Abstract
Intermittent hypoxia (IH) is a major pathological factor in the development of neural deficits associated with sleep-disordered breathing. Here we demonstrate that IH lasting 2 or 30 days, but not sustained hypoxia (SH) of the same duration, was accompanied by several posttranslational modifications of the large subunit of RNA Polymerase II, Rpb1, including hydroxylation of proline 1465, phosphorylation of serine 5 residues within the C-terminal domain, and nondegradative ubiquitylation. These modifications were found to occur in two regions of the brain, hippocampal region CA1 and the prefrontal cortex, but not in neocortex, brainstem and CA3 region of hippocampus. We also found that mice exposed to 14 or 30 days of IH, but not SH, demonstrated cognitive deficits in behavioral assays. Furthermore, by using the pheochromocytoma-derived PC12 cell line, we showed that, under in vitro IH conditions, induction of Rpb1 hydroxylation, phosphorylation, and ubiquitylation required that the von Hippel-Lindau protein be present. We hypothesize that the observed modifications of Rpb1 participate in regulating the expression of genes involved in mediating cognitive deficits evoked by chronic IH.
Keywords: intermittent hypoxia, hippocampus, prolyl hydroxylase, RNA Polymerase II, PC12 cells
INTRODUCTION
Intermittent hypoxia (IH), or repeated episodes of hypoxia followed by re-oxygenation, is associated with many human diseases and is among the prototypic consequences of sleep-disordered breathing (SDB). SDB occurs in 2% to 3% of all children (Lumeng and Chervin, 2008), and in 5% of the middle-aged, and 20% to 30% of the elderly population (Punjabi, 2008). IH has been linked to numerous morbidities in SDB, such as disorders of the central nervous system, i.e. decreased cognitive performance, depression, increased prevalence and severity of stroke, attention deficits, and excessive sleepiness (Teran-Santos et al., 1999; Beebe & Gozal, 2002; O’Brian et al., 2003a; O’Brian et al., 2003b; Morrell & Twigg, 2006; Minoguchi et al., 2007, Bassetti et al., 2006).
Cellular and biochemical changes in the human hippocampus are closely correlated with SDB (Macey et al., 2002; Morrell et al., 2003, Bartlett et al., 2004). Accordingly, our laboratories demonstrated that IH induced oxidative stress (Xhu et al., 2004), apoptosis (Gozal et al., 2001a), and specific changes in the protein profile of the hippocampal CA1 area (Gozal et al., 2002, Klein et al., 2002), which coincided with an impaired acquisition and retention of cognitive spatial tasks, as demonstrated by the Morris water maze (Gozal et al., 2001a; Gozal et al, 2001b; Gozal et al., 2003; Row et al., 2002; Row et al., 2003). We also showed that IH substantially decreased phosphorylation of CREB Ser-133, without effects on the total expression of CREB, specifically within the CA1, and not the CA3, region (Goldbart et al., 2003). In addition, IH decreases the resting potential, causes partial depolarization and diminished sodium currents (Gu et al., 2001), changes enzyme activity (Marzatico et al., 1986), and impairs the ability of CA1 neurons to induce and maintain population-spike long-term potentiation (Payne et al., 2004). However, the molecular and cellular mechanisms by which IH induces oxidative stress, causes neuronal loss of function and death, or promotes cell survival remain to be elucidated. In this study we attempted to further define molecular events associated with the IH-associated apoptosis (Gozal et al, 2001a) and changes in protein expression (Gozal et al., 2002) in the CA1 region.
Cellular mRNAs are transcribed by the RNA Polymerase II complex (RNAPII), in which the large subunit, Rpb1, has enzymatic activity. Rpb1 contains a long C-terminal domain (CTD) composed of 52 heptad repeats, each containing multiple serines that can undergo phosphorylation. Recently, we discovered that low-grade oxidative stress stimulates relocation of Rpb1 onto the DNA and hydroxylation of proline 1465 of Rpb1 (Mikhaylova et al., 2008) in the LGQLAP motif of Rpb1, a site involved in binding of the von Hippel-Lindau (pVHL)-associated E3 ubiquitin ligase (Kuznetsova et al., 2003). This hydroxylation is necessary for increased phosphorylation of Ser5 residues within the CTD (Mikhaylova et al., 2008) and is likely to be a crucial regulator of gene expression. Here we wanted to determine if IH, which is known to induce oxidative stress, regulates Rpb1 modifications in different regions of the brain.
We report that chronic IH, but not sustained hypoxia (SH), stimulated hydroxylation of P1465 and phosphorylation of Ser5 of Rpb1, specifically in the CA1 region of the hippocampus and in the prefrontal cortex but not in other regions of the brain. Likewise, mice exposed to chronic IH demonstrated cognitive deficits related to dysfunction in those brain regions. Because phosphorylation of Rpb1 on Ser5 is crucial in the regulation of gene expression, induction of this modification of Rpb1 by IH could account for changes in the expression or function of numerous proteins in those important regions. Thus, we infer that observed biochemical changes in Rpb1 might underlie the behavioral changes that occur with IH.
EXPERIMENTAL PROCEDURES
Materials
The following commercially available antibodies were used: H14 (Covance, Berkeley, CA); N20 (Santa Cruz, Santa Cruz, CA); pVHL (Ig32), anti-hemagglutinin (HA) tag (12CA5), and anti-cullin2 (Fisher Sci. Fremont, CA); and anti-Rbx1 (Invitrogen, Grand Island, NY). The antibody against hydroxylated proline within the Rpb1 peptide (HP) was custom made by Alpha Diagnostic, Inc. (San Antonio, TX). This antibody was used at a concentration of 1:500 for western blots (Mikhaylova et al., 2008). Secondary antibodies were obtained from Sigma, Cell Signaling, or Amersham. Synthetic biotinylated peptides were made by Alpha Diagnostic, Inc.
