Abstract
Exosome complexes are 3′ to 5′ exoribonucleases composed of subunits that are critical for numerous distinct RNA metabolic (ribonucleometabolic) pathways. Several studies have implicated the exosome subunits Rrp6 and Dis3 in chromosome segregation and cell division but the functional relevance of these findings remains unclear. Here, we report that, in Drosophila melanogaster S2 tissue culture cells, dRrp6 is required for cell proliferation and error-free mitosis, but the core exosome subunit Rrp40 is not. Micorarray analysis of dRrp6-depleted cell reveals increased levels of cell cycle– and mitosis-related transcripts. Depletion of dRrp6 elicits a decrease in the frequency of mitotic cells and in the mitotic marker phospho-histone H3 (pH3), with a concomitant increase in defects in chromosome congression, separation, and segregation. Endogenous dRrp6 dynamically redistributes during mitosis, accumulating predominantly but not exclusively on the condensed chromosomes. In contrast, core subunits localize predominantly to MTs throughout cell division. Finally, dRrp6-depleted cells treated with microtubule poisons exhibit normal kinetochore recruitment of the spindle assembly checkpoint protein BubR1 without restoring pH3 levels, suggesting that these cells undergo premature chromosome condensation. Collectively, these data support the idea that dRrp6 has a core exosome-independent role in cell cycle and mitotic progression.
INTRODUCTION
Exosome complexes are critical players in the processing and degradation of many RNA species (Houseley et al., 2006). Found in both archaebacteria and eukaryotes, these complexes are composed of a “core” set of subunits. With respect to the yeast and human core complexes, they consist of six RNase PH domain–containing proteins (Rrp41, Rrp42, Rrp43, Rrp45, Rrp46, and Mtr3) and three S1 domain–containing proteins (Rrp4, Rrp40, and Csl4). Our understanding of exosome complex form and function has advanced greatly though the report of the crystal structures of archaebacterial (Buttner et al., 2005; Lorentzen and Conti, 2005; Lorentzen et al., 2005) and eukaryotic exosome core (Liu et al., 2006) complexes. The RNase PH subunits assemble into a hexameric ring upon which the S1 domain subunits reside. It has been proposed that the cylindrical shape and central channel of exosome complexes have evolved as a means to tightly regulate RNA recognition and subsequent decay (Lorentzen and Conti, 2006).
Eukaryotes have compartment-specific exosome subunits and cofactors in addition to the core subunits. Although the eubacterial RNase R homolog Dis3 (also referred to as Rrp44) interacts with the yeast core (Mitchell et al., 1997; Allmang et al., 1999b; Dziembowski et al., 2007), it does not associate with either the human or trypanosome core (Chen et al., 2001; Estevez et al., 2003). Moreover, many archaebacteria lack an RNase R-like protein (Koonin et al., 2001). Thus, strictly speaking, Dis3 is not a core exosome component. Another exosome subunit missing in archaebacteria but present in eukaryotes is Rrp6, an RNase D homolog. Rrp6 associates specifically with the “nuclear” exosome complex in yeast (Allmang et al., 1999b). However, Rrp6 in fly, trypanosome, and human cells is both nuclear and cytoplasmic (Lejeune et al., 2003; Graham et al., 2006; Haile et al., 2007). Nonetheless, based on its initial purification in the yeast nuclear exosome, most if not all subsequent phenotypes associated with loss of Rrp6 have been equated with perturbation of the nuclear exosome. Remarkably, despite its numerous roles in ribonucleometabolic pathways (Briggs et al., 1998; Bousquet-Antonelli et al., 2000; Burkard and Butler, 2000; Hilleren et al., 2001; Jensen et al., 2001; Dichtl et al., 2002; Libri et al., 2002; Zenklusen et al., 2002; Das et al., 2003; Thomsen et al., 2003; Hieronymus et al., 2004; Fang et al., 2005; Kuai et al., 2005; Roth et al., 2005; Reis and Campbell, 2007), it is not essential for viability in yeast (Briggs et al., 1998). Thus, whether these nuclear functions are specific to Rrp6 and/or are occurring in the context of an exosome complex is unclear.
There is growing evidence that Rrp6 and Dis3 act independently of the exosome core. First, Drosophila Rrp6 (dRrp6) and dDis3 localize in the nuclei of S2 tissue culture cells, whereas core subunits are found largely in the cytoplasm (Graham et al., 2006). Second, ScDIS3, but no core subunit, was isolated as a high-copy suppressor of the rrp6Δ slow-growth phenotype (Abruzzi et al., 2007). Third, yeast Rrp6 and Dis3 have interaction partners that are not shared with core subunits (Noguchi et al., 1996; Gavin et al., 2002, 2006; Krogan et al., 2004; Vanacova et al., 2005). Fourth, a yeast mutant Rrp6 polypeptide that no longer interacts with the core exosome retains its 3′ end processing activities on rRNA and snoRNA transcripts (Callahan and Butler, 2008). Fifth, both Rrp6 and Dis3 have been linked to chromosome segregation and cell division (Ohkura et al., 1988; Kinoshita et al., 1991; Fomproix and Hernandez-Verdun, 1999). The only core subunit linked to these phenomena is Csl4 (Baker et al., 1998) and that effect has been shown to be indirect (van Hoof et al., 2000). It has been suggested that the mitotic function of SpRrp6 and SpDis3 is to process or degrade centromere-associated small inhibitory RNAs (siRNAs; Buhler et al., 2007; Murakami et al., 2007; Nicolas et al., 2007). Whether these results in S. pombe explain the mitotic phenotypes associated with perturbation of Rrp6 and Dis3 in other eukaryotes is unknown.
For this study, we have focused our attention on the cell biological relationship between dRrp6 and the core exosome. To this end, we knocked down dRrp6 and examined mitotic phenotypes and transcriptomic profile of depleted cells. We also investigated the subcellular distribution of dRrp6 during cell division and in response to activation of the spindle assembly checkpoint. We present evidence that dRrp6 has core-exosome–independent functions in cell cycle and mitotic progression.
MATERIALS AND METHODS
Antibodies and Reagents
Polyclonal exosome antibodies were described previously (Andrulis et al., 2002). Monoclonal anti-α-tubulin antibodies (used at 1:500 dilution) were obtained from Sigma (St. Louis, MO) and AbD Serotec (Oxford, United Kingdom). Cy2- (1:200), Texas Red– (1:200), and Cy5- (1:800) conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibodies to Aurora A (Giet et al., 2002) and BubR1 (Logarinho et al., 2004) were used as described previously. Antibodies to Mmp1 (1:500; monoclonal Ab 5H7b11) and TrioB (1:50; monoclonal Ab 9.4A) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa. Histone H1 (1:500), H2A (1:500), and H4 (1:500) antibodies and Sxl (1:500) antibodies were gifts of Drs. Peter Harte and Helen Salz (Case Western Reserve University School of Medicine), respectively. HRP-conjugated secondary antibodies for Western blots (1:20000) were obtained from Jackson ImmunoResearch Laboratories.
Double-stranded RNA Preparation and RNA Interference Treatment
Double-stranded RNA (dsRNA) was prepared as previously described (Graham et al., 2006) using primers (Supplemental Table S4) and DNA templates (Graham et al., 2006). S2 cells were treated with 15 or 30 μg dsRNA every other day over 5 d and either fixed and stained for immunofluorescence or pelleted, lysed, and then assayed by Western blot. For the growth curves, cells growing were treated with dsRNAs (30 μg) three times (days 0, 1, and 3) over 5 days, removed, and counted by hemacytometer.
