Abstract
Members of the CCN (CYR61/CTGF/NOV) family have emerged as dynamically expressed, extracellular matrix-associated proteins that play critical roles in cardiovascular and skeletal development, injury repair, fibrotic diseases and cancer. The synthesis of CCN proteins is highly inducible by serum growth factors, cytokines, and environmental stresses such as hypoxia, UV exposure, and mechanical stretch. Consisting of six secreted proteins in vertebrate species, CCNs are typically comprised of four conserved cysteine-rich modular domains. They function primarily through direct binding to specific integrin receptors and heparan sulfate proteoglycans, thereby triggering signal transduction events that culminate in the regulation of cell adhesion, migration, proliferation, gene expression, differentiation, and survival. CCN proteins can also modulate the activities of several growth factors and cytokines, including TGF-β, TNFα, VEGF, BMPs, and Wnt proteins, and may thereby regulate a broad array of biological processes. Recent studies have uncovered novel CCN activities unexpected for matricellular proteins, including their ability to induce apoptosis as cell adhesion substrates, to dictate the cytotoxicity of inflammatory cytokines such as TNFα, and to promote hematopoietic stem cell self-renewal. As potent regulators of angiogenesis and chondrogenesis, CCNs are essential for successful cardiovascular and skeletal development during embryogenesis. In the adult, the expression of CCN proteins is associated with injury repair and inflammation, and has been proposed as diagnostic or prognostic markers for diabetic nephropathy, hepatic fibrosis, systemic sclerosis, and several types of cancer. Targeting CCN signaling pathways may hold promise as a strategy of rational therapeutic design.
Keywords: angiogenesis, cancer, cardiovascular disease, chondrogenesis, fibrosis, integrin, TNFα, wound healing
INTRODUCTION
Far from being an inert scaffolding for the organization of cells into tissues, the extracellular matrix (ECM) is now recognized as a dynamic and multifunctional regulator of cell behavior (Aszodi et al., 2006). The ECM can bind and modulate the bioavailability and activity of growth factors, cytokines, chemokines, and extracellular enzymes. In addition, ECM proteins can directly interact with cell surface receptors to trigger the activation of signal transduction cascades, thereby regulating diverse cellular functions. A subset of ECM proteins, known as matricellular proteins, is dynamically expressed and does not serve obvious structural roles in the matrix (Bornstein and Sage, 2002). Rather, they function primarily to modulate cellular responses to other environmental factors. Known matricellular proteins include thrombospondins, SPARC, hevin, osteopontin, tenascin C and X, and members of the CCN family. Recent studies have shown that CCN proteins are essential regulators of embryonic development, and in the adult they play critical roles in inflammation, injury repair, fibrotic diseases, and cancer.
Members of the CCN family were first identified as secreted proteins whose synthesis was induced by mitogenic growth factors or oncogenes, or deregulated in transformed cells. The first three members described – CYR61 (cysteine-rich 61; CCN1)(O’Brien et al., 1990), CTGF (connective tissue growth factor; CCN2)(Bradham et al., 1991), and NOV (nephroblastoma overexpressed; CCN3)(Joliot et al., 1992) – provided the acronym for the CCN family. CCN4 (WISP1), CCN5 (WISP2), and CCN6 (WISP3) were subsequently identified as Wnt-inducible secreted proteins (Pennica et al., 1998), and together they comprise the family of six homologous, cysteine-rich proteins in vertebrates. CCN proteins share a modular structure, with an N-terminal secretory peptide followed by four conserved domains with sequence homologies to insulin-like growth factor binding proteins (IGFBP), von Willebrand factor type C repeat (vWC), thrombospondin type I repeat (TSP), and a carboxyl-terminal (CT) domain that contains a cysteine knot motif (Bork, 1993)(Fig. 1). Each structural module is encoded by a separate conserved exon, suggesting that CCN genes are products of exon shuffling (Brigstock, 1999; Lau and Lam, 1999). The N-terminal and C-terminal halves of the proteins are connected by a hinge region that is not conserved and is particularly sensitive to proteolysis (Kireeva et al., 1996; Dean et al., 2007). Since CCN proteins have acquired multiple names reflecting the various circumstances of their identification, a unified nomenclature has been proposed by international consensus to rename these proteins as CCN1-6 in order to minimize confusion (Brigstock et al., 2003). For example, the name CTGF (connective tissue growth factor) originally given to CCN2 implies activities and a mechanism of action akin to those of classical growth factors, a notion that has not been supported by experimental evidence to date.
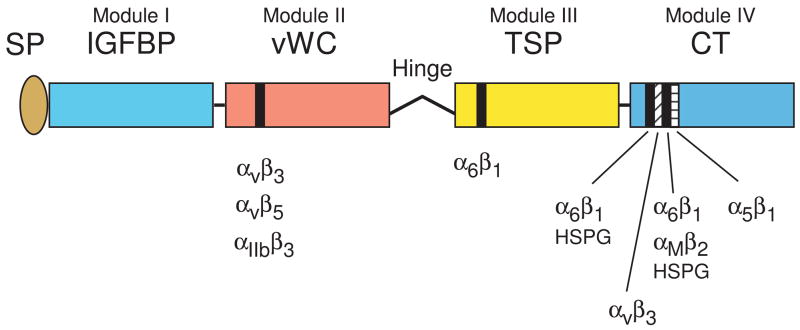
Figure 1.
Schematics of CCN protein structure and localization of their integrin binding sites. The six CCN proteins include CCN1 (CYR61), CCN2 (CTGF), CCN3 (NOV), CCN4 (WISP-1, ELM1), CCN5 (WISP-2, COP-1), and CCN6 (WISP-3). They share significant structural homology, including an N-terminal secretory signal peptide (SP), followed by modular domains (illustrated in different colors) with sequence homologies to insulin-like growth factor binding protein (IGFBP, module I), von Willebrand factor type C repeat (vWC, module II), thrombospondin type 1 repeat (TSP, module III), and a cysteine knot containing carboxyl domain (CT, module IV). Throughout the four modules are 38 cysteine residues that are highly conserved. CCN5 uniquely lacks the CT domain but conserves domains I–III. A protease-sensitive hinge region with no sequence homology among the CCN proteins separate domains II and III. Specific binding sites (black and hatched bars) for several integrins and HSPGs have been identified for CCN1 and CCN2 (Chen et al., 2000; Leu et al., 2003; Chen et al., 2004a; Leu et al., 2004; Gao and Brigstock, 2004; Gao and Brigstock, 2006).
Early studies on CCN proteins proceeded along two divergent paths: one advanced the idea that CCN proteins are polypeptide growth factors (Bradham et al., 1991; Frazier et al., 1996), while the other demonstrated their roles as ECM-associated cell adhesion molecules (Yang and Lau, 1991; Kireeva et al., 1996). The latter perspective envisions CCNs as matricellular proteins, which function primarily to modify cellular responses to other environmental factors and stimuli through interaction with cell adhesion receptors (Lau and Lam, 1999). The collective work from many laboratories in the CCN community now supports this view (Lau and Lam, 2005; Rachfal and Brigstock, 2005; Leask and Abraham, 2006; Yeger and Perbal, 2007). It should be noted that the purification of biologically active CCN proteins has presented a particular challenge, presumably due to the unusually high number of cysteine residues (~10%). The difficulty in purifying CCN proteins of high quality and the lack of unified biochemical and functional assays that define their specific activities have impeded progress in this field. It is possible that variabilities in results from different laboratories, where they exist, might be in part due to differences in methods of protein preparation.
Analyses of CCN functions are now extending the boundaries of known ECM functions. For example, CCN proteins can induce apoptosis as cell adhesion substrates, dictate the cytotoxicity of tumor necrosis factor α (TNFα), and play an essential role in hematopoietic stem cell self-renewal. In this review, we summarize the current information on CCN functions and action mechanisms, and endeavor to unravel the common threads that may underlie their seemingly disparate roles in various contexts. A recent monograph on the CCN family provides an informative resource on research in this area (Perbal and Takigawa, 2005).
RECEPTORS AND CELLULAR FUNCTIONS OF CCN PROTEINS
Receptors of CCN proteins
Like some other ECM and matricellular proteins, CCN proteins regulate diverse cellular behavior including cell adhesion, migration, differentiation, proliferation, and survival. Extensive studies have focused on identifying the signaling receptors for CCN proteins, and results of these studies support the following conclusions. First, CCNs mediate their activities primarily through interaction with cell adhesion receptors, including integrins and heparan sulfate proteoglycans (HSPGs). The CCN-integrin connection was first demonstrated by the direct binding of CCN1 to integrin αvβ3 to mediate endothelial cell adhesion (Kireeva et al., 1998). At least seven other integrins (α2β1, α5β1, α6β1, αvβ5, αIIbβ3, αMβ2, and αDβ2) have since been identified as signaling receptors mediating various CCN functions (Table I). HSPGs are known to serve as coreceptors with integrins in some contexts, and strong CCN-HSPG interaction has been documented (Yang and Lau, 1991). Indeed, CCN binding to the HSPG syndecan-4 is critical for several functions in fibroblasts (Chen et al., 2000; Chen et al., 2004b; Todorovic et al., 2005; Chen et al., 2007), whereas activities mediated through interaction with the chondroitin/dermatan sulfate proteoglycans decorin and biglycan are as yet undefined (Desnoyers et al., 2001).
Table I. Specific CCN-integrin interactions and activities they mediate.
