Abstract

Stereodefined enol derivatives of aldehydes are prepared from terminal alkynes. Specifically, terminal alkynes are known to undergo Cp2ZrCl2-catalyzed methylalumination. Here, we show that the resultant vinylalanes can be oxygenated with peroxyzinc species to generate trisubstituted enolates. Electrophilic trapping with carboxylic anydrides or silyl triflates yields trisubstituted enol esters or silanes, respectively. The tandem carbometalation/oxygenation tolerates free and protected alcohols, heterocycles, olefins and nitriles. Likewise, amination can be accomplished using azodicarboxylates. Stereodefined enol esters can undergo asymmetric dihydroxylation to yield optically-active α-hydroxy aldehydes. Reduction with NaBH4 provides the diols of 1,1-disubstituted olefins in excellent ee. An application of this methodology to the enantioselective synthesis of the insect pheromone frontalin is presented. Finally, α-hydroxy aldehydes are shown to undergo homologation to a terminal alkyne, reductive amination, oxidation and olefination. Preliminary results indicate that tandem carbometalation/amination can be accomplished with azodicarboxylates. In this way, ene-hydrazines are formed in excellent yield.
Stereodefined enol derivatives of α-branched aldehydes (4) represent valuable building blocks for organic synthesis, but limited access to them has compromised their utility. They are most often prepared from the corresponding aldehyde, although these approaches generally afford mixtures of olefin stereoisomers.1 Furthermore, current strategies for obtaining the α-substituted aldehydes themselves are limited in reaction scope and require multiple synthetic operations.2 An alternative synthesis of trisubstituted enol derivatives might involve tandem carbometalation-oxygenation of terminal alkynes (Scheme 1). In this regard, we previously documented the carbocupration-oxygenation of terminal alkynes, in which a vinyl copper intermediate (2, M = Cu) was oxidized with tBuOOLi.3 Electrophilic trapping of the resultant E-enolate (3) generated E-enol esters and silanes. However, methyl-substituted products were not accessible by this method because methyl-cupration of alkynes is not efficient.4 Accordingly, we sought a general method for obtaining methyl-substituted enol esters and ethers (4, R′=Me). As described below, we have accomplished this objective and have begun to explore the asymmetric transformations of stereodefined enol derivatives.
Scheme 1.
Negishi’s catalytic methylalumination reaction provides a complimentary method for carbometalation.5 Methylalumination-oxygenation of monosubstituted olefins has been reported,6 but analogous chemistry of alkynes is unknown (5, Table 1). Trialkyl alanes are oxidized cleanly with molecular oxygen. Therefore, our initial investigations aimed to oxidize alkenyl aluminum intermediates with O2, but these experiments proved unsuccessful. Under these conditions, incomplete conversion and over-oxidation limited yields. Results with the oxenoid tBuOOLi were more promising.7 With this reagent, we observed 65% conversion of vinylalane 5a (R = n-C10H21) to the corresponding aldehyde (E+ = H) with 2-methyl-1-dodecene accounting for the remainder of the starting material. Further evaluation of peroxymetal oxidants revealed that peroxyzinc reagents EtZnOOtBu and MeZnOOtBu effected the oxidation of 5a to the corresponding aldehyde with 85% and 98% conversions, respectively. Thus when 5a was oxidized with freshly prepared MeZnOOtBu (1.3 equiv to AlMe3) at 0°C and subsequently trapped with Bz2O, enol benzoate 4a was obtained in 78% isolated yield (Table 1, entry 1). Zinc peroxides have been used in epoxidation reactions,8 but, to the best of our knowledge, they have not been used previously to oxidize carbanions.
Table 1.
Preparation of Enol Derivatives from Terminal Alkynesa
 | ||||
|---|---|---|---|---|
| Entry | Alkyne | EX | Product | Yield (%)b |
| 1 | 1a | Bz2Oc | R = C10H21; E = Bz | 78 |
| 2 | 1b | Bz2Oc | R = CH2Ph; E = Bz | 89 |
| 3 | 1c | Bz2Oc |

