Abstract
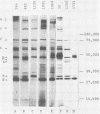
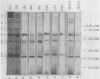
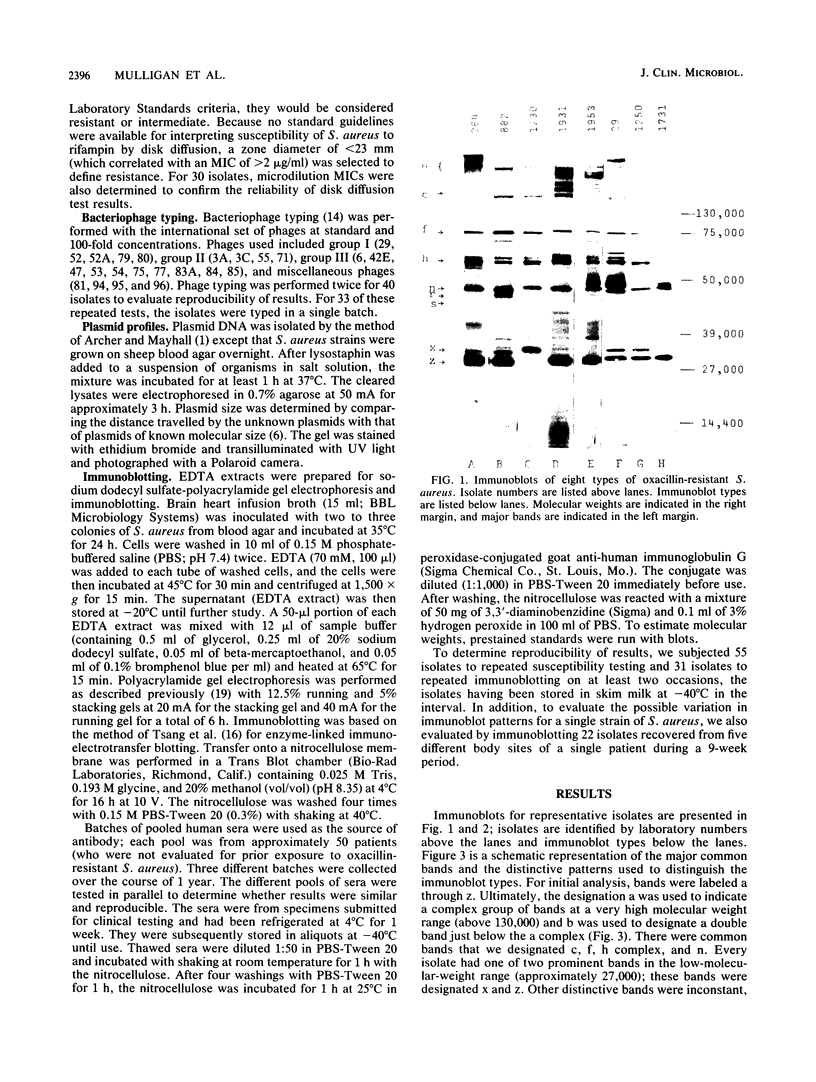
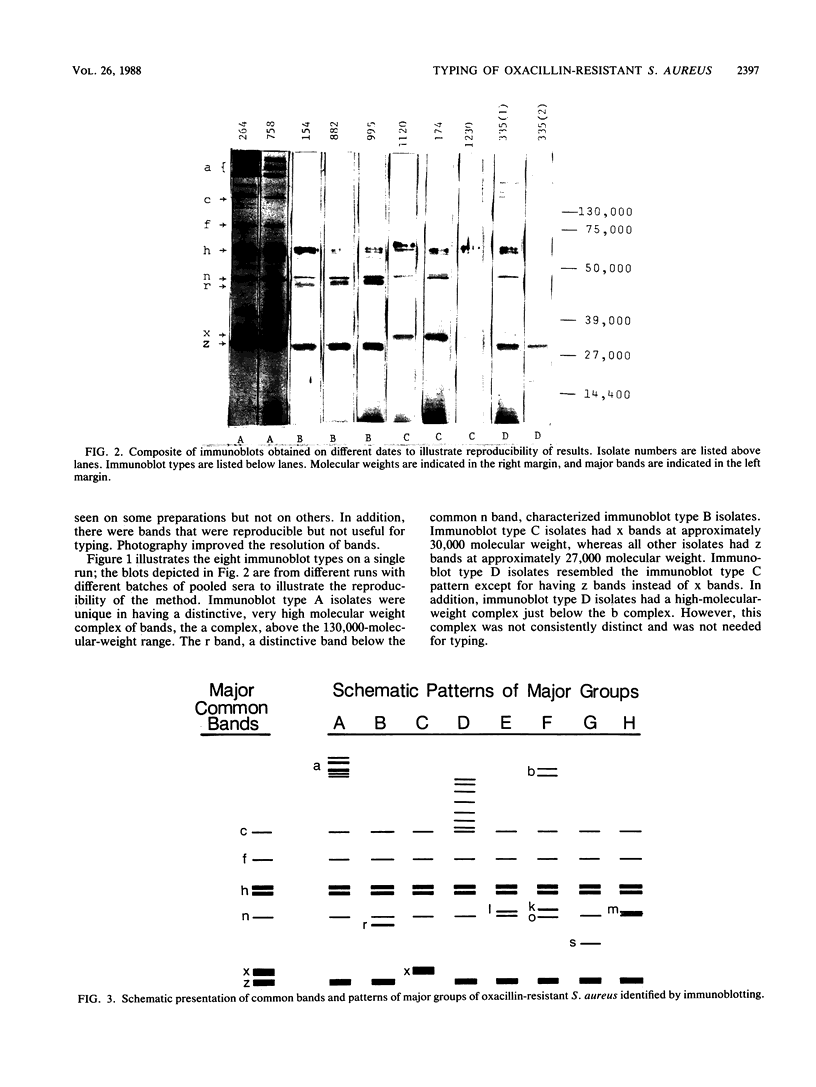
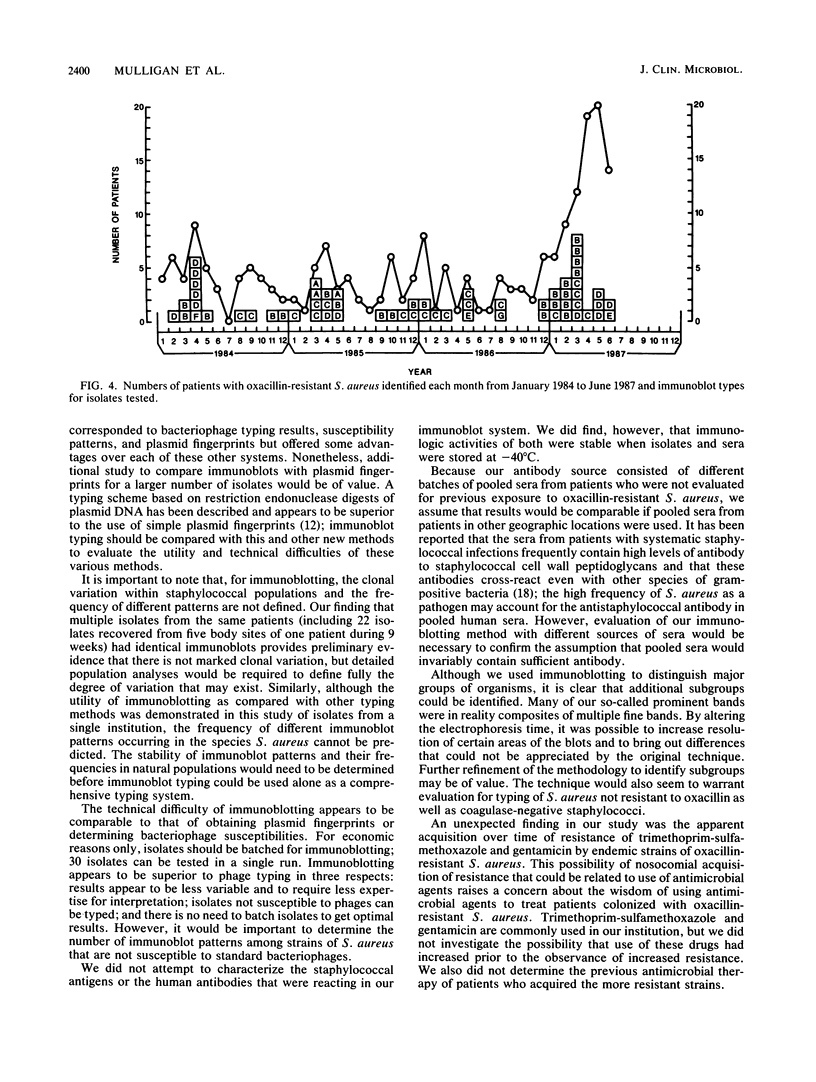
An immunoblotting system was developed for typing of oxacillin-resistant Staphylococcus aureus. Clinical isolates recovered during a 40-month period at a single institution were evaluated with this typing scheme. Results were compared with susceptibility patterns and with bacteriophage typing results for 100 clinical isolates and with plasmid fingerprints for 14 isolates. Immunoblotting was found to be a useful method with good reproducibility that distinguished seven major groups of patient isolates that were clinically and epidemiologically related. Susceptibility patterns showed specific correlations with other typing results but were inferior to immunoblotting and phage typing for differentiating major groups of organisms. Plasmid profiles failed to distinguish two major groups that were readily identified by immunoblots and phage typing. There was evidence of increasing antimicrobial resistance of endemic hospital strains. Immunoblotting correlated well with phage typing, offered an alternative method for typing isolates that could not be typed by phage typing, and was superior to susceptibility testing and plasmid profiles for distinguishing different groups of oxacillin-resistant S. aureus at our institution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Mayhall C. G. Comparison of epidemiological markers used in the investigation of an outbreak of methicillin-resistant Staphylococcus aureus infections. J Clin Microbiol. 