Abstract
Allenes can be synthesized via the direct SN2′ addition of hydride to propargylic alcohols. Previous examples of this approach, however, have involved harsh reaction conditions and have suffered from incomplete transfer of central chirality to axial chirality. Here we show that Cp2Zr(H)Cl can react with the zinc or magnesium alkoxides of propargylic alcohols to generate allenes in good yield and in high optical purity. Dialkyl-, alkyl, aryl- and diaryl-allenes are accessible by this method. Furthermore, the reaction can provide silyl-substituted allenes, tri-substituted allenes and terminal allenes.
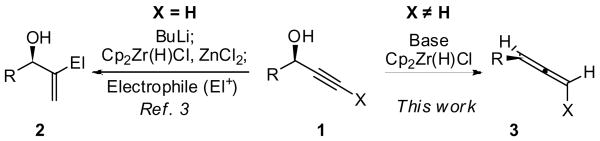
Hydrozirconation of alkynes with the Schwartz reagent [Cp2Zr(H)Cl] provides vinyl zirconium species in a stereospecific and regioselective fashion. These intermediates can react with a variety of electrophiles or participate in cross-coupling reactions.1 The least hindered vinyl zirconium product usually dominates under either kinetically or thermodynamically controlled conditions.2 However, we recently discovered that lithium alkoxides can alter the regioselectivity of these reactions. In particular, hydrozirconation of terminal propargylic alcohols (1, X = H) occurs with complete selectivity for the branched products in the presence of BuLi and ZnCl2 (Scheme 1, 1 → 2).3,4 In contrast, when internal propargylic alcohols were treated under similar reaction conditions, substantial quantities of disubstituted allenes were isolated in addition to branched allylic alcohols (Scheme 1, 1 → 3). Here we demonstrate that this direct synthesis of allenes is high-yielding, general and stereospecific.
Scheme 1.
Allenes can be prepared via SN2′ addition to propargylic alcohols or their derivatives.5 Conceptually, hydride addition is one of the most attractive routes to disubstituted allenes. Indeed, traditional hydride reagents have been used,6 and transition metal-catalyzed methods have been developed as well.7 Unfortunately, SN2 addition often competes with SN2′ addition. Furthermore, when scalemic propargylic alcohols are used as substrates, substantial deterioration of optical purity accompanies reduction.6 An alternative approach, developed by Myers and co-workers,8 involves a Mitsunobu reaction between a propargylic alcohol and a sulfonyl hydrazine. The allene is generated through a sigmatropic elimination of N2. While stereospecific and high-yielding in many cases, this protocol requires an unstable hydrazine and all the accoutrements of a Mitsunobu reaction; additionally, it has not proved generally effective for allylic, benzylic or tertiary propargylic alcohols.9
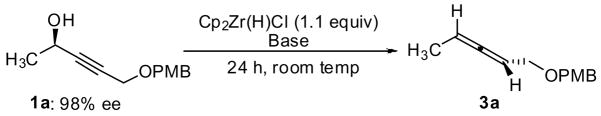
To address some limitations of existing methodology we optimized the reaction of internal propargylic alcohols with Cp2Zr(H)Cl. Initial experiments utilized alcohol 1a (98% ee) and revealed that both the yield and optical purity of the product depended strongly on the base and solvent (Table 1). For example, under conditions originally optimized for terminal propargylic alcohols,3 conversion was low (entry 1). We observed increased reactivity in hydrocarbon solvents, but inorganic salts still affected both the selectivity and efficiency of the reaction. With zinc or aluminum bases, 3a was formed in reasonable yields but in unacceptable enantiomeric excess (entries 3–4). Alternatively, the zirconium, lithium and sodium alkoxides reacted sluggishly but selectively (entries 5–7). Finally, we identified two conditions that provided the allene in good yield and excellent optical purity: deprotonation of 1 with either EtMgCl or EtZnCl (formed in situ from Et2Zn and ZnCl2) in toluene prior to hydrozirconation (entries 9, 10). Further studies revealed that the two protocols were complementary. In general, for allenes bearing two sp3-hybridized substituents, optimal results were obtained when the propargylic alcohol was deprotonated with EtMgCl. As indicated by the difference between Entries 9 and 10 in Table 1, lower ee was observed when EtZnCl was used to deprotonate these substrates.