|
82 |
| 4 | 1d | Bz2Oc | R = Ph; E = Bz | 80 |
| 5 | 1e, 1f | Bz2Oc,d |

|
4e: n = 1, 59 4f: n = 2, 79 |
| 6 | 1g | Bz2Oc |

|
71 |
| 7 | 1h | Bz2Oc |

|
83 |
| 8 | 1i, 1j | Bz2Oc |

|
4i: n = 3, 75 4j: n = 4, 76 |
| 9 | 1k | Ac2O |

|
91 |
| 10 | 1l | Ac2O |

|
97 |
| 11 | 1m | Ac2O |
|
92 |
| 12 | 1n | Ac2O |

|
90 |
| 13 | 1o | TESOTf |
|
79 |
| 14 | 1p | TESOTf |
|
83 |
1.0 equiv alkyne, 1.2–4 equiv Me3Al, 5–30 mol% Cp2ZrCl2, 2.5–30 mol%, H2O or MAO, 0.3 M in CH2Cl2; MeZnOOtBu (0.3M in Toluene, 1.3–1.4 equiv to Me3Al).
Isolated yields (chromatography not necessary for entries 10–14).
Catalytic nBu3P added.
BzCl was added after benzoylation of the enolate. See supporting information for complete experimental details.
The methylalumination-oxygenation reaction tolerates considerable functionality including protected and free alcohols, heterocycles, and olefins.9 Electrophilic trapping is not limited to benzoylation: enol acetates and TES enol ethers (entries 9–14) were prepared in high yields as well. Furthermore, in every case studied to date, the enol derivative has been isolated as a single regioisomer with a high E-isomer content (all E/Z ratios > 20/1).10
Trisubstituted, stereodefined enol derivatives of this type were previously inaccessible, and their ready availability allowed us to explore new chemistry and evaluate their synthetic utility. In particular, we envisioned an entry to chiral α-hydroxy aldehydes (7) and 1,2-diols (8) by employing the enol benzoates in catalytic asymmetric dihydroxylation (AD) reactions.11,12 As expected, the enol benzoate substrates afforded dihydroxylated products in high enantiomeric purity (Table 2, all entries ≥ 94% ee from 4). In contrast, many 1,1-disubstituted olefins (6) are poor substrates for AD; therefore, AD of the enol benzoates, followed by a reductive workup with NaBH4, presents a highly enantioselective route to these substances. Of note, controlling stereochemistry of the olefin is critical: a 9:1 E/Z mixture of 4a was converted to 8a in 81% ee under the conditions outlined in Table 2. The utility of the AD reaction was exemplified in the total synthesis of the insect pheromone (+)-frontalin (10, Scheme 2).13 Enol benzoate 9 was treated consecutively with AD-mix β and [Me4N]BH(OAc)3 to yield (+)-frontalin in 93% ee and in 49% overall yield from the commercially available alkyne 1i. In comparison, the 1,1-disubstituted olefin 11 was dihydroxylated with poor selectivity and in low yield with AD-mix β.
Table 2.
Asymmetric Dihydroxylation (AD) of Enol Benzoatesa
 | |||||
|---|---|---|---|---|---|
| Entry | R | ee (%)b from: | Product | Yield (%)c | |
| 4 | 6 | ||||
| 1 | nC10H21 | 96 | 78 | 8a | 78 |
| 2 | PhCH2 | 94 | 8b | 84 | |
| 3 |