Integrins are cell adhesion receptors that also regulate other cellular functions. They serve as the principal receptors for CCN proteins.
| Integrin | CCN protein | Activities | References |
|---|---|---|---|
| α6β1 | CCN1 CCN2 CCN3 |
cell adhesion, apoptosis in fibroblasts; adhesion, migration in VSMCs; synergism with TNFα cell adhesion in fibroblasts cell adhesion in fibroblasts, endothelial cells (ECs) |
Chen et al., 2000; Chen et al., 2001a; Lin et al., 2003; Todorovic et al., 2005; Chen et al., 2007a |
| αvβ3 | CCN1 CCN2 CCN3 |
cell adhesion, migration, DNA synthesis, cell survival in ECs; DNA synthesis in fibroblasts cell adhesion, migration, cell survival in ECs; hepatic stellate cell adhesion cell adhesion migration in ECs |
Kireeva et al., 1998; Babic et al., 1999; Grzeszkiewicz et al., 2001; Leu et al., 2002; Gao and Brigstock, 2004; Ellis et al., 2003a |
| αvβ5 | CCN1 CCN3 |
cell migration in fibroblasts, synergism with TNFα cell adhesion, migration in ECs; migration in fibroblasts |
Grzeszkiewicz et al., 2002; Lin et al., 2005b; Chen et al., 2007a |
| α5β1 | CCN2 CCN3 |
cell adhesion in chondrocytes, adhesion and migration in pancreatic stellate cells cell adhesion, migration in ECs |
Lin et al., 2003; Lin et al., 2005b; Ellis et al., 2003; Gao and Brigstock, 2006b; Hoshijima et al., 2006c |
| α2β1 | CCN1 | cell adhesion | Lin et al., 2007 |
| αIIbβ3 | CCN1 CCN2 |
cell adhesion in platelets cell adhesion in platelets |
Jedsadayanmata et al., 1999 |
| αMβ2 | CCN1 CCN2 |
cell adhesion in moncytes cell adhesion in moncytes |
Schober et al., 2002 |
| αDβ2 | CCN1 | cell adhesion | Yakubenko et al., 2006d |
Ellis, P. D., Metcalfe, J. C., Hyvonen, M., and Kemp, P. R. (2003) J. Vasc. Res. 40, 234–243
Gao, R. and Brigstock, D. R. (2006) Gut 55, 856–862
Hoshijima, M., Hattori, T., Inoue, M., Araki, D., Hanagata, H., Miyauchi, A., and Takigawa, M. (2006) FEBS Lett. 580, 1376–1382
Yakubenko, V. P., Yadav, S. P., and Ugarova, T. P. (2006) Blood 107, 1643–1650
Second, CCNs can also bind other receptors that do not typically interact with classical ECM proteins, such as the lipoprotein receptor-related proteins (LRPs)(Segarini et al., 2001). For instance, CCN2-mediated cell adhesion and modulation of Wnt signaling in some cell types depend on its binding to LRP-1 and LRP-6, respectively (Gao and Brigstock, 2003; Mercurio et al., 2004). Third, CCNs utilize distinct integrins depending on the target cell types and the activities mediated. In fibroblasts, CCN1 stimulates cell adhesion, migration, and DNA synthesis in fibroblasts through α6β1, αvβ5, and αvβ3, respectively (Table I). By contrast, it stimulates cell migration in endothelial cells and vascular smooth muscle cells (VSMCs) through binding to αvβ3 and α6β1, respectively (Lau and Lam, 2005). Finally, the distinct integrin binding sites of CCN proteins can either function in concert with or independently of one another to induce distinct cellular responses. For example, CCN1 mutants that disrupt its α6β1 binding sites specifically abrogate α6β1-dependent CCN1 activities without affecting αvβ3-mediated angiogenic functions (Leu et al., 2004). Similarly, mutation that impairs the CCN1 αvβ3 binding site abolishes αvβ3- but not α6β1-mediated functions, further establishing that these distinct integrin binding sites and their cognate signaling pathways can act independently of one another (Chen et al., 2004a). On the other hand, CCN1 must interact with both α6β1 and αvβ5 to mediate its synergism with TNFα, indicating that different integrin pathways can also function in concert to elicit distinct CCN activities (Chen et al., 2007). The interaction of CCNs with multiple receptors may contribute to their unique activities and functions.
Cell adhesion, migration, and DNA synthesis
One of the most prominent and consistent functions of CCNs is their role as cell adhesive proteins. When immobilized on solid surfaces in cell culture, CCN proteins can support the adhesion of most adherent cell types through integrins and HSPGs and induce adhesive signaling. Mechanistically, adhesion of human skin fibroblasts to CCN1 and CCN2 occurs through α6β1-HSPGs and rapidly induces the formation of α 6β1-containing focal adhesion complexes, activation of focal adhesion kinase (FAK), paxillin, Rac, actin cytoskeleton reorganization and formation of filopodia and lamellipodia (Chen et al., 2001a)(Fig. 2). These findings provide compelling evidence that CCN proteins induce adhesive signaling. Although cell adhesion to ECM proteins is known to induce transient ERK1/2 activation, adhesion to CCN1 and CCN2 uniquely induces sustained ERK1/2 activation (Chen et al., 2001a), which may promote cell cycle progression by enhancing the stability of key regulatory proteins (Murphy and Blenis, 2006). A short peptide containing the α6β1-HSPG binding sites of CCN1 is sufficient to support cell adhesion and activate sustained ERK activation (Leu et al., 2004). Additionally, CCN proteins can serve as adaptors to other ECM proteins to promote cell adhesion, as exemplified by the binding of CCN2 to fibronectin and perlecan (Nishida et al., 2003; Chen et al., 2004b).
Figure 2.
CCN1 signaling and crosstalk with TNFα. Signal transduction initiated by CCN1, a prototypical member of the family, is mediated primarily through binding to α6β1 and syndecan-4 in fibroblasts to support activities including cell adhesion, although αvβ5 is also necessary for fibroblast migration and crosstalk with TNFα (Grzeszkiewicz et al., 2001; Chen et al., 2007). Cell adhesion to CCN1 activates FAK, paxillin, and Rac1, leading to actin cytoskeleton reorganization, cell spreading, and formation of filopodia and lamellipodia (Chen et al., 2001a). Adhesion to CCN1 also induces sustained ERK activation, an activity that is mediated through binding to α6β1-HSPG (Leu et al., 2004). CCN1 induces fibroblast apoptosis by activating p53 and Bax (Todorovic et al., 2005), and converts TNFα from a pro-mitogenic factor into a potent apoptotic molecule through the Rac1-dependent generation of ROS via 5-lipoxygenase and the mitochondria (Chen et al., 2007). CCN1/TNFα-induced apoptosis occurs rapidly (within 4 hours of treatment) without requiring de novo protein synthesis, indicating that CCN1 activates this pathway directly.
In addition to supporting cell adhesion, one of the ubiquitous activities of CCN proteins is to regulate cell migration. Whereas CCN1, CCN2, and CCN3 proteins stimulate cell migration in many mesenchymal cell types (Grzeszkiewicz et al., 2001; Babic et al., 1999; Shimo et al., 1999; Lin et al., 2003; Gao and Brigstock, 2006), overexpression of CCN4 and CCN5 inhibits cell migration (Soon et al., 2003; Lake et al., 2003). Consistent with their angiogenic functions, CCN1, CCN2, and CCN3 are chemotactic (inducing directional cell migration) in microvascular endothelial cells, although CCN2 can also induce chemokinesis (random cell movement)(Babic et al., 1998; Babic et al., 1999; Lin et al., 2003).
The effect of CCN proteins on mitogenesis appears to be cell type-specific. Although CCN2 promotes DNA synthesis in chondrocytes and osteoblasts (Kubota and Takigawa, 2007b), its mitogenicity in fibroblasts has been controversial. Early studies reported that CCN2 is mitogenic in fibroblasts (Bradham et al., 1991; Frazier et al., 1996), whereas other studies showed that CCN1, CCN2, and CCN3 have no intrinsic ability to induce mitogenesis on their own, but can enhance DNA synthesis induced by other mitogenic growth factors through integrin αvβ3 (Kireeva et al., 1996; Kireeva et al., 1997; Grzeszkiewicz et al., 2001; Grotendorst et al., 2004). By contrast, the expression of CCN5, which lacks the CT domain, inhibits cell proliferation (Lake et al., 2003).
Cell survival and apoptosis
Cell adhesion to ECM molecules promotes cell survival, whereas detachment from the ECM induces rapid cell death by anoikis in many cell types. Remarkably, CCN proteins can promote apoptotic cell death while supporting cell adhesion in a cell type-specific manner (Todorovic et al., 2005). In fibroblasts, CCN1, CCN2, and CCN3 can induce apoptotic cell death as cell adhesion substrates. By contrast, endothelial cells adhered to CCNs are protected from apoptosis upon growth factor withdrawal through αvβ3, an integrin known to induce pro-survival signals (Babic et al., 1999; Leu et al., 2002). Mechanistically, CCN1 induces fibroblast apoptosis through binding to α6β1 and syndecan-4, leading to the p53-dependent activation of Bax and cytochrome c release (Todorovic et al., 2005). Therefore, CCNs can either promote cell survival or induce apoptosis in a cell type and integrin-dependent manner, suggesting a potential role in tissue remodeling.
