Abstract
Purpose
Based on the pivotal role of Ras-Raf-MAP-ERK signaling and vascular endothelial growth factor (VEGF) in papillary thyroid cancer (PTC), we conducted a phase II clinical trial of sorafenib targeting RAF and VEGF receptor kinases in PTC.
Patients and Methods
The primary end point was the objective response rate. Secondary end points included response correlation with serum thyroglobulin (Tg); functional imaging; tumor genotype; and signaling inhibition in tumor biopsies. Using a Simon minimax two-stage design, 16 or 25 chemotherapy-naïve metastatic PTC patients were to be enrolled in arm A (accessible tumor for biopsy). Arm B patients had other subtypes of thyroid carcinoma or prior chemotherapy, and did not require tumor biopsies. Patients received 400 mg orally twice per day of sorafenib. Response was assessed every 2 months using RECIST (Response Evaluation Criteria in Solid Tumors).
Results
Of 41 PTC patients, six patients had a partial response (PR; 15%; 95% CI, 6 to 29) and 23 patients (56%; 95% CI, 40 to 72) had stable disease longer than 6 months. Median duration of PR was 7.5 months (range, 6 to 14). Median progression-free survival was 15 months (95% CI, 10 to 27.5). In 14 (78%) of 18 Tg-assessable PTC patients, Tg declined more than 25%. Common grade 3 adverse events included hand-foot skin reaction, musculoskeletal pain, and fatigue. BRAF mutation was detected in 17 (77%) of 22 PTCs analyzed. Four of 10 paired tumor biopsies from PTC patients showed a reduction in levels of vascular endothelial growth factor receptor phosphorylation, ERK phosphorylation, and in VEGF expression during sorafenib therapy. No PRs were noted among non-PTC patients.
Conclusion
Sorafenib is reasonably well-tolerated therapy with clinical and biologic antitumor activity in metastatic PTC.
INTRODUCTION
The Ras-Raf-MEK-MAP-ERK kinase signaling pathway is pivotal in the development of both papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC). In PTC, activating mutations in the gene encoding the serine/threonine kinase BRAF, and genetic rearrangements involving the RET tyrosine kinase (RET/PTC oncogenes) that result in constitutive activation of this cascade, account for the majority of tumors in most populations.1–6 Similarly, constitutively activating mutations of RAS oncogenes occur in approximately 30% of FTCs,7 suggesting that this pathway plays a role in the pathogenesis and/or progression of most differentiated thyroid cancers (DTC). Recent data has also suggested that mutations in BRAF are associated with a more aggressive phenotype.8 Inhibition of tyrosine kinase–activated pathways using compounds that block receptor kinase activity directly or that inhibit the activity of downstream signaling kinases, such as MEK and PI3 kinase, induces thyroid cancer cell death in vitro and in vivo.9–12 In addition to the well-characterized roles of RAF and RET signaling in thyroid cancer, overexpression of vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) receptor is well-described in thyroid cancer, and disruption of VEGF signaling using biochemical and molecular strategies inhibits growth of thyroid cancer cells in vitro and in vivo.13–16
While initially considered a selective RAF kinase inhibitor, sorafenib is a multikinase inhibitor that targets several receptor tyrosine kinases at submicromolar concentrations; these include human VEGF receptors (VEGF-R) 1 to 3, PDGF receptor, and RET.17,18 We therefore performed a phase II study evaluating the activity of sorafenib in patients with metastatic PTC and included tumor tissue and imaging correlative studies.
PATIENTS AND METHODS
Patients
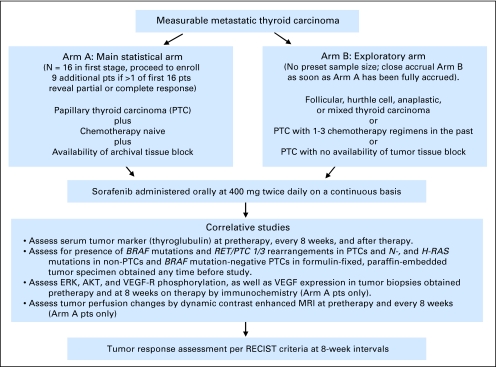
This study was approved by the institutional review board at Ohio State University. Patients were enrolled into either arm A or B (Fig 1). Patients were required to be ≥ 18 years of age with adequate performance status and measurable disease. Chemotherapy or radiation therapy was not allowed within 4 weeks before entry. Iodine-131 (131I) therapy was not allowed within 24 weeks before entry (4 weeks if negative post-treatment scan). Leukocytes ≥ 3,000/μL, absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, serum bilirubin, AST/ALT, and creatinine ≤ 1.5× upper limit of normal were required.
Fig 1.
Study design. pts, patients; VEGF-R, vascular endothelial growth factor receptor; MRI, magnetic resonance imaging; RECIST, Response Evaluation Criteria in Solid Tumors.
Sorafenib Therapy
Sorafenib (BAY 43-9006, Bayer HealthCare Pharmaceuticals, Pittsburgh, PA; NSC 724772, Onyx Pharmaceuticals, Emeryville, CA) was administered at 400 mg orally twice a day (with or without food). Blood pressure was monitored at least weekly until stable or at least the first 4 weeks. Patients were observed every 4 weeks for 1 year and every 12 weeks thereafter if stable. In the event of grade ≥ 3 or recurrent grade ≥ 2 drug-related nonhematologic toxicity or grade ≥ 2 hand-foot skin reaction (HFSR), therapy was held until the toxicity had resolved to ≤ grade 1. Dose reduction to 600 mg/d or 400 mg/d was allowed and subsequent dose re-escalation up to 800 mg/d was allowed. Sorafenib was continued until one of the following: progressive disease (PD), patient off of sorafenib for any reason for longer than 21 consecutive days, intercurrent illness that prevented further therapy, unacceptable adverse events (AEs), or patient withdrawal.
Objective Response and AEs
In the absence of validated response assessment criteria specific for antiangiogenic therapies and based on the PR observed in patients with PTC when treated with sorafenib in a phase I trial, we used RECIST (Response Evaluation Criteria in Solid Tumors) to assess the objective response.19 Computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed within 4 weeks pretreatment, every 8 weeks for the first year, and every 12 weeks thereafter. Duration of response is defined as the time period between the start of sorafenib therapy until the development of PD. The revised National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0 were utilized for AE reporting (http://ctep.cancer.gov/reporting/ctc.html).
Correlative Studies
Tumor markers.
Serum thyroglobulin (Tg) was measured at the same time patients underwent imaging studies for response assessment using same assay for all specimens until February 2006. Anti-Tg antibodies and thyrotropin concentrations were also measured simultaneously to identify potential assay interference and assess the degree of thyroid-stimulating hormone (TSH) suppression, respectively.
