac myocytes, further exacerbating the mechanical imbalance in the myocardium. Encouraging findings in animal models of cardiac hypertrophy suggest that pathological hypertrophy can be prevented or even reversed by modulating signalling pathways that are involved in the hypertrophic response, motivating the search for specific pharmacological modulators32, 33. The challenge in developing these therapeutic approaches lies in unravelling the dichotomy of physiological and compensatory hypertrophy on one hand and pathological hypertrophy on the other, as significant overlap exists between the signalling pathways that are involved in these processes.
Mechanotransduction and muscular dystrophies
In skeletal muscle cells, forces that are generated in the sarcomeres are transmitted to the ECM through a specialized protein complex that consists of dystrophin and the dystrophin-associated protein complex in the plasma membrane (Fig. 2), thereby shielding the cell membrane from excessive stress. In Duchenne muscular dystrophy, mutations in the dystrophin gene disrupt the force transmission between the cytoskeleton and the ECM, resulting in progressive muscle degeneration36. Importantly, the disruption of cytoskeletal-ECM coupling not only renders cells more susceptible to membrane damage, but also causes aberrant activation of the MAPK extracellular regulated kinase 1/2 (ERK1/2) signalling in response to stretch37. This abnormal mechanotransduction signalling could further impact cell function and viability. Recent experiments indicate that in dystrophin-deficient muscle fibres, stress-induced rupture of the more fragile plasma membrane allows influx of extracellular calcium. This causes abnormal muscle contraction and, combined with the defective coupling between the cytoskeleton and the ECM, leads to physical damage of the cytoskeleton, which subsequently results in the loss of muscle cells38. Interestingly, dystrophin is also involved in the endothelial cell response necessary for the fluid shear-stress mediated dilation of arteries. Consequently, endothelial cells from dystrophin-deficient mice display impaired mechanotransduction signalling in response to fluid shear stress, resulting in attenuated dilation of arteries and a decreased vascular density in cardiac muscle, which could further contribute to the progressive loss of muscle39.
Similarly, mutations in the cytoskeletal proteins desmin, titin, or myosin, which are important sarcomere components, result in disorganized sarcomeres and disturbed cellular mechanics, including impaired force generation and altered (passive) cytoskeletal stiffness, which can impair relaxation dynamics of myocytes. The deleterious effects of these mutations can result from direct changes in the intracellular force distribution and/or generation due to ultrastructural disorganization, but can also arise from downstream effects of altered cellular mechanosensing, as myosin and titin can function as mechanosensors40. Investigating the relative contributions of these mechanisms to muscular dysfunction could have important clinical implications, as defects in mechanotransduction pathways could potentially be attenuated with pharmacological reagents. This research is hampered, however, by the fact that mechanotransduction can directly influence cellular structure and function, making it difficult to discern cause and effect.
The recent findings that muscular dystrophies can also arise from mutations in nuclear envelope proteins (namely lamins A and C, emerin, or nesprins) further highlight the concept that disturbed intracellular structure and force transmission can contribute to muscular disease. Although these proteins are expressed in most differentiated cells, the resulting phenotypes are often muscle specific and suggest an increased sensitivity to mechanical stress in cells from affected patients. New insights have come from a mouse model of Emery-Dreifuss muscular dystrophy that lacks lamins A and C. Cells from these animals are characterized by decreased nuclear stiffness, increased nuclear fragility, and impaired activation of mechanosensitive genes, causing decreased viability in cells subjected to repetitive strain41. The increased nuclear fragility could result in nuclear rupture and cell death in mechanically stressed tissues. However, nuclear rupture can only explain a fraction of the cell death that is observed during repetitive strain application, especially in cells from emerin-null mice that have normal nuclear mechanics yet increased strain-induced apoptosis42. Therefore, it is likely the attenuated expression of mechanosensitive, anti-apoptotic genes such as Iex-1 seen in lamin A/C and emerin-deficient cells contribute to the increased cellular sensitivity to mechanical stress41,42. Currently, it remains unknown what causes the mechanotransduction defects in these cells. Although the nucleus has often been proposed as a cellular mechanosensors–for example, by altering chromatin accessibility in response to deformations–direct evidence for this function is still elusive, and future research has to address if defects in mechanotransduction signalling arise as a direct consequence of altered nuclear stiffness or if they mainly reflect broader defects in specific signalling pathways (for example, NF-κB signalling)that are modulated by nuclear lamins.
