Abstract
OBJECTIVE
To determine if the use of metformin in people with prediabetes (impaired glucose tolerance or impaired fasting glucose) would prevent or delay the onset of frank type 2 diabetes mellitus.
DATA SOURCES
MEDLINE was searched from January 1966 to the present, and articles meeting the selection criteria were hand searched.
STUDY SELECTION
Randomized controlled trials that involved administration of metformin to delay or prevent type 2 diabetes in individuals with impaired glucose tolerance or impaired fasting glucose were included. Development of diabetes was a required outcome measure; follow-up time of at least 6 months was required. Three studies met these criteria.
SYNTHESIS
The 3 studies varied in ethnicity of the population studied, in the rates of conversion to diabetes from prediabetes, and in the dose of metformin used. In general the studies were well done, although 2 of the 3 did not do true intention-to-treat analyses. A sensitivity analysis was completed by converting all data to intention-to-treat data and assuming a worst-case scenario for the people who were lost to follow-up.
CONCLUSION
Metformin decreases the rate of conversion from prediabetes to diabetes. This was true at higher dosage (850 mg twice daily) and lower dosage (250 mg twice or 3 times daily); in people of varied ethnicity; and even when a sensitivity analysis was applied to the data. The number needed to treat was between 7 and 14 for treatment over a 3-year period.
Résumé
OBJECTIF
Déterminer si l’utilisation de la metformine chez des pré-diabétiques (intolérance au glucose ou hyperglycémie à jeun) prévient ou retarde l’apparition d’un franc diabète de type 2.
SOURCES DES DONNÉES
On a consulté MEDLINE, depuis janvier 1966 jusqu’à aujourd’hui. Les articles répondant aux critères de sélection ont été repérés à la main.
CHOIX DES ÉTUDES
On a retenu les essais cliniques randomisés qui comportaient l’administration de la metformine pour retarder ou prévenir le diabète de type 2 chez des sujets présentant une intolérance au glucose ou une hyperglycémie à jeun. Le développement d’un diabète était requis comme issue mesurable; le suivi devait être d’au moins 6 mois. Trois études répondaient à ces critères.
SYNTHÈSE
Les caractéristiques ethniques des populations étudiées, les taux de conversion de pré-diabète en diabète et les doses de metformine utilisées différaient dans les études retenues. Les études étaient généralement bien faites, quoique deux d’entre elles n’avaient pas utilisé une véritable analyse respectant le principe de l’intention de traitement. On a effectué une analyse de sensibilité en transformant toutes les données comme si elles respectaient le principe de l’intention de traitement et en supposant le pire scénario pour les sujets n’ayant pas complété l’étude.
CONCLUSION
La metformine a diminué le taux de conversion du pré-diabète en diabète. Cela était vrai aux fortes doses (850 mg b.i.d.) comme aux doses plus faibles (250 mg b.i.d ou t.i.d.); chez des personnes d’origines ethniques différentes; et même après analyse de sensibilité des données. Le nombre de traitements requis variait entre 7 et 14 pour un traitement d’une durées de 3 ans.
Type 2 diabetes is a worldwide epidemic. Prevalence has tripled in the last 30 years, and diabetes is predicted to affect more than 320 million persons by 2025. The concept of pre-disease, or at least the language of pre-disease, is relatively new. Pre-disease is the recognition that the upper limits of normal (what we used to call high normal or borderline) for measurements such as blood pressure and blood glucose might pose a health risk and might be a warning that a patient is progressing toward overt hypertension or diabetes.
Prediabetes includes the concepts of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). In 2005 Wen et al reported on an 11-year follow-up of 36 000 persons.1 Those with IFG (fasting glucose levels between 6.1 and 6.9 mmol/L) had significantly increased risk of mortality related to cardiovascular disease (CVD) and diabetes compared with people with blood glucose levels below 6 mmol/L. In a detailed review of the topic, Unwin and colleagues concluded that IFG and IGT (glucose ≥ 7.8 and < 11.1 mmol/L, 2 hours after ingestion of a 75-g oral glucose load) were strongly associated with CVD. Impaired glucose tolerance was more strongly associated with CVD than IFG was.2
A number of studies have looked at lifestyle and pharmacologic interventions in people with prediabetes to determine if progression to frank diabetes can be prevented. These studies were summarized in a meta-analysis published recently in the British Medical Journal.3 Researchers concluded that, in people with IGT, lifestyle and pharmacologic interventions (various antiobesity agents and oral hypoglycemic agents) are effective in delaying the onset of type 2 diabetes. They did not look at metformin individually, but instead included it with all other oral antidiabetic agents. Metformin is recommended as first-line treatment in diabetes; it is inexpensive compared with the newer drugs, and we believe that a review looking specifically at metformin is important.