Exposure of animals to IH and SH
Male C57Bl6 mice (Jackson Labs) were exposed to either chronic IH or SH for the indicated periods of time in commercially designed chambers (Oxycycler model A44XO, Biospherix, Redfield, NY). The IH profile consisted of alternating room air and 7.8% oxygen every 150 s during daylight hours (7:00 AM to 7:00 PM) corresponding to the rest-and-sleep period of mice. The SH profile was programmed to continuously deliver 7.8% O2 to the environment throughout the duration of the exposure. For the remaining 12 hr of the sleep-wake cycle, the oxygen concentration was maintained at 21%. Control mice were exposed to circulating normoxic gas in a chamber identical to those housing IH- and SH-exposed mice. At the end of the exposure period (i.e., 14 days or 30 days), animals were sacrificed between 7:00 AM and 9:00 AM, i.e., 12 to 14 hrs after cessation of the corresponding hypoxic profile, and specific regions of the brain (hippocampus divided in CA1 and CA3 regions, prefrontal cortex, neocortex, and dorsocaudal brainstem) were surgically dissected. Mean wet tissue weights for individual tissue samples were 2 to 3 mg except for cortex samples, which weighed 5 to 6 mg. All animal experiments were performed according to protocols approved by IACUC of the University of Louisville and the University of Cincinnati.
Preparation of brain extracts
Nuclear extracts enriched for the chromatin fraction were obtained as follows: small pieces of specific frozen brain regions were first allowed to swell in cell lysis buffer for 10 min, and were then minced in the same buffer in a Dounce homogenizer to complete homogeneity. The homogenates were centrifuged at 14,000 rpm for 20 min, and the remaining pellets were extracted with 0.3 M NaCl for 30 min and digested with deoxyribonuclease and micrococcal nuclease (10 U and 37.5 U, respectively, per 100 μl of pellet volume) for 1 h to release DNA-bound RNAPII complexes. NP40 was added to a final concentration of 0.5%, and NaCl to a final concentration of 0.5 M, and the pellets were extracted for 30 min at 4°C. For western blotting, equal amounts of proteins were loaded in each lane and the samples were run on 4-20% gradient polyacrylamide gel electrophoresis using standard protocols.
Place navigation/spatial reference task in the Morris water maze
The Morris water maze was configured to test performance that reflects types of learning and memory traditionally defined as spatial reference, as previously described (Gozal et al., 2001; Kheirandish et al., 2005). In brief, one day prior to place learning, mice exposed to either IH, SH, or control normoxic conditions for 14 or 30 days were habituated to the water maze during a free swim. Place learning was assessed over 6 days of consecutive training sessions during which mice continued to be exposed to their respective hypoxia treatments. We used a spaced training regimen that has been demonstrated to elicit optimal learning in mice (Gerlai and Clayton, 1999). Each place-training session consisted of three trials separated by a 10-min intertrial interval, and such sessions occurred between 7:30 PM and 10:30 PM. At daily sessions, each animal was placed into the pool from 4 quasirandom start points and allowed a maximum of 90 seconds to escape to the platform where it was allowed to remain for 15 seconds. Mice that failed to escape were led to the platform. The position of the platform remained constant across trials. Twenty-four hours following the second, fourth, and final (6th) training session, the platform was retracted for a 30-second probe trial during which the time spent in each of the four quadrants of the maze and the number of target crossings and proximity to the previous location of the platform was recorded. Probe trials provide a measure of spatial bias developed during learning. To assess performance during place training, mean escape latencies, swim distances, and swim speeds were analyzed by 2-way analysis of variance with repeated measures on block (3 trials), followed by Newman-Keuls tests when appropriate. To assess probe performance, mean quadrant times, target crossings, and time spent in the general vicinity of the platform location were compared as well.
Cell culture and exposure of cells to IH or SH
PC12 cells overexpressing human HA-tagged pVHL, or expressing rat VHL antisense RNA were grown as described earlier (Kuznetzova et al., 2003). Experiments were timed so that the cells were 70% to 90% confluent at the time of collection. Cells were exposed to the indicated durations of repeated fluctuations of O2 from 30% to 1% O2 (in constant 5% CO2) in a hypoxic workstation (Coy Laboratory Products, Grass Lake, MI). The IH cycle was created using the manufacturer-supplied computer program to allow infusion of N2 into the chamber until O2 reached 1% in the tissue culture media in a control plate carrying the same number of cells, followed by an infusion of O2 until the oxygen level measured in the culture media was 30%. The O2 concentrations were determined in the chamber air using an O2 sensor and monitor provided with the hypoxia workstation, and O2 in the media was measured using a Dissolved Oxygen Meter (World Precision Instruments, Sarasota, FL). Both measurements were performed for the entire duration of each exposure. The exposure to SH was achieved by putting cells into the hypoxic workstation with O2 equilibrated to 1%. Full equilibration in the culture medium was achieved after 30 min of exposure. Extracts from PC12 cells were obtained as described before (Kuznetsova et al., 2003).
In vitro pVHL-peptide binding reaction
Ten micrograms of biotinylated peptide were incubated with streptavidin-coated Dynabeads (M-280, Dynal) in 300 μl of buffer containing 20 mM Tris, pH 8, 100 mM NaCl, 0.5% NP-40, and 1 mM EDTA for 1 h at room temperature. Washed beads were incubated with wild-type VHL (pRC-CMV expression vector, Invitrogen), translated in vitro using 35S-methionine and transcription/translation-coupled reticulocyte lysate (Promega). Binding reactions were washed extensively in the same buffer and analyzed for bound [35S]VHL by using SDS-PAGE. For the peptide hydroxylation step, immobilized peptides were first incubated with 100 to 200 μl of soluble nuclear extract (Kuznetsova et al., 2003) or with the remaining total nuclear pellet digested by nuclease (Kuznetsova et al., 2003). For the hydroxylation reaction, extracts were diluted to a final concentration of 100 mM NaCl.