|
96 | 8c | 59 | |
| 4 | Ph | 95 | 94d | 8d | 75 |
| 5 |
|
96 | 8g | 87 | |
| 6 |
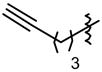
|
95 | 32 | 8qe | 75 |
All AD reactions were performed under standard conditions: AD-mix β, 0.1M in tBuOH/H2O, 0°C.
Unless noted otherwise, ee’s determined by HPLC. See supporting information for details.
Isolated yields from 4.
% ee from reference 11a.
1.0 equiv. MeSO2NH2 was added to the reaction.
Scheme 2. Total Synthesis of (+)-Frontalina.
aReagents and conditions: (a) PdCl2, CuCl, O2, DMF/H2O (7:1), quant; (b) AD-mix-β, MeSO2NH2, NaHCO3, tBuOH/H2O (1:1), 0 °C, 18 h, 85%; (c) [Me4N]BH(OAc)3, AcOH, CH3CN, 76%; (d) AD-mix-β, tBuOH/H2O, 0 °C.
The enantioenriched α-hydroxy aldehydes obtained from the dihydroxylations are useful materials for further synthetic manipulation (Scheme 3). For example, following AD, an Ohira-Bestmann homologation of aldehyde 7b provided propargylic alcohol 12 in 77% yield. Reductive amination of 7b proceeded smoothly to yield the corresponding amino alcohol (13, 84% yield).14 Alternatively, the same starting material (7b) could be oxidized to its methyl ester (14, 92%),15 or undergo olefination to afford an α,β-unsaturated ester (15, 68%, E:Z = 14.3:1).16
Scheme 3. Transformations of α-hydroxy aldehydesa.
aReagents and conditions: (a) (MeO)2POCN2COMe, K2CO3, MeOH, 0 °C-r.t.; (b) BnNH2, Toluene, 4Å MS, 105 °C; NaBH4, MeOH, 0 °C; (c) I2, KOH, MeOH, 0 °C; (d) Bu3PCH2CO2EtBr, NaHCO3, Toluene, 90 °C.
Tandem carbometalation-oxidation is not limited to carbon-oxygen bond formation. Indeed, in preliminary experiments we found that vinyl alane 5 could be aminated in high yields with azodicarboxylates (Table 3).17 Hydrogenation and deprotection of 16f provided the free amine in >90% yield.18 With access to stereodefined enol and enamine derivatives, future studies will seek to engage these materials in a variety of asymmetric transformations.
Table 3.
Synthesis of Stereodefined Ene-hydrazines (16) from Terminal Alkynes.a
 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield (%)b |
| 1 | nC10H21 | CO2iPr | 16a | 79 |
| 2 | CH2Ph | CO2iPr | 16b | 90 |
| 3 | -(CH2)3OH | CO2iPr | 16c | 86 |
| 4 | -(CH2)3OSi(iPr)3 | CO2iPr | 16d | 83 |
| 5 | 3-Thienyl | CO2iPr | 16e | 77 |
| 6 | CH2Ph | CO2tBu | 16f | 84 |
Methylalumination as in Table 1; 1.5–3 equiv azodicarboxylate. See supporting information for complete experimental details.
Isolated yields.
Supplementary Material
Acknowledgments
We thank Professor Jef De Brabander (UT Southwestern) for insightful discussions related to frontalin. Financial support was provided by the Robert A. Welch Foundation, NIGMS and the NSF (CAREER). JRD supported by a fellowship from the Frank and Sara McKnight Fund for Biochemical Research.
Footnotes
Supporting Information Available: Complete experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Wasserman HH, Keller LS. Tetrahedron Lett. 1974;15:4355. [Google Scholar]; (b) Mukaiyama T, Murakami M, Yamaguchi M. Chem Lett. 1980;9:529. [Google Scholar]
- 2.Wittig G, Frommeld HD, Suchanek P. Angew Chem Int Ed. 1963;2:683.Stork G, Dowd SR. J Am Chem Soc. 1963;85:2178.Enders D, Eichenauer H. Tetrahedron Lett. 1977;18:191.Vignola N, List B. J Am Chem Soc. 2004;126:450. doi: 10.1021/ja0392566. Reviews: Whitesell JK, Whitesell MA. Synthesis. 1983:517.Clarke ML. Current Org Chem. 2005;9:701.
- 3.Zhang D, Ready JM. Org Lett. 2005;7:5681. doi: 10.1021/ol052413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipshutz BH, Sengupta S. Org React. 1992;41:135. [Google Scholar]
- 5.(a) Negishi E, Van Horn DE, Yoshida T. J Am Chem Soc. 1985;107:6639. [Google Scholar]; (b) Wipf P, Lim S. Angew Chem Int Ed. 1993;32:1068. [Google Scholar]
- 6.(a) Shaughnessy KH, Waymouth RM. J Am Chem Soc. 1995;117:5873. [Google Scholar]; (b) Kondakov DY, Negishi E-i. J Am Chem Soc. 1995;117:10771. [Google Scholar]
- 7.Moller M, Husemann M, Boche G. J Organomet Chem. 2001;624:47. [Google Scholar]
- 8.(a) Yamamoto N. Chem Lett. 1989:1149. [Google Scholar]; (b) van der Deen H, Kellogg RM, Feringa BL. Org Lett. 2000;2:1593. doi: 10.1021/ol005843+. [DOI] [PubMed] [Google Scholar]; (c) Lewinski J, Ochal Z, Bojarski E, Tratkiewicz E, Justyniak I, Lipkowki J. Angew Chem Int Ed. 2003;42:4643. doi: 10.1002/anie.200351940. [DOI] [PubMed] [Google Scholar]; (d) Kelly AR, Lurain AE, Walsh PJ. J Am Chem Soc. 2005;127:14668. doi: 10.1021/ja051291k. [DOI] [PubMed] [Google Scholar]
- 9.Free –OH groups are benzoylated in the enol products.
- 10.Cp2ZrCl2-catalyzed methylalumination occurs with ca. 95:5 regioselectivity. However, no products derived from oxidation of the minor regioisomer were detected in the crude reaction mixtures. See Lipshutz BH, Butler T, Lower A. J Am Chem Soc. 2006;128:15396. doi: 10.1021/ja065769b.
- 11.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483.AD of vinyl sulfones: Evans P, Leffray M. Tetrahedron. 2003;59:7973.AD of enol ethers: Hashiyama T, Morikawa K, Sharpless KB. J Org Chem. 1992;57:5067.
- 12.Benzoyl substituents are known to interact favorably with AD ligands. Corey EJ, Guzman-Perez A, Noe MC. J Am Chem Soc. 1995;117:10805.Corey EJ, Noe MC. J Am Chem Soc. 1996;118:319.
- 13.Schuster C, Knollmueller M, Gaertner P. Tetrahedron: Asymm. 2006;17:2430. and references therein. [Google Scholar]
- 14.Rieger DL. J Org Chem. 1997;62:8546. doi: 10.1021/jo9705778. [DOI] [PubMed] [Google Scholar]
- 15.Yamada S, Morizono D, Yamamoto K. Tetrahedron Lett. 1992;33:4329. [Google Scholar]
- 16.Harcken C, Martin SF. Org Lett. 2001;3:3591. doi: 10.1021/ol016729+. [DOI] [PubMed] [Google Scholar]
- 17.Erdik E, Ay M. Chem Rev. 1989;89:1947. [Google Scholar]
- 18.See supporting information form complete experimental details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