The objective of this study was to determine whether the use of metformin in people with prediabetes (IFG or IGT) would prevent or delay the onset of frank type 2 diabetes mellitus.
DATA SOURCES
Literature search
MEDLINE was searched from January 1966 to the present for randomized controlled trials (RCTs) that focused on using metformin to treat prediabetes. The MeSH headings prediabetic state, glucose intolerance, and metformin were used. We also checked references of any articles and published reviews that used metformin to prevent type 2 diabetes. The search was not limited to English-language articles, but no articles written in other languages met the inclusion criteria.
Study selection
Only RCTs were selected, in order to ensure inclusion of only high-quality evidence. Studies had to involve administration of metformin to delay or prevent type 2 diabetes in a sample or subsample of individuals with IGT or IFG. Development of diabetes was a required outcome measure; follow-up time of at least 6 months was also required.
Twenty studies were identified through the literature search. Of those, we excluded 17: 13 did not focus on the development of type 2 diabetes as a primary outcome measure4–16; 4 focused on inherent risk factors influencing diabetes progression, rather than on metformin use17–20; and 1 examined metformin use in combination with rosiglitazone.21 Hand searching of the references in the remaining 2 articles22,23 and the references in a recent systematic review of all treatment strategies for prediabetes identified a third article that met our criteria.24 In total, 3 studies were included in this review (Tables 1 and 222–24).
Table 1.
Summary of methodology of reviewed trials studying the effects of metformin on prediabetes
| STUDY | DESIGN | PATIENTS | TREATMENT GROUP | CONTROL GROUP | OUTCOME OF INTEREST |
|---|---|---|---|---|---|
| Li et al,22 1999 | Double-blind, placebo-controlled RCT | 29 938 subjects from Shougang Corporation in Beijing, China, were screened with OGTT in 1992. Of those, 1165 had IGT and were rescreened in 1994. Those already taking metformin or who had renal, hepatic, or ischemic heart disease were excluded. After rescreening, 90 still had IGT. Participants included men and women aged 30–60 y | Received 250 mg metformin TID and diabetes education (information on diet, exercise, and healthy lifestyle) every 3 mo
n = 45 (33 included in primary analysis) |
Received placebo tablets identical in appearance to metformin, provided by the metformin manufacturer, and the same diabetes education as the metformin group
n = 45 (37 included in primary analysis) |
Development of diabetes after 12 mo |
| Ramachandran et al,23 2006 | RCT with 4 groups:
|
10 839 men and women, aged 33–55 y, from a middle-class Asian Indian population with no major illnesses and no pre-existing diagnosis of diabetes were screened from March 2001 to July 2002; IGT was diagnosed on basis of 2 consecutive OGTTs (FPG < 7 mmol/L; 2-h postprandial glucose 7.8–11.0 mmol/L) | Received 250 mg metformin BID
n = 136 (133 available for follow-up) |
Received usual care (standard health care advice)
n = 129 (128 available for follow-up) |
Development of diabetes after 3 y |
| Knowler et al,24 2002 | Multicentre RCT with 3 arms:
|
Adults 25 y and older (mean age 51 y) with FPG 5.3–6.9 mmol/L and 2-hr postprandial glucose of 7.8–11.0 mmol/L; 32% of patients were men; participants had an average BMI of 34 | Received 850 mg metformin BID and standard lifestyle recommendations‡ n = 1073 |
Received placebo tablets and standard lifestyle recommendations
n = 1082 |
Development of diabetes after 3 y |
BID—twice daily, BMI—body mass index, FPG—fasting plasma glucose, IGT—impaired glucose tolerance, LSM—lifestyle modification, OGTT—oral glucose tolerance test, RCT—randomized controlled trial, TID—3 times daily.