Angiogenesis
The angiogenic activity of CCNs was first described in CCN1 using the corneal micropocket implant assay (Babic et al., 1998). Subsequently, CCN1, CCN2, and CCN3 have been shown to induce angiogenesis in corneal implants (Babic et al., 1999; Lin et al., 2003), chick chorioallantoic membranes (Shimo et al., 1999), and rabbit ischemic hindlimbs (Fataccioli et al., 2002). Through direct binding to integrin αvβ3, CCN1, CCN2, and CCN3 can recapitulate angiogenic events in vitro by promoting endothelial cell adhesion, migration, proliferation, and tubule formation (Babic et al., 1999; Shimo et al., 1999; Leu et al., 2002; Lin et al., 2003). Furthermore, CCN1 stimulates integrin-dependent recruitment of CD34+ progenitor cells to endothelial cells, thereby enhancing endothelial proliferation and neovascularization (Grote et al., 2007). Consistently, Ccn1 knockout mice suffer cardiovascular defects, as discussed below (Table II). In addition to direct effects, CCNs can also regulate the expression and activities of angiogenic factors such as VEGF-A and VEGF-C (Chen et al., 2001b; Ivkovic et al., 2003; Hashimoto et al., 2002; Dean et al., 2007). A significant decrease in CCN1 and CCN3 expression is found in human placentae associated with pre-eclampsia, consistent with a role of these proteins in placental angiogenesis (Gellhaus et al., 2006). CCN proteins may play important roles in embryonic development, inflammatory diseases and tumorigenesis in part through their potent angiogenic activities (reviewed by Kubota and Takigawa, 2007a).
Table 2. Biological functions of CCN proteins.
Functions of CCNs in vivo as demonstrated by knockout, knockin, knockdown, or forced expression studies are listed below, together with related activities observed in vitro.
| Function | Effects of CCN gene alterations in vivo | Related activities in vitro | References |
|---|---|---|---|
| Angiogenesis and cardiovascular development | CCN1, CCN2, CCN3 stimulate blood vessel growth in corneal implants, or in ischemic hindlimb CCN1-null mice suffer embryonic lethality with placental vascular deficiency, embryonic vessel hemorrhage, and atrioventricular septal defects CCN1+/− mice are viable but exhibit ostium primum atrial septal defect CCN3 mutant mice show cardiac septal defects |
CCNs promote pro-angiogenic activity in microvascular endothelial cells: support cell adhesion, stimulate cell migration, enhance proliferation and survival, induce endothelial tubule formation |
Mo et al., 2002; Mo and Lau, 2006; Heath et al., 2008 Babic et al., 1998; Babic et al., 1999; Fataccioli et al., 2002; Leu et al., 2002; Lin et al., 2003 |
| Skeletal development |
CCN2-null mice are perinatal lethal, showing severe chondrodisplasia, deficient ECM production in cartilage, impaired endochondrial ossification and reduced growth plate angiogenesis CCN3 mutant mice show axial and appendicular skeletal defects and severe joint malformation CCN2 and CCN3 overexpression in osteoblasts leads to osteopenia in mice |
CCN1 promotes chondrogenesis in micromass cultures; CCN2 promotes chondrocyte proliferation and differentiation, synthesis of collagen and aggrecan, and osteogenic differentiation CCN2 binds BMP4 and inhibits its function CCN3 binds BMP2 and inhibits BMP2-induced osteogenic differentiation |
Ivkovic et al., 2003; Rydziel et al., 2007; Heath et al., 2008; Smerdel-Ramoya et al., 2008 Abreu et al., 2002; Kubota and Takigawa, 2007b |
| Cell survival | CCN1-null mice show aberrant apoptosis in vascular cells of large arteries and mesenchymal cells of the cardiac cushion tissue | CCN proteins promote endothelial cell survival |
Mo et al., 2002; Mo and Lau, 2006 Babic et al., 1999; Leu et al., 2002; Lin et al., 2003 |
| Apoptosis | CCN1 knockin mice expressing an apoptosis-defective allele in place of wild type CCN1 are resistant to TNFα-induced apoptosis | CCN proteins can unmask the cytotoxicity of TNFα by inducing ROS accumulation through 5-lipoxygenase and the mitochondria | Chen et al., 2007 |
| Fibrosis | Coinjection of both CCN2 and TGF-β, but not injection of either factor alone, induces sustained fibrosis CCN2 knockdown in the liver prevents chemically-induced liver fibrosis; ectopic expression of CCN2 in the lung render mice that are resistant to bleomycin-induced lung fibrosis to become susceptible |
CCN2 potentiates TGFβ activity, promotes matrix protein synthesis CCN2 supports the adhesion of hepatic stellate cells and oval cells, and stimulate stellate cell proliferation and oval cell migration |
Mori et al., 1999a; Paradis et al., 2002; Li et al., 2006; George and Tsutsumi, 2007; Pi et al., 2008 |
| Diabetic Nephropathy | CCN2 overexpression in podocytes in mice worsens diabetic nephropathy; CCN2 knockdown in the kidney reduces diabetic nephropathy and renal fibrosis | CCN2 promotes cell survival, matrix deposition, and inhibits glucose effect on matrix degradation in mesangial cells | Yokoi et al., 2004; Guha et al., 2007; Yokoi et al., 2008 |
| Restenosis | CCN1 down-regulation by siRNA or FOXO3a reduces neointimal hyperplasia after balloon angioplasty, an effect that is reversed by replenishment of CCN1 via gene transfer | CCN1 induced cell adhesion and migration in vascular smooth muscle cells | Lee et al., 2007; Matsumae et al., 2008; Grzeszkiewicz et al., 2002 |
| Cancer |
CCN1 overexpression in gastric cancer cells, breast cancer cells and ovarian cancer cells enhances tumorigenicity in nude mice. Ectopic or stromal expression of CCN2 promotes tumorigenicity of esophageal squamous cell carcinoma or prostate cancer cells, respectively CCN2 antibody treatment suppresses pancreatic tumor growth and breast cancer osteolytic bone metastasis Expression of CCN1 inhibits tumorigenicity of non-small cell lung carcinoma cells in nude mice, and CCN2 expression suppresses metastasis of human lung adenocarcinoma cells and colorectal cancer cells Expression of CCN4 or CCN5 inhibits tumorigenicity of murine melanoma cells or transformed fibroblasts, respectively |
CCNs promote angiogenesis, enhance cancer cell proliferation and resistance to apoptosis | Babic et al., 1998; Hashimoto et al., 1998; Zhang et al., 1998; Xie et al., 2001; Tsai et al., 2002; Gery et al., 2005; Aikawa et al., 2006; Dornhofer et al., 2006; Shimo et al., 2006; Yang et al., 2005; Deng et al., 2007 |
Chondrogenesis and osteogenesis
CCN proteins exhibit both positive and negative regulatory roles in skeletal formation, as demonstrated in animal models and cell culture experiments (Table II). The most prominent phenotype of CCN2 knockout mice is severe chondrodysplasia (Ivkovic et al., 2003), whereas CCN3 mutant mice show enhanced chondrogenesis and osteogenesis (Heath et al., 2008). Several studies suggest that CCNs can regulate chondrogenic and osteogenic differentiation. The expression of CCNs is highly regulated in chondrogenic and osteogenic differentiation by mesenchymal stem cells (MSCs) isolated from adult bone marrow and cartilage (Luo et al., 2004; Schutze et al., 2005; Si et al., 2006). CCN1 enhances chondrogenic differentiation in mouse limb bud mesenchymal cells in micromass cultures and accelerates type II collagen expression (Wong et al., 1997). Similarly, CCN2 promotes chondrogenic differentiation in micromass cultures of branchial arch mesenchymal cells and proliferation of primary chondrocytes (Nakanishi et al., 2000; Shimo et al., 2004). By contrast, CCN3 appears to inhibit chondrogenic differentiation in micromass cultures (Heath et al., 2008). CCN1 stimulates osteoblast differentiation but inhibits osteoclastogenesis, suggesting a role as a bifunctional regulator that promotes osteogenesis (Crockett et al., 2007). Although CCN2 promotes cell proliferation and differentiation of primary osteoblast in cultures (Safadi et al., 2003), overexpression of CCN2 inhibits osteoblast function and leads to osteopenia in mice (Smerdel-Ramoya et al., 2008).
Stem cell self-renewal
An intriguing new study showed that CCN3 is expressed in CD34+ pluripotent hematopoietic stem cells of human umbilical cord blood (Gupta et al., 2007). Both knockdown and add back experiments support the critical role of CCN3 in CD34+ stem cell self renewal, suggesting potential utility of CCN3 in promoting stem cell engraftment.
FUNCTIONAL AND PHYSICAL INTERACTIONS OF CCN PROTEINS WITH GROWTH FACTORS AND CYTOKINES
CCNs unmask the cytotoxicity of TNFα
CCN proteins can profoundly modify the activities of some growth factors and cytokines through functional and/or physical interactions. A particularly dramatic example is the effect of CCNs on the cytotoxicity of TNFα, which functions mainly to regulate inflammation and immunity (Aggarwal, 2003). Although TNFα can activate a death receptor and is cytotoxic to certain tumor cells, it does not trigger cell death in normal cells. Instead, it promotes cell proliferation and survival through the activation of the pro-inflammatory transcription factor NFκB, which antagonizes the TNFα-induced apoptotic pathway (Aggarwal, 2003). Thus, TNFα can induce apoptosis in normal cells only when NFκB signaling is blocked or when protein synthesis is inhibited, typically by the addition of cycloheximide in cell culture systems. How TNFα induces apoptosis in vivo is not well understood. Remarkably, the presence of CCN1, CCN2, or CCN3 can unmask the cytotoxicity of TNFα without perturbation of NFκB signaling or inhibition of de novo protein synthesis, leading to rapid apoptosis in the otherwise resistant primary human fibroblasts (Chen et al., 2007). Thus, CCNs can profoundly modify the activities of TNFα, converting it from a proliferation-enhancing factor into a potent apoptotic agent in fibroblasts. Mechanistically, CCN1 acts by binding to integrins αvβ5, α6β1, and syndecan-4, leading to the generation of reactive oxygen species (ROS) via 5-lipoxygenase and the mitochondria through a RAC1-dependent pathway (Chen et al., 2007)(Fig. 2). The high and sustained level of ROS induced by CCN1/TNFα results in the biphasic activation of JNK necessary for apoptosis, most likely through oxidative inactivation of JNK phosphatases. Importantly, mice with the genomic CCN1 locus replaced with an apoptosis-defective CCN1 allele with mutations at the α6β1/HSPG binding sites are substantially resistant to TNFα-induced apoptosis in vivo (Chen et al., 2007). These results establish CCN1 as a physiologic regulator of TNFα cytotoxicity, and suggest that CCN proteins may significantly affect the activity of TNFα during inflammatory responses. The requirement of multiple receptors for CCN1 action in this context may serve to specify the cell types targeted for elimination.