Genetic and Pharmacodynamic Studies
Paraffin-embedded tumor tissues from surgeries for primary tumor or metastasis were collected. Tumor tissue was examined for common mutations in thyroid cancer including BRAF, H1-, and N2-RAS as well as RET/PTC 1 or 3 rearrangements (Appendix, online only). Patients in arm A underwent fine needle aspiration (FNA) within 4 weeks before starting sorafenib, and at 8 weeks on therapy. Most FNAs were of metastatic neck lymph nodes sampled with ultrasound guidance using 21-gauge needles (Appendix). To determine in vivo signaling inhibition in treated patients, immunohistochemistry (IHC) was performed on FNA cell block samples collected before and on therapy to assess levels of ERK-, AKT-, and VEGFR-phosphorylation and VEGF expression (Appendix for IHC method). Results were scored by two investigators independently on a 0 to 3 scale with 0 for no immunoactivity, 1 for faint staining or staining in fewer than 50% of cells regardless of intensity, 2 for moderate staining in more than 50% of cells, and 3 for intense staining in more than 50% of cells. A signaling response was graded based on the change in staining score between the two samples on individual patients.
Functional Imaging
To study effects on tumor perfusion, dynamic contrast-enhanced (DCE) MRI scans were obtained within 4 weeks before and every 8 weeks on therapy and were evaluated by quantitative pharmacokinetic parameters.20 Fluorodeoxyglucose positron emission tomography (PET) scans were obtained at the similar time points when possible.
Statistics
The primary end point of this study was to assess the objective response rate of sorafenib in chemotherapy-naïve patients with metastatic PTC enrolled in arm A. The number of patients to be enrolled in arm B was not specified, as this arm was to explore activity of sorafenib in patients with diverse histologic types of thyroid cancers. Accrual to arm B was designed to stop as soon as arm A was fully accrued. We chose a minimax two-stage Simon design that resulted in a trial with decision to continue after 16 response-assessable patients were accrued on arm A.21 Sorafenib would be ineffective or uninteresting if the true response (PR + complete response [CR]) probability was lower than 10% and the regimen would be worthy of further study if the true response probability were ≥ 30%. If two or more patients responded in the first 16, an additional nine patients would be treated for a total of 25. If five or more patients responded of the 25, it would warrant further study.
RESULTS
Patients
Between October 2004 and August 2005, a total of 58 patients were accrued. Two patients never started therapy and are not included in the data analysis (Table 1). Data are reported through June 2007 except for Tg studies, which are included through February 2006 as a result of a change in the assay methodology after that date. A majority of patients on the study had PTC (73%), and 80% of the PTC patients were cytotoxic chemotherapy naïve. All patients had experienced 131I therapy failure or were not candidates to receive 131I as assessed by treating endocrinologist. All patients who had baseline PET scans (19 patients on arm A; 11 DTC patients on arm B, one patient with anaplastic thyroid cancer) had positive scans.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Arm A: PTC |
Arm B |
||||
|---|---|---|---|---|---|---|
| PTC |
Non-PTC |
|||||
| No. | % | No. | % | No. | % | |
| Total no. of patients | 19 | 100 | 22 | 100 | 15 | 100 |
| Median age, years | 67 | 56 | 61 | |||
| Range | 33-90 | 27-76 | 44-86 | |||
| Sex | ||||||
| Male | 11 | 58 | 10 | 45 | 10 | 67 |
| Female | 8 | 42 | 12 | 55 | 5 | 33 |
| Race | ||||||
| White | 16 | 84 | 20 | 90 | 11 | 73 |
| Hispanic, African American, or Asian | 3 | 16 | 2 | 10 | 4 | 27 |
| Pathologic type of thyroid carcinoma | ||||||
| Classic PTC | 15 | 79 | 15 | 68 | — | — |
| Follicular variant of PTC | 2 | 10 | 3 | 14 | — | — |
| Tall cell variant of PTC | 1 | 5 | 3 | 14 | — | — |
| Poorly differentiated PTC | 1 | 5 | 1 | 4 | — | — |
| Follicular | — | — | — | — | 2 | 13 |
| Hürthle cell | — | — | — | — | 9 | 60 |
| Anaplastic | — | — | — | — | 4 | 27 |
| Site of metastasis | ||||||
| Lymph node | 19 | 100 | 19 | 86 | 15 | 100 |
| Lung | 18 | 95 | 22 | 100 | 14 | 93 |
| Bone | 2 | 10 | 3 | 14 | 7 | 46 |
| Liver, kidney, or adrenal | 4 | 20 | 2 | 9 | 2 | 13 |
| Prior therapy | ||||||
| I131 | 19 | 100 | 22 | 100 | 11 | 73 |
| External beam radiation | 7 | 37 | 8 | 36 | 11 | 73 |
| Cytotoxic chemotherapy | 0 | 0 | 8 | 36 | 3 | 20 |
| Celecoxib or thalidomide | 5 | 26 | 8 | 36 | 3 | 20 |
| Other | 0 | 0 | 3 | 14 | 2 | 13 |
| Study entry Tg | ||||||
| Interpretable Tg | 11 | 59 | 11 | 50 | 10 | 67 |
| Presence of Tg antibodies | 5 | 26 | 6 | 27 | 0 | |
| Undetectable Tg | 0 | 2 | 9 | 5 | 33 | |
| Low Tg < 15 ng/mL | 1 | 5 | 3 | 14 | 0 | |
| Unsuppressed TSH | 2 | 10 | 0 | 0 | ||
| Disease status at study entry | ||||||
| Symptomatic progression in preceding 6 months | 1 | 5 | 4 | 18 | 0 | |
| RECIST progression in preceding 12 months | 7 | 37 | 11 | 50 | 10 | 67 |
| Stable disease | 8 | 42 | 6 | 27 | 5 | 33 |
| Unknown | 3 | 16 | 1 | 5 | 0 | |
| Tumor genotype | ||||||
| No. of positive BRAF mutation/No. tested | 10/12 | 7/10 | 0/6 | |||
| Median sum of target measurable lesions at baseline, cm | 6 | 13 | 12 | |||
| Average | 9 | 13 | 13 | |||
| Range | 3-29 | 3-32 | 3-29 | |||
| Median serum Tg at baseline, ng/mL | 159 | 113 | 1,074 | |||
| Average | 714 | 19,340 | 9,121 | |||
| Range | 28-6,162 | 18-188,000 | 34-49,000 | |||
Abbreviations: PTC, papillary thyroid cancer; I131, iodine-131; Tg, serum thyroglobulin; TSH, thyroid-stimulating hormone; RECIST, Response Evaluation Criteria in Solid Tumors.