Trouble in the eye
Another unexpected organ that is affected by disturbed mechanotransduction is the eye. Several recent findings suggest that modulated mechanotransduction signalling due to increased mechanical stress could significantly contribute to the pathogenesis of glaucoma and axial myopia43. In glaucoma, elevated hydrostatic pressure and altered biomechanics of the optic nerve head could initiate mechanisms that result in loss of vision. Recent experimental evidence suggests that human (and monkey)eye tissues deform in response to even minute changes in intraocular pressure44. In addition, ambient hydrostatic pressure elevations resembling intraocular conditions in glaucoma can induce apoptosis of retinal ganglion cells in vitro, similar to in vivo findings44. Moreover, human scleral fibroblasts (the primary cells that are implicated in the scleral remodelling that accompanies axial elongation during the development of myopia) express many genes that are modulated by mechanical strain application. These include genes that encode ECM proteins (such as tenascin-C), protein kinases (such as human lymphocyte specific protein tyrosine kinase (LCK)), cell receptors (such as parathyroid hormone(PTH)/PTH-related peptide receptor), cell growth and differentiation factors (such as fibroblast growth factors (FGFs), bone morphogenetic proteins(BMPs)), and transcription factors (such as Jun-B)45. Although the role of some of these proteins in ocular development and axial elongation is obscure and rather speculative, the contribution of others is more obvious. For example, activation of the PTH/PTHrP receptor by calcium regulating hormones triggers several intracellular signalling events including the activation of PKC, which is directly involved in scleral remodelling. Moreover, tenascin-C, which has been implicated in tissue remodelling during development, can act as a mediator of scleral response to stretching by increasing the synthesis of proteolytic enzymes that affect ECM remodelling. These findings suggest that increased intraocular pressure–mediated by normal mechanotransduction signalling in sclera fibroblasts–could contribute to the abnormal remodelling occurring in axial myopia.
Development and premature ageing
Mounting evidence suggests that mechanotransduction also has a critical role in development46–49. Thus, any disturbances to normal mechanotransduction mechanisms or to the cell’s physical environment can result in broad developmental defects50. A classical example to illustrate this is Kartagener’s syndrome, which is characterized by left-right reversal of the primary visceral organs. Left-right patterning in early mammalian embryos is dictated by cilia-driven leftward fluid flow during gestation, which–through mechanotransduction signalling–differentially induces expression of Nodal, a transforming growth factor (TGF)-family molecule, and a signalling cascade of other factors in the left side of the embryo. Mutations in dynein motor proteins that are primarily responsible for Kartagener’s syndrome block cilia motion within the epithelium of a midline node in the embryo and thus prevent the leftward fluid flow; in the absence of flow, the left-right patterning becomes random.
Similarly, mutations in the genes that encode the ciliary proteins polycystin-1 or the transient receptor potential channel family protein polycystin-2 (TRPP2) provide direct evidence for defects in mechanotransduction that result in kidney disease51. Polycystins form mechanosensitive ion channels in the cilia of renal epithelial cells that allow influx of calcium in response to flow-induced bending of the cilia, thus acting as a cellular flow-sensor in the kidney51. Polycystin mutations associated with loss of function leave cells unable to sense the fluid flow that normally regulates kidney morphogenesis, resulting in several types of polycystic kidney disease (PKD) that include autosomal dominant PKD, a disease characterized by progressive cyst formation that culminates in kidney destruction51, 52.