Quantification and statistical analysis of data
Films were scanned using Epson Perfection 4990 Photo scanner (Epson, Japan) with 600 DPI resolution and JPEG initial file format. The brightness and contrast were non-selectively adjusted for the entire scanned blot. The optical density of western blots was measured using ImageQuant 5.2 (Molecular Dynamics). Averaged data are expressed as mean ± SEM. Six animals were used in each group. Analysis was performed by GraphPad Instat version 3.0 for Windows using One-Way ANOVA, followed by the Tukey-Kramer multiple comparison tests. Differences having a P value less than 0.05 were considered statistically significant. To assess performance during place training, we analyzed mean escape latencies, swim distances, and swim speeds by 2-way ANOVA with repeated measures on block (3 trials), followed by Newman-Keuls tests when appropriate. To assess probe performance, we also compared mean quadrant times, target crossings, and time spent in the general vicinity of the platform location. Averaged data are presented as mean ± SEM.
RESULTS
IH but not SH stimulates increased levels of Rpb1 and its P1465 hydroxylation and Serine 5 phosphorylation in hippocampal CA1 region and prefrontal cortex
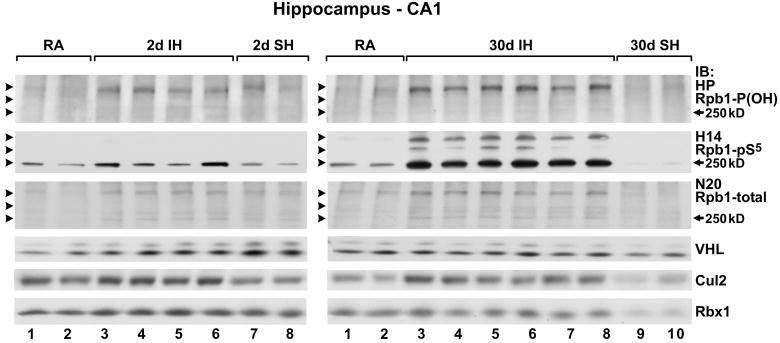
Exposure of mice (n=6 in each group) to 2 or 30 days of IH increased levels of Rpb1 in extracts from the CA1 region of the brain. This was measured using N20 antibody against the N-terminal of Rpb1 as well as antibodies detecting P1465 hydroxylation (HP) and Ser5 phosphorylation (H14) of Rpb1 (Fig. 1 and 2). Clearly, the effect was more pronounced after longer (30 days) exposure to IH. Our previous work indicated that the higher migrating forms represent nondegrading ubiquitylation of Rpb1 (Mikhaylova et al., 2008). Note that the 250 kD band is extremely weak in the case of hydroxylated Rpb1, most likely resulting from the shift towards higher molecular weights due to ubiquitylation. The levels of pVHL were not affected by IH exposure (Figs. 1 and 2D). However, the levels of Cullin2 (Cul2), a regulatory component of the pVHL-associated protein complex, were significantly induced in the CA1 region by 2 and 30 days of IH (Figs. 1 and 2E), as were the levels of Rbx-1, the actual E3 ligase in the complex (Figs. 1 and 2F).
Figure 1.
Chronic IH, but not SH, induced P1465 hydroxylation, Ser-5 phosphorylation, and ubiquitylation of Rpb1 in nuclear extracts from the hippocampal CA1 region. Western blot analysis of hydroxylated (HP, Rpb1-P(OH)), Ser-5–phosphorylated (H14, Rpb1-pS5), and total Rpb1 (N20), as well as of the components of the pVHL complex, VHL, cullin 2, and the RING finger protein Rbx1. Each lane represents an individual animal. RA, room air; IH, intermittent hypoxia; SH, sustained hypoxia. Arrowheads point towards the Rpb1 bands detected with all three different antibodies. IB: immunoblot. HP antibody was used at a concentration of 1:500; H14 antibody was used at 1:2000, and N20 at 1:500.
Figure 2.
Quantification of IH- and SH-induced modulation of different forms of Rpb1 and components of the pVHL complex in CA1 region of hippocampus. Data presented as means±SEM; * P<0.05, ** P<0.01; *** P< 0.001 as compared to controls; n=6 per group.
In contrast to IH, SH did not elicit long-term induction of hydroxylation, phosphorylation, or ubiquitylation of Rpb1. At day 2, there was an increase in the total amount of Rpb1 in the CA1 region of SH-treated animals, but that effect was short lived, and by day 30 the total levels of Rpb1 did not differ from the room-air-exposed groups (Fig. 2C). SH led also to a substantial decrease in the levels of hydroxylated (Fig. 2A) and Ser-5 phosphorylated (Fig. 2B) Rpb1 in the CA1 region, but only after 30 days of exposure. Consistent with those effects, SH coincided with a decrease in the levels of Cullin 2 (Fig. 2E) at both time points, and a decrease in Rbx-1 after 30 days of exposure (Fig. 2F). Levels of pVHL were initially increased after 2 days of SH, but returned to control levels after 30 days (Fig. 2D).
