Abstract
Neuroprotection and brain repair in patients after acute brain damage are still major unfulfilled medical needs. Pharmacological treatments are either ineffective or confounded by adverse effects. Consequently, endogenous mechanisms by which the brain protects itself against noxious stimuli and recovers from damage are being studied. Research on preconditioning, also known as induced tolerance, over the past decade has resulted in various promising strategies for the treatment of patients with acute brain injury. Several of these strategies are being tested in randomised clinical trials. Additionally, research into preconditioning has led to the idea of prophylactically inducing protection in patients such as those undergoing brain surgery and those with transient ischaemic attack or subarachnoid haemorrhage who are at high risk of brain injury in the near future. In this Review, we focus on the clinical issues relating to preconditioning and tolerance in the brain; specifically, we discuss the clinical situations that might benefit from such procedures. We also discuss whether preconditioning and tolerance occur naturally in the brain and assess the most promising candidate strategies that are being investigated.
Introduction
Organisms have evolved mechanisms to protect against tissue damage and to compensate (or even regenerate) in the event of injury. The two most elementary challenges, and thus the greatest evolutionary pressures, for living organisms are infection and deprivation of substrate or energy. Pathophysiological research has focused on mechanisms by which tissue is damaged by noxious stimuli or processes and how to prevent this injury.
To identify endogenous mechanisms of protection and repair, and to make use of these mechanisms therapeutically, biomedical investigators have developed preconditioning strategies. Preconditioning is a procedure by which a noxious stimulus near to but below the threshold of damage is applied to the tissue. Shortly after preconditioning or after a delay, the organ (and therefore the organism) develops resistance to, or tolerance of, the same, similar, or even different noxious stimuli given beyond the threshold of damage. Preconditioning thereby protects against subsequent injury.
Ischaemic brain injuries, resulting either from global or focal decreases in perfusion, are among the most common and important causes of disability and death worldwide. The consequences of global cerebral ischaemia after cardiac arrest (and successful resuscitation), focal occlusions or disruption of brain vessels (ie, stroke, including subarachnoid haemorrhage and intraparenchymatous haemorrhage), and ischaemic brain damage after cardiac or brain surgery affect many millions of people in the USA alone.1,2 Research into preconditioning aims at developing new therapeutic approaches to benefit these patients. On the one hand, preconditioning is an attractive experimental strategy to identify endogenous protective or regenerative mechanisms that can be therapeutically induced or supplemented. On the other hand, preconditioning could be used as a therapeutic technique by inducing tolerance in individuals in whom ischaemic events are anticipated, such as high-risk surgical cohorts or patients with subarachnoid haemorrhage or transient ischaemic attack. Many articles have reviewed various features of ischaemic preconditioning, tolerance, and endogenous neuroprotection in the brain.3-18 In this Review, we give a brief overview of preconditioning, including ischaemic preconditioning, and its clinical potential and discuss the therapeutic exploitation of endogenous neuroprotection. Additionally, we hope to expand the neurocentric view of preconditioning and tolerance held by neuroscientists and neurologists to include the immune system.
Induction of ischaemic tolerance
Many pathological pathways converge on shared pathways of cell injury, death, and repair. For example, although the causes of acute neurodegeneration (eg, stroke) and chronic neurodegeneration (eg, Parkinson’s disease) are different, the mechanisms of cell injury—including excitotoxicity, inflammation, and apoptosis—overlap,19 as do the pathways of survival and regeneration. Therefore, the options for inducing preconditioning and tolerance are not specific to the type of injury, which is important for the clinical adaptation of this technique. The table gives an overview of the different types of preconditioning (cross, remote, immunological, pharmacological, anaesthetic, mimetic, and effector).20-47
Table. Types of preconditioning.
| Principle | Example | |
|---|---|---|
| Cross | Preconditioning stimulus is different from the noxious stimulus against which it protects | In the earliest description of the technique20 the same stimulus was used to induce preconditioning and damage (trauma), whereas trauma was used by Janoff 21to induce protection against sterile sepsis and bowel ischaemia |
| Remote | Preconditioning of one organ or system leads to protection of a different (remote) organ | The prototypical approach for remote preconditioning is the initiation of short ischaemic intervals to a limb to protect organs such as the heart22,23 and brain.24-26 Remote preconditioning might indicate a crosstalk between the brain and the rest of the body in response to stress. Randomised clinical trials have already shown the efficacy of this strategy for the heart.27,28 Remote preconditioning is a particularly attractive strategy to protect organs that are highly susceptible to damage but that are difficult to target, such as the brain |
| Immunological | Pharmacological compounds that trigger the signalling cascades of preconditioning (without a physical stimulus) | One of the earliest examples of pharmacological preconditioning was the mild inhibition of the respiratory chain with nitropropionic acid or acetylsalicylic acid,35-38 which induced cellular changes such as those seen with hypoxia. Another relevant example is the use of the iron chelator desferrioxamine,39 which induces nuclear translocation of the transcription factor hypoxia-inducible factor 1, with consequent expression of a plethora of hypoxia-inducible genes, including erythropoietin, vascular endothelial growth factor, and hexokinase |
| Anaesthetic | Short application of any one of many different classes of anaesthetics can induce an ischaemia-protected state | Anaesthetic preconditioning is effective in the heart40,41 and brain.42-45 This protection might happen within minutes or might be delayed by many hours or days. For practical reasons, anaesthetic preconditioning is an appealing therapeutic option that has already been tested in randomised controlled trials for cardiac protection |
| Mimetics | Compounds that emulate the main danger signal can lead to preconditioning | Examples include inhibitors of mitochondrial respiration, stabilisers of hypoxia-inducible factor 1 (signal: hypoxia), or lipopolysaccharide (signal: infection)46,47 |
| Effectors | The downstream mediators of protection | These effectors include proteins such as erythropoietin, vascular endothelial growth factor, and BCL family proteins. These proteins are attractive pharmacological candidates for preventing the consequences of anticipated ischaemia |
Preconditioning can protect the brain either almost immediately after stimulation (known as early, rapid, or classical preconditioning) or after a delay of 1 to 3 days to induce protein-synthesis-dependent protection (delayed preconditioning). Most stimuli can cause both early and delayed preconditioning, and most, but not all, stimuli leave an unprotected time window between early and delayed preconditioning.24 Irrespective of the rapidity of onset, protection by preconditioning usually never lasts more than a few days. Of note, a recent study showed that a series of repetitive hypoxic preconditioning stimuli can induce neuroprotection in the retina that last many weeks. Such long-term tolerance might be associated with neuronal plasticity, including long-term potentiation, or long-lasting cellular memory associated with immune tolerance.48
Rapid preconditioning is appealing practically and clinically because this technique can be applied therapeutically in the same setting as procedures with high risks of complication, such as cardiac or brain surgery. Most of the experimental and clinical research in cardiology has thus focused on early preconditioning. Conversely, because protection conferred by delayed preconditioning seems to be more robust for the brain than that conferred early, delayed preconditioning has received more attention in neurology. However, although there are effective protocols for early preconditioning for the brain,49 there are few formal comparisons of early and delayed procedures for neuroprotection.50
The recently described event of postconditioning is commonly discussed in the context of preconditioning. Postconditioning is a form of therapeutic reperfusion by which an organ is intermittently reperfused, for example by a stuttering opening and closing of an experimentally (or clinically, as in angioplasty or in organ transplantation) occluded artery. Postconditioning is a new neuroprotective approach for lessening injury in focal ischaemia and reperfusion.51 The benefits of this procedure seem to be mediated, at least in part, by molecular pathways similar to those that control preconditioning.52 As an experimental strategy postconditioning, like preconditioning, might lead to the discovery of endogenous mechanisms of protection and repair. However, postconditioning is unlikely to be of clinical relevance as a therapeutic strategy for ischaemic brain damage, and therefore will not be discussed further in this Review.