Immunofluorescence Staining and Microscopy
Immunofluorescence staining and deconvolution microscopy was performed as previously described (Graham et al., 2006), using a Zeiss Axioplan II or a DeltaVision restoration microscope system (Applied Precision). For the Zeiss microscope, the lens was a 100× Plan-Apochromat NA 1.40 Zeiss objective. The camera was a Hamamatsu Digital CCD camera (Bridgewater, NJ). The DeltaVision system is an Olympus microscope with a similar objective (100×, UPlanSApo, NA 1.40, Melville, NY). For movies, 1) individual optical sections (0.2–0.5 μm) were acquired on the Deltavision Restoration microscope system; 2) image sections were deconvolved, using the conservative ratio method, with softWoRx software program (Applied Precision); 3) the optical sections of a given cell were converted into a volume projection, using the maximum intensity method; and 4) using the volume viewer feature, the volume projection was rotated using the interactive image rotation feature and saved as a movie file.
ATP and Cell Size Measurement
The level of cellular ATP was measured using a luciferase assay (Via Light Plus Kit, Lonza, Rockland, IL). Duplicate dsRNA experiments were performed as stated in the above section, except that on day 4 cells were harvested. The harvested cell culture volume was adjusted so the cell count would be either 2 × 105 or 4 × 105 for each independent measurement. ATP levels were measured in triplicate, according to the manufacture's instructions using an internal ATP standard. For cell size analysis, RNA interference (RNAi)-treated cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and anti-tubulin. Three-dimensional images were obtained with Deltavision software (Applied Precision, Issaquah, WA) in 0.5-μm sections. The largest cross section of each individual cell was measured using the line measurement tool. For each cell, three or four measurements (μm) were averaged to obtain the individual cell's “cell size.” For each dsRNA-treated cell type, 39 individual cells were measured. Only cells that were spherical or elliptical in shape, <13 μm in size, and had not phagocytosed were included in the count.
Karyotype Analysis
S2 cells treated with dsRNA were harvested and subjected to karyotype analysis, as described in Protocol 20.2 (Sullivan et al., 2000) but without the addition of vinblastine sulfate. Mitotic chromosomes were stained with Hoechst 33258 (0.5 μg/ml) and/or propidium iodide (1 μg/ml). Lower-resolution analysis was conducted with a Zeiss axioplan microscope (Thornwood, NY). Higher-resolution deconvolution microscopy was performed using a DeltaVision restoration microscope (Applied Precision). The color images were acquired and processed with the SoftWoRx software and Adobe Photoshop (San Jose, CA).
Cell Culture, Microtubule Drug, and RNase A Treatment
Drosophila melanogaster embryonic S2 cell lines were grown as described (Andrulis et al., 2000). Cells were treated with 10 nM taxol (Acros Organics, Morris Plains, NJ) or 2.5 μM colchicine (Acros Organics) for 1 or 2 h before fixation. For RNase A treatment, cells were adhered to coverslips, washed once in PBS, treated with RNase A (mock or 2 mg/ml) for 10 min, washed twice with PBS, fixed, washed again in PBS, and then permeabilized with saponin for immunofluorescence analysis.
Microarray Analysis
Total RNA was harvested from the dsRNA-treated cells using Trizol (Ambion, Austin, TX). Total RNA samples of 50 μg from independent, duplicate RNAi depletions were analyzed by the Gene Expression and Genotyping Core Facility on Drosophila Genome 2.0 microarrays (Affymetrix, Santa Clara, CA). Raw array data were analyzed using GCOS, Version 1.4, software (Affymetrix). To account for the nonspecific effects of dsRNA treatment, fold changes were calculated in GCOS by setting the green fluorescent protein (GFP)-treated samples as the baseline for each comparison. Results from the two Rrp6 depletions were compared with each individual GFP depletion yielding four total comparisons. The resulting signal/log ratios were converted to fold changes (shown in Supplemental Table S1), and all transcripts with fold changes below two were eliminated to exclude false positives. Only transcripts with like changes that occurred in either three or four of four comparisons were retained in the data set for further analysis. The retained transcripts were identified and grouped using Pathway Studio 5.0 (Ariadne Genomics, Rockville, MD) and Genespring GX 9.0 (Agilent,Wilmington, DE) software.
Cell Cycle Synchronization and Analysis
Log-phase S2 cells (1 × 106 cells per ml) were incubated with either 1 μM ponasterone A (pon A) or 1 μM hydroxyurea (HU) for 18 h. Cells were either harvested or released from drug by resuspending in fresh media and plated into a new dish. For release assays using HU, cell were cultured for an additional 3–6 h to obtain S-phase cells. Cells were fixed with either 90% methanol/PBS for flow cytometry or with 3.7% formaldehyde in PBS for indirect immunofluorescence. To confirm the synchrony of cell populations cells were stained with propidium iodide. Flow cytometry with was conducted using a BD Aria II (Comprehensive Flow Cytometry Core, Case Western Reserve University).
RESULTS
Depletion of dRrp6 Results in Decreased Cell Growth and Reduced Phospho-histone H3 Levels
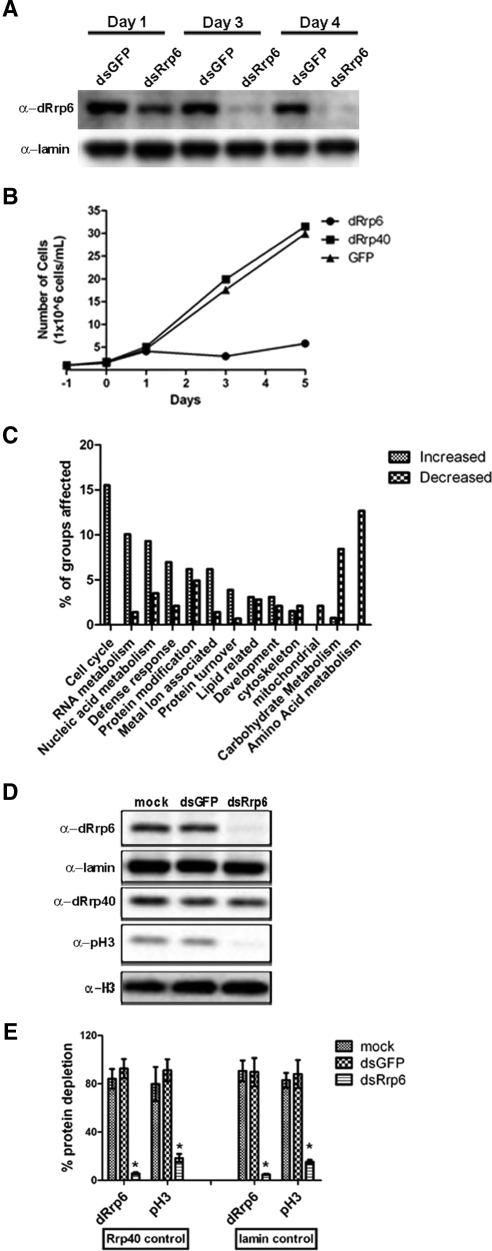
To better understand the functional relationship of dRrp6 to the core exosome, we depleted Drosophila Rrp6 (dRrp6) and the core subunit dRrp40 by RNAi in Schneider S2 tissue culture cells (Figure 1A). We observed a striking defect in cell growth in dRrp6-depleted cells after 3 d of treatment with dsRNA (Figure 1B). By comparison, treatment of S2 cells with dsRNA to the core subunit dRrp40 or to the control protein GFP had no effect on cell growth (Figure 1B) or on dRrp6 protein levels (Supplemental Figure S1A). As Rrp6 has been shown to be involved in the turnover of a large number of transcripts in yeast (Houalla et al., 2006), we suspected that our observed growth phenotype arose from a general perturbation of transcript stability.
Figure 1.