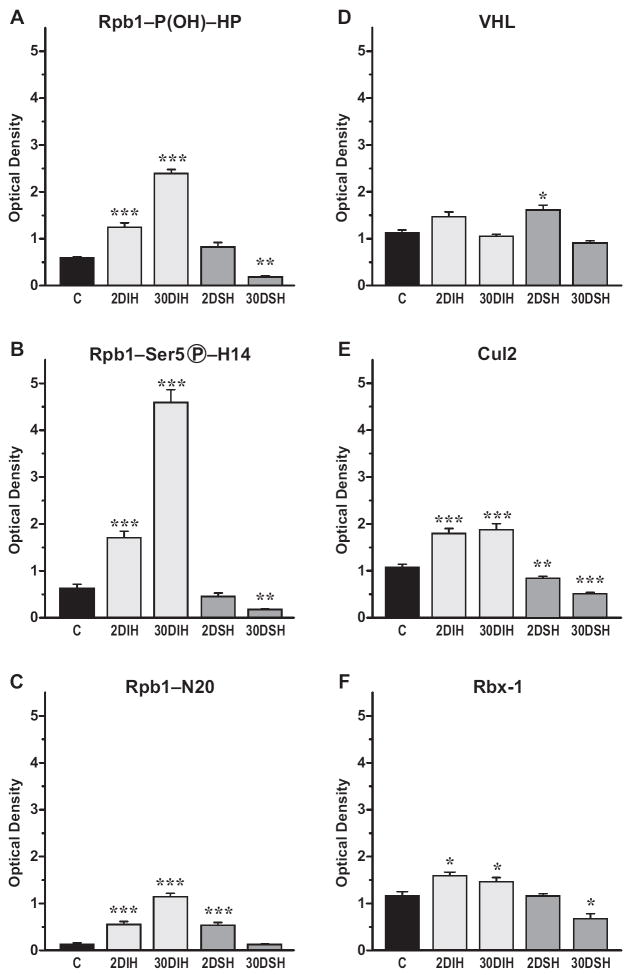
A similar effect of IH was observed in the prefrontal cortex where 2 days of IH caused substantial stimulation of the amount of Rpb1 as well as its P1465 hydroxylation, phosphorylation, and ubiquitylation detected with N20, HP, and H14 antibodies (Fig. 3). In contrast to the CA1 region, those changes were maximal on day 2 for Rpb1 detected with all three antibodies. These levels remained the same after 30 days of exposure (Fig. 3B and C) with the exception of Rpb1 detected with N20 antibody, which showed a decrease in the total level of Rpb1 (Fig. 3D). Matched exposures to SH had no effect on the overall levels or hydroxylation and phosphorylation of Rpb1 in the prefrontal cortex, as compared to mice that had been exposed to room air. The only exception was a significant decrease in Rpb1 detected with N20 antibody after a 30-day exposure.
Figure 3.
Chronic IH induces P1465 hydroxylation, Ser5 phosphorylation, and increased total Rpb1 in nuclear extracts from prefrontal cortex. A. Western blot analysis using indicated antibody; B. Quantification of averaged results; n=6 per group. Labeling as in Figs. 1 and 2.
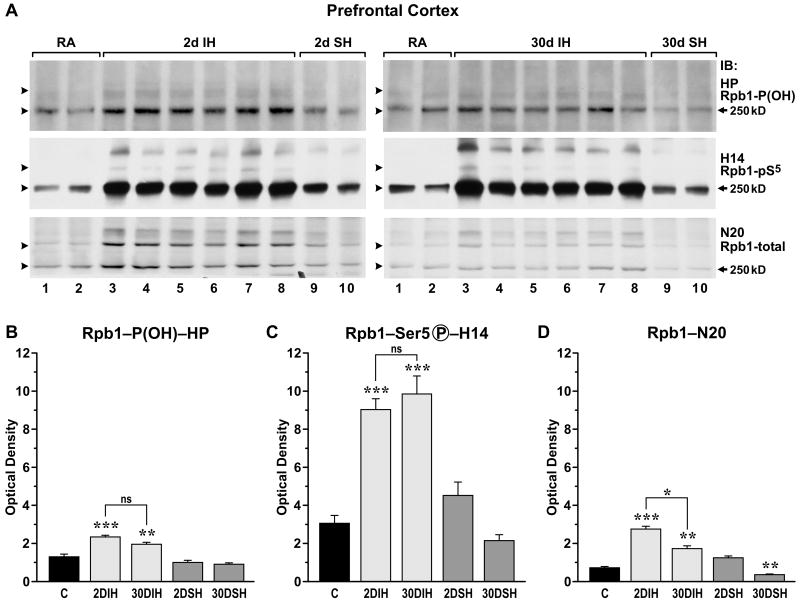
In contrast to the effects on the hippocampal CA1 region and prefrontal cortex, we detected no effects on Rpb1 in the brain neocortex (Fig. 4), brainstem, or hippocampal region CA3 (data not shown) after 2 days of either IH or SH. After 30 days of IH exposure Ser5 phosphorylation was reduced in the neocortex, and the total levels of Rpb1 and Rpb1 hydroxylated on P1465 did not differ from room air levels (Fig. 4A). Interestingly, 30 days of SH had the same inhibitory effect on Ser5 phosphorylation of Rpb1 as 30 days of IH (Fig. 4A). In addition, 30 days of SH led to decreases in the levels of pVHL, Cullin2, and Rbx-1.
Figure 4.
(A) In extracts obtained from cortex, unlike in the hippocampus, there is no difference in hydroxylation, phosphorylation, or ubiquitylation of Rpb1 in mice exposed to IH or SH for 2 days as compared to mice exposed to room air. However, phosphorylation of Rpb1 is inhibited after 30 days of exposure to either IH or SH. Western blot analysis of nuclear extracts probed with the indicated antibodies. (B) Western blot analysis comparing constitutive levels of pVHL, Cullin2, and Rbx 1 in extracts from different parts of the brain. Labeling as in Fig1.
Levels of pVHL, Cul2, and Rbx 1 were similar in different parts of the brain (Fig. 4B), indicating that differences in constitutive levels of those regulatory proteins were not responsible for the observed differences in the effects of IH and SH on the modifications of Rpb1.