Genomic reprogramming
The induction of ischaemic tolerance is accompanied by substantial change in gene expression, suggesting that preconditioning stimulates a fundamental genomic reprogramming of cells that confers cytoprotection and survival.6 The genomic response after ischaemic preconditioning is a signature of the complex interplay of multiple signalling pathways. These highly specialised pathways in different cell types of the brain seem to refine the cellular and systemic response to combat the noxious stimulus. Hundreds of genes are either upregulated or downregulated in response to ischaemic preconditioning stimuli.53-55 Changes in gene expression differ between harmful ischaemia and ischaemic preconditioning. Preconditioning seems to attenuate the response to ischaemia.54 Tolerance induced by ischaemic preconditioning changes the expression of genes involved in the suppression of metabolic pathways, immune responses, ion-channel activity, and blood coagulation.54
Gene expression is regulated by transcription factors but also depends on epigenetic mechanisms such as DNA methylation and histone modification, which modify the chromatin structure that controls access of transcription factors to regulatory loci. Inhibition of DNA methylation and increased histone acetylation have neuroprotective effects in experimental models of stroke.56 Ischaemic preconditioning induces substantial changes in acetylation of the H3 and H4 histones, which are associated with neuroprotection (Yildrim F, Meisel A, unpublished). These changes seem to facilitate widespread changes in transcription and support the concept of genomic reprogramming by preconditioning. Pharmacological inhibition of histone deacteylases with trichostatin A leads to increased histone acetylation and has a neuroprotective effect.56-62 Inhibition of histone deacteylases increases the production and function of regulatory T cells63 and might thereby augment protective immune mechanisms associated with endogenous tolerance. Epigenetic mechanisms of gene regulation might therefore provide another avenue of therapeutic neuroprotection.62
Hypoxia-inducible factor: a regulator of ischaemic preconditioning?
One of the key regulators of the genomic response after ischaemic preconditioning is the transcriptional activator hypoxia-inducible factor (HIF). This protein is a heterodimer with an unstable α-subunit (HIFα) and a stable β-subunit (HIFβ).64 HIF is regulated by an evolutionarily conserved pathway mediated by oxygen-dependent post-translational hydroxylation of HIFα. Under typical oxygen conditions, HIFα becomes hydroxylated at two prolyl residues by members of the prolyl-4 hydroxylase domain family, generating a binding site for a component of the ubiquitin ligase complex. Polyubiquitination tags HIFα for proteasomal degradation. The activity of prolyl-4 hydroxylase domain proteins is fully dependent on oxygen. Under hypoxic conditions, HIFα is not degraded; it accumulates, dimerises with the HIFβ subunit, and transactivates about 100 genes. These genes encode proteins involved in oxygen transport (erythropoietin), angiogenesis (vascular endothelial growth factor [VEGF] and angiopoietin-2), vasomotor control (adrenomedullin and β-adrenergic receptors), cell survival (VEGF and erythropoietin), pH regulation (carbonic anhydrases), and energy metabolism (glucose transporters and glycolytic enzymes).64 HIF might thereby support tissue oxygenation and cellular energy preservation after cerebral ischaemia. Ischaemic preconditioning activates HIF1—the best known member of the HIF family—and its target genes.65-71 HIF1 activation is neuroprotective,47 whereas a neuron-specific HIF1α deletion exacerbates brain injury in an experimental model of stroke.72 Furthermore, HIF and its target gene VEGF are upregulated in neurons that are connected to, but remote from, the infarcted area.73 Because neurons projecting to an infarcted lesion are at risk of delayed neuronal death through loss of trophic support (eg, growth factors and excitation) by surrounding cells in the damaged area, upregulation of trophic cytokines might prevent neuronal death and support axonal sprouting into the penumbra.74
By contrast with the events in focal cerebral ischaemia,72 neuron-specific HIF1α deletion decreased brain damage in a mouse model of global ischaemia.75 Proapoptotic genes are downregulated, whereas prototypical HIF-dependent neuroprotective genes are regulated similarly in the absence and presence of HIF1α in the brain, suggesting a functional redundancy of their transcriptional control in this experimental strategy.75 This suggestion is supported by the observation that the transcriptional response to hypoxia is controlled not only by HIF1α but also by EPAS1 (endothelial PAS domain protein 1; formerly known as HIF2α).76,77 Additionally, HIF1α knockdown mice maintain their ability to develop ischaemic tolerance as a result of ischaemic preconditioning,72 supporting the conclusion that HIF1α is not essential for conferring robust neuroprotection. However, the situation is much more complex because HIF transactivates not only prosurvival factors but also proapoptotic proteins such as BNIP3.78,79 As with other regulating factors involved in the stress response, HIF coordinates both cell survival and death mechanisms.47
HIF1 indisputably has an important role in the complex transcriptional response to hypoxic-ischaemic brain damage; however, whether the effects of HIF1 activation are predominantly beneficial for the CNS is still unclear.80 Therefore, a comprehensive understanding of the prosurvival and pro-death mechanisms of HIF is crucial for developing new pharmacological strategies for stroke treatment. The prolyl-4 hydroxylase domain proteins, which regulate HIF, are promising targets.
Of the three isoforms, prolyl-4 hydroxylase domain 2 is most important for HIF regulation;81 the neuroprotective effect of inhibitors of these proteins is dependent on the ability of those inhibitors to activate HIF1.72 Prolyl-4 hydroxylases belong to the iron (II) and 2-oxoglutarate-dependent family of oxygenases.82 Chelators of iron such as desferrioxamine induce not only HIF1 and its target genes but also neuroprotection.39,66,83 Because iron chelators might have many unwanted side-effects, more specific HIF-stabilising prolyl-4 hydroxylase domain antagonists that compete with the binding of the natural cofactors might provide neuroprotection in stroke.47,84 Some of these small-molecule inhibitors such as FG-2216 are being investigated in phase II clinical trials in anaemic patients with chronic kidney disease.
Improving outcome after stroke
The endogenous response aimed at improving outcome after decreased substrate delivery to the brain (ie, ischaemia), which is at least partly mediated by HIF, relies on four basic actions: increased substrate delivery, decreased energy use, antagonised mechanisms of damage, and improved recovery. Preconditioning can modulate all four of these actions.
Increased substrate delivery
Substrate delivery to the brain depends mainly on cerebral perfusion. At the cellular level, delivery is mainly affected by substrate storage and uptake. Perfusion changes in cerebral blood flow might be implicated in ischaemic tolerance. Many studies investigating cerebral perfusion in animals that did and did not receive preconditioning in the acute phase after detrimental cerebral ischaemia showed no notable increases in cerebral blood flow in the tolerant state.85-88 By contrast with the acute phase, an increase in cerebral blood flow in the subacute phase after ischaemia is seen in the penumbra of cerebral ischaemic lesions in animals that have received preconditioning.89 Furthermore, ischaemic tolerance is associated with the preservation of microvascular perfusion during stroke.90 In immature brains, preconditioning attenuates the decrease in cerebral blood flow during severe ischaemia. The preservation of cerebral blood flow is accompanied by the induction of many genes involved in vasoregulation and angiogenesis, as well as by an increased vascular density as early as 24 h after preconditioning.91 Angiogenesis has an important role in neuroregeneration after stroke67,92 and the growth of new vessels is stimulated by the VEGF and erythropoietin cytokines that are regulated by HIF1.67,93,94 Adrenomedullin, another HIF1-regulated cytokine, is also involved in ischaemic tolerance.95 This cytokine improves recovery after stroke and is associated with an induction of angiogenesis and increased cerebral blood flow.96,97 Ischaemic preconditioning enhances angiogenesis within the first 2 weeks of stroke in the ischaemic penumbra.98
The decrease in post-ischaemic brain oedema by ischaemic preconditioning has been attributed, at least in part, to the protective effects of preconditioning on cerebral endothelial cells.99 Post-ischaemic microvascular endothelial apoptosis is mitigated by ischaemic preconditioning via the induction of the phosphoinositide 3 (PI3)-Akt kinase pathway.100 The kinase activates survivin, which antagonises apoptosis-inducing factor.100-102 The PI3-Akt kinase pathway is crucial for inducing the tolerant state in the CNS103,104 and is mainly activated by growth factors and cytokines (such as VEGF and erythropoietin), which induce antiapoptotic mechanisms. Nitric oxide generated by endothelial nitric oxide synthase is important for vascular function, and nitric oxide has vasodilatory, anti-inflammatory, and antithrombotic properties. Augmentation of nitric oxide production increases cerebral blood flow, which can lead to neuroprotection during brain ischaemia. Endothelial nitric oxide synthase regulated by the PI3-Akt kinase signalling cascade contributes to ischaemic tolerance.105
Astrocytic glycogen, which provides the main energy reserve in the brain during cerebral ischaemia,106 is neuroprotective.107,108 Preconditioning in immature brains increases glycogen and delays energy depletion caused by ischaemia,109 which suggests that glycogen contributes to energy preservation during cerebral ischaemia. Glucose transport is crucial for neuronal survival during anoxia. Preconditioning increases glucose transport activity, whereas blocking glucose uptake in neurons abrogates anoxic tolerance induced by preconditioning.110
Metabolic downregulation?