Phenotypic and transcriptomic consequences of dRrp6 depletion. (A and B) Drosophila S2 cells were cultured in media with 30 μg dsRNAs to dRrp6, dRrp40, and GFP, and cells were removed and analyzed for protein levels and cell number after 1, 3, and 5 d. (C) Major classes of transcripts affected in dRrp6-depleted cells as determined by microarray analysis. (D) dRrp6-depleted cells exhibit diminished phospho-histone H3 levels. (E) Quantitative determination of reduction of phospho-histone H3 levels. Data are from three independent experiments.
To address this possibility, we performed microarray analysis on polyA+ mRNA isolated from dRrp6-depleted cells. After applying stringent criteria to exclude false positives in our microarray study, we obtained a data set of 134 increased transcripts and 100 decreased transcripts (Supplemental Tables S1–S3). When these were identified and grouped, the major group of transcripts that were stabilized in these cells were those related to cell cycle progression, followed by those related to RNA metabolism and nucleic acid metabolism (Figure 1C, Supplemental Table S4). Notably, the most stabilized transcripts were those of histones H2A, H2B, and H3 (Supplemental Tables S1–S3).
Although the link between histone mRNA stability and cell cycle progression is just beginning to be investigated (Houalla et al., 2006; Reis and Campbell, 2007), that between histone protein levels and the cell cycle is well established (Zhao, 2004; Vidanes et al., 2005; Muller et al., 2007). We thus determined whether a change in histone protein levels were affected in dRrp6-depleted cells. Despite the stabilization of histone transcripts in these cells (Supplemental Tables S1–S3), there was no effect on histone (H1, H2A, H3, and H4) protein levels compared with the loading controls lamin and dRrp40 (Figure 1D; Supplemental Figure S1B). In contrast, we observed a striking reduction of phosphorylated form of histone H3 (pH3), a marker of mitotic chromosome condensation (Johansen and Johansen, 2006). The levels of pH3 are reduced 5–10-fold (Figure 1E). These results, taken together, suggest that loss of cell growth in dRrp6-depleted cells may be a consequence of diminished or aberrant mitotic progression.
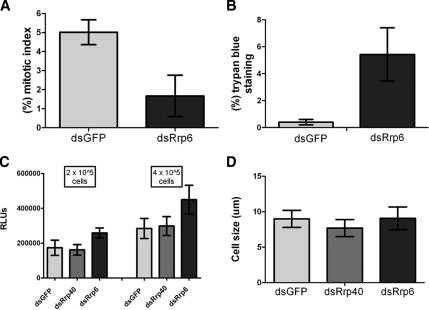
To determine whether loss of cell growth was accompanied by a quantitative reduction in the frequency of mitotic cells, we scored cells undergoing mitosis. Indeed, the mitotic index was reduced (Figure 2A). Because this phenotype could arise from a loss of cell proliferation or from apoptosis, we performed several experiments to differentiate between these two possibilities. First, we observed that dRrp6 dsRNA-treated cells exhibited an increased frequency of trypan blue staining (Figure 2B), consistent with cell permeabilization. Interestingly, however, these cells did not exhibit nuclear blebbing, showed only a modest effect on cytosolic ATP levels (Figure 2C), and did not vary in size (Figure 2D). These data indicate that depletion of dRrp6 was not inducing apoptosis but rather causing a loss of cell proliferation.
Figure 2.
Cells depleted of dRrp6 show diminished mitosis but do not exhibit signs of apoptosis. (A) To determine mitotic index, dsRNA-treated cells were fixed and stained with antibodies to α-tubulin and the DNA dye, DAPI. Cells were scored as mitotic if they showed clear chromosome condensation and diagnostic centrosomal and microtubule patterns. (B) S2 cells were stained with trypan blue, which only stains cells with a permeabilized membrane. (C) ATP levels are not diminished in dRrp6 RNAi-treated cells. Results are obtained from two independent experiments that were conducted in triplicate; error bars, ±SDs. (D) Depletion of dRrp6 does not affect cell size. No cell blebbing, a phenotypic indicator of apoptosis, was detected in the RNAi-treated cell populations.
Depletion of dRrp6 Results in Chromosome Segregation Defects In Vivo
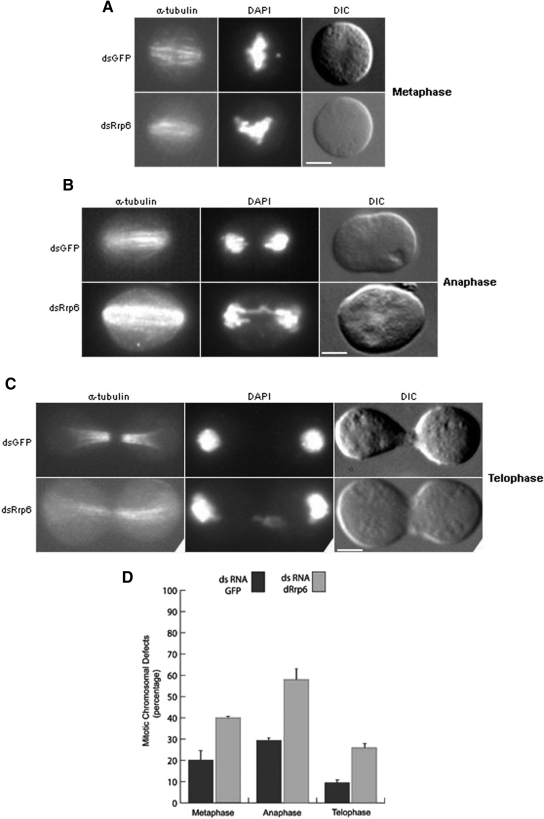
Our data indicate that Drosophila Rrp6 is essential for cell proliferation and important for mitosis. We thus characterized mitotic events in more detail to understand dRrp6 function. Notably, dRrp6-depleted mitotic cells revealed chromosomal abnormalities that were increased above the general background defects observed in S2 cells. For example, dRrp6-depleted cells showed incompletely congressed and aligned chromosomes (Figure 3A), anaphase chromosomal bridges (Figure 3B), and mis-partitioned chromosomes (Figure 3C). Scoring of more than 500 cells from two independent experiments demonstrated a 2–3-fold increase in these aberrant mitotic events (Figure 3D).
Figure 3.
Reduction in dRrp6 leads to chromosome segregation defects in vivo. (A–C) Typical examples of mitotic defects in metaphase, anaphase, and telophase that are pronounced in the dRrp6-depleted cells compared with GFP dsRNA-treated control. Bar, 5 μm. (D) Cells treated with dsRNA either to dRrp6 or GFP were scored for mitotic defects. Quantitative analysis was carried out on at least two independent RNAi experiments examining >500 mitotic cells each. The low background defects seen in the GFP dsRNA-treated cells are observed even in the absence of RNAi treatment.
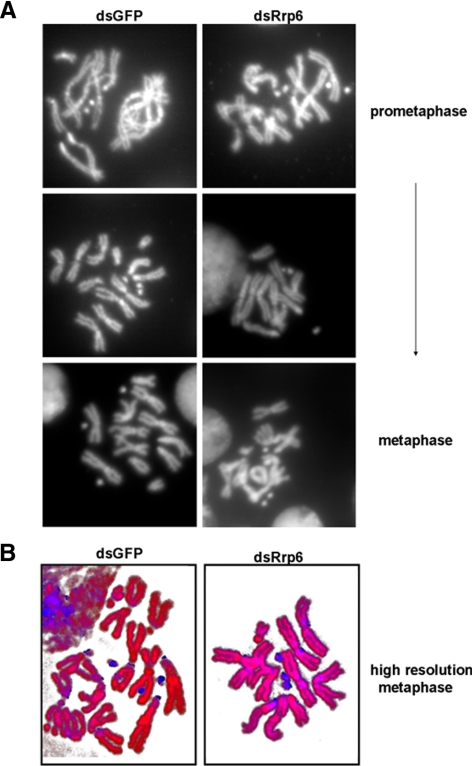
To determine whether these mitotic phenotypes arise from defects in chromosomal packaging or architecture, we examined the karyotypes (chromosome spreads) of dRrp6- or GFP-depleted cells. We did not observe an effect on chromosome condensation or structure in any of the spreads (Figure 4A). Analysis of the chromosomes via high-resolution microscopy with DAPI and propidium iodide did not reveal any blatant chromosome abnormalities as well (Figure 4B). Thus, chromosome structure appears unaffected in dRrp6-depleted cells.