Altogether, these data indicate that IH, but not SH, increases overall levels of Rpb1, its P1465 hydroxylation, Ser5 phosphorylation, and nondegradative ubiquitylation specifically in CA1 region of hippocampus and the prefrontal cortex.
IH but not SH adversely affects spatial reference task performance in the Morris water maze
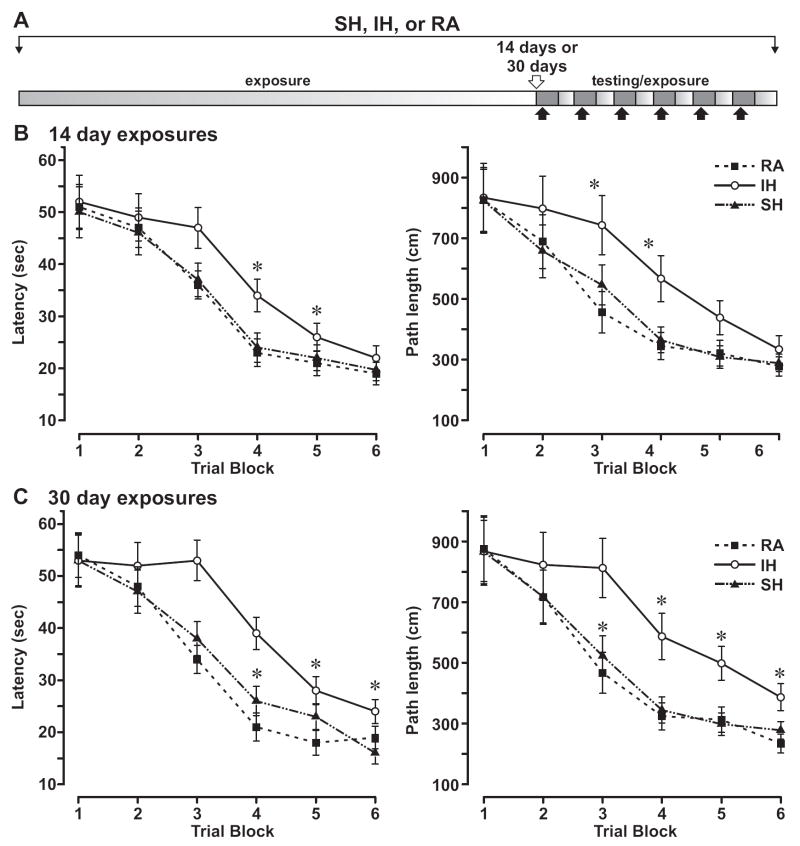
To further investigate whether conditions that led to the observed biochemical changes could also induce functional neural impairment, mice were exposed to 14 or 30 days of IH, SH, or standard room air and then assessed for spatial task acquisition and retention in a Morris water maze. Mice exposed to IH clearly showed substantial deficits (Fig. 5). There was a significant interaction for both latency and path length, and post-hoc analyses revealed that the IH-exposed mice were impaired with regard to measures of task acquisition, as compared to either SH or normoxic controls. No significant group differences were observed over the final day of training with exception of path length measured after 30 days of exposure (Fig. 5), indicating that all groups were able to reach a similar level of performance by the end of training. No significant differences emerged in swimming speeds over the trials among the 3 groups (data not shown). On measures of spatial bias obtained during the probe trial conducted 24 hours following completion of training, no significant group differences were observed in the percentage of time spent in the platform quadrant, or in the relative proximity to target platform location. However, IH-exposed mice had fewer platform crossings than the other 2 groups (data not shown, p<0.003). Mice exposed to SH for 30 days did not exhibit detectable changes in their ability to acquire or retain a standard place-training discrimination task. Indeed, SH mice displayed normal spatial learning and probe trial performances compared to normoxic mice.
Figure 5.
Spatial learning deficits in mice exposed to IH but not to SH. Top panel shows schematics of place-training session schedules for 6 days, with filled areas representing 12-hour periods of darkness, open areas corresponding to 12-hour daylight periods, and arrows to timing of water maze sessions in early darkness period. Mice were exposed to room air (filled squares, dashed line), IH (open circles, solid line), or SH (closed triangles, double-dashed line) for 14 (A) or 30 days (B). Mean latencies (sec, left) and swim distances (cm, right) to locate the target platform during place training. Data are expressed as mean ± SEM; * P<0.01 vs. controls; n=18 per group; each block represents 3 training sessions.
These data indicate that chronic IH, but not SH, elicits behavioral cognitive impairment originating within the hippocampus and prefrontal cortex.
The von Hippel-Lindau tumor suppressor is required for IH-induced P1465 hydroxylation, Serine 5 phosphorylation, and ubiquitylation
Because our previous work indicated that pVHL activity is instrumental for hydroxylation and Ser5 phosphorylation of Rpb1 in response to low-grade oxidative stress in renal cancer cells (Mikhaylova et al., 2008), we wanted to determine here if IH-induced modifications of Rpb1 involve pVHL activity.