We included groups 1 and 3 in our review
We included groups 1 and 2 in our review.
Intention-to-treat analysis was used. We also did a worst-case-scenario sensitivity analysis.
Table 2.
Summary of results from reviewed trials: Development of diabetes outcomes.
| STUDY | OUTCOME | EER n/N (%) | CER n/N (%) | RRR % (95% CI) | ARR % (95% CI) | NNT N (95% CI) | YATES CORRECTED P VALUE | COMMENTS |
|---|---|---|---|---|---|---|---|---|
| Li et al,22 1999 (Primary analysis of 70 participants) | Development of diabetes at 12 mo | 1/33 (3.0) | 6/37 (16.2) | 81.3 (−9.5 to 97.0) | 13.2 (−0.9 to 17.9) | 7.6 (5.5 to infinity) | .15 | Our 2-way contingency table analysis did not show a statistically significant difference in development of diabetes based on χ2 test |
| Li et al,22 1999 (Intention-to- treat and worst-case- scenario sensitivity analysis) | Development of diabetes at 12 mo | 6/45 (13.3) | 8/45 (17.8) | 25 (−9.3 to 71.0) | 4.4 (−9.0 to 17.2) | 22.5 (5.8 to infinity) | .77 | As expected the sensitivity analysis is even less statistically significant |
| Ramachandran et al,23 2006 (Analysis excludes those lost to follow- up) | Development of diabetes at 3 y | 52/128 (40.6) | 73/133 (54.9) | 26 (4.4 to 42.8) | 14.3 (2.1 to 26.0) | 7.0 (3.8 to 46.7) | .029 | Shows a statistically significant and, by our assessment, clinically significant decrease in progression to diabetes; lack of placebo control is of some concern |
| Ramachandran et al,23 2006 (Intention-to- treat and worst-case- scenario sensitivity analysis) | Development of diabetes at 3 y | 53/129 (41.1) | 73/136 (53.7) | 23.5 (1.2 to 40.9) | 12.6 (0.6 to 24.2) | 7.9 (4.1 to 178.0) | .054 | Statistical significance at the .05 level is not quite reached using Yates correction for χ2; however, the 95% CIs suggest statistical significance |
| Knowler et al,24 2002 (Analysis from article) | Development of diabetes at 3 y | 233/1073 (21.7) | 313/1082 (28.9) | 24.9 (13.1 to 35.2) | 7.2 (3.5 to 10.8) | 13.9 (9.2 to 28.2) | < .001 | The large sample size contributes to the highly statistically significant outcome; the 7.2% absolute difference is clinically significant, especially on a population basis |
| Knowler et al,24 2002 (Worst-case- scenario sensitivity analysis) | Development of diabetes at 3 y | 237/1073 (22.1) | 313/1082 (28.9) | 23.6 (1.7 to 34.0) | 6.8 (3.2 to 10.5) | 14.6 (9.6 to 31.6) | < .001 | Worst-case- scenario sensitivity analysis did not appreciably change results because almost all participants were available for follow-up |
ARR—absolute risk reduction, CER—control event rate, CI—confidence interval, EER—experimental event rate, NNT—number needed to treat, RRR—relative risk ratio.
SYNTHESIS
Both authors reviewed the 3 articles independently. Although we were not blinded to the authors or citation sources of the articles, we were not familiar with the authors of any of the excluded or included articles. The critical appraisal process considered the validity of the methods, the strength of the results, the study populations, and how well the results could be applied to clinical practice. The methods and results are reported for the 3 studies in Tables 1 and 2.22–24
The study by Li et al enrolled 90 participants, 45 in each group.22 In their primary analysis, however, Li et al excluded patients from both groups if they did not comply with treatment (metformin or placebo), if they were lost to follow-up, or if they had gastrointestinal side effects. This left just 70 patients who were analyzed for outcomes in the primary analysis: 33 in the metformin group and 37 in the placebo group. The authors did perform what they referred to as an intention-to-treat analysis but still excluded the 5 participants (3 metformin, 2 placebo) who were lost to follow-up. They reported follow-up outcomes on those excluded for non-compliance and side effects but not on those lost to follow-up. We report on their primary analysis of 70 participants, but we also conducted an intention-to-treat analysis including the 5 participants who were lost to follow-up. We structured it as a worst-case scenario: the 3 participants in the metformin group were assumed to have developed diabetes and the 2 in the placebo group were assumed to have not. Our reanalysis of their primary analysis does not show a statistically significant difference as they reported. As expected, our intention-to-treat, worst-case-scenario sensitivity analysis also did not show a statistically significant difference.