1983 Aug;18(2):395–399. doi: 10.1128/jcm.18.2.395-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. A., Edberg S. C., David G. Infectious disease in the sella turcica. Rev Infect Dis. 1986 Sep-Oct;8(5):747–755. doi: 10.1093/clinids/8.5.747. [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Landry M., Deetz T. R., DuPont H. L. Epidemiologic studies of an outbreak of nosocomial methicillin-resistant Staphylococcus aureus infections. Infect Control. 1981 Mar-Apr;2(2):110–116. doi: 10.1017/s0195941700053881. [DOI] [PubMed] [Google Scholar]
- Crossley K., Landesman B., Zaske D. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. II. Epidemiologic studies. J Infect Dis. 1979 Mar;139(3):280–287. doi: 10.1093/infdis/139.3.280. [DOI] [PubMed] [Google Scholar]
- Gilbert G. L., Asche V., Hewstone A. S., Mathiesen J. L. Methicillin-resistant Staphylococcus aureus in neonatal nurseries. Two years' experience in special-care nurseries in Melbourne. Med J Aust. 1982 May 29;1(11):455–459. [PubMed] [Google Scholar]
- Locksley R. M., Cohen M. L., Quinn T. C., Tompkins L. S., Coyle M. B., Kirihara J. M., Counts G. W. Multiply antibiotic-resistant Staphylococcus aureus: introduction, transmission, and evolution of nosocomial infection. Ann Intern Med. 1982 Sep;97(3):317–324. doi: 10.7326/0003-4819-97-3-317. [DOI] [PubMed] [Google Scholar]