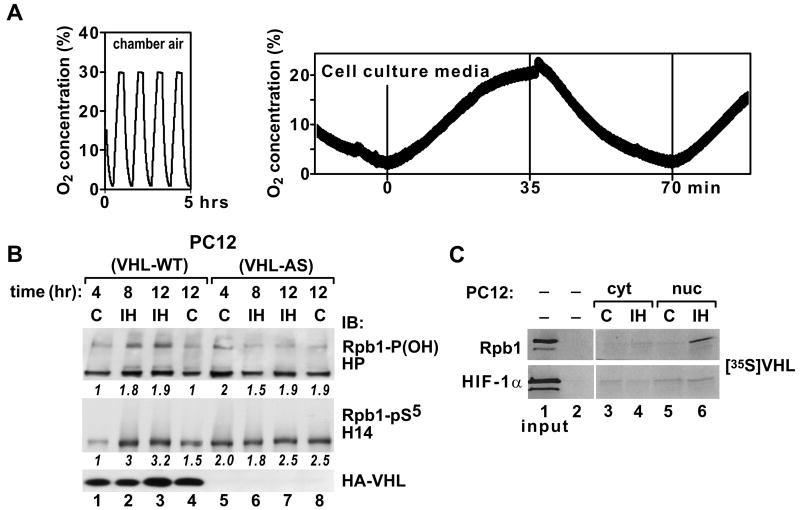
To evaluate the role of the pVHL complex in the hydroxylation and ubiquitylation of Rpb1, we employed neuroendocrine PC12 cells with manipulated pVHL levels (Kuznetzova et al., 2003). PC12 cells overexpressing pVHL (VHL-WT) or underexpressing pVHL (VHL-AS) were exposed to repeated fluctuations of O2 from 1% to 30%. In each cycle, equilibration of the cell culture media to the desired O2 level lasted approximately 30 to 35 min (Fig. 6A). IH stimulated P1465 hydroxylation, Ser5 phosphorylation, and ubiquitylation of Rpb1 only in nuclear extracts from cells expressing wild-type pVHL, but not from cells where expression of endogenous pVHL was substantially reduced by VHL antisense RNA (Fig. 6B). P1465 hydroxylation of Rpb1 by IH was additionally confirmed by the in vitro hydroxylation of an Rpb1 peptide in extracts obtained from cells exposed to IH (Fig. 6C). In this experiment, a synthetic 36-amino acid Rpb1 peptide or a control HIF-α peptide (Kuznetsova et al., 2008) containing the element undergoing hydroxylation, LGQLAP, was incubated with either cytoplasmic (cyt) or nuclear (nuc) extracts from PC12 cells (VHL-WT) exposed to normoxia or 8 h of IH. The successful hydroxylation of peptide was detected based on the peptide’s ability to capture exogenous VHL protein that had been in vitro translated in the presence of [35S]-labeled methionine. Clearly, nuclear, but not cytoplasmic, extract from cells exposed to IH had the ability to hydroxylate P1465, thus enabling the peptide to capture [35S]-labeled pVHL. In contrast, there was no effect of IH on hydroxylation of an HIF-α peptide under similar conditions (Fig. 6C). These data support results from cell culture experiments and whole-animal exposure to IH by providing in vitro evidence that IH specifically stimulates hydroxylation of P1465 on Rpb1.
Figure 6.
P1465 hydroxylation and ubiquitylation of Rpb1 correlate with the status of pVHL in pheochromocytoma cells (PC12). (A) Schematic representation of the fluctuations in O2 level in the environmental chamber (left) and in the cell culture medium (right) during exposure of cells to the IH paradigm. (B) Western blot (and quantification of the optical density in arbitrary units) of indicated extracts shows that IH stimulates P1465 hydroxylation, Ser5 phosphorylation, and ubiquitylation of Rpb1 in PC12 cells expressing pVHL (VHL-WT), but not in cells with pVHL knockdown (VHL-AS). (C) Hydroxylation of the Rpb1 peptide, but not the HIF-1α peptide, is induced upon incubation of peptides with extract from VHL-WT cells exposed to IH. Lane 1, input corresponding to 1% of [35S]pVHL; lanes 2 and 3, capture of [35S]pVHL after the peptides were incubated with cytoplasmic extracts (cyt) from control (C) or IH-exposed cells; lanes 4 and 5, capture of [35S]pVHL after the peptides were incubated with nuclear extracts (nuc) from control (C) or IH-exposed cells. In B and C, a representative example of at least three independent experiments is shown.
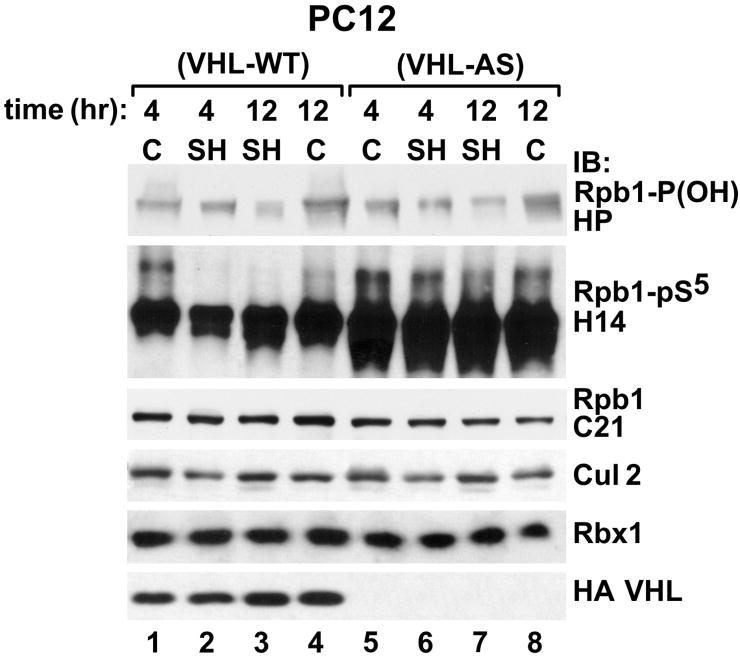
In contrast to IH, SH did not induce hydroxylation or phosphorylation of Rpb1, but it actually decreased both of them in PC12 VHL-WT cells (Fig. 7). P1465 hydroxylation and Ser5 phosphorylation were not affected in VHL-AS cells (Fig. 7). These data support a connection between P1465 hydroxylation and Ser5 phosphorylation in cells expressing wild-type pVHL but not in cells without VHL (Mikhaylova et al. 2008).