Physical interactions with growth factors and their functional consequences
CCN proteins may also modulate the bioavailability and signal transduction of growth factors. Substantial evidence indicates that CCN2 potentiates transforming growth factor-β (TGF-β) actions. CCN2 has been reported to bind TGF-β through the vWC domain and as a result enhance the binding of TGF-β to all three TGF-β receptors (Abreu et al., 2002)(Fig. 3). In addition to enhancing the effective concentration of TGF-β, CCN2 also modifies TGF-β signaling. Some 30% of the genes that are normally inducible by TGF-β are no longer responsive to TGF-β in CCN2-null or CCN2-knockdown cells, indicating that CCN2 is required for a subset of TGF-β responses (Wang et al., 2004; Shi-wen et al., 2006). In vivo, CCN2 cooperates with TGF-β to induce a sustained level of fibrotic response that is not achieved by either factor alone (Mori et al., 1999).
Figure 3.
Interaction of CCN proteins with other molecules. CCN proteins interact with a variety of cell surface receptors and extracellular ligands, including various integrins, HSPGs, and LRPs (Lau and Lam, 2005; Gao and Brigstock, 2003; Mercurio et al., 2004). Receptors that interact with CCN proteins are shown schematically below the four conserved CCN domains and extracellular proteins that bind CCNs are shown above, aligned with the interacting CCN domains. Whereas CCN3 binds the receptor Notch (Sakamoto et al., 2002), CCN2 has been shown to bind BMPs and TGF-β through the vWC domain and VEGF through the TSP and CT domains (Abreu et al., 2002; Inoki et al., 2002). CCN2 also binds ECM proteins such as fibronectin and perlecan through the CT domain (Nishida et al., 2003; Chen et al., 2004b).
In contrast to promoting the functions of TGF-β, CCNs appear to inhibit the activities of bone morphogenetic proteins (BMPs). CCN2 binds BMP-4 through the vWC domain and in so doing inhibits BMP-4 binding to its receptors (Abreu et al., 2002). Microinjection of CCN1 mRNA into Xenopus embryos also inhibits BMP signaling (Latinkic et al., 2003; Mercurio et al., 2004), and injection of CCN6 RNA curtails the phenotypic effects of BMP-2b overexpression in zebrafish (Nakamura et al., 2007). Likewise, CCN3 binds and antagonizes BMP-2, inhibiting BMP-2 induced Smad signaling and osteogenic differentiation (Rydziel et al., 2007). Consistent with the idea that CCN2 and CCN3 can act as BMP antagonists, transgenic mice that overexpress CCN2 or CCN3 in osteoblasts develop osteopenia (Rydziel et al., 2007; Smerdel-Ramoya et al., 2008). Thus, CCN proteins may directly interact with and regulate the functions of TGF-β and BMPs.
In addition to the aforementioned interactions, CCN2 can bind VEGF165 at two binding sites in the TSP and CT domains (Inoki et al., 2002). CCN2-VEGF interaction inhibits VEGF binding to its receptor VEGFR2 and the angiogenic activity of both molecules, whereas proteolysis of the complex by matrix metalloproteinases (MMPs) releases the bound VEGF in an active form (Hashimoto et al., 2002; Dean et al., 2007). Thus, CCN2 may act to fine tune the bioavailability of VEGF, releasing it for angiogenic action only in contexts where MMPs are being secreted and activated, such as during tissue remodeling and wound repair. Another example of bioavailability regulation is illustrated by the ability of CCN1 to displace ECM-bound bFGF, thereby enhancing bFGF-induced DNA synthesis (Kolesnikova and Lau, 1998).
Functional interaction with Wnt and Notch signaling
CCN1 and CCN2 are transcriptionally activated by the Wnt3A/β-catenin signaling pathway during osteogenic differentiation, but have opposing effects on this pathway. Knockdown of CCN1 inhibited Wnt3A-induced osteogenic differentiation (Si et al., 2006). Microinjection of CCN1 mRNA into Xenopus embryos induces expression of Wnt/β-catenin transcriptional targets and the formation of a partial or complete secondary body axes, an effect similar to ectopic Wnt-signaling (Latinkic et al., 2003). However, injection of CCN1 mRNA also inhibits secondary axes-induction by Xwnt8, suggesting that CCN1 can both mediate and antagonize Wnt signaling (Latinkic et al., 2003). By contrast, overexpression of CCN2 inhibited Wnt3A-induced osteogenic differentiation (Luo et al., 2004). CCN2 also inhibits Wnt signaling in Xenopus, most likely through direct interaction with the Wnt-coreceptor LRP-6 through the CT domain (Mercurio et al., 2004). Recent studies also showed that CCN6 antagonizes Wnt-signaling in zebrafish embryonic development (Nakamura et al., 2007). Thus, while CCN1 can mediate or activate Wnt-signaling in some instances, CCN2 and CCN6 can antagonize it in a context-dependent manner.
CCN3 interacts with Notch1 through its CT domain, and suppresses myogenic differentiation in vitro (Sakamoto et al., 2002). CCN3 and Notch1 are concomitantly expressed in presomitic mesoderm, suggesting that their interaction may contribute to Notch signaling and the resulting inhibition of myogenesis (Sakamoto et al., 2002).
REGULATION OF CCN GENE EXPRESSION BY GROWTH FACTORS, HORMONES, AND ENVIRONMENTAL STRESSES
The expression of CCN genes is exquisitely sensitive to environmental perturbations, including the availability of growth factors, hormones, and cytokines, and exposure to oxygen deprivation, UV, and mechanical forces. Both CCN1 and CCN2 were first identified in differential expression screens for immediate-early genes that are transcriptionally activated by serum (Lau and Lam, 1999) or TGF-β (CCN1 and CCN2 were named βIG-M1 and βIG-M2 in this study)(Brunner et al., 1991) without requiring de novo protein synthesis. These genes are also highly responsive to induction by a variety of mitogenic signals and external stimuli, including fibroblast growth factor, platelet-derived growth factor, phorbol esters, and cAMP (O’Brien et al., 1990). In the CCN1 promoter, a serum response element (SRE) is essential for transcriptional activation by serum or platelet-derived growth factor in fibroblasts (Latinkic et al., 1991). A 2 kb fragment of the CCN1 promoter that includes the SRE is sufficient to confer faithful developmental and pathological (wound healing) expression in transgenic mice, thereby defining the critical promoter for CCN1 transcriptional regulation in vivo (Latinkic et al., 2001).
TGF-β exerts strong regulation of CCN gene expression, including transcriptional activation of CCN1, CCN2, CCN4, and CCN5, and repression of CCN3 (Lafont et al., 2002; Parisi et al., 2006). The full activation of CCN2 by TGF-β requires three CCN2 promoter elements: a SMAD binding site, a tandem repeat of an ETS element, and an element important for basal transcriptional activity (Leask et al., 2003; Van Beek et al., 2006). Recently, a novel mechanism of regulation has been described in which the metalloproteinase MMP3 binds a CCN2 enhancer element and augments its expression in a transcription factor-like manner in chondrocytes (Eguchi et al., 2008). Intracellular MMP3 synergizes with TGF-β to activate the CCN2 promoter, suggesting an interaction between MMP3 and SMAD signaling.
Several hormones also upregulate CCN expression. Angiotensin II enhances CCN1 expression in VSMCs in vitro and in rat aorta ex vivo (Hilfiker et al., 2002). CCN2 is upregluated by endothelin-1 in fibroblasts, VSMCs, and cardiac myocytes, consistent with its roles in fibrotic and vascular functions (Kemp et al., 2004; Xu et al., 2004; Rodriguez-Vita et al., 2005). Estrogen is also a potent inducer of CCN1 expression. In the mammary adenocarcinoma cell line MCF-7, which is dependent on estrogen for growth, blockade of CCN1 activity by neutralizing antibodies abrogated estrogen-dependent DNA synthesis (Sampath et al., 2001). In addition, CCN1 transcription is transiently induced by dihydroxy-vitamin D3 (1,25-(OH)2D3), which promotes osteoblast differentiation (Schutze et al., 1998).