Treatment Administered
The details of duration, dose, and tolerance of therapy are outlined in Table 2. Fifty-four patients went off study for PD (n = 35), AEs (n = 14), or other reasons (n = 5). A total of 32 patients were alive, whereas 24 patients died. A majority of patients (20 of 24) died from PD (PTC, n = 13; Hürthle cell carcinoma [HTC], n = 4); anaplastic thyroid cancer, n = 3). Additional reasons that contributed to death in PTC patients were acute myeloid leukemia (n = 1), Aspergillus pneumonia (n = 1), hip fracture due to accident (n = 1), and sudden death (n = 1).
Table 2.
Treatment Administered
| Parameter | Arm A: PTC | Arm B |
||
|---|---|---|---|---|
| PTC | HTC/FTC | ATC | ||
| No. of patients | 19 | 22 | 11 | 4 |
| Median duration of therapy, months | 14 | 10 | 8 | 2 |
| Range | 0.25-32 | 0.25-33 | 0.25-26 | 0.5-10 |
| Duration of therapy in all patients, months | 262 | 296 | 124 | 16 |
| Therapy, mg/d dose/months | ||||
| 800 | 84 | 186 | 60 | 16 |
| 600 | 90 | 70 | 56 | 0 |
| 400 | 88 | 40 | 8 | 0 |
| No. of patients with dose reduction | 11 | 10 | 8 | 0 |
| Median time to dose reduction, months | 1.5 | 4.5 | 1.7 | NA |
| Range | 1-12 | 0.5-26 | 0.5-9.5 | NA |
| Reasons for dose reduction | ||||
| Hand-foot skin reaction | 6 | 6 | 2 | 0 |
| Diarrhea and weight loss | 2 | 3 | 1 | 0 |
| Hypertension | 2 | 0 | 1 | 0 |
| Fatigue | 1 | 0 | 0 | 0 |
| Arthralgia | 0 | 1 | 1 | 0 |
| Musculoskeletal chest pain | 0 | 0 | 2 | 0 |
| Mouth pain | 0 | 0 | 1 | 0 |
Abbreviations: PTC, papillary thyroid cancer; HTC, Hürthle cell carcinoma; FTC, follicular thyroid cancer; ATC, anaplastic thyroid cancer; NA, not available.
Objective Response
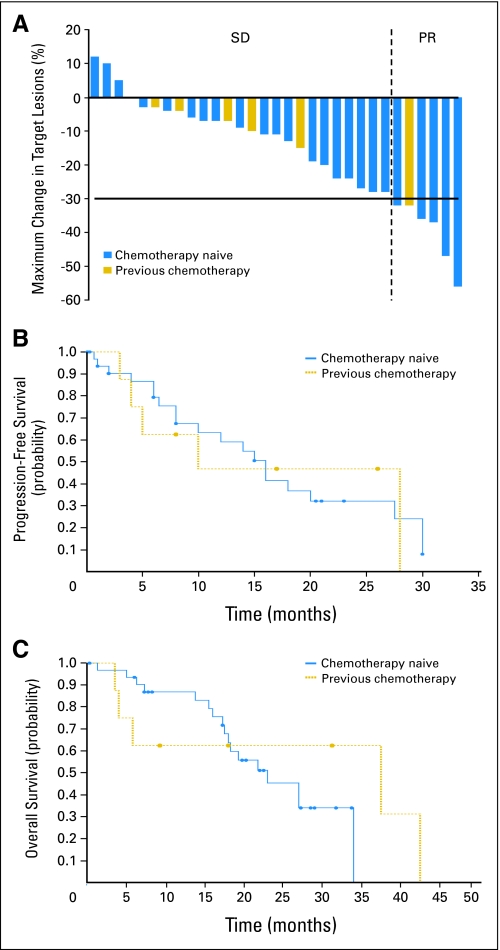
Objective response, Kaplan-Meier Analysis of progression-free survival (PFS), and overall survival data are described inTable 3 and Figures 2A to 2C. Although PRs in three patients in the first stage of patients in arm A had been noted, nine additional patients to arm A were not enrolled onto the second stage of trial as there were already 14 chemotherapy-naïve PTC patients on arm B. Response data in PTC patients are analyzed in two groups: chemotherapy-naïve PTC patients on arm A and B; PTC patients with prior chemotherapy on arm B. Of note, among four response-assessable patients with follicular variant of PTC, two patients had PRs of 23 and 26 months duration, and two patients had SD of 10 or 21 months duration. In general, patients with SD (> 6 months duration) also had improvement in nontarget lesions, serum Tg, and disease-related symptoms if present at study entry. In several cases, CT scans of patients with SD also revealed development of a hypoattenuated area in the index lesions suggesting necrosis that might be associated with a response to sorafenib (Appendix Fig A1, online only).
Table 3.
Tumor Response According to RECIST
| Parameter | PTC Arm A and Chemotherapy- Naïve PTC Arm B Patients |
Arm B |
||||||
|---|---|---|---|---|---|---|---|---|
| PTC (patients with prior chemotherapy) |
HTC or FTC |
ATC |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Total patients | 33 | 8 | 11 | 4 | ||||
| Assessable patients | 28 | 85 | 8 | 100 | 10 | 91 | 4 | |
| Best response by RECIST | ||||||||
| Complete response | 0 | 0 | 0 | 0 | ||||
| PR* | 5 | 15 | 1 | 13 | 0 | 0 | ||
| SD | 19 | 57 | 6 | 75 | 9 | 82 | 1 | 25 |
| Progressive disease | 4 | 12 | 1 | 12 | 1 | 9 | 3 | 75 |
| Durable SD, ≥ 6 months | 19 | 57 | 4 | 50 | 6 | 54 | 1 | 25 |
| Objective response, % | 15 | 13 | — | — | — | — | ||
| 95% CI | 5 to 32 | 0.3 to 53 | ||||||
| Median time to PR, months | 12 | 20 | — | — | — | — | ||
| Range | 2 to 12 | |||||||
| Median duration of PR† | 9 | 6 | — | — | — | — | ||
| Range | 6 to 14 | |||||||
| PFS | ||||||||
| Kaplan-Meier estimate of median PFS, months | 16 | 10 | 4.5 | |||||
| 95% CI | 8 to 27.5 | 4 to 28 | 2 to 16 | |||||
| Kaplan-Meier estimate of 1-year PFS rate, % | 59 | 47 | 30 | |||||
| 95% CI | 40 to 78 | 10 to 83 | 5 to 55 | |||||
| OS | ||||||||
| Median Kaplan-Meier median estimate, months | 23 | 37.5 | 24. 2 | |||||
| 95% CI | 18 to 34 | 4 to 42.5 | 11 to 37.5 | |||||
| Kaplan-Meier 1-year estimate, % | 87 | 63 | 64 | |||||
| 95% CI | 75 to 99 | 29 to 96 | 38 to 90 | |||||
Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors; PTC, papillary thyroid cancer; HTC, Hürthle cell carcinoma; FTC, follicular thyroid cancer; ATC, anaplastic thyroid cancer; PR, partial response; SD, stable disease; PFS, progression-free survival; SD, stable disease.