The study by Ramachandran et al enrolled 531 participants randomly assigned to 1 of 4 different groups.23 We compared the metformin-only group with the usual care (control) group. No placebo was used in the control group, which meant that blinding patients to treatment was not possible. Of 129 participants enrolled in the control group and 136 in the metformin group, only 128 and 133, respectively, were available for follow-up and analysis. As in the study by Li et al, we carried out intention-to-treat, worst-case-scenario sensitivity analysis. The significant difference seen in the analysis reported in the study was still minimal in the sensitivity analysis.
The study by Knowler et al from the Diabetes Prevention Program (DPP) Research Group enrolled 3234 persons who were randomly assigned to 1 of 3 groups.24 We compared the placebo-controlled group (n = 1082) with the metformin group (n = 1073). This study appears to have been well done and was well reported; it also had a substantially larger sample size than the other 2 studies. The authors did an intention-to-treat analysis, meaning they included all those who were enrolled in the study in the assessment of outcomes. However, in order to have treated all studies equally, we decided to conduct a worst-case-scenario sensitivity analysis for this study as well. The authors did not report the actual number of participants lost to follow-up in each group. They did report that 99.6% of the full study population was alive at the end of the follow-up period. We assumed that this percentage was equally distributed across both groups and used this to calculate the number of people lost to follow-up in each group. We used these numbers in our worst-case-scenario analysis.
Four meta-analyses were performed. Figure 122–24 shows the results of the meta-analysis that includes the findings from all 3 studies as the authors reported them. Figure 222–24 reports on the results of the 3 studies when intention-to-treat, worst-case-scenario sensitivity analysis was used. Figure 322,23 reports on the 2 studies in which a lower dose of metformin was used (sensitivity analysis data). Figure 422,24 reports on the 2 studies that used a placebo control (sensitivity analysis data).
Figure 1.
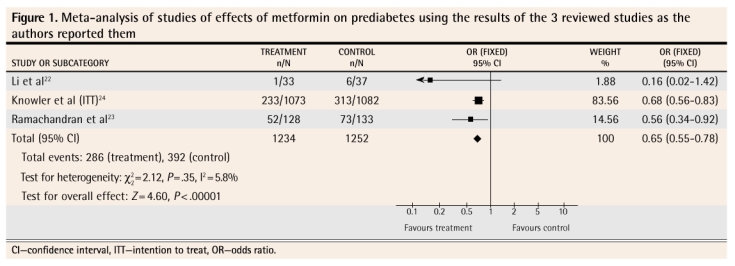
Meta-analysis of studies of effects of metformin on prediabetes using the results of the 3 reviewed studies as the authors reported them
Figure 2.
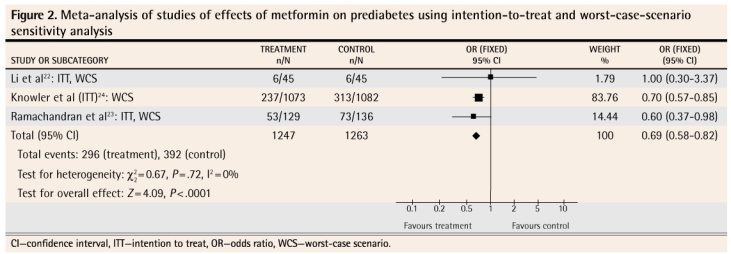
Meta-analysis of studies of effects of metformin on prediabetes using intention-to-treat and worst-case-scenario sensitivity analysis
Figure 3.