A striking aspect of CCN gene expression is its sensitivity to environmental stress. For example, CCN1 is induced by exposure to UV light (Quan et al., 2006). CCN1 and CCN2 are transcriptionally induced under hypoxia, a condition that favors blood vessel growth by the induction of several angiogenic factors, including VEGF, through the action of hypoxia-inducible factor-1α (HIF-1α). HIF-1α interacts with c-Jun/AP-1 and thereby contribute to CCN1 transcription in hypoxic conditions (Kunz et al., 2003). In CCN2, both HIF-1α-dependent transcription and enhanced mRNA stability through a sequence element in the 3′UTR contribute to its expression under hypoxia (Higgins et al., 2004; Kondo et al., 2006). Mechanical force is another form of stress that induces CCN gene expression. CCN1 and CCN2 expression is rapidly up-regulated by tensile forces and mechanical stretch in fibroblasts, chondrocytes and VSMCs, hemodynamic forces in endothelial cells, and hydrostatic pressure in mesangial cells (reviewed by Chaqour and Goppelt-Struebe, 2006). Promoter analysis showed that a binding site for Egr-1 is critical for stretch-induced activation of CCN1 in VSMCs (Grote et al., 2004). Mechanical stress also up-regulates CCN1 and CCN2 expression in both cardiac and skeletal muscles (Hilfiker-Kleiner et al., 2004; Kivela et al., 2007). In humans, a single bout of strenuous exercise is sufficient to enhance expression of CCN1 and CCN2 in skeletal muscles due to mechanical stretch rather than a temporary hypoxic condition after exercise (Kivela et al., 2007).
Inflammation and tissue injury constitute other forms of stress that induce CCN expression. In postnatal development and in the adult, CCN proteins generally expressed at a low level in most tissues, but become elevated again in sites of inflammation and injury repair (Igarashi et al., 1993; Chen et al., 2001b; Latinkic et al., 2001). Consistently, CCN expression is induced by inflammatory cytokines such as IL-1 and TNFα (Cooker et al., 2007; Gashaw et al., 2008), as well as by bacterial and viral infections (Kim et al., 2004; Wiedmaier et al., 2008).
CCN PROTEINS IN EMBRYONIC DEVELOPMENT
Targeted gene disruptions in mice have been accomplished for CCN1, CCN2, CCN3, and CCN6. With the exception of CCN6-null mice, which show no observable phenotypic change, the resulting phenotypes establish a critical role for CCNs in cardiovascular and skeletal development. Early studies showed that CCN1 expression is tightly associated with the skeletal, cardiovascular, and neuronal systems during embryogenesis (O’Brien and Lau, 1992), suggesting a role for CCN1 in the development of these organ systems. These notions are supported by the finding that targeted disruption of CCN1 in mice results in embryonic lethality with cardiovascular defects (Mo et al., 2002; Mo and Lau, 2006). Approximately 30% of CCN1-null embryos fail to form chorioallantoic fusion at E8.5 and die by E9.5, whereas the remaining embryos perish at mid-gestation from placental vascular insufficiency, loss of embryonic vessel integrity leading to hemorrhage, and cardiac atrioventricular septal defect (AVSD). The observed defect in chorioallantoic fusion, a process known to involve α4 integrin and VCAM-1, implicates a role for CCN1 in cell adhesion events between the allantois and the chorion. CCN1-null embryos are defective in vessel bifurcation at the chorionic plate, leading to a paucity of sprouting vessels that penetrate into the labyrinth and thus an undervascularized placenta (Mo et al., 2002). Furthermore, the large vessels in CCN1-null embryos lack a discrete basement membrane, and the vascular cells are disorganized and apoptotic, leading to rupture and hemorrhage (Mo et al., 2002). CCN1-null mice perish too early in development to fully assess whether CCN1 deficiency may impair skeletal development, although no obvious skeletal defects were observed by the time of their embryonic deaths.
CCN1-null embryos exhibit severe defects in atrioventricular valvuloseptal morphogenesis, resulting in a common atrioventricular valve orifice that is the hallmark of complete AVSD (Mo and Lau, 2006). This phenotype is in part due to precocious apoptosis in mesenchymal cells of the endocardial cushion tissue, which must fuse with the atrial and ventricular septa to undergo extensive remodeling. Although CCN1+/− mice are largely viable, they display persistent ostium primum atrial septal defects in 20% of the adult. Human AVSD is a common group of congenital disorders that is frequently associated with Down’s syndrome, whereas non-syndromic AVSDs are inherited with autosomal dominance (Sheffield et al., 1997). The atrial septal defects due to CCN1 haploinsufficiency are similar to those observed in some human patients with mutations in AVSD1, a susceptibility gene for non-syndromic AVSD identified by linkage analysis (Sheffield et al., 1997). Remarkably, the human CCN1 gene maps to chromosome 1p21-31 (Jay et al., 1997), precisely the same location as AVSD1 (Sheffield et al., 1997). These findings suggest that CCN1 may be a candidate gene for human AVSD.
As discussed above, CCN1 and CCN2 share many similarities in their activities and patterns of expression. Despite these similarities, targeted disruptions of CCN1 and CCN2 in mice show distinct phenotypes. While CCN1 is essential for cardiovascular development, CCN2-null mice are neonatal lethal due to respiratory defects as a secondary consequence of severe skeletal malformations (Ivkovic et al., 2003). CCN2 deficiency results in generalized chondrodysplasia throughout the appendicular and axial skeleton due to decreased growth plate angiogenesis, aberrant ECM metabolism, and defective endochondrial ossification (Ivkovic et al., 2003). These findings are consistent with a wealth of in vitro data showing the roles of CCN2 in chondrogenesis and endochondrial ossification (reviewed by (Kubota and Takigawa, 2007b). CCN2-null embryos also suffer from pulmonary hypoplasia, with reduced cell proliferation and increased apoptosis in the lung (Baguma-Nibasheka and Kablar, 2008). Loss of CCN2 perturbed differentiation of type II alveolar epithelial cells, resulting in excessive glycogen retention and diminished lamellar body and nuclear size, although surfactant synthesis was not affected. Although CCN2 is also highly expressed in the developing cardiovascular system, no prominent cardiovascular defects were observed in CCN2-null mice (Ivkovic et al., 2003; Chuva de Sousa Lopes SM et al., 2004).
Mice with targeted disruption of CCN3 have been constructed in which exon 3 was replaced with a TK-neomycin cassette to generate mice that produce no full length CCN3 but express a very low level of mutant CCN3 that lacks the VWC domain (Heath et al., 2008). While <50% of homozygous CCN3 mutant mice are viable, they show deficiencies in tissues also impaired in CCN1 and CCN2 null mice, including defects in the appendicular and axial skeleton, severe joint malformation, and abnormal remodeling of the endocardial cushions with associated cardiac septal defects. Premature tissue degeneration was also observed in the lens of CCN3 mutant mice, with cataracts developing in adults older than 6 months.
Loss-of-function mutations in CCN6 (WISP3) in humans cause the autosomal recessive skeletal disease progressive pseudorheumatoid dysplasia, a juvenile-onset degenerative disease of the joint (Hurvitz et al., 1999). However, the CCN6 mRNA is undetectable in mouse tissues by RNA blotting or in situ hybridization, and CCN6-null mice exhibit no observable phenotype (Kutz et al., 2005). Mice that over-express CCN6 are also normal. Therefore, CCN6 appears to play different roles in mice and in humans, and is not essential for skeletal growth or homeostasis in mice (Kutz et al., 2005).
Although CCN1, CCN2, and CCN3 are prominently expressed in the neuronal system during development, no neuronal phenotypes have been reported in mice with targeted disruptions of these genes to date. The specific functions of CCN protein in neuronal cells or neuronal development are currently unknown.
FUNCTIONS OF CCN PROTEINS IN WOUND HEALING AND DISEASE
Wound healing
Expression of CCNs is tightly regulated during injury repair in many organs, including the liver following partial hepatectomy (Ujike et al., 2000), the heart after myocardial infarction (Hilfiker-Kleiner et al., 2004; Chuva de Sousa Lopes SM et al., 2004), and in granulation tissue during cutaneous wound healing (Igarashi et al., 1993; Chen et al., 2001b; Lin et al., 2005b). In the initial phase of injury repair, an abundance of CCN2 is released from the α granules of platelets (Kubota et al., 2004; Cicha et al., 2004). CCN1 and CCN2 can both support the adhesion of activated platelets through direct binding to integrin αIIbβ3 (Jedsadayanmata et al., 1999), and serve as adhesion substrates to invading inflammatory cells such as monocytes through integrin αMβ2 (Schober et al., 2002). In later stages of wound healing, CCN proteins are highly induced in the granulation tissue during its remodeling. In this context, CCN proteins may synergize with TGF-β in matrix remodeling, and they may also interact with TNFα to trigger apoptosis of fibroblasts (Chen et al., 2007).
CCN1 and CCN2 expression is also elevated during fracture repair in the long bones throughout the reparative phase of the callus, notably in proliferating chondrocytes and osteoblasts (Hadjiargyrou et al., 2000; Nakata et al., 2002). Furthermore, blockade of CCN1 by antibodies inhibits bone fracture healing in mice (Athanasopoulos et al., 2007) and recombinant CCN2 protein promotes the repair of articular cartilage in a rat osteoarthritis model (Nishida et al., 2004). These studies suggest that CCN proteins may play important roles in the homeostasis of bone and cartilage tissues.
Fibrosis
CCN2 overexpression is strongly associated with fibrosis of various organs, and may potentiate the activity of TGF-β (Table II). For example, injection of either TGF-β or CCN2 alone in the skin induces only transient granulation tissue formation, whereas application of both TGF-β and CCN2 together produces a sustained fibrotic response (Mori et al., 1999). Likewise, mice resistant to bleomycin-induced lung fibrosis, a condition that is TGF-β-dependent, can be made susceptible by overexpression of CCN2 concomitant with bleomycin treatment (Bonniaud et al., 2004). It has been hypothesized that TGF-β may initiate the fibrotic response, and CCN2 cooperates with TGF-β to maintain and exacerbate fibrosis (Takehara, 2003).