Partial response was confirmed at 2 months in four patients and at 3 months in two patients.
One patient has partial response of at least 14 months duration as evaluated at the last response assessment and is still on therapy; 14 months duration of PR has been taken as duration of response in this case.
Fig 2.
(A) Maximum percentage of tumor reduction for target lesions for by Response Evaluation Criteria in Solid Tumors in all papillary thyroid cancer (PTC) patients who had stable disease (SD) or partial response (PR). With intent to treat analysis, six PRs (15%), 25 SDs (61%), and five PDs (12%) were noted in total of 41 patients with PTCs (33 chemotherapy naïve, eight with prior chemotherapy). Each bar represents an individual patient. Chemotherapy naïve (blue) or prior chemotherapy (gold) status is noted by different color of the bar. (B) Kaplan-Meier analysis of progression-free survival (PFS) among all PTC patients (n = 41) who received at least one dose of sorafenib. For PTC chemotherapy-naïve patients (n = 33), median PFS is 16 months with 95% CI of 8 to 27.5. For PTC patients with prior chemotherapy (n = 8), median PFS is 10 months with 95% CI of 4 to 28. Using log-rank test to compare the curves for PFS, no statistically significant difference (P = .8627) was found in PFS between PTC groups. (C) Kaplan-Meier analysis of overall survival (OS). OS among all PTC patients (n = 41) who received at least one dose of sorafenib. For PTC chemotherapy-naïve patients (n = 33), median OS is 23 months with 95% CI of 18 to 34). For PTC patients with prior chemotherapy, median OS is 37.5 months with 95% CI of 4 to 42.5. Using log-rank test to compare the curves for OS, no statistically significant difference (P = .4787) was found in OS between PTC groups.
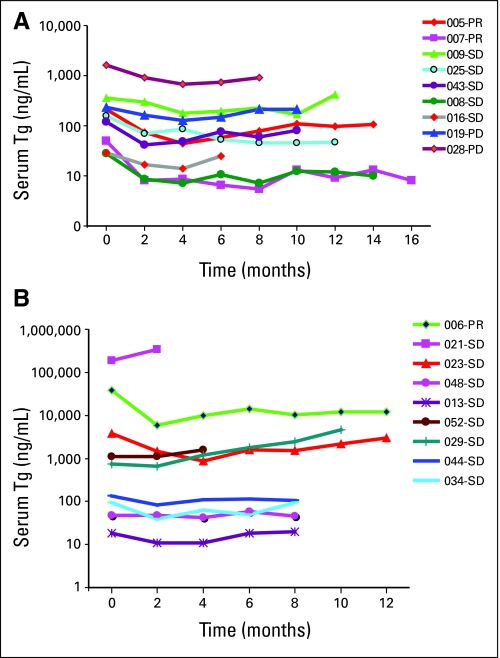
Tumor Marker Response
Serum Tg response in DTC patients is outlined inTable 4 and individual responses over time are shown in PTC patients in Appendix Figures A2A to 2B (online only). Tg responders are classified as ≥ 25% reduction in serum Tg compared to baseline Tg when noted on two consecutive tests obtained 8 weeks apart. Although dramatic sustained decreases in serum Tg levels were observed in some patients with PRs and SDs, neither baseline Tg nor Tg response consistently correlated with degree or duration of objective response.
Table 4.
Tg Best Response
| Parameter | PTC Arm A and Chemotherapy Naïve PTC Arm B |
Arm B |
||||
|---|---|---|---|---|---|---|
| PTC Patients With Prior Chemotherapy |
HTC or FTC Patients |
|||||
| No. | % | No. | % | No. | % | |
| Interpretable Tg at baseline | 19 | 4 | 10 | |||
| Not assessable | 5* | 26 | 0 | 0 | ||
| Assessable for Tg response | 14 | 74 | 4 | 100 | 10 | 100 |
| Tg responders | 12 | 64 | 2 | 50 | 4 | 40 |
| ≥ 75% decrease | 3 | 16 | 1 | 25 | 0 | |
| ≥ 50-74% decrease | 6 | 32 | 1 | 25 | 1 | 10 |
| ≥ 25-50% decrease | 3 | 16 | 0 | 0 | 3 | 30 |
| Tg nonresponders | 2 | 10 | 2 | 50 | 6 | 60 |
| > 0-25% decrease | 1 | 5 | 1 | 25 | 2 | 20 |
| > 0% increase | 1 | 5 | 1 | 25 | 4 | 40 |
Abbreviations: Tg, serum thyroglobulin; PTC, papillary thyroid cancer; HTC, Hürthle cell carcinoma; FTC, follicular thyroid cancer.
NOTE. Best response was observed either at 2 or 4 months on therapy.
One of these five patients had serial Tg uninterpretable due to nonsuppressed thyroid-stimulating hormone during follow-up, while four patients were off study prior to 8 weeks.
AEs
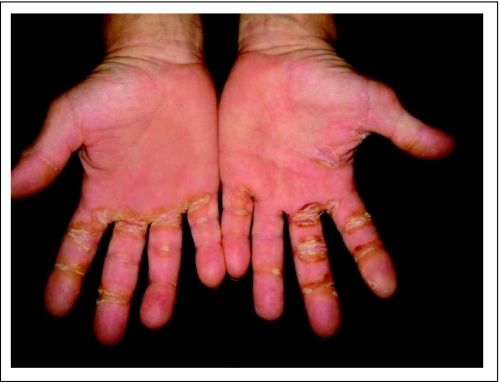
Sorafenib was generally well tolerated. However, a dose reduction was necessary to improve tolerance in 52% of patients. Grade 1 to 3 AEs are described in Tables 5 and 6. The most common (≥ 5% frequency) grade 3 AEs included hand or foot pain (12%), arthralgia (11%), fatigue (16%), HFSR (7%; Appendix Fig A3, online only), musculoskeletal chest pain (7%), and asymptomatic hyponatremia (5%). Grade 4 AEs were rare and included pericardial effusion (2%) and reversible neutropenia (4%). The grade 5 event of sudden death (n = 1) was unlikely attributed to sorafenib in a 68 year-old man with follicular variant of PTC who had metastasis to the lungs and paratracheal lymph nodes who died at 21 months on the study. The patient achieved PR on the study and had required dose reduction to 400 mg per day of sorafenib because of grade 3 HFSR. The patient who developed acute myeloid leukemia had received 523 mCi 131I and radiation to his neck mass. Aspergillus pneumonia occurred in a patient who had received multiple chemotherapies and was on 30 mg of oral prednisone daily for 3 years.