Figure 4.
Loss of dRrp6 does not elicit blatant chromosomal abnormalities. (A) Chromosome spreads of GFP- or dRrp6-dsRNA—treated cells taken at from early prometaphase to metaphase cells. Although S2 cells are generally polyploid, we did not observe any qualitative or quantitative changes in our karyotype analysis of control and experimental cell populations. (B) Nucleic acid staining pattern is unaffected in dRrp6-depleted cells. Chromosome spreads were stained with DAPI (blue) and propidium iodide (red).
Dynamic Chromosomal Association of dRrp6 during Mitosis
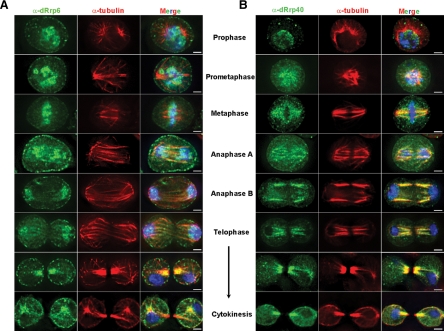
The mitotic phenotypes associated with dRrp6 depletion could arise as a secondary consequence of perturbing one or more ribonucleometabolic pathways. However, we reasoned that if dRrp6 functioned directly in cell division, we should observe it associating with mitotic structures. To address this issue, we immunolocalized endogenous dRrp6 as a function of mitotic stage. dRrp6 is extremely dynamic during mitosis, with the major localization patterns presented in Figure 5A and detailed here, several of the minor patterns presented in Supplemental Figure S2, and all localization data summarized in Table 1. During prophase, dRrp6 is found either in the nucleus or it redistributes to the cytoplasm and separating centrosomes, accumulating on the pericentriolar material (PCM). dRrp6 localizes to a large number of foci that are often arranged along the astral microtubules (MTs) and the cell cortex. At prometaphase, dRrp6 is detected on the condensed chromosomes and infrequently enveloping the chromosomes on the presumptive perichromosomal layer (PCL), a ribonucleoprotein structure that sheaths the chromosomes during mitosis (Van Hooser et al., 2005). In metaphase, dRrp6 localizes to either the spindle and PCL or, in a nonoverlapping manner, to the condensing chromosomes. At anaphase, dRrp6 transitions exclusively to the chromosomes. During telophase, dRrp6 antibodies stain the spindle remnant (nascent midbody) or both the chromosomes and the spindle remnant. During cytokinesis, dRrp6 is observed on the midbody, astral MTs, the PCM, and cell cortex, yet is not enriched in the nascently forming nuclei. Thus dRrp6 dynamically redistributes during mitosis. Importantly, these immunofluorescent signals (and those described below) were only observed when primary and secondary antibodies were used together, were not observed with preimmune sera, and were not due to bleed-through between channels; these findings validate and extend upon the antibody specificity documented previously (Andrulis et al., 2002; Graham et al., 2006).
Figure 5.
dRrp6 and dRrp40 differentially and dynamically redistribute during distinct mitotic stages. (A) S2 cells were fixed and stained with anti-dRrp6 antibodies. Images representing each stage of mitosis were captured and catagorized. Images in this and subsequent panels show single Z-sections through individual cells. (B) S2 cells were fixed and stained with anti-dRrp40 antibodies. Bar, 2 μm.
Table 1.
Quantitative distribution analysis of dRrp40 and dRrp6 as a function of mitosis and cell division
| Prophase |
Prometaphase |
Metaphase |
Anaphase |
Telophase |
Cytokinesis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dRrp6 (n = 12) | dRrp40 (n = 11) | dRrp6 (n = 22) | dRrp40 (n = 32) | dRrp6 (n = 15) | dRrp40 (n = 13) | dRrp6 (n = 18) | dRrp40 (n = 11) | dRrp6 (n = 7) | dRrp40 (n = 7) | dRrp6 (n = 11) | dRrp40 (n = 10) | |
| Nucleus | 40 | 63 | ||||||||||
| Cytoplasm and centrosomal | 60 | 18 | ||||||||||
| Cytoplasm | N/A | 18 | ||||||||||
| Perichromosomal | 18 | 16 | ||||||||||
| Perichromosomal and spindle | N/A | 84 | 40 | 46 | ||||||||
| Chromosomes | 82 | N/A | 60 | N/A | 100 | N/A | ||||||
| Spindle | N/A | 54 | N/A | N/A | ||||||||
| Spindle remnant | N/A | 18 | ||||||||||
| Spindle remnant and chromosomes | N/A | 82 | ||||||||||
| Nascent nuclei | 29 | N/A | ||||||||||
| Nascent nuclei and midbody | 29 | 29 | ||||||||||
| Cytoplasm and midbody | 42 | 71 | 55 | 30 | ||||||||
| Cytoplasm, centrosomal, and midbody | 45 | 60 | ||||||||||
| Nascent nuclei, centrosomal, and midbody | N/A | 10 | ||||||||||
Values are expressed as percent; N/A, not applicable. Only deconvolved images captured with the Deltavision Restoration system were used in this categorization. Representative images of the localization patterns for these different categories are shown in Supplemental Figure S1.
To confirm these observations, we set out to examine the distribution of an epitope-tagged form of dRrp6 described in previous work (Graham et al., 2006). Interestingly, cells overexpressing epitope-tagged dRrp6 stopped growing and were not found in mitosis (Supplemental Figure S3). In contrast, control cells grew unimpeded. This result supports the idea that the phenotypes associated with dRrp6 depletion (Figure 3) could arise from perturbation of cell cycle–related functions and interactions.
Core Exosome Subunits are Associated with Microtubules during Mitosis
We sought to determine whether these localization patterns were specific to dRrp6. We thus performed indirect immunofluorescence on S2 cells with antibodies to the core exosome subunit dRrp40 because this subunit is not required for S2 cell proliferation (Figure 1B). dRrp40 colocalized predominantly with the MTs in all mitotic stages (Figure 5B), although other, nonexclusive distribution patterns were also observed (Supplemental Figure S2; Table 1). For example, in some cells, dRrp40 associated both with the spindle and the PCL in prometaphase and metaphase as well as to the spindle remnant and the chromosomes during anaphase. Although some of the subtle differences observed at specific mitotic stages could simply reflect slight temporal changes in their chromosomal and microtubular associations, our data shows clear differential distribution of dRrp6 and dRrp40 throughout the whole of mitosis.