Figure 7.
pVHL is necessary for inhibition of P1465 hydroxylation and Ser5 phosphorylation by SH in PC12 cells. Exposure to the indicated duration of 1% O2 repressed P1465 hydroxylation and Ser5 phosphorylation in PC12 cells expressing wild-type pVHL, but not in cells with diminished levels of pVHL due to the expression of VHL antisense mRNA. Western blots were probed with the indicated antibodies.
DISCUSSION
In this work we have demonstrated that steady-state levels of Rpb1 and its three major modifications, hydroxylation of P1465, phosphorylation of serine 5, and ubiquitylation, are induced by IH. Moreover, we show that these modifications are specific to regions of the brain functionally affected during exposure to IH, the CA1 region of the hippocampus, and the prefrontal cortex, and that these biochemical changes coincide with functional neurological impairment. Importantly, the Rpb1 modifications occurred specifically in those areas of the brain that were previously demonstrated to suffer IH-induced loss of neurons, that is, the CA1 region of the hippocampus and the prefrontal cortex (Gozal et al., 2001a; Gozal et al, 2001b; Gozal et al., 2003; Row et al., 2002; Row et al., 2003). The modifications also occur in those regions where neuronal death, which occurs during relatively short exposures to IH (days), is replaced by partial neurogenesis in the CA1 and dentate gyrus after weeks of IH (Gozal et al., 2003; Zhu et al., 2005). This suggests that the biochemical changes observed in the CA1 region, which began after 48h of exposure but were more prominent after 30 days, could either cause or result from neuronal loss and/or neuronal regeneration in parts of the brain sensitive to IH.
These IH-related modifications of Rpb1 are likely to require the presence of pVHL. This idea arose from experiments using cell lines, such as PC12 cells with manipulated levels of pVHL (used here) or renal clear cell carcinoma cell lines with reconstituted wild-type pVHL in which we showed that P1465 hydroxylation, Ser5 phosphorylation, and ubiquitylation of Rpb1 in response to oxidative stress required the presence of pVHL (Mikhaylova et al., 2008). In that study we showed that pVHL-dependent hydroxylation of Rpb1 was primarily mediated by proline hydroxylase 1 (PHD1) while opposed by proline hydroxylase 2 (PHD2). We also demonstrated that hydroxylation of P1465 was necessary for the oxidative stress-induced phosphorylation of serine 5 residues within the CTD. Interestingly, we also showed, in a proteomic analysis using 2-d gel electrophoresis, that oxidative stress induced an increase in the intensity of 72 spots while only 14 spots were decreased by oxidative stress in VHL(+) cell extracts. These data are consistent with the stimulating effect of IH on protein synthesis in the CA1 region. In fact, IH induced expression of 32 proteins in the CA1 region, but only 7 proteins in CA3 regions (Gozal et al., 2002). Thus, although the evidence presented here is indirect, it is logical to propose that induction and modification of Rpb1 are major regulators of gene expression specifically in brain regions vulnerable to IH.
The molecular mechanism of this regulation of gene expression by Rpb1 modifications remains to be elucidated. However, phosphorylation of the CTD, particularly on Ser5, is required for the RNAPII complex to associate with multiple regulatory protein factors, including cap- and polyA-binding proteins and splicing factors, which are necessary for posttranscriptional RNA processing, transport, and translation (Maniatis and Reed, 2002). The CTD is also required for the binding of enzymes acting on DNA or chromatin, such as topoisomerase I, DNA (cytosine-5) methyltransferase 1, or poly(ADP-ribose)polymerase-1 (Carty & Greenleaf, 2002). Thus, phosphorylation of Ser5 represents an important and incompletely understood mechanism of gene expression regulation, which may control not only transcription but also splicing, mRNA transport, and translation in different cell systems (Maniatis and Reed, 2002).
Our results also demonstrated that, in contrast to IH, SH had a less consistent and overall inhibitory effect on Rpb1 modifications in the brain. It inhibited hydroxylation and phosphorylation of Rpb1 in extracts from the CA1 region after 30 days of treatment, but did not affect these modifications in the prefrontal cortex. This coincided with the lack of neurological impairment measured in our behavioral tests. Thus, the data support the specificity of IH in eliciting cellular and molecular damage and modifications of Rpb1 in specific regions of the hippocampus. These data are also in agreement with our cell culture model wherein PC-12 cells exposed to SH did not undergo extensive apoptosis, as compared to cells exposed to IH (Gozal et al., 2005).
SH had a strong inhibitory effect on P1465 hydroxylation and Ser5 phosphorylation of Rpb1 in PC12 cells. This effect was dependent on the presence of pVHL, as demonstrated by its occurrence in PC-12 cells expressing wild-type pVHL, but not in cells expressing antisense VHL mRNA. The data obtained from PC12 cells are consistent with the finding that the proline hydroxylases hydroxylating P1465 require molecular oxygen and that lowering the partial pressure of oxygen inhibits their activity (Epstein et al., 2001). Similar results were reported for HIF-αs for which the decrease in O2 and consequent inhibition of prolyl hydroxylases specifically resulted in dissociation from pVHL, inhibition of ubiquitylation, and degradation of HIF-αs (Epstein et al., 2001). These data are also consistent with our previously published data showing that hydroxylation of Rpb1 requires the presence of pVHL (Mikhaylova et al., 2008). The reason for the differences between the results obtained from the brain tissue and PC12 cells in response to SH is not clear. However, one potential explanation is that PC12 cells have an intrinsic ability to sense O2 level, as described by us previously in the context of tyrosine hydroxylase gene expression regulation by hypoxia, while neurons do not have this ability (Czyzyk-Krzeska et al., 1994).
Overall, we have identified novel modifications of the large subunit of RNA Polymerase II that are differentially regulated by IH and SH in the specific regions of the brain affected by IH. It is very likely that these modifications in RNA Polymerase II participate in the regulation of gene expression that is involved in adaptations to both types of hypoxia.