Disturbed mechanotransduction can also underlie several other diseases that are not traditionally approached from a biophysical perspective. One such example is Hutchinson-Gilford progeria syndrome (HGPS), a progeroid disorder caused by mutations in the LMNA gene, which encodes lamin A. Patients with HGPS appear normal at birth, but fail to thrive shortly thereafter and die in their early teens. Arteriosclerosis is the leading cause of death in HGPS patients53, and post-mortem analysis of vascular tissue from patients with HGPS and from a mouse model of HGPS have revealed extensive loss of vascular smooth muscle cells and an unusual susceptibility to hemodynamic stress54,55. Mechanotransduction in vascular cells in response to fluid shear stress and strain from vessel expansion is a critical protective mechanism against arteriosclerosis and can mediate apoptosis, proliferation, and ECM secretion in healthy vascular smooth muscle cells56. Recent experiments from our laboratory revealed that fibroblasts from patients with HGPS have decreased viability when subjected to repetitive mechanical strain, and that cells from patients with HGPS lack the strain-induced proliferation response seen in cells from healthy controls57. These findings suggest that increased cellular sensitivity of vascular cells subjected to normal fluid shear stress and vessel expansion could be a possible mechanism for the progressive loss of smooth muscle cells and severe arteriosclerosis in HGPS patients. Although increased cellular sensitivity to mechanical strain is certainly not the only factor in HGPS, our experiments suggest that it could have an important role in the development of severe arteriosclerosis that ultimately leads to lethal stroke or myocardial infarction in human patients55. Furthermore, it could contribute to defects in other mechanically loaded tissue, perhaps causing the bone abnormalities and skeletal muscle dystrophy that are characteristic for HGPS.
Cancer cells have lost their touch
Perhaps the most intriguing of all the mechanotransduction diseases is cancer. In the past decade, sudden changes in ECM mechanics, ECM remodelling, and the resultant disturbance in cytoskeletal tension and mechanotransduction signalling have emerged as important factors that can promote malignant transformation, tumorigenesis and metastasis58–60. In addition to a combination of genetic mutations and increased oncogene activity, cytoskeletal reorganizations–particularly those that are manifested by alterations in the tensional force generated by the actin-myosin apparatus in the cell–play a pivotal role in the morphological changes adopted by tumour cells as they develop invasive phenotypes. One of the main regulators of cytoskeletal tension is the Rho family of GTPases. Among its many targets, Rho functions via Rho kinase (ROCK) to regulate myosin-II light chain phosphorylation through inhibitory phosphorylation of myosin phosphatase. Although studies on Rho activity in tumours have yielded contradictory results (some reports provided substantial evidence which supports the notion that tumours have increased Rho activity and exhibit more cytoskeletal tension, whereas others reported decreased Rho activity in solid tumours61–66) it has become apparent that cytoskeletal tension significantly impacts signalling pathways that are implicated in cancer progression. Discrepancy in these studies may be partly attributable to the multi-variant experimental conditions used and to the limitations that are associated with two dimensional monolayer cell cultures.
Furthermore, several studies have shown that cytoskeletal tension in tumours is influenced by ECM stiffness2, 58, 67. Tumours are generally much stiffer than the surrounding normal tissue. Concurrent changes in tissue stiffness, tumour growth due to proliferating cells, and/or elevated interstitial fluid pressure all combine to affect the physical environment of cancerous cells inside the tumour and the adjacent normal cells68. This altered physical environment can modulate the fate of these cells through mechanotransduction (Fig. 5). For example, higher ECM stiffness can result in disruption of normal epithelial cell polarity inducing mammary epithelial cells to fill the cystic lumens in breast cancer2. Paszek and coworkers investigated whether the increased tissue stiffness that is observed in mammary tumours in three-dimensional (3D) matrices promotes the malignant phenotype by influencing integrins, cell surface receptors that connect specific ECM molecules to the cytoskeleton2 (Fig. 2). They found that matrix stiffness (exogenous force) and cytoskeletal tension (endogenous force) functionally cooperate in a ‘mechano-circuit’ that modulates phenotypic transformations in tumours by coupling the mechanosensing role of integrins in relaying external physical cues to Rho and ERK signalling pathways. As such, the stiffer matrix disturbs epithelial morphogenesis by causing force-dependent aggregation and clustering of integrins, thus resulting in elevated Rho-ROCK-dependent cytoskeletal tension that amplifies the formation and stabilization of focal adhesions assembly and This increase in cell-generated force and in focal adhesion assembly was accompanied by focal adhesion kinase (FAK) signalling, ROCK-mediated disruption of adherens junctions, enhanced growth factor-dependent ERK activation driving tumour cell proliferation, and disruption of basal polarity, hence abrogating lumen formation and remodelling mammary tissue architecture. Disrupting Rho or ERK signalling to reduce cytoskeletal tension to normal levels resulted in significant reduction in tumour cell proliferation and repression of the malignant phenotype. Pertaining to this study, recent experiments demonstrated that both integrins and Rho-mediated regulation of intracellular tension are needed to promote the invasive behaviour of fibroblasts and cancer cells in co-cultures69, 70.
