Abstract
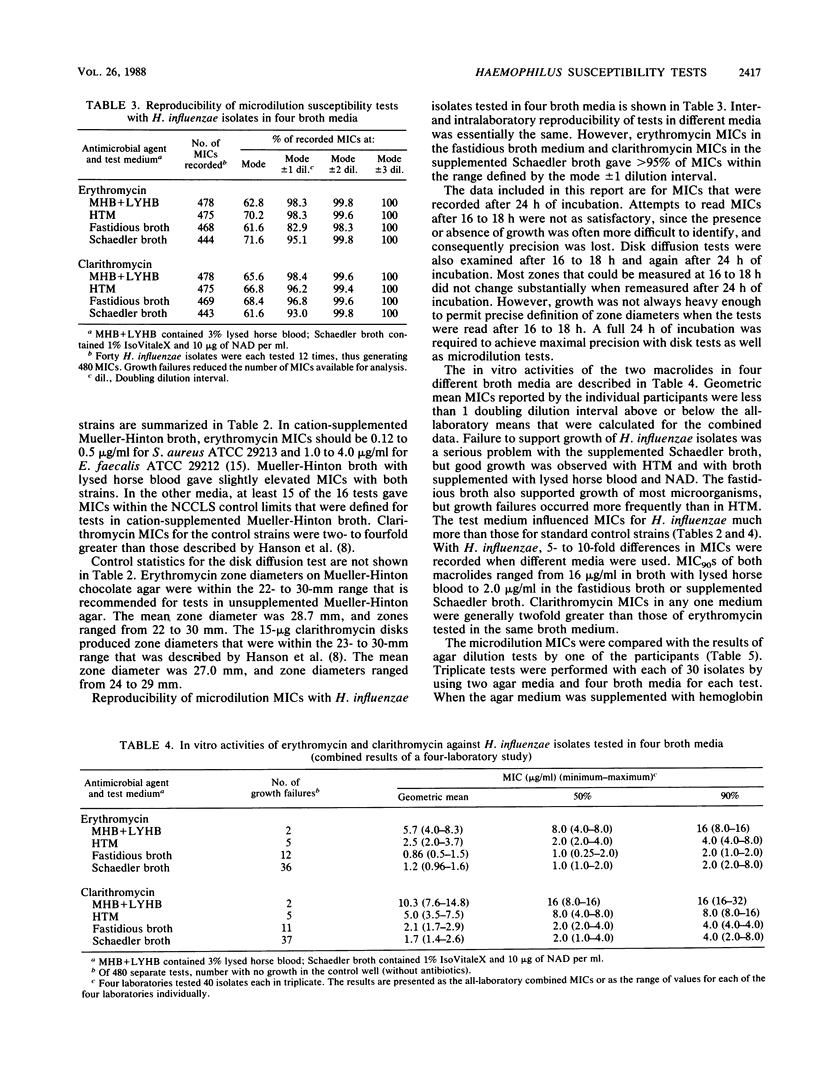
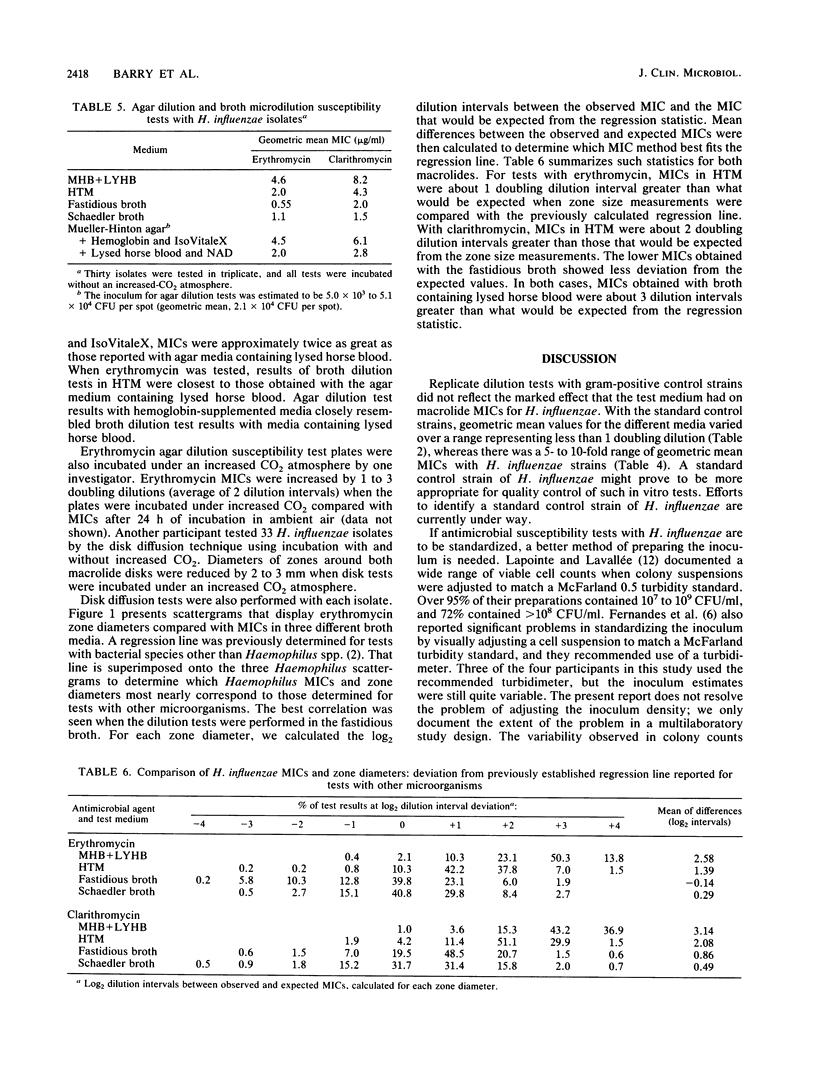
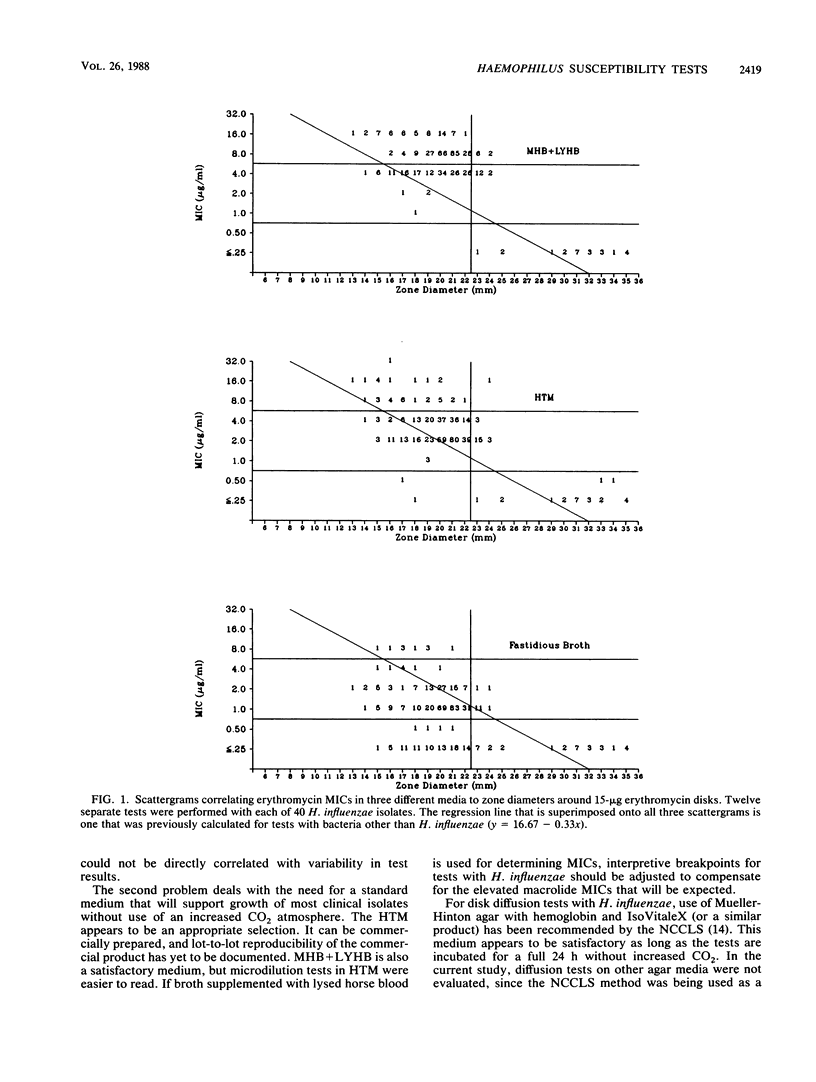
Four separate laboratories performed antimicrobial susceptibility tests with 40 Haemophilus influenzae isolates, each tested in triplicate. Erythromycin and a new macrolide, clarithromycin (A-56268; TE-031), were tested by the disk diffusion method, by the agar dilution procedure in two different media, and by broth microdilution tests in four different media. Erythromycin MICs for 90% of the strains were 16 micrograms/ml in Mueller-Hinton broth with 3% lysed horse blood and NAD, 4.0 micrograms/ml in hemophilus test medium, and 2.0 micrograms/ml in supplemented Schaedler broth or in the fastidious broth medium from Beckman Instruments, Inc. Clarithromycin MICs were generally 1 doubling dilution greater than erythromycin MICs in each of the media. Erythromycin disk tests corresponded best with MICs determined in the fastidious broth medium. In that same medium, clarithromycin MICs were about 1 doubling dilution greater than what would be expected from the results of disk tests. Because there were fewer growth failures, hemophilus test medium is recommended for microdilution tests with H. influenzae. Incubation of all tests for a full 24 h without an increased CO2 atmosphere was needed to achieve maximal precision of the tests. Interlaboratory and intralaboratory reproducibility of all tests was satisfactory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Cotton J. L., Jones R. N., Packer R. R. Evaluation of a proprietary broth medium for microdilution susceptibility testing of nutritionally fastidious bacteria. J Clin Microbiol. 