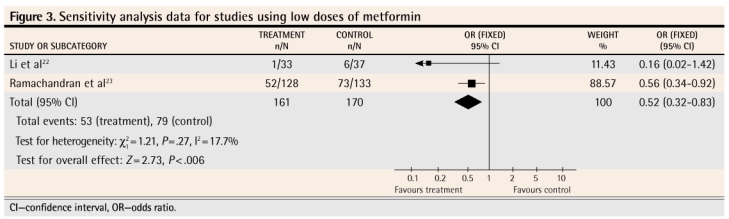
Sensitivity analysis data for studies using low doses of metformin
Figure 4.
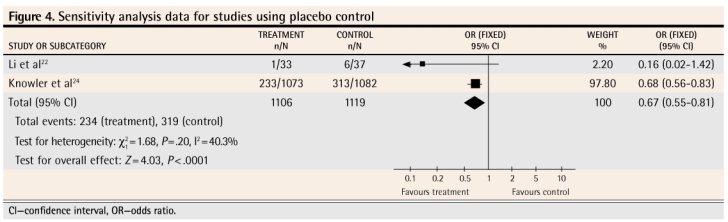
Sensitivity analysis data for studies using placebo control
DISCUSSION
The meta-analysis of the results of these 3 studies as they were presented by the authors shows that metformin, used for up to 3 years, does decrease the likelihood that prediabetes will progress to diabetes (Figure 122–24). In order to counteract the potential bias caused by lack of intention-to-treat analysis in 2 of the studies and the effect of patients who were lost to follow-up in all of the studies, we conducted a sensitivity analysis. We restructured the numbers to reflect a true intention-to-treat scenario (we included all patients enrolled in each study in the results calculations for each study) and a worst-case scenario, in which those lost to follow-up in the intervention groups were assumed to have progressed to diabetes and those lost to follow-up in the control groups were assumed not to have progressed to diabetes. Even when stacking the odds against a significant effect, the meta-analysis still showed that metformin decreased the likelihood of progression to diabetes (Figure 222–24). We also performed 2 other meta-analyses. The first included only the 2 studies where a lower dose of metformin was used (Figure 322,23) and revealed that the lower dose also had a significant effect, at least in the ethnic subgroups (Indian and Chinese) in which these studies were conducted. The other meta-analysis included only the 2 studies where a placebo (Figure 422,24) was used in control groups; this also showed a significant effect of treatment.
We also calculated the numbers-needed-to-treat (NNTs) for these 4 meta-analyses. For the meta-analysis in Figure 1,22–24 the NNT is 12 (95% confidence interval [CI] 9 to 21); for the meta-analysis in Figure 2,22–24 the NNT is 12 (95% CI 9 to 22); for the meta-analysis in Figure 3,22,23 the NNT is 7 (95% CI 4 to 32); for the meta-analysis in Figure 4,22,24 the NNT is 14 (95% CI 9 to 27). All of these meta-analyses are dominated by studies that followed patients for 3 years; hence the NNTs apply to treatment over a 3-year period.
It is important to note the variation in overall rates of progression to diabetes in these 3 groups. The study conducted in China22 had an overall rate of conversion to diabetes of 10%; the study in India23 a rate of 48%; the DPP study,24 in which ethnicity was mixed (55% white, 20% African American, and only 5% Asian), a rate of conversion to diabetes midway between the other 2 studies at 24%. This fits with the recognized higher prevalence of diabetes and metabolic syndrome in people of South Asian decent.
It is difficult to know from this review whether the relative effectiveness of the lower dosage of metformin (250 mg twice or 3 times daily) compared with the higher dosage (850 mg twice daily) used in the DPP study would hold true for all people. The 2 studies that used lower dosages were conducted in China and India, where conversion rates to diabetes are different from that in the study using the higher metformin dosage. In the 2 studies that used lower dosages, only the Indian study (in which the overall conversion rate was much higher) showed a statistically significant difference in rates of conversion between treatment and control. It is possible that the effectiveness of the lower dosage is somehow related to genetics or ethnicity.
Limitations
The main limitation of this paper is the difficulty of applying the results to clinical practice. The studies do show that patients with prediabetes who take metformin are less likely to have blood glucose levels in the diabetic range after 3 years. It is possible, however, that this is simply a treatment effect and not a prevention of progression at all. It is likely because of this possibility that most primary care physicians are not yet prescribing metformin for their patients with prediabetes, but using lifestyle treatment instead. It is also probably because of this possibility that guideline-generating groups are not yet recommending that prediabetes be treated with metformin as a matter of course.