Figure 5. Mechanotransduction in cancer cells.
Schematic representation of how increased extracellular matrix (ECM) stiffness and altered cytoskeletal tension can contribute to tumour formation. Increased ECM stiffness may arise from fibrosis, or in response to increased cytoskeletal tension, caused for examples by oncogene (Ras)-driven ERK activation. The increased ECM stiffness is sensed by focal adhesions and activates integrins and focal adhesion kinase, thereby promoting focal adhesion assembly and stimulating the Rho-ROCK pathway. ROCK activation increases cytoskeletal tension by increasing myosin light chain (MLP) phosphorylation, which could result in further increases in ECM stiffness due to cellular mechanotransduction signalling, completing a self-enforcing (i.e., positive) feedback loop. Cross-talk between the Rho-ROCK pathway and the epidermal growth-factor receptor (EGFR)-Ras-ERK pathway, as well as modulation of Growth factor-dependent ERK activation by integrins, results in increased proliferation. ERK activation can also increase cytoskeletal tension through ROCK, further complementing the cross-talk between cytoskeletal tension and proliferative pathways. In breast cancer cells, the combined action of increased contractility and proliferation, triggered by increased extracellular matrix stiffness, may drive the undifferentiated and proliferative phenotype of mammary epithelial cancer cells and result in tumour formation. Decreasing Rho-mediated cytoskeletal contractility or ERK activity is sufficient to revert EGFR-transformed cells that form disorganized and invasive colonies into phenotypically normal cells that form polarized and growth-arrested acini in 3D culture 2. Figure modified with permission from Ref. (58).
All cells, with the exception of haematopoietic cells, need to adhere to a solid substrate for normal cell-cycle progression and survival. Notably, cancer cells lose this dependency on anchorage and cell/surface tension as they become able to invade other tissues71, 72. This hallmark of metastatic cells, that is, their ability to break through the basal lamina, infiltrate blood vessels, exit the blood vessels, and form new tumours, requires finely regulated biomechanical interactions between the cancer cell and its physical milieu. For example, adhesion of melanoma cells to the endothelial cells lining of blood vessels (a critical step to extravasation and metastasis) is in part regulated by hydrodynamic shear rate by mediating melanoma-leukocyte aggregation, which enhances adhesion of tumour cells to the endothelium73. Moreover, although tumours are stiffer, metastatic cells can be distinguished from non-invasive cancer cells and normal cells by reduced cytoskeletal stiffness and increased deformability60,74,75. Recent evidence suggests that cell deformability strongly correlates with passage time through narrow pores and with enhanced metastatic potential in mouse melanoma cells76. Thus, increased cellular and nuclear deformability could enable metastatic cancer cells passage through size-limiting pores and blood vessels, resulting in enhanced metastatic spreading. Although some of these studies to measure the stiffness of cancer cells had technical limitations (e.g., measurements of normally adherent cells performed in suspension, or only small cell numbers tested), the experiments overall illustrate that many cancer cells are characterized by altered physical properties and that biomechanical measurements of cells isolated from pleural effusions may potentially have significant diagnostic and prognostic value.