1986 Nov;24(5):701–704. doi: 10.1128/jcm.24.5.701-704.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. Disk diffusion and disk elution tests with A-56268 and erythromycin. Eur J Clin Microbiol. 1987 Feb;6(1):109–111. doi: 10.1007/BF02097213. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. In vitro activity of a new macrolide, A-56268, compared with that of roxithromycin, erythromycin, and clindamycin. Antimicrob Agents Chemother. 1987 Feb;31(2):343–345. doi: 10.1128/aac.31.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D., Bailer R., McDonald E., Pintar J., Ramer N., Swanson R., Gade E. Susceptibility testing of macrolide antibiotics against Haemophilus influenzae and correlation of in vitro results with in vivo efficacy in a mouse septicemia model. Antimicrob Agents Chemother. 1987 Aug;31(8):1243–1250. doi: 10.1128/aac.31.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd-Reising S., Hindler J. A., Young L. S. In vitro activity of A-56268 (TE-031), a new macrolide antibiotic, compared with that of erythromycin and other antimicrobial agents. Antimicrob Agents Chemother. 1987 Apr;31(4):640–642. doi: 10.1128/aac.31.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. W., Bailer R., Gade E., Rode R. A., Fernandes P. B. Regression analysis, proposed interpretative zone size standards, and quality control guidelines for a new macrolide antimicrobial agent, A-56268 (TE-031). J Clin Microbiol. 