Whenever a systematic review is conducted, there is the possibility of missing important published articles and unpublished data. Our search of MEDLINE was exhaustive, and it is unlikely we missed any RCTs indexed in that database. It is possible that other databases, such as EMBASE, might have indexed articles that were not included in MEDLINE. Our hand checking of references in the articles we retrieved and the huge overlap between MEDLINE and EMBASE makes it unlikely that an important published article was missed. We did not approach investigators working in the field to see if they had unpublished data that met our criteria. The possibility of unpublished data exists, but we believe this possibility is remote because a randomized trial of sufficient power and quality to have met our inclusion criteria would have been expensive to complete and publication would, in all likelihood, have been sought.
Future research
There is uncertainty about the effect of metformin: is the effect seen in these studies a treatment effect or a preventive effect? A study of sufficient power needs to be conducted that duplicates the effects of metformin shown in these studies; that study then needs to switch both groups to placebo to see if the benefit disappears within a few weeks in the group that previously had metformin. If the effect disappears it would mean we are simply seeing a treatment effect—that is, the metformin was keeping the blood glucose in the nondiabetic range rather than slowing the course of diabetes.
Conclusion
It seems that, even when applying a worst-case-scenario sensitivity analysis, the effectiveness of metformin on rates of conversion from prediabetes to diabetes remains. The NNT for treatment over a 3-year period is between 7 and 14. This compares very favourably with many other treatments and on a population basis could have an important effect on diabetes and its complications. It is probably best to use a metformin dosage of 850 mg twice daily except in people of South Asian descent, for whom this dosage might be higher than needed and might lead to side effects.
EDITOR’S KEY POINTS
A number of studies have looked at lifestyle and pharmacologic interventions in people with prediabetes to determine if progression to frank diabetes can be prevented, but none has looked at metformin individually.
This meta-analysis showed that treatment with metformin decreased the likelihood of progression to diabetes. Even when applying a worst-case-scenario sensitivity analysis, the effectiveness of metformin on rates of conversion from prediabetes to diabetes remained.
A study needs to be conducted, however, to see if the benefits seen in these studies represent a treatment effect or a preventive effect. If patients treated with metformin were switched to placebo, would the benefit disappear within a few weeks?
POINTS DE REPÈRE DU RÉDACTEUR
Plusieurs études ont examiné le rôle du mode de vie et de certains médicaments chez des pré-diabétiques afin d’établir si on peut empêcher la conversion d’un pré-diabète en franc diabète, mais aucune n’a vérifié l’effet de la metformine administrée seule.
Cette méta-analyse a montré qu’un traitement à la metformine diminue la probabilité d’une progression vers un diabète. Même avec une analyse de sensibilité utilisant le pire scénario, la metformine demeure efficace pour réduire le taux de conversion d’un pré-diabète en diabète.
Une étude sera toutefois nécessaire pour savoir si l’avantage observé dans ces études correspond à un effet de traitement ou à un effet préventif. Si on remplaçait la metformine par un placebo, cet avantage disparaîtrait-il en quelques semaines?
Footnotes
Cet article a fait l’objet d’une révision par des pairs.
Competing interests
None declared
Contributors
Ms Lilly and Dr Godwin contributed to concept and design of the study, the literature review, selection and analysis of the studies, interpretation of the analysis, and preparing the manuscript for submission.
This article has been peer reviewed.