Metabolic downregulation, as well as channel arrest, are major mechanisms of hibernation and anoxic tolerance in vertebrate species.111 Preconditioning induces evolutionarily conserved responses to low blood flow and oxygen availability that also occur during hibernation.6,104,112,113 Preconditioning regulates genes that decrease ATP use or lead to channel arrest and thereby might decrease energy metabolism.54,114 However, direct measurement of brain energy metabolism has not shown a clear metabolic downregulation after preconditioning.114
Ischaemic preconditioning preserves mitochondrial membrane integrity and function after severe ischaemia.115-117 Several mechanisms that refine mitochondrial function have been described.3,11 Tolerance, at least in part, is based on HIF1-dependent reprogramming of the basal cellular metabolism that involves mitochondrial genes.118,119 HIF might actively downregulate oxidative metabolism by lowering mitochondrial biogenesis, augmenting glycolysis, decreasing metabolite entry into the citric acid cycle, promoting removal of free-radical-generating mitochondria by autophagy,120,121 and optimising respiratory efficiency by the differential regulation of cytochrome oxidase subunits.119
Antagonism of mechanisms of damage
Neuronal excitotoxicity is important in the pathophysiology of cerebral ischaemia. Excessive extracellular concentrations of the excitatory neurotransmitter glutamate are neurotoxic.122 Ischaemic preconditioning might induce tolerance either by lowering excessive glutamate release or by increasing glutamate uptake. This preconditioning inhibits excitatory pathways through the downregulation of NMDA and AMPA receptors123,124 and ameliorates excitotoxicity.125 Additionally, a shift from excitatory glutamate-mediated neurotransmission to inhibitory GABA-mediated neurotransmission was seen in the tolerant state.114,125 Glutamate uptake by specific transporters is the most effective mechanism to maintain glutamate concentrations below excitotoxic concentrations.126 The glial glutamate transporter 1-excitatory amino acid transporter 2 (GLT1-EAAT2) is involved in the bulk of glutamate transport in the brain,127 and specific overexpression of the GLT1-EAAT2 transporter in astrocytes is neuroprotective.128 β-lactam antibiotics induce neuroprotection via upregulation of the glial GLT1-EAAT2 glutamate transporter. Ischaemic preconditioning causes upregulation of this transporter in astrocytes via the ligand-activated transcription factor peroxisome proliferator-activated receptor γ (PPARγ), leading to a subsequent decrease in ischaemia-induced glutamate release129,130 and brain ischaemic tolerance.131 In human beings, increased plasma concentrations of the endogenous PPARγ receptor agonist 15d-prostaglandin J2 are associated with better outcome in ischaemic stroke.132 Agonists of the PPARγ receptor are also neuroprotective in experimental models of stroke.133,134
The neuronal cellular environment—including astrocytes, microglia, and endothelium—protects neurons against ischaemia. Several cytokines that act in an autocrine and paracrine manner are involved in endogenous neuroprotection. For example, preconditioning stimulates the expression of several HIF1-dependent cytokines (eg, erythropoietin, VEGF, and adrenomedullin). Neuroprotection induced by preconditioning is mediated partly by these cytokines,70,71,95,135 which exert neuroprotective effects via the PI3-Akt kinase pathway, thereby blocking proapoptotic mechanisms either by upregulation or activation of antiapoptotic proteins such as BCL2L1 (formerly known as BCLXL), BCL2, and survivin or by inactivation of proapoptotic regulators such as BAD.70,117
Improved recovery
Ischaemic preconditioning induces the expression of gene programmes involved not only in cytoprotection but also in restorative mechanisms such as neurogenesis and angiogenesis. In adult life, neurons are continuously generated from the progenitor cells in the subventricular zone of the lateral ventricles and the subgranular zone in the hippocampal dentate gyrus. Neurogenesis can be triggered by tissue injury, and ischaemic stroke enhances endogenous neural progenitor cell proliferation and differentiation.136-140 Although most studies found inflammation and microglia activation detrimental to the survival of the new neurons, recent experimental evidence indicates that, under certain circumstances, microglia can be beneficial and can support neurogenesis, progenitor proliferation, survival, migration, and differentiation.141 Strategies to increase endogenous neurogenesis might be desirable to improve recovery.142
Ischaemic preconditioning encourages cell survival and differentiation of neural progenitor cells.98,143,144 Proliferation and differentiation of progenitor cells is mainly controlled by growth factors.93,136,145 Ischaemic preconditioning stimulates the expression of many growth factors, including insulin-like growth factor 1, fibroblast growth factor 2, transforming growth factor β1, epidermal growth factor, brain-derived neurotrophic factor, erythropoietin, VEGF, glial-derived growth factor, and platelet-derived growth factor A.69-71,93,135,143,146-149 Such growth factors are involved in ischaemic tolerance and improve outcome in experimental stroke.135,150-157 Although these growth factors have antiapoptotic and anti-inflammatory effects, part of their effect might be attributable to their role as mediators of a neurorestorative response. The presence of newly generated glial and neuronal precursors might be a neurorestorative response to ischaemic preconditioning.143,158 The proposed mechanisms for endogenous neurogenesis include neuronal replacement, self-regeneration, and release of neurotrophic substances.159 Angiogenesis, which is augmented by ischaemic preconditioning98 and stimulated by growth factors,93,155 plays an important part in neuroregeneration after stroke.92 Proliferating endogenous progenitor cells seem to have not only neurorestorative properties but also direct neuro-protective effects that are essential to the establishment of tolerance after preconditioning.160 The discovery of endogenous regenerative capacity in the mature CNS will improve our understanding of how brains can heal themselves and might provide new therapeutic strategies.161
Immunological tolerance
Activation of the innate immune response is a consequence of stroke, and preclinical data indicate that inflammation in the immediate post-stroke period contributes to ischaemic brain injury.162 Clinical data attribute a detrimental role to post-stroke inflammation. The most convincing of these data came from a trial aimed at preventing leucocyte influx into ischaemic brain tissue. The protein used in this trial, however, induced a systemic inflammatory response associated with worse outcome.163 Furthermore, patients who develop infection after stroke have worse stroke outcome; this association, in part, indicates that patients with more severe strokes are at high risk of infection, but also suggests that the systemic inflammatory response accompanying an infection has a detrimental effect on the ischaemic brain.164
Inflammatory stimuli can also induce ischaemic tolerance. For example, preconditioning with lipopolysaccharide, a potent trigger of the innate immune response, stimulates anti-inflammatory and suppresses proinflammatory pathways.6 The production of proinflammatory cytokines is initiated by signalling cascades involving Toll-like receptors, which recognise host-derived molecules released from damaged tissue as well as common molecular motifs of invading pathogens. These proteins can exacerbate ischaemic injury;165-167 for example, a polymorphism in the gene that encodes Toll-like receptor 4 is associated with ischaemic stroke.168 Receptors 2, 4, and 9 are mediators of ischaemic tolerance in the brain,7,169,170 and others might be involved.9 Ligands of Toll-like receptors, such as lipopolysaccharide, induce a state of tolerance to subsequent ischaemia.171 Although recent data indicate that stimulation of Toll-like receptors leads to specific genomic reprogramming that is different from the gene expression pattern induced by ischaemic preconditioning, the degree of protection is similar. Toll-like receptors are thus promising targets for neuroprotection.7,171 Small doses of lipopolysaccharide or other agonists of these receptors given before an ischaemic insult can be protective, whereas the inflammatory response associated with infection after stroke can be detrimental. The timing and dose of stimulus application are therefore important to mediate the effects of preconditioning.