Table 1.
Effect of additives on the reduction of propargylic alcohols with Cp2Zr(H)Cl.
 | |||||
|---|---|---|---|---|---|
| Entry | Base | Equiv | Solvent | Yield (%)a | Ee (%) |
| 1 | MeLi + ZnCl2 | 1/6 | THF | <5 | -- |
| 2 | Me2Zn | 1 | THF | 15 | -- |
| 3 | Me2Zn | 1 | Benzene | 58 | 64 |
| 4 | Me2AlCl | 1 | Benzene | ~50 | 78 |
| 5b | None | -- | Benzene | 40 | 93 |
| 6 | MeLi | 1 | Benzene | 15 | 95 |
| 7 | NaH | 1 | Benzene | <20 | 90 |
| 8 | EtMgCl | 1 | Benzene | 70d | 98 |
| 9 | EtMgCl | 1 | Toluene | 70d | 98 |
| 10c | Et2Zn + ZnCl2 | 0.5/0.5 | Toluene/THFe | 72d | 90 |
Yield determined by 1H NMR except as indicated.
2 equiv Cp2Zr(H)Cl.
1.6 equiv Cp2Zr(H)Cl.
Isolated yield.
30:1 Tol:THF
For allenes connected to one or two sp2-hybridized carbons, deprotonation of the propargylic alcohol with EtZnCl was preferred. The use of only one equivalent of EtZnCl was critical as racemization of the allene was observed in the presence of excess zinc salts.10 Additionally, the use of EtMgCl with aryl- or vinyl-substituted substrates led to over-reduction of the allene. For both procedures, toluene was found to offer the optimal balance of reactivity and selectivity; halogenated solvents displayed higher reactivity, but the allenes were isolated with lower ee. Finally, under optimized conditions, little or no allylic alcohol was isolated, indicating that the regioselectivity of the hydrometalation is high.
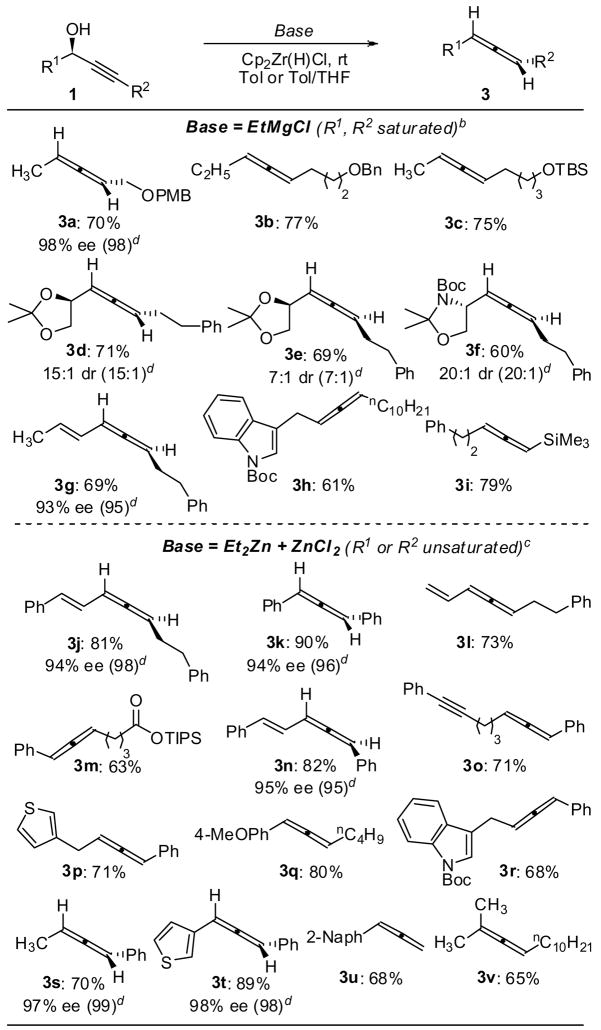
Having identified conditions to convert propargylic alcohols into enantiomerically enriched allenes, we evaluated the generality of the reduction (Figure 1). Benzyl and silyl ethers were tolerated under the reaction conditions (3a–3c), as were acetals and aminals (3d–3f). Neither carbamates (3f, 3h, 3r) nor silyl esters (3m) interfered with the reduction. Various aromatic and heteroaromatic rings remained intact. Additionally, we detected no hydrozirconation of olefins (3g, 3j, 3l) or other alkynes (3o) present in the substrates. Finally, we have used the hydrozirconation to prepare silyl-substituted allenes (3i), allenes derived from tertiary alcohols (3v) and terminal allenes (3u), although the latter showed some evidence of polymerization under the reaction conditions.