The core exosome subunits dRrp4, dRrp46, and dCsl4 were likewise enriched on the mitotic spindle (Supplemental Figure S4A), almost coincident with MTs. Association of these subunits with MTs may be independent of RNA, because RNase A treatment does not affect the MT staining pattern of dRrp40 (Supplemental Figure S4B). These core exosome subunits do not interact with either α- or γ-tubulin in whole cell extracts (Supplemental Figure S5). Collectively these cell biological data strengthen the idea that dRrp6 is functioning independently of the core exosome and directly in chromosome segregation. Indeed, depletion of dRrp6 did not affect the interphase or mitotic localization patterns of dRrp40 or dCsl4 (data not shown). Given this evidence distinguishing dRrp6 from the core, we examined how dRrp6 functionally and cytologically responds to mitotic stress.
dRrp6 and Core Subunit Response to and Function in the Spindle Assembly Checkpoint
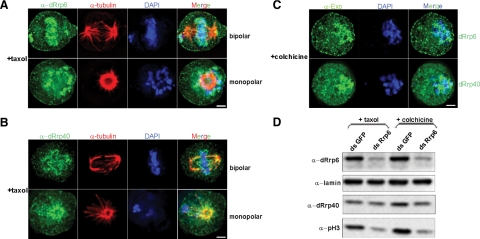
The spindle assembly checkpoint (SAC) ensures accurate chromosome segregation (Musacchio and Salmon, 2007). Because we observed chromosomal defects in dRrp6-depleted cells (Figure 3) and the chromosomal localization pattern of dRrp6 during mitosis was distinct from other exosome subunits (Figure 5A; Supplemental Figure S2 and Supplemental Table S1), we tested whether dRrp6 redistributed in response to checkpoint activation. To address this, we exploited the fact that the SAC can be activated either by unattached kinetochores or by loss of tension across the spindle. In response to taxol, a drug that creates loss of spindle tension, we observed robust accumulation of dRrp6 on chromosomes that were either aligned on the metaphase plate or had not yet been aligned (Figure 6A, bipolar; Movie S1). Notably, we did not observe any of the minor dRrp6 localization patterns observed during normal mitoses (Supplemental Figure S2; Table 1), indicating that checkpoint activation drives dRrp6 exclusively to the condensed chromosomes. In contrast, taxol treatment reveals dRrp40 localized on the kinetochore MTs and the PCL (Figure 6B, bipolar; Movie S2), reflecting the normal distribution pattern found during mitosis (Figure 5; Supplemental Figure S2; Table 1). This differential distribution between dRrp6 and dRrp40 was also observed in taxol-treated prometaphase cells or cells containing a monopolar spindle (Figure 6, A and B, monopolar; Movie S3), indicating sensitive yet different signaling pathways regulating the targeting of these two exosome subunits in response to loss of spindle tension. However, in response to colchicine, a drug that depolymerizes MTs and thus activates the SAC by producing unattached kinetochores, both dRrp6 and dRrp40 localize similarly on and around chromosomes (Figure 6C). This may reflect a different, perhaps shared, response pathway from that observed with taxol treatment. Alternatively, this could represent chromosome-proximal dRrp6 and dRrp40 aggregation in the absence of MTs. The dRrp40 recruitment pattern to chromosomes does not appear to reflect loss of core exosome stability as neither taxol nor colchicine affect exosome subunit interactions in cell extracts (Supplemental Figure S5).
Figure 6.
Relationship of dRrp6 to the spindle assembly checkpoint. (A) Metaphase cells that had been treated with taxol were stained with antibodies to dRrp6 or dRrp40 and compared with α-tubulin and DAPI. Bar, 2 μm. (B) Striking dRrp6 and dRrp40 prometaphase distribution revealed through microtubule stabilization. Note how particles of dRrp40 decorate the microtubules extending to the unaligned chromosome. dRrp40 also “sheaths” the unaligned chromosome, whereas dRrp6 is “upon” the chromosomes. Bar, 2 μm. (C) Disruption of MTs elicits accumulation of dRrp6 and dRrp40 around chromosomes with unattached kinetochores. Bar, 2 μm. (D) Taxol or colchicine treatment of dRrp6-depleted cells does not affect the reduced levels of pH3.
We inquired whether the SAC was affected in dRrp6-depleted cells. As a cellular marker for checkpoint activation, we visualized BubR1, a checkpoint protein that localizes to kinetochores in cells that are responding to the SAC (Logarinho et al., 2004). We did not observe a change in BubR1 recruitment frequency under any condition where dRrp6 was depleted compared with mock-depleted cells (Supplemental Figure S6A). Importantly, dRrp6 depletion had no affect on the levels of either BubR1 or the mitotic serine/threonine kinase Aurora A or on other control proteins (Supplemental Figure S6B). Thus, although dRrp6 may respond to the SAC, it does not appear important for assembly or stability of at least one checkpoint protein on unattached kinetochores.
Loss of dRrp6 quantitatively affected pH3 levels and mitotic index but did not affect the number of cells exhibiting BubR1 recruitment in response to the SAC. These results suggest that, in dRrp6-depleted cells, either MT poisons were bypassing the need for pH3 and condensing chromosomes prematurely (and thus allowing us to observe BubR1 recruitment) or that the poisons were restoring the wild-type pH3 levels and entering into mitosis in an appropriate temporal manner. To examine these alternatives, we performed Western blotting on extracts isolated from dRrp6- or mock-depleted cells that had been treated with either taxol or colchicine. The correlated reduction between dRrp6 and pH3 levels was still observed in cells treated with either taxol or colchicine (Western blot in Figure 6D and quantitated in Supplemental Figure S7). These data indicate that dRrp6-depleted cells, when treated with MT poisons, overcome the low levels of pH3 and undergo premature chromosome condensation. It is worth noting that this phenotype has been observed in a study with hRrp6 (Fomproix and Hernandez-Verdun, 1999). In sum, our data suggest that dRrp6 functions in genome surveillance and that this is an important mechanism undergirding proper cell cycle control and, as shown here, mitotic progression.
Dynamic dRrp6 Localization as a Function of Cell Cycle Stage
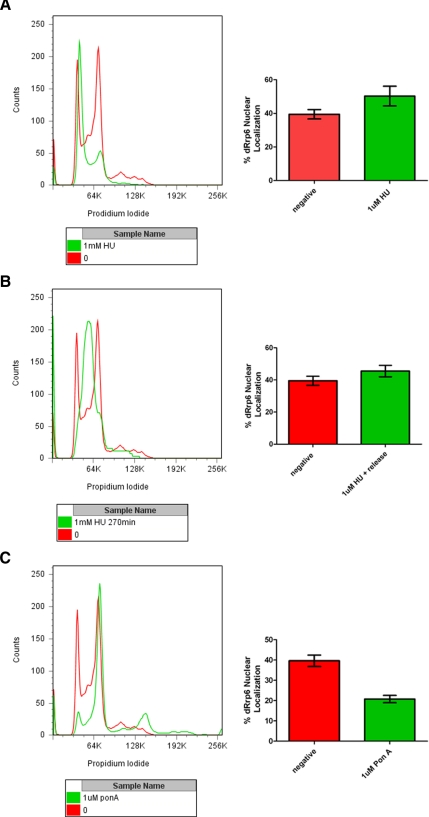
The decrease in cell proliferation, pH3 levels, and the frequency of mitotic passage and concomitant increase in chromosome segregation defects and premature chromosome condensation are all consequences of dRrp6 depletion. Whether these phenotypes arise due to specific and direct roles for dRrp6 in mitosis, as suggested by our cell biological analysis, or are due to alteration of dRrp6 function in other stages of the cell cycle is an unanswered question. To address this, we synchronized cells in S-phase with hydroxyurea (HU) or at the G2/M transition using ponasterone A (pon A) and quantitatively determined the cell-to-cell nucleoplasmic accumulation of dRrp6 during the treatment with the drug or following release (despite our efforts, we were unable to achieve release S2 cells from the G2/M block induced by pon A). After the HU-induced synchronization of S2 cells, we observed a statistically significant increase in the number of cells manifesting dRrp6 nuclear accumulation (Figure 7A) that diminished after recovery (Figure 7B). G2/M synchronization elicited the opposite effect, with a twofold decrease in cells exhibiting nuclear dRrp6 (Figure 7C). These observations suggest that dRrp6 nucleocytoplasmic distribution varies as a consequence of cell cycle stage and help promote the idea that the dRrp6 depletion-induced mitotic phenotypes may be due to a breakdown in proper dRrp6 localization and function outside of mitosis.