Cerebral ischaemia might activate adaptive immunity and innate immunity. The adaptive immune response is characterised by lymphocytes that recognise and respond to specific antigens; however, this response depends on the environment in which the lymphocyte was initially familiarised to the antigen.172 For a lymphocyte to become activated to an antigen, that antigen must have been encountered in the context of the MHC II and a co-stimulatory signal must be received. The cytokine milieu at the time of encounter then drives the differentiation into different effector cell types. For example, T-helper-1 cells, which are important for cell-mediated immune reactions, develop in the presence of interferon γ, and T-helper-2 cells, which are important for humoral immunity and the response to parasitic infection, develop in the presence of interleukin 4.173 T-helper-17 cells are newly described CD4-positive effector cells associated with autoimmune disease. These cells develop in the presence of interleukin 6 and transforming growth factor β1. If the cell encounters its antigen in the presence of transforming growth factor β1 but not interleukin 6, a regulatory T cell develops. A separate type of regulatory T cell develops in the presence of interleukin 10. These inducible regulatory T cells secrete cytokines that modulate the immune response (either transforming growth factor β1 or interleukin 10) and are distinct from naturally present regulatory T cells, which function through direct cell contact.173
Because inflammation seems to worsen stroke outcome, induction of regulatory T cells to limit CNS inflammation presents a new opportunity for stroke treatment. Such regulatory T cells can be induced with mucosal tolerance, which can be described as immunological preconditioning when done before an ischaemic insult. Although these cells are activated in an antigen-specific way, they secrete cytokines that modulate the immune response in an antigen non-specific way. Inducible regulatory T cells can thus be used to control the immune response wherever their cognate antigen is present, irrespective of whether that antigen brings about the immune response, an event referred to as bystander suppression.174 In experimental models of stroke, regulatory T cells primed to the CNS antigens myelin basic protein and myelin oligodendrocyte glycoprotein were associated with decreased infarct size for both antigens.175-177 Animals with an increased number of regulatory T cells specific to myelin basic protein also have improved long-term outcome as assessed by several behavioural measures.178 Induction of regulatory T cells to the vascular antigen E-selectin is associated with similar benefits, decreasing the risk of stroke in spontaneously hypertensive stroke-prone rats and reducing infarct volume.179,180
There are dynamic changes in the cytokine milieu of the brain after stroke, suggesting that different types of T-effector cells and regulatory T cells could emerge depending on the timing of the lymphocyte-antigen encounter. Experimental data indicate that endogenous regulatory T cells are upregulated after stroke.181,182 There are limited data on the development of T-effector cells after stroke but, under healthy conditions, T-helper-1 cells directed towards the brain (ie, myelin basic protein) are distinctly unusual.181 If an animal has a systemic inflammatory stimulus at the time of stroke, however, the situation changes. For example, lipopolysaccharide, a component of the gram-negative bacterial cell wall and a Toll-like receptor 4 agonist, predisposes animals to develop a T-helper-1 response to myelin basic protein.181 This effect of lipopolysaccharide is probably multifactorial, but upregulation of costimulatory signals on antigen-presenting cells and an increase in interferon γ secretion could be important. This autoimmune response is associated with worse stroke outcome, which suggests at least one explanation for why individuals who develop an infection after stroke have worse outcome.164
Clinical use of preconditioning
Preconditioning has been successful as an experimental procedure to identify mechanisms for brain protection and regeneration. Important examples of strategies to modulate these mechanisms include erythropoietin, activators of mitochondrial KATP channels, and volatile anaesthetics.
Because of the high risk of neurological complications associated with coronary artery bypass grafting and carotid endarterectomy, patients scheduled for these procedures could potentially benefit from therapeutic preconditioning. These complications include stroke and cognitive deficits, which result either from haemodynamic compromise or embolism (both macroembolism or microembolism).183,184 However, late cognitive decline after coronary artery bypass grafting is not specific to the use of cardiopulmonary bypass. Characteristics of patients, particularly the degree of pre-existing vascular disease in the brain, might have a more important role for patients undergoing these procedures.185 Nevertheless, neuroprotective strategies such as preconditioning, or the use of mimetics or preconditioning effectors could be initiated before surgery. The approach might be useful in subarachnoid haemorrhage because about 20-30% of patients have delayed neurological ischaemic deficits associated with vasospasm several days after the initial event.186 Similarly, up to 10% of patients with transient ischaemic attack will have a stroke within a month of the event.187
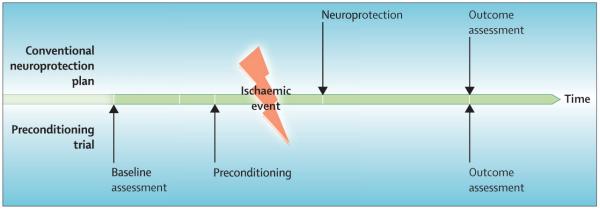
By contrast with conventional neuroprotection trials, in which neuroprotective drugs are given after the ischaemic event, preconditioning strategies have the advantage of antagonising the deleterious mechanisms at the earliest possible time, because the intervention precedes the injury. Moreover, a baseline assessment of neurological status before patients have an ischaemic insult is included (figure 1). The disadvantage of such a clinical trial strategy, which has been proposed as a promising approach to provide clinical proof of concept for neuroprotection studies in stroke research,188 is that a large sample size is needed because of the overall low rates of neurological complication.
Figure 1. Comparison of conventional and preconditioning neuroprotection trials.
Note that the preconditioning trial enables a complete baseline assessment and leads to organ protection before ischaemia (or trauma).
Several clinical trials are underway to test the safety and efficacy of preconditioning strategies for protecting the brain against anticipated damage. For example, one randomised controlled trial aims to determine whether remote ischaemic preconditioning (by thigh cuff inflation; see below) decreases subclinical cerebral and myocardial damage in patients undergoing carotid endarterectomy.189 A pharmacological preconditioning strategy (ie, preloading) is being tested by treating patients with subarachnoid haemorrhage with erythropoietin α to prevent the delayed ischaemic neurological deficits and their consequences.190 Several trials have been or are assessing post-treatment of cerebral ischaemia with preconditioning effectors such as erythropoietin,191 nitric oxide,192,193 and interleukin 1 receptor antagonist.194
Preconditioning is an invaluable technique in basic neuroprotection research and lends itself to new designs of clinical trials. Short episodes of spontaneous ischaemia (such as angina or transient ischaemic attack) might induce endogenous protection against subsequent longer and potentially deleterious episodes of organ ischaemia. For example, when an acute myocardial infarction is preceded by preinfarction angina, the resultant infarction is milder, with fewer cardiac arrhythmias and better left ventricular function than those without preceding angina.195 This observation has motivated the therapeutic use of brief balloon inflations before longer coronary interventions to protect the heart (early preconditioning approach) and promoted the development of delayed preconditioning mimetic compounds such as nitro glycerin.196 Both strategies have been successfully tested in randomised controlled trials.196,197 Data from two retrospective studies of stroke showed that in patients with previous transient ischaemic attack the severity of subsequent stroke was attenuated and the outcome better than those without a preceding event.198,199 In a small prospective study, despite similar size and severity of the perfusion deficit, patients with previous transient ischaemic attack had smaller initial diffusion lesions and final infarct volumes and showed clinical deficits that were less severe than in those without a preceding event.200 Together with other clinical evidence,201,202 these observations suggest that endogenous preconditioning triggered by a transient ischaemic attack is present in the human brain. A criticism of these studies, however, is that patients with previous transient ischaemic attack might fundamentally differ from those without, particularly with respect to cause of stroke. This problem is further exacerbated by the heterogeneity of stroke and the people who have it; furthermore, some transient ischaemic attacks are associated with MRI evidence of infarction and others are not.203 Two other retrospective studies showed no association between previous transient ischaemic attack and low stroke severity.204,205 Therefore, whether the clinical event of a transient ischaemic attack is neuroprotective and associated with less brain injury from subsequent strokes is unclear.
Clinical use: challenges and opportunities
A central belief in preconditioning research is that the preconditioning stimulus must be sub-threshold and should not cause damage. The postulated dose response of the preconditioning stimulus therefore ranges from no response at low intensities to the protected state at higher intensities; a further increase in stimulus intensity will cause overt damage. The therapeutic range of preconditioning is narrow.4,71 Most preconditioning studies are short in duration, with limited periods of survival (up to 1 week); these studies might therefore have missed the maturation of damage. Furthermore, few studies have used techniques sensitive enough to detect subtle structural injury. Increasingly, sophisticated brain imaging suggests that transient ischaemic attack (ie, a potential preconditioning equivalent) commonly leads to structural damage to the brain.203 Sommer206 postulated that “ischemic PC [preconditioning] necessarily involves some form of brain damage, leading to functional impairment with behavioral deficits, although without any lesion”. If true, titration of the preconditioning stimuli so that it has an effect but does not cause damage to the tissue might not only be difficult but potentially impossible. Whether preconditioning mimetics, such as mild inhibition of mitochondrial respiration by aspirin35 or activation of mitochondrial KATP channels,207 are non-toxic is unclear. Because of these potential problems, preconditioning effectors could be a safer way to provide endogenous brain protection than preconditioning mimetics or preconditioning itself. Another caveat is that medication such as anti-inflammatory drugs or statins could interfere with signalling cascades involved in the induction of preconditioning and therefore could diminish (or even potentiate) protective responses to preconditioning.