Figure 1. Synthesis of Allenes from Propargylic Alcohols.a.
aIsolated yields. Allenes are drawn such that the original alkyne substituent (R2) is on the right. See supporting information for experimental details. b1 equiv EtMgCl; 0.2 M in toluene; 1.1 equiv Cp2ZrHCl. c0.5 equiv each Et2Zn, ZnCl2; 0.2 M in toluene:THF 30:1; 1.6 equiv. Cp2ZrHCl. dValues in parentheses represent ee or dr of starting material.
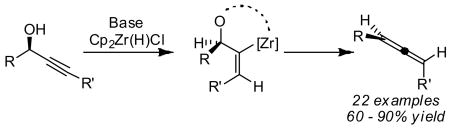
About half of our substrates were optically active,11 and in every case studied to date, the allenes were isolated with nearly the same optical purity as the starting propargylic alcohols (Figure 1). The absolute stereochemistry of the allenes was assigned based on optical rotation.12 The conversion of central chirality to axial chirality is consistent with a cis addition of Zr-H to the alkyne followed by a syn-elimination of Cp2ZrO (eq 1).13
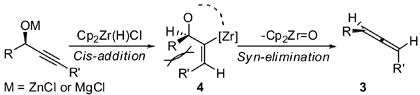 |
(1) |
The difference in reactivity between terminal and internal propargylic alcohols is noteworthy. Our previous study demonstrated that the vinyl metal species derived from terminal propargylic alcohols is sufficiently stable to be trapped with various electrophiles (Scheme 1).3 In contrast, we demonstrate here that the corresponding vinyl metal species formed from internal propargylic alcohols (4) eliminates rapidly to form allene. We interpret this difference as a manifestation of A1,3 strain as indicated in eq 1. Furthermore, substrates with weaker C-O bonds (e.g. benzylic alcohols) suffer elimination even in the context of terminal alkynes (see 3u).
In summary, we have identified two complementary sets of conditions that generate allenes in high yield and with high stereochemical purity. The method provides access to dialkyl-, aryl-alkyl- and diaryl-substituted allenes in excellent enantiomeric or diastereomeric ratios. This approach provides direct and stereospecific access to allenes from free propargylic alcohols and therefore represents an attractive alternative to non-selective reductive methods and substitution of propargylic esters.
Supplementary Material
Acknowledgments
We thank Donghui Zhang for key initial experiments. Financial support was provided by NIGMS (R01-GM074822) and the Welch Foundation.
Footnotes
Supporting Information Available: Complete experimental details and characterization data for new compounds. Time-course experiments related to racemization studies. This material is available free of charge via the internet (http://pubs.acs.org).