Figure 7.
Cell synchronization analysis reveals distinct cell cycle stage-dependent nuclear dRrp6 accumulation. (A) S2 cells were synchronized with hydroxyurea (HU), which arrests cells at the G1/S transition. A representative flow cytometric analysis is presented in the left panel. Quantitative analysis from three independent immunofluorescence experiments showing the percentage of cells exhibiting nuclear dRrp6 (right panel). We never observed a complete HU-induced G1/S block (note minor green peak corresponding to G2/M cells). (B) Recovery from HU block and passage through S-phase elicits a reduction in cells staining for nuclear dRrp6 as compared with those blocked with HU. Panels as described above. (C) G2/M block induced with pon A elicits a twofold reduction in cells manifesting nuclear dRrp6. Panels are as described above. Again, a complete pon A block was never observed (note minor green peaks corresponding to G1/S and polyploidy cells).
DISCUSSION
This study provides several lines of evidence for core-exosome–independent roles for dRrp6 in cell cycle progression in Drosophila. First, we show that dRrp6, but not the core exosome subunit dRrp40, is required for S2 cell proliferation. Second, microarray analyses reveal that cell cycle transcripts (especially histones) represent the largest class of mRNAs stabilized by dRrp6 depletion. Third, loss of Rrp6 is accompanied by reduction of pH3. Fourth, loss of dRrp6 elicits chromosome segregation defects in vivo. Fifth, we show that dRrp6 associates predominantly with condensed mitotic chromosomes throughout mitosis, whereas core subunits localize along microtubules. Sixth, core exosome association with MTs are independent of dRrp6 and RNA. Finally, in response to loss of spindle tension, dRrp6, but not dRrp40, is recruited exclusively to condensed mitotic chromosomes. These findings may be relevant in eukaryotic cells that undergo nuclear envelope breakdown and assemble an extra-nuclear mitotic spindle, but not in those with a closed mitosis, such as budding yeast, where RRP6 is not an essential gene (Briggs et al., 1998).
Rrp6 Function Independent of the Nuclear Exosome
Although Rrp6 is the defining component of the nuclear exosome in yeast, our data in fly cells indicate that it can act independently of the core complex in the context of mitosis. Indeed, the dynamic and differential localization patterns of dRrp6 from other subunits is quite intriguing. This suggests that its spatiotemporal function may be important for specific functions at particular subcellular organelles and structures. Alternatively, these distinct localization patterns could indicate the movement or partitioning of dRrp6 molecules within the dividing cells. It will be useful to determine whether dRrp6 is enzymatically active in mitosis, to identify its protein partners, and to uncover the mechanisms regulating its exosome-independent movement and deposition. By extension, whether Rrp6 acts more generally in a core exosome-independent manner is unknown, but certainly an important question. To date, several yeast studies have singularly used an rrp6Δ mutant to argue that the nuclear exosome is functioning in a particular pathway (Briggs et al., 1998; Allmang et al., 1999a,b; Hilleren et al., 2001; Kuai et al., 2005; Vodala et al., 2008). However, Rrp6 has functions distinct from those of other exosome subunits, in 5.8S rRNA processing (Briggs et al., 1998; Allmang et al., 1999a,b). Most, if not all, studies related to nuclear mRNA surveillance in yeast have been shown to require Rrp6 but, importantly, core exosome subunits have neither been tested nor shown to contribute to this function (e.g., Hilleren et al., 2001; Libri et al., 2002; Zenklusen et al., 2002; Das et al., 2003; Thomsen et al., 2003; Hieronymus et al., 2004; Fang et al., 2005; Kuai et al., 2005; Roth et al., 2005; Davis and Ares, 2006; Vodala et al., 2008). Finally, and consistent with our findings here, recent data have shown that yeast Rrp6 has RNA processing functions independent of the core exosome (Callahan and Butler, 2008).
A comparison between published microarray data for yeast rrp6Δ mutant (Houalla et al., 2006) and our data yielded information that was limited but interesting. For example, of 234 transcripts (134 increased, 100 decreased) changed greater than twofold in our study, only 40 had readily identifiable yeast orthologues in yeast. Despite this shortcoming, a clear finding was that histone mRNAs and heat-shock genes were up-regulated in both studies (Houalla et al., 2006; this work). More targeted studies have also indicated that histone mRNAs have increased half lives in rrp6Δ cells (Canavan and Bond, 2007; Reis and Campbell, 2007). The most striking difference between the two microarray data sets was the proportion of total mRNAs affected that were decreased (our study, 43% compared with yeast, 17%). These similarities and differences may reflect, respectively, the conserved functions of Rrp6 in all eukaryotes and those functions evolved in metazoan cells.
The observation that dRrp6 overexpression affected cell cycle progression was also notable. In this regard, it would be extremely interesting to perform a microarray analysis on RNA isolated from the dRrp6-overexpressing S2 cells line and then compare and contrast the changed transcripts in these cells to those transcripts affected in the dRrp6-depletion microarray data set. Moreover, it will be useful to test other exosome subunit genes in these pathways to determine whether these functions ascribed to the nuclear exosome are indeed properties shared with the core complex or are core exosome-independent functions for Rrp6.
Models for Rrp6 Function in Cell Cycle Progression
How might Rrp6 function in the cell cycle? Its dynamic cell cycle and mitotic distribution patterns, its phenotypic effects on chromosomes, and the transcriptomic changes caused by its depletion, lead us to present three models. In the first model, we propose that dRrp6 acts both locally and globally to catabolize RNAs, and that loss of this control leads to loss of cell proliferation. In this explanatory scheme, the phenotype arising from the dRrp6 depletion is not reducible to one RNA or pathway but arises from a general and eminently unpredictable set of transcripts that fall below a critical threshold at which the cell ceases to progress through mitosis effectively. Indeed, there are many transcripts whose stability is regulated tightly in a cell cycle–dependent manner. Given that the threshold of dRrp6 protein levels appears to be a determining feature of whether or not these mitotic effects are observed (cf. this work and Goshima et al., 2007), when dRrp6 falls below this threshold, its effects will be observed. Alternatively, given its functions in mRNA metabolism in yeast (Hilleren et al., 2001; Libri et al., 2002; Hieronymus et al., 2004; Luna et al., 2005; Wyers et al., 2005; Davis and Ares, 2006), Rrp6 could play a critical role in the poorly understood mechanisms controlling genome-wide ribonucleometabolic shutdown during mitosis in eukaryotes.
In contrast to this view is a second model: dRrp6-dependent modulation of specific soluble mRNAs is the source of the cell cycle slowdown and defects in mitotic entry and transit. Indeed, consistent with our observations, yeast studies support a role for Rrp6 in regulating histone mRNA levels (Houalla et al., 2006; Reis and Campbell, 2007). Histone transcripts and proteins have many relationships with checkpoints and cell cycle progression (Zhao, 2004; Vidanes et al., 2005; Muller et al., 2007). It is possible that the phenotypes observed here indeed arise from the stabilization of these transcripts alone; this remains to be tested. Alternatively, there may be another class of mRNAs whose levels may be crucial for allowing mitotic passage. For example, a yeast RNase has been implicated in cell cycle progression through CLB2 mRNA stability (Gill et al., 2004). Finally, dRrp6 depletion could stabilize a set of small regulatory RNAs that interfere with specific mRNAs encoding proteins required for mitotic entry and/or passage; this would be consistent with our observation that ∼100 mRNAs are down-regulated in the dRrp6 depletion.