Immunological preconditioning and other interventions that modulate the immune response to induce neuroprotection and enhance repair pose specific challenges. First, the immune response can stimulate recovery at more delayed time points after stroke;208 therefore, prolonged immunomodulation could interfere with these important restorative processes. Most animal studies focus on early stroke outcomes (days) and might therefore miss important intervention effects weeks to months after treatment. Second, most immunomodulatory drugs are non-specific and affect the immune response in the brain and elsewhere. Because patients with stroke are already predisposed to infection,209 drugs that might further increase this risk are of limited benefit. The strategy of inducing immunological tolerance to CNS antigens is appealing210 because this approach would lead to an immunomodulatory response only where the antigen is present and accessible to the immune system, thus localising the effects of the therapy to the brain during times of compromise of the blood-brain barrier. However, studies of mucosal tolerance for the treatment of multiple sclerosis show that slight differences in antigen treatment could affect both the efficacy and safety of this strategy. For example, mucosal delivery of antigen can give rise to a T-helper-1 response to that antigen. Such a response could potentially lead to encephalitis, as was seen in the vaccine trials of amyloid β for Alzheimer’s disease.211 However, if the CNS immune response was completely suppressed, the susceptibility to infection such as progressive multifocal leukoencephalopathy might increase, as was seen in the studies of natalizumab for multiple sclerosis.212,213
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed by use of search terms that included “preconditioning”, “ischemic tolerance”, “neuroprotection”, “brain repair”, “brain ischemia”, “brain hypoxia”, and “stroke” (“tolerance” and “preconditioning” were common modifiers), with various search periods (from January, 1980, to December, 2008). The full list of search terms is available from the author on request. The bibliographies of the most recent articles were also screened to fi nd other previously unidentified articles. Only papers published in English were reviewed. Further references were obtained from personal reprint files.
Additional factors need to be understood before considering preconditioning for clinical application, even if safe strategies are identified. For example, preconditioning stimuli and effectors in the neuroprotective signalling cascades might be specific to sex,44,214 dependent on age,215-217 and affected by diet110,218 and medical comorbidities.219,220 Because the target population for preventive neuroprotection is usually elderly patients with several medical problems, investigators who undertake preclinical studies are advised to take these factors into consideration.
Despite these challenges, several promising strategies to elicit endogenous brain protection are under clinical development. As patients’ safety is the main concern, compounds or strategies that are already in use for other indications in which they have shown safety are highly attractive candidates. For example, HIF1 mediates the activation of a large cassette of genes involved in the adaptation to hypoxia and ischaemia.47 The iron chelator desferrioxamine is clinically approved for various indications, including thalassaemia and other iron-overload syndromes. Erythropoietin is approved for treatment of anaemia and seems safe and effective for critically ill patients who are anaemic and have had trauma.221 Various inhalational anaesthetics used in human beings (eg, sevoflurane) induce preconditioning and tolerance against brain ischaemia and act as brain protectants after ischaemia in preclinical experiments.43,222,223 These compounds are safe and effective in eliciting early preconditioning in patients undergoing coronary artery bypass graft surgery in randomised controlled trials.224 These drugs also elicit delayed preconditioning in human beings.225,226 Another promising approach in clinical testing is the induction of early remote preconditioning in patients undergoing carotid endarterectomy. In a randomised controlled trial, patients who receive ischaemic preconditioning have a thigh cuff inflated on one leg until flow in the pedal arteries stops.189 After 5 min the cuffis moved to the opposite thigh. The cycle is repeated so that each leg has two 5-min periods of ischaemia followed by 5 min of reperfusion. Another attractive concept, albeit still in an early clinical development stage, is the induction of mucosal tolerance (immunological preconditioning) against E-selectin via a nasal spray to lower stroke events and reduce the effect of cerebral ischaemia.227
Conclusions
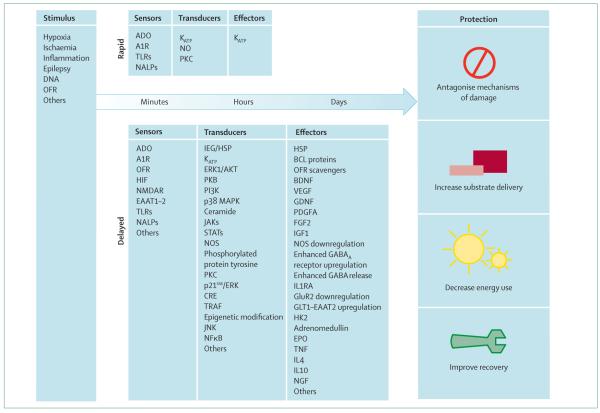
Preconditioning (ie, induced tolerance) is an experimental technique in which protective and regenerative mechanisms of the brain can be isolated from deleterious mechanisms. The molecular signalling cascades of endogenous brain protection—from stimulus and sensor to transducers and effectors—are being identified (figure 2). Research has led to the discovery of several promising strategies for the treatment of patients with acute CNS injury. Additionally, studies of preconditioning have led to the preloading concept of preventively inducing protection in patients who are at high risk of damage to the brain in the near future. Preconditioning-derived strategies include haemopoietic cytokines, immunological tolerance, and physical measures such as remote ischaemic preconditioning. Some of these interventions seem to be safe and effective in protecting the ischaemic heart, and they are now being tested in randomised clinical trials to protect the brain. However, there are still many uncertainties about this therapeutic approach, including which doses of preconditioning or mimetics are safe and effective, and whether transient ischaemic attacks are an ischaemic preconditioning equivalent in human beings. Even in cardiology, where preconditioning strategies have been clinically studied and applied for more than a decade, conclusive evidence for the efficacy and safety of preconditioning or the use of mimetics is scarce. Consequently, therapeutic guidelines for the treatment of heart disease do not yet advocate its use, and there is still little clinical evidence for the use of preconditioning to protect the brain. Such basic factors need to be resolved before randomised controlled trials of preconditioning for the treatment of cerebral ischaemia can be done.
Figure 2. Signalling cascades of preconditioning.
Various stimuli lead to protection via modules of sensors, transducers, and effectors. Adapted from Dirnagl et al,4 with permission from Elsevier. A1R=adenosine receptor type 1. ADO=adenosine. Akt=a serine/threonine kinase family. BDNF=brain-derived neurotrophic factor. CRE=cyclic AMP response element. EAAT=excitatory amino-acid transporter. EPO=erythropoeitin. ERK=extracellular signal-regulated kinase. FGF2=fibroblast growth factor 2. GDNF=glia-derived growth factor. GluR2=glutamate receptor subunit 2. GLT=glutamate transporter. HIF=hypoxia-inducible factor. HK2=hexokinase 2. HSP=heat shock protein. IEG=immediate early gene. IGF1=insulin-like growth factor 1. IL=interleukin. IL1RA=interleukin 1 receptor antagonist. JAK=janus kinase. JNK=c-Jun N-terminal kinase. KATP=ATP-sensitive potassium channel. MAPK=mitogen-activated protein kinase. NALP=NACHT-containing, LRR-containing, and pyrin-domain-containing protein. NFκB=nuclear factor κB. NGF=nerve growth factor. NMDAR=NMDA receptor. NO=nitric oxide. NOS= nitric oxide synthase. OFR=oxygen free radicals. PDGFA=platelet-derived growth factor receptor A. PI3K=phosphoinositide-3 kinase. PKB=protein kinase B. PKC=protein kinase C. STAT=signal transducer and activator of transcription.TNF=tumour necrosis factor. TLR=Toll-like receptor. TRAF=TNF receptor-associated factor. VEGF=vascular endothelial growth factor.
Acknowledgments
UD and AM are supported by the Hermann and Lilly Schilling Foundation, the European Union’s Seventh Framework Programme (FP7/2008-2013) under grant agreements 201024 and 202213 (European Stroke Network), the Helmholtz Gemeinschaft für Forschungseinrichtungen, the German Ministry for Health and Education, and the Deutsche Forschungsgemeinschaft. KB is supported by the National Institute of Neurological Disorders and Stroke grants RO1NS056457 and RO1NS049197.
Footnotes
Conflicts of interest
AM and UD are the patent inventors of a patent application on a neuroprotective compound (an erythropoeitin derivative) that has been filed to the European Patent Office (PCT/EP2006/004564). Charite Universitätsmedizin Berlin is the patent owner. KB is the patent inventor of US patent 08/994,293, which focuses on the use of mucosal tolerance for the treatment of stroke. The US Department of Health is the patent owner.