Clearly, cancer is not exclusively caused by defective mechanotransduction signalling, as deregulation of cell-cycle control, defects in DNA-damage repair, suppression of apoptosis, and altered adhesion and/or migration all contribute to this multifaceted disease. However, many of these cellular functions involved in tumorigenesis and metastasis are modulated by mechanotransduction. Hence, altered mechanotransduction signalling could be a critical component in tumour formation and metastatic progression. Improved cell culture modalities that would permit studying tumour progression in a 3D context would greatly enhance our ability to decipher the effects of mechanotransduction aberrations in cancer progression.
Conclusions and future perspectives
The above examples demonstrate that the mechanotransduction feedback loop that couple cellular structure and function and that modulates cellular structure and the extracellular environment play a critical role in the maintenance of normal tissue function. Moreover, events that disrupt these feedback loops, either by affecting cellular mechanosensing, intracellular mechanotransduction signalling, or intracellular or extracellular force distribution, can result in various clinical phenotypes.
One challenge that remains is to tease apart the contribution of defects in structural or motor proteins to their role in mechanotransduction. Often, structure, function, and mechanotransduction are tightly linked, as the examples of myosin, titin (both recently identified as potential cellular mechanosensors), talin, or lamins illustrate. Talin and vinculin are structural components of the focal adhesion complex, linking integrins to actin filaments (Fig. 2). Molecular dynamic models suggest that force-induced conformational changes in talin can activate a cryptic vinculin binding site, enabling subsequent recruitment of vinculin to reinforce focal adhesion sites77. Similarly, lamins were first identified as nuclear intermediate filaments, but subsequently shown to have an important role in transcriptional regulation and DNA and RNA synthesis78. These examples highlight our still limited understanding of the cellular structure-function relationships, that is, we still do not fully understand how the 3D organization of the cytoskeleton and the nucleus affect cellular functions such as DNA and RNA transcription. Future research should focus on how changes in intracellular structure, through induced deformations or remodelling, for example, can modulate these cellular functions and processes.
Understanding these processes may also provide us with new clues in the search for the elusive cellular mechanosensors and the question of how cells manage to sense their physical environment. The many reports of putative mechanosensing proteins suggest that multiple mechanisms exist, even within single cell types, so that the interplay of redundant or complementary mechanotransduction pathways has to be viewed in a ‘systems biology’ context3. This will be especially important when designing treatment approaches for mechanotransduction diseases. Although mutations in structural proteins may require replacing affected genes or cells by targeted gene or stem-cell therapy–currently a challenging and daunting task–an alternative approach for at least some of these diseases could be to correct downstream signalling pathways that are disturbed by altered mechanotransduction signalling. For some dominant negative mutations, another strategy could be based on the possible redundancy of related proteins; it might be possible to reduce levels of the mutant protein by RNA interference. Such an approach has been proposed for a lamin A mutation associated with HGPS, as studies in a lamin A-deficient mouse model suggest that lamin C might be sufficient to maintain cellular function without an increase in cellular sensitivity79. Alternatively, some cellular mechanical defects can potentially be directly addressed by small molecules treatment approaches. For example, the membrane sealant poloxamer 188 has been successfully applied to reduce damage to the plasma membrane in a mouse model of Duchenne Muscular Dystrophy, resulting in significant improvement in cellular function in cardiac and skeletal muscle80, 81.
The past years have provided increasing evidence that the finely tuned feedback between cells and their physical environment is critical for many important cellular functions, ranging from differentiation to proliferation and viability. Events that interfere with these cellular mechanotransduction processes may thus result in diseases that affect a variety of tissues and organs. Studying the mechanisms that underlie these diseases may lead us to new treatment strategies, improved tissue-engineering design, and enhanced biomaterials, and will also provide us with an opportunity to learn more about mechanotransduction and mechanobiology in normal cells and physiology.