Finally, a third, “mitoticentric” model posits that Rrp6 regulates specific RNA molecules that reside exclusively on mitotic structures. In this model, perturbation of these RNAs could clog or interfere with assembly/disassembly of these structures. There are numerous RNA substrates during mitosis that could be candidate Rrp6 targets. For example, ribosomal RNAs associate with the PCL (Van Hooser et al., 2005). Many mRNAs and rRNAs reside on asters and spindles, where they are required for assembly of these structures in Xenopus cell-free extracts (Blower et al., 2005). Moreover, mRNAs associate with centrosomes, where they may be functionally important for centrosome propagation in surf clam oocytes (Alliegro et al., 2006). Furthermore, mRNAs are asymmetrically partitioned on centrosomes during mollusk embryonic cleavages (Lambert and Nagy, 2002). Finally, SpRrp6 and SpDis3 processes or degrade centromeric siRNAs that regulate kinetochore function (Murakami et al., 2007; Nicolas et al., 2007); whether these RNAs underlie the mechanism of metazoan Rrp6 action on mitotic chromosomes is unknown.
Future work will be required to differentiate among these models. Although we cannot exclude the possibility that dRrp6 could have RNA-independent functions, we consider this unlikely. Rather, we theorize an essential, conserved, RNA-based control system through which ribonucleases affect proper cell cycle progression.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Peter Harte, Helen Salz, Claudio Sunkel (Institute for Molecular and Cell Biology, Porto, Portugal), David Glover (University of Cambridge, Cambridge, United Kingdom), and Paul Fisher (Stony Brook University, Stony Brook, NY) for antibodies, Drs. David MacDonald and Piet de Boer (both from the Case Western Reserve University School of Medicine) for microscopes, and Dr. Alan Tartakoff and members of the Andrulis lab for discussions and manuscript review. This work is supported by Grant GM072820 from the National Institutes of Health (E.D.A). E.D.A. is a Mount Sinai Health Care Foundation Scholar.
Abbreviations used:
- MT
microtubule
- pH3
phospho-histone H3
- PCL
perichromosomal layer
- PCM
pericentriolar material
- RNAi
RNA interference
- SAC
spindle assembly checkpoint.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0825) on February 18,2009.
REFERENCES
- Abruzzi K., Denome S., Olsen J. R., Assenholt J., Haaning L. L., Jensen T. H., Rosbash M. A novel plasmid-based microarray screen identifies suppressors of rrp6Delta in Saccharomyces cerevisiae. Mol. Cell. Biol. 2007;27:1044–1055. doi: 10.1128/MCB.01299-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliegro M. C., Alliegro M. A., Palazzo R. E. Centrosome-associated RNA in surf clam oocytes. Proc. Natl. Acad. Sci. USA. 2006;103:9034–9038. doi: 10.1073/pnas.0602859103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C., Kufel J., Chanfreau G., Mitchell P., Petfalski E., Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999a;18:5399–5410. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmang C., Petfalski E., Podtelejnikov A., Mann M., Tollervey D., Mitchell P. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 1999b;13:2148–2158. doi: 10.1101/gad.13.16.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrulis E. D., Guzman E., Doring P., Werner J., Lis J. T. High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev. 2000;14:2635–2649. doi: 10.1101/gad.844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrulis E. D., Werner J., Nazarian A., Erdjument-Bromage H., Tempst P., Lis J. T. The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature. 2002;420:837–841. doi: 10.1038/nature01181. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Harris K., Zhang K. Mutations synthetically lethal with cep1 target S. cerevisiae kinetochore components. Genetics. 1998;149:73–85. doi: 10.1093/genetics/149.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower M. D., Nachury M., Heald R., Weis K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell. 2005;121:223–234. doi: 10.1016/j.cell.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Bousquet-Antonelli C., Presutti C., Tollervey D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell. 2000;102:765–775. doi: 10.1016/s0092-8674(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Briggs M. W., Burkard K. T., Butler J. S. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem. 1998;273:13255–13263. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- Buhler M., Haas W., Gygi S. P., Moazed D. RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell. 2007;129:707–721. doi: 10.1016/j.cell.2007.03.038. [DOI] [PubMed] [Google Scholar]
- Burkard K. T., Butler J. S. A nuclear 3′-5′ exonuclease involved in mRNA degradation interacts with Poly(A) polymerase and the hnRNA protein Npl3p. Mol. Cell Biol. 2000;20:604–616. doi: 10.1128/mcb.20.2.604-616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner K., Wenig K., Hopfner K. P. Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol. Cell. 2005;20:461–471. doi: 10.1016/j.molcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Callahan K. P., Butler J. S. Evidence for core exosome independent function of the nuclear exoribonuclease Rrp6p. Nucleic Acids Res. 2008;36:6645–6655. doi: 10.1093/nar/gkn743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan R., Bond U. Deletion of the nuclear exosome component RRP6 leads to continued accumulation of the histone mRNA HTB1 in S-phase of the cell cycle in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35:6268–6279. doi: 10.1093/nar/gkm691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Gherzi R., Ong S. E., Chan E. L., Raijmakers R., Pruijn G. J., Stoecklin G., Moroni C., Mann M., Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- Das B., Butler J. S., Sherman F. Degradation of normal mRNA in the nucleus of Saccharomyces cerevisiae. Mol. Cell. Biol. 2003;23:5502–5515. doi: 10.1128/MCB.23.16.5502-5515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Ares M., Jr Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2006;103:3262–3267. doi: 10.1073/pnas.0507783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl B., Blank D., Ohnacker M., Friedlein A., Roeder D., Langen H., Keller W. A role for SSU72 in balancing RNA polymerase II transcription elongation and termination. Mol. Cell. 2002;10:1139–1150. doi: 10.1016/s1097-2765(02)00707-4. [DOI] [PubMed] [Google Scholar]
- Dziembowski A., Lorentzen E., Conti E., Seraphin B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol. 2007;14:15–22. doi: 10.1038/nsmb1184. [DOI] [PubMed] [Google Scholar]
- Estevez A. M., Lehner B., Sanderson C. M., Ruppert T., Clayton C. The roles of intersubunit interactions in exosome stability. J. Biol. Chem. 2003;278:34943–34951. doi: 10.1074/jbc.M305333200. [DOI] [PubMed] [Google Scholar]
- Fang F., Phillips S., Butler J. S. Rat1p and Rai1p function with the nuclear exosome in the processing and degradation of rRNA precursors. RNA. 2005;11:1571–1578. doi: 10.1261/rna.2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomproix N., Hernandez-Verdun D. Effects of anti-PM-Scl 100 (Rrp6p exonuclease) antibodies on prenucleolar body dynamics at the end of mitosis. Exp. Cell Res. 1999;251:452–464. doi: 10.1006/excr.1999.4578. [DOI] [PubMed] [Google Scholar]
- Gavin A. C., et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gavin A. C., et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–147. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- Giet R., McLean D., Descamps S., Lee M. J., Raff J. W., Prigent C., Glover D. M. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T., Cai T., Aulds J., Wierzbicki S., Schmitt M. E. RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation. Mol. Cell Biol. 2004;24:945–953. doi: 10.1128/MCB.24.3.945-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S. S., Zhang N., Scholey J. M., Vale R. D., Stuurman N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 2007;316:417–421. doi: 10.1126/science.1141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. C., Kiss D. L., Andrulis E. D. Differential distribution of exosome subunits at the nuclear lamina and in cytoplasmic foci. Mol. Biol. Cell. 2006;17:1399–1409. doi: 10.1091/mbc.E05-08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile S., Cristodero M., Clayton C., Estevez A. M. The subcellular localisation of trypanosome RRP6 and its association with the exosome. Mol. Biochem. Parasitol. 2007;151:52–58. doi: 10.1016/j.molbiopara.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hieronymus H., Yu M. C., Silver P. A. Genome-wide mRNA surveillance is coupled to mRNA export. Genes Dev. 2004;18:2652–2662. doi: 10.1101/gad.1241204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren P., McCarthy T., Rosbash M., Parker R., Jensen T. H. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature. 2001;413:538–542. doi: 10.1038/35097110. [DOI] [PubMed] [Google Scholar]