Genetic evidence has further endorsed the notion that CCN2 plays an important role in liver fibrosis and systemic sclerosis. Knockdown of CCN2 by siRNA prevents liver fibrosis induced by CCl4 or N-nitrosodimethylamine in rats, showing that CCN2 plays a critical role (Li et al., 2006; George and Tsutsumi, 2007). Consistent with the role of CCN2 in fibrosis, its elevated expression has been noted in hepatic stellate cells of the fibrous septa in fibrotic and cirrhotic livers in experimental models and human patients (Rachfal and Brigstock, 2003). In vitro, CCN2 supports the adhesion of hepatic stellate cells and oval cells, and stimulate stellate cell proliferation and oval cell migration (Paradis et al., 2002; Pi et al., 2008). In addition, CCN2 is prominently overexpressed in systemic sclerosis or scleroderma, in which TGF-β and CCN2 both appear to play a role (Takehara, 2003). A polymorphism in the human CCN2 promoter that relieves Sp3-mediated transcriptional repression has been found to be significantly associated with scleroderma (Fonseca et al., 2007). The N-terminal cleavage products of CCN2 are present at elevated levels in the plasma and dermal interstitial fluid of scleroderma patients, and serves as a marker for the disease (Dziadzio et al., 2005). Together, these studies underscore the important roles of CCN2 in fibrosis, and suggest that CCN2 may be a potential target for antifibrotic therapy (Leask, 2008).
Diabetic nephropathy
The critical role of CCN2 in renal disease is supported by the findings that its overexpression in podocytes worsens diabetic nephropathy in mice (Yokoi et al., 2008), whereas reduction of CCN2 expression by antisense oligonucleotides ameliorates renal tubulointerstitial fibrosis and attenuates nephropathy in mouse models of diabetes (Yokoi et al., 2004; Guha et al., 2007). In vitro, CCN2 promotes mesangial cell survival, stimulates matrix deposition, and inhibits high glucose effect on matrix degradation. Several recent studies have independently concluded that plasma or renal CCN2 level is a useful risk marker for diabetic nephropathy (Jaffa et al., 2008; Nguyen et al., 2008; Thomson et al., 2008).
Vascular diseases
Both CCN1 and CCN2 are over-expressed in VSMCs of atherosclerotic lesions, or in retenosis after balloon angioplasty (Hilfiker et al., 2002; Grzeszkiewicz et al., 2002; Schober et al., 2002). Suppression of CCN1 expression by either siRNA or FOXO3a-mediated repression results in reduced neointimal hyperplasia after balloon angioplasty, an effect that is reversed by replenishment of CCN1 via gene transfer (Lee et al., 2007; Matsumae et al., 2008). These findings underscore a critical role for CCN1 in vascular injury repair, and suggest that inhibition of CCN1 may potentially prevent restenosis after vascular interventions (Matsumae et al., 2008).
Cancer
A large body of work on CCN proteins in cancer support the following observations: 1. CCNs are aberrantly expressed in cancers of a broad range of tissues; 2. ectopic expression of CCN genes can either enhance or suppress the tumorigenicity of tumor cells, depending on the specific cancer; 3. in some cases, anti-CCN therapy inhibits tumor growth or metastasis; and 4. CCN gene expression may serve as a diagnostic or prognostic marker for certain malignancies (Table II). Aberrant expression of CCNs is observed in cancers of numerous organs and tissues, including (but not limited to) breast, colorectal, gallbladder, gastric, ovarian, pancreatic, and prostate cancers, gliomas, hepatocellular carcinoma, non-small cell lung and squamous cell carcinoma, lymphoblastic leukemia, melanoma, and cartilaginous tumors (reviewed by Menendez et al., 2003; O’Kelly and Koeffler, 2005; Kleer et al., 2007; Yeger and Perbal, 2007).
One of the mechanisms by which CCNs may promote tumor growth is the enhancement of tumor angiogenesis. Consistent with the angiogenic activity of CCN1, its overexpression in gastric adenocarcinoma cells enhances the tumorigenicity of these cells in nude mice, resulting in tumors that are more vascularized than tumors of control cells (Babic et al., 1998). Likewise, increased tumorigenicity and tumor vascular density in vivo have been observed upon ectopic expression of CCN1 in MCF7 breast cancer cells and ovarian cancer cells (Menendez et al., 2003; O’Kelly and Koeffler, 2005). Another pro-tumorigenic mechanism of CCNs is the enhancement of cell survival. Forced expression of CCN1 in breast cancer cells confers resistance to apoptosis by upregulation of the anti-apoptotic protein XIAP (Lin et al., 2004), and promotes resistance to the pro-apoptotic anti-cancer drug Taxol through an integrin αvβ3-dependent mechanism (Menendez et al., 2005).
Recent studies have found that CCN2 is overexpressed in human pancreatic cancer, and CCN2-specific monoclonal antibody therapy inhibits pancreatic tumor growth, lymph node metastasis, and tumor angiogenesis in rodent models (Aikawa et al., 2006; Dornhofer et al., 2006). CCN2 is part of a gene signature that specifies osteolytic bone metastasis of breast cancer (Kang et al., 2003), and CCN2 neutralizing antibodies suppress breast cancer osteolytic bone metastasis and microvascularization (Shimo et al., 2006). These results support a critical role for CCN2 in pancreatic cancer and metastatic breast cancer, and suggest targeting CCN2 as potential anti-cancer therapy.
Paradoxically, CCNs may also inhibit tumor growth. For example, CCN1 suppresses tumor growth of non-small cell lung carcinoma in nude mice (Tong et al., 2001). CCN2 inhibits metastasis and invasion of human lung adenocarcinoma through a CRMP-1 dependent mechanism (Chang et al., 2004), and suppresses metastasis of colorectal cancer (Lin et al., 2005a). CCN4 (Elm1) and CCN5 (COP1) were identified as genes that inhibited tumor growth of K-1175 murine melanoma cells and transformed fibroblasts in immunodeficient mice, respectively (Hashimoto et al., 1998; Zhang et al., 1998). Likewise, CCN6, which can modulate IGF signaling, suppresses inflammatory breast cancer tumor growth (Kleer et al., 2007).
While the angiogenic and apoptotic activities of CCN proteins may respectively promote or inhibit tumor growth, the roles of CCN proteins in cancer are likely complex and may involve multiple downstream effectors. Understanding how CCN proteins function to either promote or inhibit tumorigenesis will require further investigation. Nevertheless, the strong correlation of CCN expression in various cancers have underscored their prognostic values, and suggests that CCN signaling pathways may be useful targets for novel anti-cancer therapy.
CONCLUSIONS AND FUTURE PROSPECTS
The CCN family of proteins has emerged as ECM-associated, multifunctional regulators of development and injury repair. On the cellular level, CCN proteins regulate cell adhesion, migration, proliferation, differentiation, apoptosis, and survival, acting primarily through direct binding to integrins, with HSPGs and LRPs as coreceptors in some contexts. In addition, they also interact with and modulate the bioavailability and/or activity of growth factors and cytokines, including TGF-β, TNFα, VEGF, BMPs, and members of the Wnt family, thereby contributing to the seemingly bewildering array of processes that CCN proteins appear to influence. CCN proteins are potent regulators of angiogenesis and chondrogenesis, and play important roles in injury repair, fibrotic diseases, and cancer. Recent studies have underscored their unique ability to either promote cell death or survival as cell adhesion molecules, and to profoundly regulate the cytotoxicity of inflammatory cytokines such as TNFα. While CCN1 has been shown to be a physiologic regulator of TNFα, the full effects of CCN proteins on inflammation have yet to be thoroughly explored. Although the functional domains of CCN proteins have been delineated and several specific integrin binding sites have been identified, the three dimensional structures of CCN proteins are still unknown. Their unusually high number of conserved cysteine residues portends an interesting tertiary structure that may impact on their function.
Gene targeting studies to date have established the critical roles of CCNs in cardiovascular and skeletal development. However, since knockouts of CCN1 and CCN2 in mice result in embryonic or perinatal lethality, many aspects of their roles in later stages of development and in disease progression have yet to be uncovered. Therefore, tissue-specific and conditional ablations of CCN genes will be needed to unravel their roles in specific organs and tissues. In addition, analysis of mice with allelic replacements in which CCN genes are replaced by mutant alleles that abrogate specific binding sites for their receptors or other interacting proteins will likely illuminate CCN functions through specific signaling pathways (Chen et al., 2007). Given the association of CCN proteins with injury repair, it is likely that conditional ablation of CCNs will reveal defects in the physiological response to challenges of injury or stress. In this regard, RNAi-mediated knockdowns via viral vectors are already yielding useful information about the causal roles of CCNs in disease. Although aberrant expression of CCN proteins underlies pathologies such as fibrosis and cancer, their specific functions in these contexts are not well understood. Further investigation on their mechanisms of action in disease will be necessary in contemplating the CCN signaling pathways as targets of rational therapeutic designs.
Acknowledgments
We thank Vladislava Juric for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (CA46565, GM78492, HL81390) to L.F.L.