Acknowledgments
We apologize to all those authors whose papers we could not cite because of space limitations. We thank Drs. Richard T. Lee and Parth Patwari for insightful discussions and helpful comments. This work was supported by National Institutes of Health grants HL082792, NS059348, the American Heart Association grant 0635359N, and a research grant from the Progeria Research Foundation.
Glossary Terms
- Sensory cells
Cells involved in the sensory reception of touch or hearing, often using specialized cellular structures such as hair bundles or proteins (e.g., stretch-activated ion channels) to detect applied forces and deformations
- Vascular smooth muscle cells
Non-striated muscle cells found in the medial layer of arteries and arterioles involved in regulating blood pressure and vessel tone
- Mechanosensitive proteins
Proteins that are directly involved in sensing forces or deformations. Microscopic forces result in conformational changes in these proteins, thereby altering their affinity to binding partners or ion conductivity and initiating downstream signalling pathways
- Stereocilila
Finger-like cytoplasmic extensions projecting from the apical end of the inner ear’s hair cells into the cochlear fluid that respond to fluid movement and changes in fluid pressure to mediate various sensory functions including hearing
- Motor proteins
Proteins such as dynein or kinesin that generate intracellular forces required for molecular transport orcell tension and contractility
- Deafness genes
A set of genes, including the cadherin 23 gene, that encode a group of proteins called “tip links” found in the hair cells in the inner ear and that play a central role in the conversion of physical stimuli into electrochemical signals (Figure 1). Mutations in these genes cause certain types of deafness
- Stretch-sensitive ion channels
Ion channels that can change their conformation from closed to open in response to mechanical strain in the membrane
- Sarcomeres
Basic functional units in striated muscle cells consisting mostly of thick myosin filaments and thin actin filaments to generate forces
- Aortic stenosis
A condition that is characterized by abnormal narrowing of the valve opening between the left ventricle and the aorta in the heart, restricting blood flow and impeding the ability of the heart to pump blood to the body
- Ventricular wall stress
The mechanical stress, i.e. force per unit area, within the myocardium. Decrease in wall thickness, e.g., following loss of myocytes after infarction, can result in increased left ventricular stress and damage to remaining myocytes
- Interstitial fibrosis
A progressive condition that is characterized by fibrous connective tissue replacing normal tissue such as muscle which is lost by injury or by infection and infiltration of inflammatory cells into the small spaces between tissues
- Hemodynamic load
The quantity of forces generated from the cardiac output and physical resistances due to the flow of blood in the circulation
- Glaucoma
A disease, in which the optic nerve is permanently damaged due to abnormally high pressure of the fluid within the eye resulting in impaired or complete loss of vision
- Axial myopia
Near-or short-sightedness associated with an increase in the eye’s axial length
- Intraocular pressure
The pressure inside the eyeball that is generated by the resistance to the outward fluid flow of the aqueous humor. This pressure helps maintain the shape of the eye, but can result in glaucomaif it is too high
- Cilia
Hair-like projection on the outer surface of some cell types and unicellular organisms. Beating in unison in wave-like motion, cilia serve multiple functions including in mechanosensing, motility, and feeding
- Focal adhesions
Dynamic protein complexes at the plasma membrane involved in connecting the extracellular matrix to the actin cytoskeleton. Focal adhesions consist of integrins, talin, paxillin, several signalling molecules such as focal adhesion kinase (FAK); several of these protein are thought to act as mechanosensors and to participate in mechanotransduction signalling
- Pleural effusions
Are fluids that collect in the space between the lungs and the chest wall
References
- 1.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. This paper presents an elegant study linking matrix stiffness and cytoskeletal tension to cancer progression. As a component of a “mechano-regulatory circuit” that includes integrins, Rho, and ERK, disruption of tensional homeostasis can promote malignant phenotype in a model of breast cancer. [DOI] [PubMed] [Google Scholar]
- 3.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 4.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–75. doi: 10.1038/nrm1890. This is a comprehensive review of the literature on how physical cues in the microenvironment of a eukaryotic cell are sensed and converted to biochemical signals that define cell shape and regulate cell growth, differentiation, and survival. [DOI] [PubMed] [Google Scholar]