References
- 1.Kurtzke JF. The current neurologic burden of illness and injury in the United States. Neurology. 1982;32:1207–14. doi: 10.1212/wnl.32.11.1207. [DOI] [PubMed] [Google Scholar]
- 2.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 3.Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–44. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 5.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50:307–20. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 6.Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38:680–85. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- 7.Stevens SL, Stenzel-Poore MP. Toll-like receptors and tolerance to ischaemic injury in the brain. Biochem Soc Trans. 2006;34:1352–55. doi: 10.1042/BST0341352. [DOI] [PubMed] [Google Scholar]
- 8.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 9.Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling-a unifying theme in ischemic tolerance. J Cerebr Blood Flow Metab. 2004;24:1288–304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 10.Simon R, Henshall D, Stoehr S, Meller R. Endogenous mechanisms of neuroprotection. Epilepsia. 2007;48:72–73. doi: 10.1111/j.1528-1167.2007.01356.x. [DOI] [PubMed] [Google Scholar]
- 11.Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev. 2008;88:211–47. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:291–99. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Pinzon MA, Dave KR, Raval AP. Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antiox Redox Signal. 2005;7:1150–57. doi: 10.1089/ars.2005.7.1150. [DOI] [PubMed] [Google Scholar]
- 14.O’Duffy AE, Bordelon YM, McLaughlin B. Killer proteases and little strokes-how the things that do not kill you make you stronger. J Cerebr Blood Flow Metabol. 2007;27:655–68. doi: 10.1038/sj.jcbfm.9600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Develop Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- 16.Kirino T. Ischemic tolerance. J Cerebr Blood Flow Metabol. 2002;22:1283–96. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 17.Dawson VL, Dawson TM. Mining for survival genes. Biochem Soc Trans. 2006;34:1307–09. doi: 10.1042/BST0341307. [DOI] [PubMed] [Google Scholar]
- 18.Dawson VL, Dawson TM. Neuronal ischaemic preconditioning. Trends Pharmacol Sci. 2000;21:423–24. doi: 10.1016/s0165-6147(00)01560-1. [DOI] [PubMed] [Google Scholar]
- 19.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–97. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 20.Noble RL. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am J Physiol. 1943;138:346–51. [Google Scholar]
- 21.Janoff A. Alterations in lysosomes (intracellular enzymes) during shock; effects of preconditioning (tolerance) and protective drugs. Int Anesthesiol Clinics. 1964;2:251–69. doi: 10.1097/00004311-196402000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–46. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 23.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 24.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–75. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Tokuno S, Hinokiyama K, Tokuno K, Lowbeer C, Hansson L-O, Valen G. Spontaneous ischemic events in the brain and heart adapt the hearts of severely atherosclerotic mice to ischemia. Arterioscler Thrombosis Vasc Biol. 2002;22:995–1001. doi: 10.1161/01.atv.0000017703.87741.12. [DOI] [PubMed] [Google Scholar]
- 27.Ali ZA, Callaghan CJ, Lim E, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–79. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 29.Bensard DD, Brown JM, Anderson BO, et al. Induction of endogenous tissue antioxidant enzyme activity attenuates myocardial reperfusion injury. J Surg Res. 1990;49:126–31. doi: 10.1016/0022-4804(90)90250-6. [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Brown JM, Ao L, et al. Myocardial gene reprogramming associated with a cardiac cross-resistant state induced by LPS preconditioning. Am J Physiol. 1998;275:C475–83. doi: 10.1152/ajpcell.1998.275.2.C475. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann C, Ginis I, Furuya K, et al. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res. 2001;895:59–65. doi: 10.1016/s0006-8993(01)02028-5. [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–81. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- 33.Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg. 2005;103:715–23. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- 34.Kawano T, Kunz A, Abe T, et al. iNOS-derived NO and nox2-derived superoxide confer tolerance to excitotoxic brain injury through peroxynitrite. J Cerebr Blood Flow Metab. 2007;27:1453–62. doi: 10.1038/sj.jcbfm.9600449. [DOI] [PubMed] [Google Scholar]
- 35.Riepe MW, Kasischke K, Raupach A. Acetylsalicylic acid increases tolerance against hypoxic and chemical hypoxia. Stroke. 1997;28:2006–11. doi: 10.1161/01.str.28.10.2006. [DOI] [PubMed] [Google Scholar]
- 36.Riepe MW, Esclaire F, Kasischke K, et al. Increased hypoxic tolerance by chemical inhibition of oxidative phosphorylation: “chemical preconditioning”. J Cerebr Blood Flow Metab. 1997;17:257–64. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Weih M, Bergk A, Isaev NK, et al. Induction of ischemic tolerance in rat cortical neurons by 3-nitropropionic acid: chemical preconditioning. Neurosci Lett. 1999;272:207–10. doi: 10.1016/s0304-3940(99)00594-7. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand F, Liao W, Busch C, et al. Respiratory chain inhibition induces tolerance to focal cerebral ischemia. J Cerebr Blood Flow Metab. 1999;19:1229–37. doi: 10.1097/00004647-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Prass K, Ruscher K, Karsch M, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cerebr Blood Flow Metab. 2002;22:520–25. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 40.De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–93. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- 41.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007;21:212–19. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 42.Kapinya KJ, Lowl D, Futterer C, et al. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson AN. Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci. 2007;80:1157–75. doi: 10.1016/j.lfs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cerebr Blood Flow Metab. 2007;27:1377–86. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opin Pharmacol. 2008;8:104–10. doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiq A, Aminova LR, Ratan RR. Prolyl 4-hydroxylase activity-responsive transcription factors: from hydroxylation to gene expression and neuroprotection. Front Biosci. 2008;13:2875–87. doi: 10.2741/2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratan RR, Siddiq A, Smirnova N, et al. Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med. 2007;85:1331–38. doi: 10.1007/s00109-007-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Investig Ophthalmol Visual Sci. 2007;48:1735–43. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]
- 49.Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cerebr Blood Flow Metab. 1999;19:757–61. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–27. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2:313–18. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 52.Pignataro G, Meller R, Inoue K, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cerebr Blood Flow Metab. 2008;28:232–41. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 53.Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–38. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- 54.Stenzel-Poore MP, Stevens SL, Xiong Z, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 55.Tang Y, Pacary E, Freret T, et al. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Endres M, Meisel A, Biniszkiewicz D, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–81. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endres M, Fan G, Meisel A, Dirnagl U, Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12:3763–66. doi: 10.1097/00001756-200112040-00032. [DOI] [PubMed] [Google Scholar]
- 58.Faraco G, Pancani T, Formentini L, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol. 2006;70:1876–84. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- 59.Meisel A, Harms C, Yildirim F, et al. Inhibition of histone deacetylation protects wild-type but not gelsolin-deficient neurons from oxygen/glucose deprivation. J Neurochem. 2006;98:1019–31. doi: 10.1111/j.1471-4159.2006.04016.x. [DOI] [PubMed] [Google Scholar]
- 60.Yildirim F, Gertz K, Kronenberg G, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol. 2008;210:531–42. doi: 10.1016/j.expneurol.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 61.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 62.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 63.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 64.Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Ruscher K, Isaev N, Trendelenburg G, et al. Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci Lett. 1998;254:117–20. doi: 10.1016/s0304-3940(98)00688-0. [DOI] [PubMed] [Google Scholar]
- 66.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–96. [PubMed] [Google Scholar]
- 67.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–76. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–47. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 69.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prass K, Scharff A, Ruscher K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–86. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 72.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–32. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stowe AM, Plautz EJ, Nguyen P, et al. Neuronal HIF-1 alpha protein and VEGFR-2 immunoreactivity in functionally related motor areas following a focal M1 infarct. J Cereb Blood Flow Metab. 2008;28:612–20. doi: 10.1038/sj.jcbfm.9600560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008;39:1380–88. doi: 10.1161/STROKEAHA.107.499962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Helton R, Cui J, Scheel JR, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiesener MS, Jurgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. Faseb J. 2003;17:271–73. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 77.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aminova LR, Chavez JC, Lee J, et al. Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. doi: 10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z, Yang X, Zhang S, Ma X, Kong J. BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia. Stroke. 2007;38:1606–13. doi: 10.1161/STROKEAHA.106.475129. [DOI] [PubMed] [Google Scholar]
- 80.Aminova LR, Siddiq A, Ratan RR. Antioxidants, HIF prolyl hydroxylase inhibitors or short interfering RNAs to BNIP3 or PUMA, can prevent prodeath effects of the transcriptional activator, HIF-1alpha, in a mouse hippocampal neuronal line. Antioxid Redox Signal. 2008;10:1989–98. doi: 10.1089/ars.2008.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Diff er. 2008;15:635–41. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- 82.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 83.Zaman K, Ryu H, Hall D, et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–30. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siddiq A, Ayoub IA, Chavez JC, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–43. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsushima K, Hakim AM. Transient forebrain ischemia protects against subsequent focal cerebral ischemia without changing cerebral perfusion. Stroke. 1995;26:1047–52. doi: 10.1161/01.str.26.6.1047. [DOI] [PubMed] [Google Scholar]
- 86.Barone FC, White RF, Spera PA, et al. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–50. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 87.Alkayed NJ, Goyagi T, Joh HD, et al. Neuroprotection and P450 2 C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–84. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 88.Kunz A, Park L, Abe T, et al. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–93. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao L, Nowak TS. CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2006;26:1128–40. doi: 10.1038/sj.jcbfm.9600269. [DOI] [PubMed] [Google Scholar]
- 90.Dawson DA, Furuya K, Gotoh J, Nakao Y, Hallenbeck JM. Cerebrovascular hemodynamics and ischemic tolerance: lipopolysaccharide-induced resistance to focal cerebral ischemia is not due to changes in severity of the initial ischemic insult, but is associated with preservation of microvascular perfusion. J Cereb Blood Flow Metab. 1999;19:616–23. doi: 10.1097/00004647-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab. 2007;27:928–38. doi: 10.1038/sj.jcbfm.9600408. [DOI] [PubMed] [Google Scholar]
- 92.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–38. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X, Deng J, Boyle DW, Zhong J, Lee WH. Potential role of IGF-I in hypoxia tolerance using a rat hypoxic-ischemic model: activation of hypoxia-inducible factor 1alpha. Pediatr Res. 2004;55:385–94. doi: 10.1203/01.PDR.0000111482.43827.40. [DOI] [PubMed] [Google Scholar]
- 95.Tixier E, Leconte C, Touzani O, Roussel S, Petit E, Bernaudin M. Adrenomedullin protects neurons against oxygen glucose deprivation stress in an autocrine and paracrine manner. J Neurochem. 2008;106:1388–403. doi: 10.1111/j.1471-4159.2008.05494.x. [DOI] [PubMed] [Google Scholar]