Table 6.
Laboratory AEs (possible, probable, or definite attribution to the drug)
| AEs | Grade 1 and 2 AEs |
Grade 3 AEs |
||||||
|---|---|---|---|---|---|---|---|---|
| Arm A (n = 19) |
Arm B (n = 37) |
Arm A (n = 19) |
Arm B (n = 37) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic | ||||||||
| Neutropenia | 2 | 11 | 2 | 5 | — | — | — | — |
| Anemia | 7 | 37 | 14 | 39 | — | — | — | — |
| Lymphopenia | 1 | 5 | 4 | 11 | — | — | — | — |
| Thrombocytopenia | 1 | 5 | 1 | 3 | — | — | — | — |
| Leucopenia | 7 | 37 | 13 | 35 | 1 | 5 | 1 | 3 |
| Liver enzyme elevation | ||||||||
| Alkaline phosphatase | 3 | 16 | 4 | 11 | — | — | — | — |
| ALT | 8 | 42 | 14 | 39 | — | — | — | — |
| AST | 9 | 47 | 17 | 46 | — | — | — | — |
| Bilirubin | 1 | 5 | 2 | 6 | — | — | — | — |
| LDH | 8 | 42 | 17 | 47 | — | — | — | — |
| Serum chemistry | ||||||||
| Hypocalcemia | 8 | 42 | 22 | 59 | 0 | 2 | 5 | |
| Hyponatremia | 11 | 58 | 22 | 59 | 3 | 16 | 0 | |
| Hypokalemia | 3 | 16 | 3 | 8 | — | — | — | — |
| Low albumin | 0 | 2 | 6 | — | — | — | — | |
| Elevated creatinine | 1 | 5 | 2 | 6 | — | — | — | — |
| Hyperglycemia | 1 | 5 | 3 | 8 | — | — | — | — |
Abbreviations: AE, adverse event; LDH, lactate dehydrogenase.
Table 5.
Clinical AEs (possible, probable, or definite attribution to the drug)
| AEs | Grade 1 and 2 AEs |
Grade 3 AEs |
||||||
|---|---|---|---|---|---|---|---|---|
| Arm A |
Arm B |
Arm A |
Arm B |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 19 | 37 | 19 | 37 | ||||
| Constitutional | ||||||||
| Fatigue | 14 | 74 | 23 | 62 | 2 | 11 | 7 | 19 |
| Weight loss | 11 | 58 | 32 | 89 | 1 | 5 | 2 | 5 |
| Anorexia | 11 | 53 | 21 | 57 | — | — | — | — |
| Taste changes | 4 | 21 | 8 | 22 | — | — | — | — |
| GI | ||||||||
| Ileus | — | — | — | — | — | — | 1 | 3 |
| Colon perforation | — | — | — | — | 1 | 5 | — | — |
| Diarrhea | 15 | 79 | 25 | 68 | 1 | 5 | 1 | 3 |
| Stomatitis | 2 | 11 | 6 | 17 | 1 | 5 | — | — |
| Pain tongue or tooth | 2 | 11 | 5 | 14 | 1 | 5 | — | — |
| Pain abdomen or rectal | 17 | 89 | 18 | 49 | 1 | 5 | 2 | 6 |
| Nausea | 10 | 53 | 21 | 58 | — | — | — | — |
| Vomiting | 7 | 37 | 3 | 8 | — | — | — | — |
| Heartburn | 7 | 37 | 15 | 42 | — | — | — | — |
| Flatulence | 15 | 79 | 24 | 65 | — | — | — | — |
| Dry mouth | 1 | 5 | 2 | 6 | — | — | — | — |
| Musculoskeletal | ||||||||
| Proximal myopathy | 1 | 5 | — | — | ||||
| Hand-foot skin reaction | 11 | 58 | 20 | 56 | 2 | 11 | 2 | 5 |
| Back pain | — | — | 4 | 11 | 1 | 5 | 1 | 3 |
| Chest pain | 1 | 5 | 3 | 8 | — | — | 4 | 11 |
| Scalp pain | 5 | 26 | 8 | 22 | — | — | 1 | 3 |
| Pain (general) | 2 | 11 | 5 | 14 | 1 | 5 | 1 | 3 |
| Hand or foot pain | 14 | 74 | 12 | 33 | 1 | 5 | 6 | 16 |
| Arthralgia | 13 | 68 | 21 | 58 | 1 | 5 | 5 | 14 |
| Myalgia | 2 | 11 | 4 | 11 | — | — | — | — |
| Muscle cramps | 10 | 53 | 10 | 28 | — | — | — | — |
| Dermatologic | ||||||||
| Skin rash | 14 | 74 | 28 | 76 | 1 | 5 | 1 | 3 |
| Flushing | 6 | 32 | 12 | 32 | — | — | — | — |
| Brown skin spots | 3 | 16 | 6 | 16 | — | — | — | — |
| Dry skin | 16 | 84 | 31 | 84 | — | — | — | — |
| Pruritis | 15 | 79 | 28 | 75 | — | — | — | — |
| Nail changes | 13 | 68 | 20 | 54 | — | — | — | — |
| Skin sores | 4 | 21 | 2 | 5 | — | — | — | — |
| Alopecia | 15 | 79 | 29 | 78 | — | — | — | — |
| Vascular | ||||||||
| Hypertension | 8 | 42 | 14 | 38 | 1 | 5 | 1 | 3 |
| Hemoptysis | 1 | 5 | 1 | 3 | 0 | 2 | 6 | |
| Epistaxis | 1 | 5 | 1 | 3 | — | — | — | — |
| Retinal hem/vein occlusion | 1 | 5 | 1 | 3 | — | — | — | — |
| Gum bleeding | 0 | 1 | 3 | — | — | — | — | |
| Tumor bleeding | 0 | 1 | 3 | — | — | — | — | |
| Wound healing (slow) | 0 | 1 | 3 | — | — | — | — | |
| Cardiac | ||||||||
| Left ventricular dysfunction | — | — | — | — | 0 | 1 | 3 | |
| Atrial fib or SVT | 0 | 2 | 6 | 0 | 1 | 3 | ||
| Sinus bradycardia | 0 | 1 | 3 | — | — | — | — | |
| Palpitation | 1 | 5 | 2 | 6 | — | — | — | — |
| Neurological | ||||||||
| Syncope | — | — | — | — | 0 | 1 | 3 | |
| Anxiety | 0 | 1 | 3 | — | — | — | — | |
| Dizziness | 2 | 11 | 5 | 14 | — | — | — | — |
| Headache | 3 | 16 | 6 | 17 | — | — | — | — |
| Neuropathy (sensory) | 4 | 21 | 8 | 22 | — | — | — | — |
| Respiratory | ||||||||
| Cough | 0 | 1 | 3 | — | — | — | — | |
| Dyspnea | 3 | 16 | 5 | 14 | — | — | — | — |
| Hoarseness | 2 | 11 | 2 | 6 | — | — | — | — |
| Endocrine changes | ||||||||
| Irregular menses | 0 | 2 | 6 | — | — | — | — | |
| Infection | ||||||||
| Infection | 0 | 2 | 6 | — | — | 1 | 3 | |
| Abscess | 2 | 11 | 0 | — | — | — | — | |
| Osteomyelitis-actinomycosis | — | — | — | — | 0 | 1 | 3 | |
| Other tumors | ||||||||
| Acute myeloid leukemia | — | — | — | — | 1 | 5 | 0 | |
| Keratocanthoma | — | — | — | — | 0 | 2 | 5 | |
NOTE. Worst grade experienced by patient on study is counted in the above table. Please see text for grade 4-5 AEs.