- 6.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30:339–65. doi: 10.1146/annurev.neuro.29.051605.112917. This paper provides a detailed and mechanistic review on the process of mechanotransduction in hearing. It describes the constituents of this process from the mechanosensory elements in the inner ear to the genetic determinants, mutations in which are attributed for loss of hearing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–8. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev. 2004;84:1097–153. doi: 10.1152/physrev.00043.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. This paper offers an overview of the signaling pathways implicated in cardiaomyocyte mechanotransduction and discusses the diverse responses of a cardiac myocyte in its adaptation to alterations in its mechanical environment. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–85. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 12.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–60. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–71. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258–64. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt S, Kuhn H, Gessner C, Seyfarth HJ, Wirtz H. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol. 2007;37:699–705. doi: 10.1165/rcmb.2006-0429OC. [DOI] [PubMed] [Google Scholar]
- 16.Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–12. [PubMed] [Google Scholar]
- 17.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 18.Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr Biol. 2002;12:492–7. doi: 10.1016/s0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 20.Klein-Nulend J, Bacabac RG, Veldhuijzen JP, Van Loon JJ. Microgravity and bone cell mechanosensitivity. Adv Space Res. 2003;32:1551–9. doi: 10.1016/S0273-1177(03)90395-4. [DOI] [PubMed] [Google Scholar]
- 21.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–6. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 22.Affonce DA, Lutchen KR. New perspectives on the mechanical basis for airway hyperreactivity and airway hypersensitivity in asthma. J Appl Physiol. 2006;101:1710–9. doi: 10.1152/japplphysiol.00344.2006. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest. 2003;111:691–9. doi: 10.1172/JCI17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheson LA, Maksym GN, Santerre JP, Labow RS. Cyclic biaxial strain affects U937 macrophage-like morphology and enzymatic activities. J Biomed Mater Res A. 2006;76:52–62. doi: 10.1002/jbm.a.30448. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin MF, Sohn DD, Schmid-Schonbein GW. Recoil and stiffening by adherent leukocytes in response to fluid shear. Biophys J. 2008;94:1046–51. doi: 10.1529/biophysj.107.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji JY, Jing H, Diamond SL. Hemodynamic regulation of inflammation at the endothelial-neutrophil interface. Ann Biomed Eng. 2008;36:586–95. doi: 10.1007/s10439-008-9465-4. [DOI] [PubMed] [Google Scholar]
- 27.Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes--hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–64. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansman JB, Franco-Obregon A. Mechanosensitive ion channels in skeletal muscle: a link in the membrane pathology of muscular dystrophy. Clin Exp Pharmacol Physiol. 2006;33:649–56. doi: 10.1111/j.1440-1681.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 30.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103:16–23. doi: 10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 31.Marian AJ. Genetic determinants of cardiac hypertrophy. Curr Opin Cardiol. 2008;23:199–205. doi: 10.1097/HCO.0b013e3282fc27d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–39. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 34.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer BM. Thick filament proteins and performance in human heart failure. Heart Fail Rev. 2005;10:187–97. doi: 10.1007/s10741-005-5249-1. [DOI] [PubMed] [Google Scholar]
- 36.Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med. 2007;17:55–9. doi: 10.1016/j.tcm.2006.12.002. This study provides a neat example of how abnormalities in structural proteins which are implicated in intracellular force transmission and in cell-matrix coupling can result in disease. It describes how mutations in the dystrophin glycoprotein complex contribute to the etiology of cardiac and skeletal myopathy. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–13. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- 38.Claflin DR, Brooks SV. Direct observation of failing fibers in muscles of dystrophic mice provides mechanistic insight into muscular dystrophy. Am J Physiol Cell Physiol. 2008;294:C651–8. doi: 10.1152/ajpcell.00244.2007. [DOI] [PubMed] [Google Scholar]
- 39.Loufrani L, et al. Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin. Arterioscler Thromb Vasc Biol. 2004;24:671–6. doi: 10.1161/01.ATV.0000118683.99628.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–25. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammerding J, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–8. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lammerding J, et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–91. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90:383–8. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–38. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 45.Cui W, Bryant MR, Sweet PM, McDonnell PJ. Changes in gene expression in response to mechanical strain in human scleral fibroblasts. Exp Eye Res. 2004;78:275–84. doi: 10.1016/j.exer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–7. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 47.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–44. doi: 10.1038/nrm2222. In addition to providing a concise review of the role of cell surface mechanics in embryonic and tissue morphogenesis, this paper extends questions that are of vital importance to our understanding of developmental phenomena. [DOI] [PubMed] [Google Scholar]