- 96.Dogan A, Suzuki Y, Koketsu N, et al. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1997;17:19–25. doi: 10.1097/00004647-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Exp Neurol. 2006;197:521–30. doi: 10.1016/j.expneurol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 98.Lee SH, Kim YJ, Lee KM, Ryu S, Yoon BW. Ischemic preconditioning enhances neurogenesis in the subventricular zone. Neuroscience. 2007;146:1020–31. doi: 10.1016/j.neuroscience.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 99.Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab. 2003;23:1348–55. doi: 10.1097/01.WCB.0000091762.61714.FE. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol. 2007;292:H2573–81. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]
- 101.Conway EM, Zwerts F, Van Eygen V, et al. Survivin-dependent angiogenesis in ischemic brain: molecular mechanisms of hypoxia-induced up-regulation. Am J Pathol. 2003;163:935–46. doi: 10.1016/S0002-9440(10)63453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce WJ. Cerebrovascular effects of ischemic preconditioning: endothelial survivin joins the fray. Am J Physiol Heart Circ Physiol. 2007;292:H2559–60. doi: 10.1152/ajpheart.00367.2007. [DOI] [PubMed] [Google Scholar]
- 103.Yano S, Morioka M, Fukunaga K, et al. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:351–60. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Hillion JA, Li Y, Maric D, et al. Involvement of Akt in preconditioning-induced tolerance to ischemia in PC12 cells. J Cereb Blood Flow Metab. 2006;26:1323–31. doi: 10.1038/sj.jcbfm.9600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashiguchi A, Yano S, Morioka M, et al. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004;24:271–79. doi: 10.1097/01.WCB.0000110539.96047.FC. [DOI] [PubMed] [Google Scholar]
- 106.Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- 107.Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:162–69. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- 108.Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–10. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brucklacher RM, Vannucci RC, Vannucci SJ. Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci. 2002;24:411–17. doi: 10.1159/000069051. [DOI] [PubMed] [Google Scholar]
- 110.Yu S, Zhao T, Guo M, et al. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 2008;1211:22–29. doi: 10.1016/j.brainres.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 111.Nilsson GE, Lutz PL. Anoxia tolerant brains. J Cereb Blood Flow Metab. 2004;24:475–86. doi: 10.1097/00004647-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Stenzel-Poore MP, Stevens SL, Simon RP. Genomics of preconditioning. Stroke. 2004;35:2683–86. doi: 10.1161/01.STR.0000143735.89281.bb. [DOI] [PubMed] [Google Scholar]
- 113.Lee YJ, Miyake S, Wakita H, et al. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–62. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39:2910–17. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA. Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab. 2001;21:1401–10. doi: 10.1097/00004647-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 116.Dave KR, DeFazio RA, Raval AP, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–82. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyawaki T, Mashiko T, Ofengeim D, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:4892–97. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aragones J, Schneider M, Van Geyte K, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–80. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 119.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–22. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 120.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–42. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang H, Bosch-Marce M, Shimoda LA, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor-still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- 123.Aizenman E, Sinor JD, Brimecombe JC, Herin GA. Alterations of N-methyl-D-aspartate receptor properties after chemical ischemia. J Pharmacol Exp Ther. 2000;295:572–77. [PubMed] [Google Scholar]
- 124.Tanaka H, Calderone A, Jover T, et al. Ischemic preconditioning acts upstream of GluR2 down-regulation to afford neuroprotection in the hippocampal CA1. Proc Natl Acad Sci USA. 2002;99:2362–67. doi: 10.1073/pnas.261713299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dave KR, Lange-Asschenfeldt C, Raval AP, et al. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–73. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- 126.Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15:461–73. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 127.Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–91. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 128.Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ. Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia-ischemia. Neuroscience. 2008;155:1204–11. doi: 10.1016/j.neuroscience.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romera C, Hurtado O, Botella SH, et al. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci. 2004;24:1350–57. doi: 10.1523/JNEUROSCI.1596-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romera C, Hurtado O, Mallolas J, et al. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27:1327–38. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- 131.Zhang M, Li WB, Geng JX, et al. The upregulation of glial glutamate transporter-1 participates in the induction of brain ischemic tolerance in rats. J Cereb Blood Flow Metab. 2007;27:1352–68. doi: 10.1038/sj.jcbfm.9600441. [DOI] [PubMed] [Google Scholar]
- 132.Blanco M, Moro MA, Davalos A, et al. Increased plasma levels of 15-deoxy Delta prostaglandin J2 are associated with good outcome in acute atherothrombotic ischemic stroke. Stroke. 2005;36:1189–94. doi: 10.1161/01.STR.0000166054.55993.e5. [DOI] [PubMed] [Google Scholar]
- 133.Shimazu T, Inoue I, Araki N, et al. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–59. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- 134.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–96. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 135.Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–07. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–15. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yagita Y, Kitagawa K, Ohtsuki T, et al. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–96. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 138.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 139.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 140.Zhang R, Zhang Z, Wang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–48. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 141.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.052. published online July 3. DOI:10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 142.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–96. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 143.Naylor M, Bowen KK, Sailor KA, Dempsey RJ, Vemuganti R. Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochem Int. 2005;47:565–72. doi: 10.1016/j.neuint.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 144.Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–70. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 145.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–29. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sakaki T, Yamada K, Otsuki H, Yuguchi T, Kohmura E, Hayakawa T. Brief exposure to hypoxia induces bFGF mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress. Neurosci Res. 1995;23:289–96. doi: 10.1016/0168-0102(95)00954-x. [DOI] [PubMed] [Google Scholar]
- 147.Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–82. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- 148.Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–97. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 149.Lee TH, Yang JT, Ko YS, Kato H, Itoyama Y, Kogure K. Influence of ischemic preconditioning on levels of nerve growth factor, brain-derived neurotrophic factor and their high-affinity receptors in hippocampus following forebrain ischemia. Brain Res. 2008;1187:1–11. doi: 10.1016/j.brainres.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 150.Sakata M, Yanamoto H, Hashimoto N, et al. Induction of infarct tolerance by platelet-derived growth factor against reversible focal ischemia. Brain Res. 1998;784:250–55. doi: 10.1016/s0006-8993(97)01345-0. [DOI] [PubMed] [Google Scholar]
- 151.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–49. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yanamoto H, Nagata I, Sakata M, et al. Infarct tolerance induced by intra-cerebral infusion of recombinant brain-derived neurotrophic factor. Brain Res. 2000;859:240–48. doi: 10.1016/s0006-8993(00)01966-1. [DOI] [PubMed] [Google Scholar]
- 154.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–37. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 156.Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–32. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu J, Narasimhan P, Yu F, Chan PH. Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke. 2005;36:1264–69. doi: 10.1161/01.STR.0000166180.91042.02. [DOI] [PubMed] [Google Scholar]
- 158.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 159.Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76:223–31. doi: 10.1002/jnr.20040. [DOI] [PubMed] [Google Scholar]
- 160.Maysami S, Lan JQ, Minami M, Simon RP. Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab. 2008;28:1104–13. doi: 10.1038/jcbfm.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kokovay E, Shen Q, Temple S. The incredible elastic brain: how neural stem cells expand our minds. Neuron. 2008;60:420–29. doi: 10.1016/j.neuron.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 162.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Enlimomab Acute Stroke Trial Investigators Use of anti-ICAM-1 therapy in ischemic stroke: results of the enlimomab acute stroke trial. Neurology. 2001;57:1428–34. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 164.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–53. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 165.Lehnardt S, Lehmann S, Kaul D, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 166.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 168.Lin YC, Chang YM, Yu JM, Yen JH, Chang JG, Hu CJ. Toll-like receptor 4 gene C119A but not Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis. 2005;180:305–09. doi: 10.1016/j.atherosclerosis.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 169.Hua F, Ma J, Ha T, et al. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stevens SL, Ciesielski TM, Marsh BJ, et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–47. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.07.067. published online August 12. DOI:10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 173.Awasthi A, Murugaiyan G, Kuchroo V. Interplay between effector Th17 and regulatory T cells. J Clin Immunol. 2008;28:660–70. doi: 10.1007/s10875-008-9239-7. [DOI] [PubMed] [Google Scholar]
- 174.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–98. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–15. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- 176.Becker KJ, McCarron RM, Ruetzler C, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci USA. 1997;94:10873–78. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–32. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 178.Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–82. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33:2156–64. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- 180.Chen Y, Ruetzler C, Pandipati S, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci USA. 2003;100:15107–12. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye Z-C. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–44. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–31. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 183.Gottesman RF, Wityk RJ. Brain injury from cardiac bypass procedures. Semin Neurol. 2006;26:432–39. doi: 10.1055/s-2006-948324. [DOI] [PubMed] [Google Scholar]
- 184.de Borst GJ, Moll FL, van de Pavoordt HD, Mauser HW, Kelder JC, Ackerstaf RG. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg. 2001;21:484–89. doi: 10.1053/ejvs.2001.1360. [DOI] [PubMed] [Google Scholar]
- 185.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–90. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 186.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–72. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 187.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–06. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 188.Donnan GA. The 2007 Feinberg lecture: a new road map for neuroprotection. Stroke. 2008;39:242–42. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- 189.Does remote ischaemic preconditioning reduce heart and cerebral damage following carotid endarterectomy? [accessed Feb 13, 2009];A randomised controlled trial. ISRCTN98544942. http://www.controlled-trials.com/ISRCTN98544942.