Acknowledgments
This work was supported in part by the following grants: NIH HL58687, HL66312, NCI CA122346, DoD W81XWH-07-02-0026 (to M.F.C-K). DG is supported by NIH grants SCOR 2P50-HL-60296 (Project 2) and RO1-HL-086662, The Children’s Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
We thank J. Striet and A. Gibson for technical assistance, G. Doerman for preparing the figures, and Dr. M. Daston for editorial assistance.
ABBREVIATIONS
- IH
intermittent hypoxia
- SH
sustained hypoxia
- RA
room air
- SDB
sleep disordered breathing
- pVHL
von Hippel-Lindau protein
- VHL-WT
wild type VHL
- VHL-AS
VHL antisense RNA
- RNAPII
RNA Polymerase II
- Rpb1
large subunit of RNA Polymerase II
- CTD
C-terminal domain
- Ser5
serine 5 in CTD
- P1465
Proline 1465 in Rpb1
- Cul2
cullin 2
- HP
hydroxylated proline
- PC12
pheochromocytoma cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartlett DJ, Rae C, Thompson CH, Byth K, Joffe DA, Enright, Grunstein RR. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med. 2004;5:593–596. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Carty SM, Greenleaf AF. Hyperphosphorylated C-terminal repeat domain bv associating proteins in the nuclear proteome link transcription to DNA/chromatin modification and RNA processing. Mol Proteomics. 2002;1:598–610. doi: 10.1074/mcp.m200029-mcp200. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem. 1994;269:760–764. [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda I, Tian YM, Masson N, Hamilton D, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, Payne RS, Cheng Z, Brittian KR, Gozal D. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003;122:585–590. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohniach GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001a;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, Sachleben LR, Jr, Guo SZ. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. Eur J Neurosci. 2003;18:2335–2342. doi: 10.1046/j.1460-9568.2003.02947.x. [DOI] [PubMed] [Google Scholar]
- Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett. 2001b;305:197–201. doi: 10.1016/s0304-3940(01)01853-5. [DOI] [PubMed] [Google Scholar]
- Gozal E, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleben LR, Jr, Brittian KR, Guo SZ, Cai J, Klein JB. Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem. 2002;83:331–345. doi: 10.1046/j.1471-4159.2002.01134.x. [DOI] [PubMed] [Google Scholar]
- Gozal E, Sachleben LR, Jr, Rane MJ, Vega C, Gozal D. Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol Cell Physiol. 2005;288:535–542. doi: 10.1152/ajpcell.00270.2004. [DOI] [PubMed] [Google Scholar]
- Gu XQ, Haddad GG. Decreased neuronal excitability in hippocampus neurons of mice exposed to cyclic hypoxia. J Appl Physiol. 2001;91:1245–1250. doi: 10.1152/jappl.2001.91.3.1245. [DOI] [PubMed] [Google Scholar]
- Kheirandish L, Row BW, Li RC, Brittian KR, Gozal D. Apolipoprotein E deficient mice exhibit increased vulnerability to intermittent hypoxia-induced spatial learning deficits. Sleep. 2005;28:1412–1417. doi: 10.1093/sleep/28.11.1412. [DOI] [PubMed] [Google Scholar]
- Klein J, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleban LR, Guo S-Z, Cai J, Gozal E. Proteomic identification of a novel protein regulated in CA1 and CA3 hippocampal regions during intermittent hypoxia. Resp Physiol Neurobiol. 2002;136:91–103. doi: 10.1016/s1569-9048(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, Sanchez Y, Conaway JW, Conaway RC, Czyzyk-Krzeska MF. von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA. 2003;100:2706–2711. doi: 10.1073/pnas.0436037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Resp Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Marzatico F, Curti D, Dagni F, Taglietti M, Benzi G. Brain enzyme adaptation to mild normobaric intermittent hypoxia. J Neurosci Res. 1986;16:419–428. doi: 10.1002/jnr.490160209. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- Mikhaylova O, Ignacak ML, Barankiewicz TJ, Harbaugh SV, Ying Y, Maxwell PH, Schneider M, Van Geyte K, Carmeliet P, Revelo MP, Wyder M, Greis KD, Meller J, Czyzyk-Krzeska MF. The von Hippel-Lindau tumor suppressor protein and Egl 9-type proline hydroxylases regulate the large subunit of RNA Polymerase II in response to oxidative stress. Mol Cell Biol. 2008;28:2701–2717. doi: 10.1128/MCB.01231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O’Donnell CP, Adachi M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Resp Crit Care Med. 2007;175:612–617. doi: 10.1164/rccm.200608-1141OC. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin ARC, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Twigg G. Neural consequences of sleep-disordered breathing: the role of intermittent hypoxia. Adv Exp Med Biol. 2006;588:75–88. doi: 10.1007/978-0-387-34817-9_8. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial earning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Hobrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, Hume B, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year old children with parentally reported symptoms of attention-deficit disorder. Pediatrics. 2003;111:554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ivanenko A, Crabtree VM, Holbrook CR, Bruner JL, Klaus CJ, Gozal D. Sleep disturbances in children with attention deficit hyperactive disorder. Ped Res. 2003;54:237–243. doi: 10.1203/01.PDR.0000072333.11711.9A. [DOI] [PubMed] [Google Scholar]
- Payne RS, Goldbart A, Gozal D, Schurr A. Effect of intermittent hypoxia on long-term potentiation in rat hippocampal slices. Brain Res. 2004;1029:195–199. doi: 10.1016/j.brainres.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Punjab NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Ped Res. 2002;52:449–453. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Resp Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neurosci. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhao T, Li H, Zhao H, Wu L, Ding A, Fan W, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res. 2005;1055:1–6. doi: 10.1016/j.brainres.2005.04.075. [DOI] [PubMed] [Google Scholar]