References
- 1.Wen CP, Cheng TY, Tsai SP, Hsu HL, Wang SL. Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care. 2005;28(11):2756–61. doi: 10.2337/diacare.28.11.2756. [DOI] [PubMed] [Google Scholar]
- 2.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19(9):708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 3.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. doi: 10.1136/bmj.39063.689375.55. Epub 2007 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi K, Tomizawa H, Ishikawa J, Hoshide S, Numao T, Fukuda T, et al. Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetes. Hypertens Res. 2007;30(1):23–30. doi: 10.1291/hypres.30.23. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians—result of Indian Diabetes Prevention Programme. Diabetes Res Clin Pract. 2007;76(2):215–8. doi: 10.1016/j.diabres.2006.08.009. Epub 2006 Sep 18. [DOI] [PubMed] [Google Scholar]
- 6.Maji D, Roy RU, Das S, Hoshide S, Numao T, Fukuda T, et al. Prevention of type 2 diabetes in the prediabetic population. J Indian Med Assoc. 2005;103(11):609–11. [PubMed] [Google Scholar]
- 7.Gubern C, Lopez-Bermejo A, Biarnes J, Vendrell J, Ricart W, Fernández-Real JM. Natural antibiotics and insulin sensitivity: the role of bactericidal/ permeability-increasing protein. Diabetes. 2006;55(1):216–24. [PubMed] [Google Scholar]
- 8.Biarnes J, Fernándes-Real JM, Fernandez-Castaner M, del Mar García M, Soler J, Ricart W. Differential regulation of insulin action and tumor necrosis factor alpha system activity by metformin. Metabolism. 2005;54(2):235–9. doi: 10.1016/j.metabol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005;241(1):334–42. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288(5):E930–4. doi: 10.1152/ajpendo.00522.2004. Epub 2005 Jan 4. [DOI] [PubMed] [Google Scholar]
- 11.Caballero AE, Delgado A, Aguilar-Salinas CA, Herrera AN, Castillo JL, Cabrera T, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2004;89(8):3943–8. doi: 10.1210/jc.2004-0019. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Saenz JL, Trujilo-Arriaga HM, Rivas-Vilchis JF, Mendez-Francisco JD, Alarcon-Aguilar FJ, Roman-Ramos R. Crossover and double blind study with metformin and rosiglitazone in impaired glucose tolerance subjects. Proc West Pharmacol Soc. 2003;46:143–7. [PubMed] [Google Scholar]
- 13.Morel Y, Golay A, Perneger T, Lehmann T, Vadas L, Pasik C, et al. Metformin treatment leads to an increase in basal, but not insulin-stimulated, glucose disposal in obese patients with impaired glucose tolerance. Diabet Med. 1999;16(8):650–5. doi: 10.1046/j.1464-5491.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheen AJ, Letiexhe MR, Lefebvre PJ. Short administration of metformin improves insulin sensitivity in android obese subjects with impaired glucose tolerance. Diabet Med. 1995;12(11):985–9. doi: 10.1111/j.1464-5491.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 15.Widen EI, Eriksson JG, Groop LC. Metformin normalizes nonoxidative glucose metabolism in insulin-resistant normoglycemic first-degree relatives of patients with NIDDM. Diabetes. 1992;41(3):354–8. doi: 10.2337/diab.41.3.354. [DOI] [PubMed] [Google Scholar]
- 16.Linday LA. Trivalent chromium and the diabetes prevention program [review] Med Hypotheses. 1997;49(1):47–9. doi: 10.1016/s0306-9877(97)90251-6. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, et al. Body size and shape changes and the risk of diabetes in the Diabetes Prevention Program. Diabetes. 2007;56(6):1680–5. doi: 10.2337/db07-0009. Epub 2007 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florez JC, Jablonski KA, Kahn SE, Franks PW, Dabelea D, Hamman RF, et al. Type 2 diabetes-associated missense polymorphisms KCNJ11 E23K and ABCC8 A1369S influence progression to diabetes and response to interventions in the Diabetes Prevention Program. Diabetes. 2007;56(2):531–6. doi: 10.2337/db06-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relationship of body size and shape to the development of diabetes in the diabetes prevention program. Obesity (Silver Spring) 2006;14(11):2107–17. doi: 10.1038/oby.2006.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28(4):888–94. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinman B, Harris SB, Gerstein HC, Young TK, Raboud JM, Neuman J, et al. Preventing type 2 diabetes using combination therapy: design and methods of the Canadian Normoglycaemia Outcomes Evaluation (CANOE) trial. Diabetes Obes Metab. 2006;8(5):531–7. doi: 10.1111/j.1463-1326.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 22.Li CL, Pan CY, Lu JM, Zhu Y, Wang JH, Deng XX, et al. Effect of metformin on patients with impaired glucose tolerance. Diabet Med. 1999;16(6):477–81. doi: 10.1046/j.1464-5491.1999.00090.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;48(2):289–97. doi: 10.1007/s00125-005-0097-z. Epub 2006 Jan 4. [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]