- Peacock J. E., Jr, Marsik F. J., Wenzel R. P. Methicillin-resistant Staphylococcus aureus: introduction and spread within a hospital. Ann Intern Med. 1980 Oct;93(4):526–532. doi: 10.7326/0003-4819-93-4-526. [DOI] [PubMed] [Google Scholar]
- Peacock J. E., Jr, Moorman D. R., Wenzel R. P., Mandell G. L. Methicillin-resistant Staphylococcus aureus: microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J Infect Dis. 1981 Dec;144(6):575–582. doi: 10.1093/infdis/144.6.575. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Shlaes D. M., Keys T. F., Serkey J., Kirkley B., Kim C., Currie-McCumber C. A., Hall G. Nosocomial clonal dissemination of methicillin-resistant Staphylococcus aureus. Elucidation by plasmid analysis. Arch Intern Med. 1987 Mar;147(3):521–524. [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P. The emergence of methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):440–442. doi: 10.7326/0003-4819-97-3-440. [DOI] [PubMed] [Google Scholar]
- Wergeland H. I., Endresen C. Antibodies to various bacterial cell wall peptidoglycans in human and rabbit sera. J Clin Microbiol. 1987 Mar;25(3):540–545. doi: 10.1128/jcm.25.3.540-545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H., Mulligan M. E., Finegold S. M. Polyacrylamide gel electrophoresis patterns produced by Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S229–S234. doi: 10.1093/clinids/6.supplement_1.s229. [DOI] [PubMed] [Google Scholar]
- Yu V. L., Goetz A., Wagener M., Smith P. B., Rihs J. D., Hanchett J., Zuravleff J. J. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91–96. doi: 10.1056/NEJM198607103150204. [DOI] [PubMed] [Google Scholar]