1987 Jun;25(6):1079–1082. doi: 10.1128/jcm.25.6.1079-1082.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L., Kalin M. Comparative in vitro activity of A-56268 against respiratory tract pathogens. Eur J Clin Microbiol. 1987 Aug;6(4):494–496. doi: 10.1007/BF02013122. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Fuchs P. C., Thornsberry C. Disk diffusion susceptibility testing of two macrolide antimicrobial agents: revised interpretive criteria for erythromycin and preliminary guidelines for roxithromycin (RU 965). J Clin Microbiol. 1986 Aug;24(2):233–239. doi: 10.1128/jcm.24.2.233-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A., Howell A. W. Improved medium for antimicrobial susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Nov;25(11):2105–2113. doi: 10.1128/jcm.25.11.2105-2113.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J. R., Lavallée C. Antibiotic interaction of amoxycillin and clavulanic acid against 132 beta-lactamase positive Haemophilus isolates: a comparison with some other oral agents. J Antimicrob Chemother. 1987 Jan;19(1):49–58. doi: 10.1093/jac/19.1.49. [DOI] [PubMed] [Google Scholar]
- Lorian V., Sabath L. D. Effect of pH on the activity of erythromycin against 500 isolates of gram-negative bacilli. Appl Microbiol. 1970 Nov;20(5):754–756. doi: 10.1128/am.20.5.754-756.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