- 190. [accessed 13 Feb, 2009];Treating patients with aneurysmal subarachnoid hemorrhage (SAH) with epoetin alfa (EPO) NCT00626574. http://clinicaltrials.gov/ct2/show/NCT00626574.
- 191.Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 192.ENOS Trial Investigators Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122) Int J Stroke. 2006;1:245–49. doi: 10.1111/j.1747-4949.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 193.Kobayashi A, Czlonkowska A, Grabska K, et al. Efficacy of nitric oxide in stroke—a randomized trial. Characteristics of patients recruited in Poland. Neurol Neurochir Pol. 2008;42:99–104. [PubMed] [Google Scholar]
- 194.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–72. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rezkalla SH, Kloner RA. Preconditioning in humans. Heart Fail Rev. 2007;12:201–06. doi: 10.1007/s10741-007-9037-y. [DOI] [PubMed] [Google Scholar]
- 196.Jneid H, Chandra M, Alshaher M, et al. Delayed preconditioning-mimetic actions of nitroglycerin in patients undergoing exercise tolerance tests. Circulation. 2005;111:2565–71. doi: 10.1161/CIRCULATIONAHA.104.515445. [DOI] [PubMed] [Google Scholar]
- 197.Argaud L, Rioufol G, Lievre M, et al. Preconditioning during coronary angioplasty: no influence of collateral perfusion or the size of the area at risk. Eur Heart J. 2004;25:2019–25. doi: 10.1016/j.ehj.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 198.Weih M, Kallenberg K, Bergk A, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30:1851–54. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 199.Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–94. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- 200.Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–21. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- 201.Sitzer M, Foerch C, Neumann-Haefelin T, et al. Transient ischaemic attack preceding anterior circulation infarction is independently associated with favourable outcome. J Neurol Neurosurg Psychiatr. 2004;75:659–60. doi: 10.1136/jnnp.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Castillo J, Moro MaA, Blanco M, et al. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54:811–19. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- 203.Oppenheim C, Lamy C, Touz E, et al. Do transient ischemic attacks with diffusion-weighted imaging abnormalities correspond to brain infarctions? AJNR Am J Neuroradiol. 2006;27:1782–87. [PMC free article] [PubMed] [Google Scholar]
- 204.Johnston SC. Ischemic preconditioning from transient ischemic attacks? Data from the Northern California TIA Study. Stroke. 2004;35:2680–82. doi: 10.1161/01.STR.0000143322.20491.0f. [DOI] [PubMed] [Google Scholar]
- 205.Della Morte D, Abete P, Gallucci F, et al. Transient ischemic attack before nonlacunar ischemic stroke in the elderly. J Stroke Cerebrovasc Dis. 2008;17:257–62. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sommer C. Ischemic preconditioning: postischemic structural changes in the brain. J Neuropathol Exp Neurol. 2008;67:85–92. doi: 10.1097/nen.0b013e3181630ba6. [DOI] [PubMed] [Google Scholar]
- 207.Gaspar T, Snipes JA, Busija AR, et al. ROS-independent preconditioning in neurons via activation of mitoK(ATP) channels by BMS-191095. J Cerebr Blood Flow Metab. 2008;28:1090–103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- 208.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 209.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–73. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 210.Faria AMC, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after A{beta}42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 212.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 213.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 214.von Arnim CAF, Etrich SM, Timmler M, Riepe MW. Gender-dependent hypoxic tolerance mediated via gender-specific mechanisms. J Neurosci Res. 2002;68:84–88. doi: 10.1002/jnr.10195. [DOI] [PubMed] [Google Scholar]
- 215.Nguyen LT, Rebecchi MJ, Moore LC, Glass PSA, Brink PR, Liu L. Attenuation of isoflurane-induced preconditioning and reactive oxygen species production in the senescent rat heart. Anesth Analg. 2008;107:776–82. doi: 10.1213/ane.0b013e318180419d. [DOI] [PubMed] [Google Scholar]
- 216.Boengler K, Buechert A, Heinen Y, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–55. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 217.He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–74. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- 218.Niccoli G, Altamura L, Fabretti A, et al. Ethanol abolishes ischemic preconditioning in humans. J Am Coll Cardiol. 2008;51:271–75. doi: 10.1016/j.jacc.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 219.Purcell JE, Lenhard SC, White RF, Schaeffer T, Barone FC, Chandra S. Strain-dependent response to cerebral ischemic preconditioning: differences between spontaneously hypertensive and stroke prone spontaneously hypertensive rats. Neurosci Lett. 2003;339:151–55. doi: 10.1016/s0304-3940(02)01476-3. [DOI] [PubMed] [Google Scholar]
- 220.Iliodromitis EK, Zoga A, Vrettou A, et al. The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis. 2006;188:356–62. doi: 10.1016/j.atherosclerosis.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 221.Napolitano LM, Fabian TC, Kelly KM, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65:285–97. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- 222.Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: a redox sensitive mechanism? Neuroreport. 2002;13:1431–35. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- 223.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cerebr Blood Flow Metab. 2007;27:1108–28. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Meco M, Cirri S, Gallazzi C, Magnani G, Cosseta D. Desflurane preconditioning in coronary artery bypass graft surgery: a double-blinded, randomised and placebo-controlled study. Eur J Cardio-Thoracic Surg. 2007;32:319–25. doi: 10.1016/j.ejcts.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 225.Lucchinetti E, Aguirre J, Feng J, et al. Molecular evidence of late preconditioning after sevoflurane inhalation in healthy volunteers. Anesth Analg. 2007;105:629–40. doi: 10.1213/01.ane.0000278159.88636.aa. [DOI] [PubMed] [Google Scholar]
- 226.Lucchinetti E, Ambrosio S, Aguirre J, et al. Sevoflurane inhalation at sedative concentrations provides endothelial protection against ischemia-reperfusion injury in humans. Anesthesiology. 2007;106:262–68. doi: 10.1097/00000542-200702000-00013. [DOI] [PubMed] [Google Scholar]
- 227. [accessed Feb 13, 2009];E-selectin nasal spray to prevent stroke recurrence. NCT00012454. http://clinicaltrials.gov/ct2/show/NCT00012454?term=NCT00012454&rank=1.