Abbreviations: AE, adverse event; SVT, supraventricular tachycardia.
Tumor Genotype
Overall, 17 of 22 PTC patients with DNA of sufficient quality for analysis had activating mutations in exon 15 of BRAF Table 1). Fourteen (64%) of 22 had a V600E mutation while three (14%) had a K601E mutation.22 Eight of nine PTC patients with interpretable results from more than one histological sample had concordant BRAF mutation status. In one case, a V600E mutation was detected in one of two tissue samples. None of the six examined non-PTC tissues had a BRAF mutation. RET/PTC 1 and 3 rearrangements were not identified in the 20 PTC cases from which adequate RNA could be isolated. Activating N2-RAS and H1-RAS mutations were not identified in the samples from six patients with non-PTC histologies or in BRAF mutation–negative PTCs (n = 4). Because of the high frequency of BRAF mutations in the study population, statistical comparison of results on the basis of BRAF mutation status was not possible.
Pharmacodynamic Studies
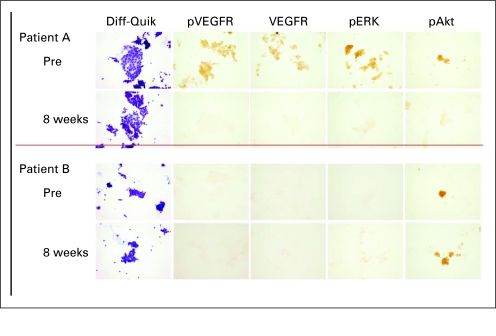
To determine if sorafenib inhibited signaling in tumor tissues, FNA samples obtained before and 8 weeks after initiation of therapy on arm A patients were analyzed for levels of immunoactive pVEGFR; VEGF expression, and ERK phosphorylation (pERK) and AKT phosphorylation (pAKT) by IHC. Paired samples from 10 of these patients were able to be analyzed. Based on the grade of signaling response, four of 10 had a major reduction in levels of immunoactive pVEGFR, pERK, and VEGF (reduction of ≥ 2 scoring levels) with therapy while pAKT immunoactivity was reduced in two of these four cases. In all patients in which high (grade 2 or 3) pVEGFR and pERK levels were detected basally, inhibition was noted on therapy. pAKT was variably detected and inhibited. Several cases with a BRAF mutation did not demonstrate basal pERK or pVEGFR immunoactivity, thereby demonstrating heterogeneity in the degree of pathway activation even in tumors with BRAF-activating mutations (Appendix Fig A4, online only). Inhibition of VEGFR would be predicted to increase VEGF expression. However, sorafenib also inhibits targets that have been shown to upregulate VEGF expression in cell systems, which may in part account for the reduced immunoactive VEGF on therapy.23,24
Tumor Perfusion Response
In 10 of 14 assessable PTC patients, the 8- or 16-week on therapy DCE-MRI scans revealed a median decrease of 46% (range, 27% to 92%) in exchange rate (Kep[min−1]; Kep, exchange rate constant) in the index lesions compared to baseline while no change was noted in Kep in the remaining four patients. Of note, no objective response occurred in any of the four patients who did not reveal change in Kep. Furthermore, a median decrease of 34% (range, 9% to 67%) in amplitude (Amp [arbitrary units]) was noted among 13 assessable PTC patients while one patient had a 16% increase in Amp. Median duration of the decrease in Kep was 4 months (range, 2 to 8). Neither baseline Kep values nor decrease in Kep was correlated with objective tumor response. Among eight patients who had assessable results for paired biopsies and serial DCE-MRIs, there was no correlation between pVEGFR levels or signaling response of pVEGFR and pharmacokinetic parameters of DCE-MRIs. Of note, index lesions for biopsies and DCE-MRIs were not the same in a majority of patients.
PET Imaging Response
In 14 patients with PTC in arm A with assessable PET scans, the median number of time points for PET imaging obtained per patient was 3 (range, 2 to 5) and the median number of index lesions per patient at baseline was 8 (range, 1 to 20). No clear correlation was noted between PET response (% changes in standardized uptake value maximum [SUVmax] and metabolic volume compared with pretherapy) and objective tumor response. No consistent pattern of changes in SUVmax and metabolic volume existed among several index lesions in a given patient.