ABBREVIATIONS
- AVSD
atrioventricular septal defects
- BMP
bone morphogenetic protein
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- HIF-1α
hypoxia-inducible factor-1α
- HSPG
heparan sulfate proteoglycan
- LRP
lipoprotein receptor-related protein
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- ROS
reactive oxygen species
- SRE
serum response element
- TGF-β
transforming growth factor β
- TNFα
tumor necrosis factor α
- TPA
12-O-tetradecanoyl-phorbol 13-acetate
- VSMCs
vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn. 2008;237:485–493. doi: 10.1002/dvdy.21433. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A, Chinn J, Neubauer M, Purchio AF. Identification of a gene family regulated by transforming growth factor-β. DNA Cell Biol. 1991;10:293–300. doi: 10.1089/dna.1991.10.293. [DOI] [PubMed] [Google Scholar]
- Chang CC, Shih JY, Jeng YM, Su JL, Lin BZ, Chen ST, Chau YP, Yang PC, Kuo ML. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. J Natl Cancer Inst. 2004;96:364–375. doi: 10.1093/jnci/djh059. [DOI] [PubMed] [Google Scholar]
- Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and CTGF induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001a;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen CC, Mo FE, Lau LF. The angiogenic inducer Cyr61 induces a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001b;276:47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- Chen N, Leu SJ, Todorovic V, Lam SCT, Lau LF. Identification of a novel integrin αvβ3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004a;279:44166–44176. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Abraham DJ, Shi-wen X, Pearson JD, Black CM, Lyons KM, Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004b;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M. Activated human platelets release connective tissue growth factor. Thromb Haemost. 2004;91:755–760. doi: 10.1160/TH03-09-0602. [DOI] [PubMed] [Google Scholar]
- Cooker LA, Peterson D, Rambow J, Riser ML, Riser RE, Najmabadi F, Brigstock D, Riser BL. TNF-alpha, but not IFN-gamma, regulates CCN2 (CTGF), collagen type I, and proliferation in mesangial cells: possible roles in the progression of renal fibrosis. Am J Physiol Renal Physiol. 2007;293:F157–F165. doi: 10.1152/ajprenal.00508.2006. [DOI] [PubMed] [Google Scholar]
- Crockett JC, Schutze N, Tosh D, Jatzke S, Duthie A, Jakob F, Rogers MJ. The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of alphavbeta3 and alphavbeta5. Endocrinology. 2007;148:5761–5768. doi: 10.1210/en.2007-0473. [DOI] [PubMed] [Google Scholar]
- Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, Overall CM. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, Tong XJ, Xie D. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282:36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- Dornhofer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacamuli R, Hockel M, Le Q, Longaker M, Yang G, Koong A, Giaccia A. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- Dziadzio M, Usinger W, Leask A, Abraham D, Black CM, Denton C, Stratton R. N-terminal connective tissue growth factor is a marker of the fibrotic phenotype in scleroderma. QJM. 2005;98:485–492. doi: 10.1093/qjmed/hci078. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T, Ibaragi S, Sasaki A, Kuboki T, Takigawa M. Novel transcription factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol. 2008;28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fataccioli V, Abergel V, Wingertsmann L, Neuville P, Spitz E, Adnot S, Calenda V, Teiger E. Stimulation of angiogenesis by cyr61 gene: a new therapeutic candidate. Hum Gene Ther. 2002;13:1461–1470. doi: 10.1089/10430340260185094. [DOI] [PubMed] [Google Scholar]
- Fonseca C, Lindahl GE, Ponticos M, Sestini P, Renzoni EA, Holmes AM, Spagnolo P, Pantelidis P, Leoni P, McHugh N, Stock CJ, Shi-wen X, Denton CP, Black CM, Welsh KI, du Bois RM, Abraham DJ. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N Engl J Med. 2007;357:1210–1220. doi: 10.1056/NEJMoa067655. [DOI] [PubMed] [Google Scholar]
- Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tussue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin αvβ3 and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Gashaw I, Stiller S, Boing C, Kimmig R, Winterhager E. Premenstrual Regulation of the Pro-Angiogenic Factor CYR61 in Human Endometrium. Endocrinology. 2008;149:2261–2269. doi: 10.1210/en.2007-1568. [DOI] [PubMed] [Google Scholar]
- Gellhaus A, Schmidt M, Dunk C, Lye SJ, Kimmig R, Winterhager E. Decreased expression of the angiogenic regulators CYR61 (CCN1) and NOV (CCN3) in human placenta is associated with pre-eclampsia. Mol Hum Reprod. 2006;12:389–399. doi: 10.1093/molehr/gal044. [DOI] [PubMed] [Google Scholar]
- George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803. doi: 10.1038/sj.gt.3302929. [DOI] [PubMed] [Google Scholar]
- Gery S, Xie D, Yin D, Gabra H, Miller C, Wang H, Scott D, Yi WS, Popoviciu ML, Said JW, Koeffler HP. Ovarian carcinomas: CCN genes are aberrantly expressed and CCN1 promotes proliferation of these cells. Clin Cancer Res. 2005;11:7243–7254. doi: 10.1158/1078-0432.CCR-05-0231. [DOI] [PubMed] [Google Scholar]
- Grote K, Bavendiek U, Grothusen C, Flach I, Hilfiker-Kleiner D, Drexler H, Schieffer B. Stretch-inducible expression of the angiogenic factor CCN1 in vascular smooth muscle cells is mediated by Egr-1. J Biol Chem. 2004;279:55675–55681. doi: 10.1074/jbc.M406532200. [DOI] [PubMed] [Google Scholar]
- Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood. 2007;110:877–885. doi: 10.1182/blood-2006-07-036202. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates human skin fibroblasts migration through integrin αvβ5 and enhances mitogenesis through integrin αvβ3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz TM, Lindner V, Chen N, Lam SCT, Lau LF. The angiogenic factor CYR61 supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316:590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res. 2000;15:1014–1023. doi: 10.1359/jbmr.2000.15.6.1014. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Shindo-Okada N, Tani M, Nagamachi Y, Takeuchi K, Shiroishi T, Toma H, Yokota J. expression of the Elm1 gene, a nove gene of the CCN (Connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses in vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med. 1998;187:289–296. doi: 10.1084/jem.187.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkotter HJ, Schieffer B, Drexler H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254–260. doi: 10.1161/01.cir.0000021426.87274.62. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, Drexler H. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227–2233. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van HW, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van HE, Rezai-Delui H, Legius E, Le MM, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23:94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffa AA, Usinger WR, McHenry MB, Jaffa MA, Lipstiz SR, Lackland D, Lopes-Virella M, Luttrell LM, Wilson PW. Connective Tissue Growth factor and Susceptibility to Renal and Vascular Disease Risk in Type 1 Diabetes. J Clin Endocrinol Metab. 2008;93:1893–1900. doi: 10.1210/jc.2007-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay P, Berge-Lefranc JL, Marsollier C, Mejean C, Taviaux S, Berta P. The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p. Oncogene. 1997;14:1753–1757. doi: 10.1038/sj.onc.1200986. [DOI] [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/Mouse connective tissue growth factor is mediated through integrin αIIbβ3. J Biol Chem. 1999;274:24321–24327. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2004;37:603–606. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Kim SM, Park JH, Chung SK, Kim JY, Hwang HY, Chung KC, Jo I, Park SI, Nam JH. Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. J Virol. 2004;78:13479–13488. doi: 10.1128/JVI.78.24.13479-13488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Lam SCT, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both signaling cell adhesion molecules: comparison of activities, metablism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela R, Kyrolainen H, Selanne H, Komi PV, Kainulainen H, Vihko V. A single bout of exercise with high mechanical loading induces the expression of Cyr61/CCN1 and CTGF/CCN2 in human skeletal muscle. J Appl Physiol. 2007;103:1395–1401. doi: 10.1152/japplphysiol.00531.2007. [DOI] [PubMed] [Google Scholar]
- Kleer CG, Zhang Y, Merajver SD. CCN6 (WISP3) as a new regulator of the epithelial phenotype in breast cancer. Cells Tissues Organs. 2007;185:95–99. doi: 10.1159/000101308. [DOI] [PubMed] [Google Scholar]
- Kolesnikova TV, Lau LF. Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene. 1998;16:747–754. doi: 10.1038/sj.onc.1201572. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kubota S, Mukudai Y, Moritani N, Nishida T, Matsushita H, Matsumoto S, Sugahara T, Takigawa M. Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-untranslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene. 2006;25:1099–1110. doi: 10.1038/sj.onc.1209129. [DOI] [PubMed] [Google Scholar]
- Kubota S, Kawata K, Yanagita T, Doi H, Kitoh T, Takigawa M. Abundant retention and release of connective tissue growth factor (CTGF/CCN2) by platelets. J Biochem (Tokyo) 2004;136:279–282. doi: 10.1093/jb/mvh126. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007a;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007b;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kunz M, Moeller S, Koczan D, Lorenz P, Wenger RH, Glocker MO, Thiesen HJ, Gross G, Ibrahim SM. Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61 in melanoma cells. J Biol Chem. 2003;278:45651–45660. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol. 2005;25:414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont J, Laurent M, Thibout H, Lallemand F, Le Bouc Y, Atfi A, Martinerie C. The expression of novH in adrenocortical cells is down-regulated by TGFbeta 1 through c-Jun in a Smad-independent manner. J Biol Chem. 2002;277:41220–41229. doi: 10.1074/jbc.M204405200. [DOI] [PubMed] [Google Scholar]