References
- 1.(a) Schwartz J, Labinger JA. Angew Chem Int Ed. 1976;88:402. [Google Scholar]; (b) Negishi E, Takahashi T. Synthesis. 1988:1. [Google Scholar]; (c) Wipf P, Jahn H. Tetr ahedron. 1996;52:12853. [Google Scholar]; (d) Lipshutz BH, Pfeiffer SS, Noson K, Tomioka T. In: Titanium and Zirconium in Organic Synthesis. Marek I, editor. Chap 4. Wiley-VCH; Weinheim: 2002. [Google Scholar]; (e) Wipf P, Kendall C. Topics Organomet Chem. 2005;8:1. [Google Scholar]
- 2.Hart DW, Blackburn TF, Schwartz J. J Am Chem Soc. 1975;97:679. [Google Scholar]
- 3.(a) Zhang DH, Ready JM. J Am Chem Soc. 2007;129:12088. doi: 10.1021/ja075215o. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu X, Ready JM. Tetrahedron. 2008;64:6955. doi: 10.1016/j.tet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branched vinyl zirconium species from oxidative addition to vinyl halides: Takahashi T, Kotora M, Fischer R, Nishihara Y, Nakajima K. J Am Chem Soc. 1995;117:11039.
- 5.(a) Ohno H, Nagaoka Y. In: Tomioka in Modern Allene Chemistry. Chap 4 Krause N, Hashmi SK, editors. Vol. 1. Wiley-VCH; Weinheim: 2004. [Google Scholar]; (b) Brummond KM, DeForrest JE. Synthesis. 2007:795. [Google Scholar]
- 6.Claesson A, Olsson LI. J Am Chem Soc. 1979;101:7302. [Google Scholar]
- 7.(a) Tabuchi T, Inanaga J, Yamaguchi M. Tetrahedron Lett. 1986;27:5237. [Google Scholar]; (b) Deutsch C, Lipshutz BH, Krause N. Angew Chem Int Ed. 2007;46:1650. doi: 10.1002/anie.200603739. [DOI] [PubMed] [Google Scholar]; (c) Lo VKY, Wong MK, Che CM. Org Lett. 2008;10:517. doi: 10.1021/ol702970r. [DOI] [PubMed] [Google Scholar]
- 8.Myers AG, Zheng B. J Am Chem Soc. 1996;118:4492. [Google Scholar]
- 9.(a) Clay MD, Fallis AG. Angew Chem Int Ed. 2005;44:4039. doi: 10.1002/anie.200500484. [DOI] [PubMed] [Google Scholar]; (b) Regas D, Riz JM, Afonso MM, Palenzuela JA. J Org Chem. 2006;71:9153. doi: 10.1021/jo061582r. [DOI] [PubMed] [Google Scholar]
- 10.See Supporting Information for details.
- 11.Synthesis of optically active substrates: Gao Y, Klunder JM, Hanson RM, Masamune H, Ko SY, Sharpless KB. J Am Chem Soc. 1987;109:5765.Burgess K, Jennings LD. J Am Chem Soc. 1991;113:6129.Frantz DE, Fassler R, Carreira EM. J Am Chem Soc. 2000;122:1806.Takita R, Yakura K, Ohshima T, Shibasaki M. J Am Chem Soc. 2005;127:13720. doi: 10.1021/ja053946n.
- 12.Optically active allenes 3k and 3s are known. Other simple allenes were assigned according to: Lowe G. Chem Commun. 1965:411.
- 13.Elimination of Cp2MO (M = Zr, Ti) to from allenes: Yoshida T, Negishi E. J Am Chem Soc. 1981;103:1276.Buchwald SE, Grubbs RH. J Am Chem Soc. 1983;105:5490.Tucker CE, Greve B, Klein W, Knochel P. Organometallics. 1994;13:94.Takahashi R, Hara R, Huo S, Ura Y, Lesse MP, Suzuki N. Tetrahedron Lett. 1997;38:8723.Petasis NA, Hu YH. J Org Chem. 1997;62:782.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.