DISCUSSION
The RAS-RAF signaling pathway, VEGF-R, and PDGF-R play a critical role in the pathogenesis of advanced-stage PTC. To our knowledge, this is one of the first phase II clinical trials conducted using an oral small molecule multikinase inhibitor in patients with iodine-refractory metastatic PTC. In our trial, we decided a priori that sorafenib was worthy of further study if the true response probability or target response rate was 30% or higher. Our results did not meet this level of response with only five (15%) of 33 patients demonstrating PR. However, this finding may underestimate sorafenib's antitumor activity. Clinical benefit is noted based on PRs and SD ≥ 6 months in 23 of PTC patients (56%), and is inferred from the decreases in serum Tg levels. The lack of correlation between the serum Tg response and the objective response is possibly due to small sample size as well as the definition of objective response based on anatomic imaging. Limitations of RECIST are well recognized for assessing response to novel antiangiogenic and kinase inhibitors.25–27
The dose and schedule of sorafenib used in our study is generally well tolerated, although dose reductions were necessary in 52% of patients, suggesting variability of pharmacokinetics or pharmacogenomics among our patients. The higher frequency of dose reductions in our study might be a result of relatively lower acceptability of chronic toxicities by thyroid cancer patients as well as a result of specific criteria required for dose reduction in our study. Of note, tumor response was generally maintained despite dose reductions. Highly important is the early recognition of potentially serious toxicities, such as ruptured bowel, tumor bleeding, keratoacanthoma, or uncontrolled hypertension. Toxicities observed in our study are similar to other phase II monotherapy studies of sorafenib.27–29 However, an increased thyroid hormone requirement was not observed in our study.28 Keratoacanthoma possibly related to sorafenib is uncommon and the mechanism remains unclear.30 In general, grade 1 and 2 AEs are considered acceptable AEs in the cancer community and dose reductions are not typically considered until grade 3 or 4 AEs. However, CTCAE criteria may not be applicable to oral targeted therapies using continuous dosing such as sorafenib, as patients may not tolerate chronic grade 2 AEs and therefore modification of CTCAE may be necessary.31
The results of studies performed on serial tumor biopsies are consistent with the inhibitory action of sorafenib on RAS-RAF kinase signaling. Furthermore, decreases in pVEGFR levels in tumor biopsies and Kep on DCE-MRIs in response to sorafenib verifies its potential as an antiangiogenic therapy. The lack of correlation between imaging and tumor tissue may represent heterogeneity of tumor signaling, the heterogeneity of tumor response in metastases at different locations in a given patient, or the multiple targets of this drug. Because of the small number of BRAF mutation–negative PTC patients, this study does not clarify if a BRAF mutation predicts response to sorafenib. However, the spectrum of responses from PR to PD in BRAF mutation–positive patients, and the correlative FNA results in which the basal degree of pathway activation varied, suggest that there may be additional factors besides BRAF that determine the response to sorafenib.
Another phase II study of sorafenib using similar dose/schedule in 30 patients with advanced thyroid cancer reported higher PR (23%) and median progression-free survival (21 months) compared with our study.28 Such discrepancy may be due to differences in patient characteristics (tumor burden, BRAF mutation status), dose intensity, and/or frequency of response evaluations. Several kinase inhibitors such as axitinib and motesanib that have activity against the VEGF-Rs and PDGF-Rs in common also have significant antitumor activity.32,33 Indeed, the new class of oral kinase inhibitors targeting the VEGF-R pathway may offer an improved option of therapy over traditional strategies for patients with progressive radioiodine-refractory PTC.
In summary, sorafenib is generally well tolerated and displays clinical activity against metastatic PTC, but close monitoring and aggressive toxicity management are essential. To optimize the efficacy of sorafenib, future studies will need to focus on the mechanisms of efficacy and resistance, and the testing of combination therapies in patients based on preclinical data.
Acknowledgment
We thank Huiling He, MD, and Zhongyuan Li, MPharm, for their assistance with SSCP studies.
Appendix
After dissection of archival tumor on slides, DNA was extracted using QIAamp DNA mini kit (Qiagen, Valencia, CA) according to the manufacturer's instruction. Sequences of H1- and N2-RAS oncogene regions were amplified using previously described conditions and primers (Vasko V, Ferrand M, Di Cristofaro J, et al: J Clin Endocrinol Metab 88:2745-2752, 2003). Exon 15 of BRAF was amplified in a nested polymerase chain reaction (PCR) protocol with the first amplification using a protocol and primers as previously described (Nikiforova MN, Kimura E, Gandhi M, et al: J Clin Endocrinol Metab 88:5399-5404, 2003) and second amplification using inner primers (forward 5′-TGC TTG CTC TGA TAG GAA AAT G-3′, and reverse 5′-CCA CAA AAT GGA TCC AGA CA-3′) using the following reaction conditions (94°C 10 minutes, 38 cycle of 94°C 0.5 minutes, 58°C 1 minute, and 72°C 1 minute). PCR products were electrophoresed, purified using a QIAEX II Gel Extraction Kit (Qiagen), and sequenced using an Applied Biosystem 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). In cases where sequencing results were uncertain or inconsistent between samples from an individual patient, DNA was reamplified using the above protocol and single stranded conformation polymorphism analysis was performed to detect mutations as previously described (Salvatore G, Giannini R, Faviana P, et al: J Clin Endocrinol Metab 89:5175-5180, 2004). To detect RET/PTC1 or 3 rearrangements, total RNA was extracted from paraffin samples using Pinpoint Slide RNA Isolation (ZYMO Research, Orange, CA). Reverse- transcriptase PCR of the RNA was performed for RET/PTC 1 and 3 as previously described (Di Cristofaro J, Vasko V, Savchenko V, et al: Endocr Relat Cancer 12:173-183, 2005).
In addition to conventional smears stained with Romanowsky or Papanicolaou stain, fine needle aspiration material was rinsed in CytoRich Red cytologic preservative using the Autocyte Prep (B-D Diagnostics, Burlington, NC) protocol. Cell-block preparations made from rinsed cells using a thrombin clot method were then formalin-fixed and paraffin-embedded using standard histologic protocol. Plus glass slides (Fisher Scientific, Pittsburgh, PA) were used to facilitate cell adhesion. One hematoxylin and eosin stained slide was examined to document presence of tumor, and at least eight unstained cell-block slides were submitted for molecular testing.
Slides were prepared from cell blocks as previously described (Vasko V, Saji M, Hardy E, et al: J Med Genet 41:161-170, 2004) and incubated overnight at 4°C with antibodies against phospho-Akt (Ser473; #3787), phospho-p44/42 MAPK (#9101) from Cell Signaling Technology (Beverly, MA), phospho-VEGF receptors 2/3 (#pc460) from Calbiochem (La Jolla, CA), or with anti-VEGF antibodies from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Immunostaining was performed with Vectastain Universal Quik kit (Vector Laboratories, Burlingame, CA) as previously reported (Di Cristofaro J, Vasko V, Savchenko V, et al: Endocr Relat Cancer 12:173-183, 2005). Peroxidase activity was revealed in 3, 3 diaminobenzidine and negative controls were prepared by omission of primary antibodies. All experiments were performed simultaneously for each antibody to eliminate inter-assay variance.
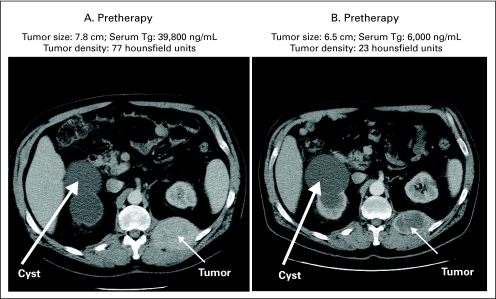
Fig A1.