- Lake AC, Bialik A, Walsh K, Castellot JJ., Jr CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol. 2003;162:219–231. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, Mohun TJ, Smith JC. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130:2429–2441. doi: 10.1242/dev.00449. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Mo FE, Greenspan JA, Copeland NG, Gilbert DJ, Jenkins NA, Lau LF. Promoter function of the angiogenic inducer Cyr61 gene in transgenic mice: tissue specificity, inducibility during wound healing, and role of the serum response element. Endocrinology. 2001;142:2549–2557. doi: 10.1210/endo.142.6.8208. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, O’Brien TP, Lau LF. Promoter function and structure of the growth factor-inducible immediate early gene cyr61. Nucleic Acids Res. 1991;19:3261–3267. doi: 10.1093/nar/19.12.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Lau LF, Lam SCT. Integrin-mediated CCN functions. In: Perbal B, Takigawa M, editors. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. pp. 61–79. [Google Scholar]
- Leask A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008;20:1409–1414. doi: 10.1016/j.cellsig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K, Kim HS. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Chen N, Chen CC, Todorovic V, Bai T, Juric V, Liu Y, Yan G, Lam SCT, Lau LF. Targeted mutagenesis of the matricellular protein CCN1 (CYR61): selective inactivation of integrin α6β1-heparan sulfate proteoglycan coreceptor-mediated cellular activities. J Biol Chem. 2004;279:44177–44187. doi: 10.1074/jbc.M407850200. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Lam SCT, Lau LF. Proangiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin α6β1 binding site in the angiogenic Inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801–33808. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ, Chau YP, Kuo ML. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005a;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005b;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chang CC, Chen ST, Chang HL, Su JL, Chau YP, Kuo ML. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J Biol Chem. 2004;279:24015–24023. doi: 10.1074/jbc.M402305200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, Kuo ML. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem. 2007;282:34594–34604. doi: 10.1074/jbc.M706600200. [DOI] [PubMed] [Google Scholar]
- Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M, Kita T, Tanaka M. CCN1 Knockdown Suppresses Neointimal Hyperplasia in a Rat Artery Balloon Injury Model. Arterioscler Thromb Vasc Biol. 2008;28:1077–1083. doi: 10.1161/ATVBAHA.108.162362. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Mehmi I, Griggs DW, Lupu R. International Congress on Hormonal Steroids and Hormones and Cancer: The angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr Relat Cancer. 2003;10:141–152. doi: 10.1677/erc.0.0100141. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24:761–779. doi: 10.1038/sj.onc.1208238. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo FE, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circulation Research. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Weidinger G, Liang JO, quilina-Beck A, Tamai K, Moon RT, Warman ML. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. J Clin Invest. 2007;117:3075–3086. doi: 10.1172/JCI32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/endo.141.1.7267. [DOI] [PubMed] [Google Scholar]
- Nakata E, Nakanishi T, Kawai A, Asaumi K, Yamaai T, Asano M, Nishida T, Mitani S, Inoue H, Takigawa M. Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24) during fracture healing. Bone. 2002;31:441–447. doi: 10.1016/s8756-3282(02)00846-3. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Tarnow L, Jorsal A, Oliver N, Roestenberg P, Ito Y, Parving HH, Rossing P, van Nieuwenhoven FA, Goldschmeding R. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008 doi: 10.2337/dc07-2469. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- O’Brien TP, Lau LF. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth & Differentiation. 1992;3:645–654. [PubMed] [Google Scholar]
- O’Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kelly J, Koeffler HP. Thr role of CCN1 in tumorigenesis and cancer progression. In: Perbal B, Takigawa M, editors. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. pp. 273–291. [Google Scholar]
- Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82:767–774. doi: 10.1097/01.lab.0000017365.18894.d3. [DOI] [PubMed] [Google Scholar]
- Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone. 2006;38:671–677. doi: 10.1016/j.bone.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B, Takigawa M. CCN Proteins: A New Family of Cell Growth and Differentiaion Regulators. London: Imperial College Press; 2005. [Google Scholar]
- Pi L, Ding X, Jorgensen M, Pan JJ, Oh SH, Pintilie D, Brown A, Song WY, Petersen BE. Connective tissue growth factor with a novel fibronectin binding site promotes cell adhesion and migration during rat oval cell activation. Hepatology. 2008;47:996–1004. doi: 10.1002/hep.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ, Fisher GJ. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1–9. doi: 10.1016/s1386-6346(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, Egido J. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97:125–134. doi: 10.1161/01.RES.0000174614.74469.83. [DOI] [PubMed] [Google Scholar]
- Rydziel S, Stadmeyer L, Zanotti S, Durant D, Smerdel-Ramoya A, Canalis E. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem. 2007;282:19762–19772. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr, Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Sampath D, Winneker RC, Zhang Z. Cyr61, a member of the ccn family, is required for mcf-7 cell proliferation: regulation by 17beta-estradiol and overexpression in human breast cancer. Endocrinology. 2001;142:2540–2548. doi: 10.1210/endo.142.6.8186. [DOI] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Schutze N, Lechner A, Groll C, Siggelkow H, Hufner M, Kohrle J, Jakob F. The human analog of murine cysteine-rich protein 61 is a 1α, 25-dihydroxyvitamin D3 responsive immediate early gene in human fetal osteoblasts: regulation by cytokines, growth factors, and serum. Endocrinology. 1998;139:1761–1770. doi: 10.1210/endo.139.4.5954. [DOI] [PubMed] [Google Scholar]
- Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 2005;3:5. doi: 10.1186/1478-811X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarini PR, Nesbitt JE, Li D, Hayes LG, Yates JR, III, Carmichael DF. The low density lipoprotein receptor-related protein/alpha 2-Macroglobulin receptor is a receptor for connective tissue growth factor (CTGF) J Biol Chem. 2001;276:40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Pierpont ME, Nishimura D, Beck JS, Burns TL, Berg MA, Stone EM, Patil SR, Lauer RM. Identification of a complex congenital heart defect susceptibility locus by using DNA pooling and shared segment analysis. Hum Mol Genet. 1997;6:117–121. doi: 10.1093/hmg/6.1.117. [DOI] [PubMed] [Google Scholar]
- Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006;281:10715–10726. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kanyama M, Wu C, Sugito H, Billings PC, Abrams WR, Rosenbloom J, Iwamoto M, Pacifici M, Koyama E. Expression and roles of connective tissue growth factor in Meckel’s cartilage development. Dev Dyn. 2004;231:136–147. doi: 10.1002/dvdy.20109. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kubota S, Yoshioka N, Ibaragi S, Isowa S, Eguchi T, Sasaki A, Takigawa M. Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J Bone Miner Res. 2006;21:1045–1059. doi: 10.1359/jbmr.060416. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Canalis E. Skeletal overexpression of connective tissue growth factor (ctgf) impairs bone formation and causes osteopenia. Endocrinology. 2008 doi: 10.1210/en.2008-0254. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- Takehara K. Hypothesis: pathogenesis of systemic sclerosis. J Rheumatol. 2003;30:755–759. [PubMed] [Google Scholar]
- Thomson SE, MCLennan SV, Kirwan PD, Heffernan SJ, Hennessy A, Yue DK, Twigg SM. Renal connective tissue growth factor correlates with glomerular basement membrane thickness and prospective albuminuria in a non-human primate model of diabetes: possible predictive marker for incipient diabetic nephropathy. J Diabetes Complications. 2008;22:284–294. doi: 10.1016/j.jdiacomp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Xie D, O’Kelly J, Miller CW, Muller-Tidow C, Koeffler HP. Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer. J Biol Chem. 2001;276:47709–47714. doi: 10.1074/jbc.M107878200. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Bogart DF, Castaneda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:8178–8185. doi: 10.1038/sj.onc.1205682. [DOI] [PubMed] [Google Scholar]
- Ujike K, Shinji T, Hirasaki S, Shiraha H, Nakamura M, Tsuji T, Koide N. Kinetics of expression of connective tissue growth factor gene during liver regeneration after partial hepatectomy and D-galactosamine-induced liver injury in rats. Biochem Biophys Res Commun. 2000;277:448–454. doi: 10.1006/bbrc.2000.3693. [DOI] [PubMed] [Google Scholar]
- Van Beek JP, Kennedy L, Rockel JS, Bernier SM, Leask A. The induction of CCN2 by TGFbeta1 involves Ets-1. Arthritis Res Ther. 2006;8:R36. doi: 10.1186/ar1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JF, Olson ME, Ma L, Brigstock DR, Hart DA. Connective tissue growth factor siRNA modulates mRNA levels for a subset of molecules in normal and TGF-beta 1-stimulated porcine skin fibroblasts. Wound Repair Regen. 2004;12:205–216. doi: 10.1111/j.1067-1927.2004.012113.x. [DOI] [PubMed] [Google Scholar]
- Wiedmaier N, Muller S, Koberle M, Manncke B, Krejci J, Autenrieth IB, Bohn E. Bacteria induce CTGF and CYR61 expression in epithelial cells in a lysophosphatidic acid receptor-dependent manner. Int J Med Microbiol. 2008;298:231–243. doi: 10.1016/j.ijmm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wong M, Kireeva ML, Kolesnikova TV, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev Biol. 1997;192:492–508. doi: 10.1006/dbio.1997.8766. [DOI] [PubMed] [Google Scholar]
- Xie D, Miller CW, O’Kelly J, Nakachi K, Sakashita A, Said JW, Gornbein J, Koeffler HP. Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. J Biol Chem. 2001;276:14187–14194. doi: 10.1074/jbc.M009755200. [DOI] [PubMed] [Google Scholar]
- Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth & Differentiation. 1991;2:351–357. [PubMed] [Google Scholar]
- Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007;1:159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, Yoshioka T, Saito Y, Ogawa Y, Kuwabara T, Sugawara A, Nakao K. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131–6141. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]