- 48.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 49.Moore KA, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–81. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 50.Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008;103:234–43. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 52.Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–8. doi: 10.1016/j.cell.2004.07.007. This review sums up the literature on polycystin proteins and their role as mechanically gated channels in mediating mechanosensation in kidney cells as well as in regulation of gene expression. [DOI] [PubMed] [Google Scholar]
- 53.Al-Shali KZ, Hegele RA. Laminopathies and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1591–5. doi: 10.1161/01.ATV.0000136392.59656.8b. [DOI] [PubMed] [Google Scholar]
- 54.Stehbens WE, Delahunt B, Shozawa T, Gilbert-Barness E. Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc Pathol. 2001;10:133–6. doi: 10.1016/s1054-8807(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 55.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 56.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–93. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–6. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Wolf K, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 60.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–38. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 62.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 63.Horiuchi A, et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–70. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 64.Lozano E, Betson M, Braga VM. Tumor progression: Small GTPases and loss of cell-cell adhesion. Bioessays. 2003;25:452–63. doi: 10.1002/bies.10262. [DOI] [PubMed] [Google Scholar]
- 65.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 66.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 67.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 68.Sarntinoranont M, Rooney F, Ferrari M. Interstitial stress and fluid pressure within a growing tumor. Ann Biomed Eng. 2003;31:327–35. doi: 10.1114/1.1554923. [DOI] [PubMed] [Google Scholar]
- 69.Hebner C, Weaver VM, Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol. 2008;3:313–39. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- 70.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 71.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–8. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 72.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 73.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng. 2008;36:661–71. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cross SE, et al. Nanomechanical properties of glucans and associated cell-surface adhesion of Streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology. 2007;153:3124–32. doi: 10.1099/mic.0.2007/007625-0. [DOI] [PubMed] [Google Scholar]
- 75.Guck J, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–98. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ochalek T, Nordt FJ, Tullberg K, Burger MM. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res. 1988;48:5124–8. [PubMed] [Google Scholar]
- 77.Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech. 2007;40:2096–106. doi: 10.1016/j.jbiomech.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 78.Mattout-Drubezki A, Gruenbaum Y. Dynamic interactions of nuclear lamina proteins with chromatin and transcriptional machinery. Cell Mol Life Sci. 2003;60:2053–63. doi: 10.1007/s00018-003-3038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fong LG, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–52. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasuda S, et al. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–9. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 81.Ng R, Metzger JM, Claflin DR, Faulkner JA. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol. 2008;295:C146–50. doi: 10.1152/ajpcell.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perozo E. Gating prokaryotic mechanosensitive channels. Nat Rev Mol Cell Biol. 2006;7:109–19. doi: 10.1038/nrm1833. [DOI] [PubMed] [Google Scholar]
- 83.Holt JR, Corey DP. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc Natl Acad Sci U S A. 2000;97:11730–5. doi: 10.1073/pnas.97.22.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haque F, et al. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–51. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–35. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- 86.Nonaka S, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 87.Dong C, Slattery MJ, Liang S, Peng HH. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech. 2005;2:145–59. [PMC free article] [PubMed] [Google Scholar]