- Houalla R., Devaux F., Fatica A., Kufel J., Barrass D., Torchet C., Tollervey D. Microarray detection of novel nuclear RNA substrates for the exosome. Yeast. 2006;23:439–454. doi: 10.1002/yea.1369. [DOI] [PubMed] [Google Scholar]
- Houseley J., LaCava J., Tollervey D. RNA-quality control by the exosome. Nat. Rev. Mol. Cell. Biol. 2006;7:529–539. doi: 10.1038/nrm1964. [DOI] [PubMed] [Google Scholar]
- Jensen T. H., Boulay J., Rosbash M., Libri D. The DECD box putative ATPase Sub2p is an early mRNA export factor. Curr. Biol. 2001;11:1711–1715. doi: 10.1016/s0960-9822(01)00529-2. [DOI] [PubMed] [Google Scholar]
- Johansen K. M., Johansen J. Regulation of chromatin structure by histone H3S10 phosphorylation. Chromosome Res. 2006;14:393–404. doi: 10.1007/s10577-006-1063-4. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Goebl M., Yanagida M. The fission yeast dis3+ gene encodes a 110-kDa essential protein implicated in mitotic control. Mol. Cell Biol. 1991;11:5839–5847. doi: 10.1128/mcb.11.12.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Wolf Y. I., Aravind L. Prediction of the archaeal exosome and its connections with the proteasome and the translation and transcription machineries by a comparative-genomic approach. Genome Res. 2001;11:240–252. doi: 10.1101/gr.162001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J., et al. High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell. 2004;13:225–239. doi: 10.1016/s1097-2765(04)00003-6. [DOI] [PubMed] [Google Scholar]
- Kuai L., Das B., Sherman F. A nuclear degradation pathway controls the abundance of normal mRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2005;102:13962–13967. doi: 10.1073/pnas.0506518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. D., Nagy L. M. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682–686. doi: 10.1038/nature01241. [DOI] [PubMed] [Google Scholar]
- Lejeune F., Li X., Maquat L. E. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol. Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- Libri D., Dower K., Boulay J., Thomsen R., Rosbash M., Jensen T. H. Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol. Cell. Biol. 2002;22:8254–8266. doi: 10.1128/MCB.22.23.8254-8266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Greimann J. C., Lima C. D. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell. 2006;127:1223–1237. doi: 10.1016/j.cell.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Logarinho E., Bousbaa H., Dias J. M., Lopes C., Amorim I., Antunes-Martins A., Sunkel C. E. Different spindle checkpoint proteins monitor microtubule attachment and tension at kinetochores in Drosophila cells. J. Cell Sci. 2004;117:1757–1771. doi: 10.1242/jcs.01033. [DOI] [PubMed] [Google Scholar]
- Lorentzen E., Conti E. Structural basis of 3′ end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core. Mol. Cell. 2005;20:473–481. doi: 10.1016/j.molcel.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Lorentzen E., Conti E. The exosome and the proteasome: nano-compartments for degradation. Cell. 2006;125:651–654. doi: 10.1016/j.cell.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Lorentzen E., Walter P., Fribourg S., Evguenieva-Hackenberg E., Klug G., Conti E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat. Struct. Mol. Biol. 2005;12:575–581. doi: 10.1038/nsmb952. [DOI] [PubMed] [Google Scholar]
- Luna R., Jimeno S., Marin M., Huertas P., Garcia-Rubio M., Aguilera A. Interdependence between transcription and mRNP processing and export, and its impact on genetic stability. Mol. Cell. 2005;18:711–722. doi: 10.1016/j.molcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′→5′ exoribonucleases. Cell. 1997;91:457–466. doi: 10.1016/s0092-8674(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Muller B., Blackburn J., Feijoo C., Zhao X., Smythe C. Are multiple checkpoint mediators involved in a checkpoint linking histone gene expression with DNA replication? Biochem. Soc. Trans. 2007;35:1369–1371. doi: 10.1042/BST0351369. [DOI] [PubMed] [Google Scholar]
- Murakami H., Goto D. B., Toda T., Chen E. S., Grewal S. I., Martienssen R. A., Yanagida M. Ribonuclease activity of Dis3 is required for mitotic progression and provides a possible link between heterochromatin and kinetochore function. PLoS ONE. 2007;2:e317. doi: 10.1371/journal.pone.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nicolas E., Yamada T., Cam H.P., Fitzgerald P.C., Kobayashi R., Grewal S.I. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat. Struct. Mol. Biol. 2007 doi: 10.1038/nsmb1239. [DOI] [PubMed] [Google Scholar]
- Noguchi E., et al. Dis3, implicated in mitotic control, binds directly to Ran and enhances the GEF activity of RCC1. EMBO J. 1996;15:5595–5605. [PMC free article] [PubMed] [Google Scholar]
- Ohkura H., Adachi Y., Kinoshita N., Niwa O., Toda T., Yanagida M. Cold-sensitive and caffeine-supersensitive mutants of the Schizosaccharomyces pombe dis genes implicated in sister chromatid separation during mitosis. EMBO J. 1988;7:1465–1473. doi: 10.1002/j.1460-2075.1988.tb02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C. C., Campbell J. L. Contribution of Trf4/5 and the nuclear exosome to genome stability through regulation of histone mRNA levels in Saccharomyces cerevisiae. Genetics. 2007;175:993–1010. doi: 10.1534/genetics.106.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K. M., Wolf M. K., Rossi M., Butler J. S. The nuclear exosome contributes to autogenous control of NAB2 mRNA levels. Mol. Cell Biol. 2005;25:1577–1585. doi: 10.1128/MCB.25.5.1577-1585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W., Ashburner M., Hawley R. S. Drosophila Protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Thomsen R., Libri D., Boulay J., Rosbash M., Jensen T. H. Localization of nuclear retained mRNAs in Saccharomyces cerevisiae. RNA. 2003;9:1049–1057. doi: 10.1261/rna.5170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A., Staples R. R., Baker R. E., Parker R. Function of the ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell. Biol. 2000;20:8230–8243. doi: 10.1128/mcb.20.21.8230-8243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser A. A., Yuh P., Heald R. The perichromosomal layer. Chromosoma. 2005;114:377–388. doi: 10.1007/s00412-005-0021-9. [DOI] [PubMed] [Google Scholar]
- Vanacova S., Wolf J., Martin G., Blank D., Dettwiler S., Friedlein A., Langen H., Keith G., Keller W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidanes G. M., Bonilla C. Y., Toczyski D. P. Complicated tails: histone modifications and the DNA damage response. Cell. 2005;121:973–976. doi: 10.1016/j.cell.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Vodala S., Abruzzi K. C., Rosbash M. The nuclear exosome and adenylation regulate posttranscriptional tethering of yeast GAL genes to the nuclear periphery. Mol. Cell. 2008;31:104–113. doi: 10.1016/j.molcel.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F., et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Zenklusen D., Vinciguerra P., Wyss J. C., Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol. Cell. Biol. 2002;22:8241–8253. doi: 10.1128/MCB.22.23.8241-8253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Coordination of DNA synthesis and histone gene expression during normal cell cycle progression and after DNA damage. Cell Cycle. 2004;3:695–697. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.