Illustration of development of hypoattenuation in tumor lesion on computed tomography scan in response to sorafenib in a patient with papillary thyroid cancer. Tg, thyroglobulin.
Fig A2.
(A) Serum thyroglobulin (Tg) levels with therapy in patients with papillary thyroid cancer (PTC) on arm A (n = 9). (B) Serum thyroglobulin levels with therapy in patients with PTC on arm B (n = 9). Patients 023, 048, 013, 029, 044 were cytotoxic chemotherapy-naïve PTC patients while 006, 021, 052, 034 had prior cytotoxic chemotherapy. 005-PR, patient No. 005 who has partial response (PR) by Response Evaluation Criteria in Solid Tumors (RECIST); 006-PR, patient No. 006 in arm B who has partial response (PR) by RECIST; SD, stable disease; PD, progressive disease.
Fig A3.
Typical severe hand-foot syndrome affecting palms with erythema, skin peeling, and cracking attributed to sorafenib.
Fig A4.
Fine needle aspiration samples were obtained from two patients (cases A and B) before initiation of sorafenib (0) and 8 weeks after treatment was initiated (8 weeks). Both of these patients had a BRAF V600E mutation. In case A, high levels of immunoactive phosphorylated vascular endothelial growth factor receptor (pVEGFR), VEGF, and phosphorylated ERK (pERK) and phosphorylated AKT (pAKT) were detected before treatment and the levels were reduced on therapy. Diff-Quik (Dade Behring, Newark, DE) staining confirms the presence of thyroid cancer cells. Case B demonstrated no evidence of immunactive pVEGFR, VEGF, or pERK before therapy. pAKT was detected but was not changed during treatment with sorafenib.
Footnotes
Supported by Grants No. R21 CA112903, U01 CA76576, and NO1-CM57018 from the National Cancer Institute, Bethesda, MD.
Presented in part at the 13th International Thyroid Congress, Buenos Aires, Argentina, October 30-November 4, 2005; and at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, June 2-6, 2006.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard T. Kloos, AstraZeneca (C); Matthew D. Ringel, AstraZeneca (C), Amgen (C), Exelixis (U); Manisha H. Shah, Exelixis (C) Stock Ownership: None Honoraria: Manisha H. Shah, Bayer-Onyx Pharmaceuticals Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Richard T. Kloos, Matthew D. Ringel, Michael V. Knopp, John J. Wright, Manisha H. Shah
Financial support: John J. Wright, Michael Grever, Manisha H. Shah
Administrative support: Michael Grever, Manisha H. Shah
Provision of study materials or patients: Richard T. Kloos, Matthew D. Ringel, Manisha H. Shah
Collection and assembly of data: Richard T. Kloos, Matthew D. Ringel, Michael V. Knopp, Nathan C. Hall, Mark King, Robert Stevens, Jiachao Liang, Paul E. Wakely Jr, Motoyasu Saji, Jennifer Rittenberry, Daria Arbogast, Minden Collamore, Manisha H. Shah
Data analysis and interpretation: Richard T. Kloos, Matthew D. Ringel, Michael V. Knopp, Nathan C. Hall, Mark King, Robert Stevens, Jiachao Liang, Paul E. Wakely Jr, Vasyl V. Vasko, Motoyasu Saji, Lai Wei, Michael Grever, Manisha H. Shah
Manuscript writing: Richard T. Kloos, Matthew D. Ringel, Jiachao Liang, John J. Wright, Manisha H. Shah
Final approval of manuscript: Richard T. Kloos, Matthew D. Ringel, Michael V. Knopp, Mark King, Robert Stevens, Manisha H. Shah
REFERENCES
- 1.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22:6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 3.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 4.Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 5.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 6.Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxford) 2004;61:239–243. doi: 10.1111/j.1365-2265.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 7.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: Evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 8.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang B, Knauf JA, Smith EP, et al. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006;12:1785–1793. doi: 10.1158/1078-0432.CCR-05-1729. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore G, De Falco V, Salerno P, et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006;12:1623–1629. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 11.Park JI, Strock CJ, Ball DW, et al. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol Cell Biol. 2003;23:543–554. doi: 10.1128/MCB.23.2.543-554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito J, Kohn AD, Roth RA, et al. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid. 2001;11:339–351. doi: 10.1089/10507250152039073. [DOI] [PubMed] [Google Scholar]
- 13.Turner HE, Harris AL, Melmed S, et al. Angiogenesis in endocrine tumors. Endocr Rev. 2003;24:600–632. doi: 10.1210/er.2002-0008. [DOI] [PubMed] [Google Scholar]
- 14.Tuttle RM, Fleisher M, Francis GL, et al. Serum vascular endothelial growth factor levels are elevated in metastatic differentiated thyroid cancer but not increased by short-term TSH stimulation. J Clin Endocrinol Metab. 2002;87:1737–1742. doi: 10.1210/jcem.87.4.8388. [DOI] [PubMed] [Google Scholar]
- 15.Lin JD, Chao TC. Vascular endothelial growth factor in thyroid cancers. Cancer Biother Radiopharm. 2005;20:648–661. doi: 10.1089/cbr.2005.20.648. [DOI] [PubMed] [Google Scholar]
- 16.Vieira JM, Santos SC, Espadinha C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors in thyroid carcinomas of follicular origin: A potential autocrine loop. Eur J Endocrinol. 2005;153:701–709. doi: 10.1530/eje.1.02009. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 18.Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Knopp MV, von Tengg-Kobligk H, Choyke PL. Functional magnetic resonance imaging in oncology for diagnosis and therapy monitoring. Mol Cancer Ther. 2003;2:419–426. [PubMed] [Google Scholar]
- 21.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Trovisco V, Vieira de Castro I, Soares P, et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 23.Milanini J, Vinals F, Pouyssegur J, et al. P42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem. 1998;273:18165–18172. doi: 10.1074/jbc.273.29.18165. [DOI] [PubMed] [Google Scholar]
- 24.Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000;19:139–145. doi: 10.1023/a:1026506011458. [DOI] [PubMed] [Google Scholar]
- 25.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J Natl Cancer Inst. 2000;92:179–181. doi: 10.1093/jnci/92.3.179. [DOI] [PubMed] [Google Scholar]
- 26.Tuma RS. Sometimes size doesn't matter: Reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98:1272–1274. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 27.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 28.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 30.Kong HH, Cowen EW, Azad NS, et al. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–172. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgerly M, Fojo T. Is there room for improvement in adverse event reporting in the era of targeted therapies? J Natl Cancer Inst. 2008;100:240–242. doi: 10.1093/jnci/djm324. [DOI] [PubMed] [Google Scholar]
- 32.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 33.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]