Abstract
Mitochondrial outer membrane permeabilization (MOMP) is a critical step in apoptosis and is regulated by Bcl-2 family proteins. In vitro systems using cardiolipin-containing liposomes have demonstrated the key features of MOMP induced by Bax and cleaved Bid; however, the nature of the “pores” and how they are formed remain obscure. We found that mitochondrial outer membranes contained very little cardiolipin, far less than that required for liposome permeabilization, despite their responsiveness to Bcl-2 family proteins. Strikingly, the incorporation of isolated mitochondrial outer membrane (MOM) proteins into liposomes lacking cardiolipin conferred responsiveness to cleaved Bid and Bax. Cardiolipin dependence was observed only when permeabilization was induced with cleaved Bid but not with Bid or Bim BH3 peptide or oligomerized Bax. Therefore, we conclude that MOM proteins specifically assist cleaved Bid in Bax-mediated permeabilization. Cryoelectron microscopy of cardiolipin-liposomes revealed that cleaved Bid and Bax produced large round holes with diameters of 25–100 nm, suggestive of lipidic pores. In sum, we propose that activated Bax induces lipidic pore formation and that MOM proteins assist cleaved Bid in this process in the absence of cardiolipin.
INTRODUCTION
Bcl-2 family proteins are critical regulators of apoptosis, and their major site of action is on the mitochondrial outer membrane (MOM) (Cory and Adams, 2002; Kuwana and Newmeyer, 2003; Youle and Strasser, 2008). They share multiple BH (Bcl-2 homology) domains (BH1–3) and regulate apoptosis predominantly by controlling the permeability of the MOM. Proapoptotic Bcl-2 family members promote permeabilization, which leads to the release of apoptogenic proteins, including cytochrome c (Liu et al., 1996), SMAC/DIABLO (Du et al., 2000; Verhagen et al., 2000), and Omi/HtrA (Suzuki et al., 2001), from the intermembrane space. Antiapoptotic family members inhibit this process. Proapoptotic multidomain Bcl-2 family members Bax and Bak are essential effectors of mitochondrial outer membrane permeabilization (MOMP) (Lindsten et al., 2000; Wei et al., 2001), and when they are activated, they change the conformation and oligomerize in the membrane to promote MOMP (Nechushtan et al., 1999, 2001; Antonsson et al., 2001; Kuwana et al., 2002).
The molecular mechanisms that underlie MOMP have been a subject of intensive study, because this step is deemed to have high potential for therapeutic intervention in cancer (Lowe et al., 2004; Brown and Attardi, 2005; Fesik, 2005; Green and Kroemer, 2005). Once MOMP occurs, cells permanently cease to proliferate (Xiang et al., 1996; McCarthy et al., 1997; Chipuk and Green, 2005; Brown et al., 2007; Knudson and Brown, 2008), except under unusual circumstances (Colell et al., 2007). However, the molecular mechanism whereby the MOM is permeabilized upon Bax and Bak activation remains obscure. Bax-mediated membrane permeabilization has been studied in in vitro liposome systems that faithfully recapitulate some aspects of MOMP. Such studies have revealed a size-indiscriminatory release of contents upon the addition of proapoptotic Bax and caspase-8–cleaved Bid and inhibition of this release by antiapoptotic family members (Basanez et al., 2002; Kuwana et al., 2002, 2005; Roucou et al., 2002b; Yethon et al., 2003; Terrones et al., 2004). It is also established that liposomes require at least 7 mol % of cardiolipin in their membranes to undergo optimal permeabilization induced by cleaved Bid and Bax (Kuwana et al., 2002; Yethon et al., 2003; Terrones et al., 2004). The nature of this permeabilization remains unclear. Some studies suggest that it may involve the formation of proteinaceous channels by Bax molecules, either by themselves (Saito et al., 2000) or together with other MOM-resident membrane proteins, such as voltage-dependent anion channel (VDAC) (Shimizu et al., 1999, 2000). However, that double knockout mice lacking VDAC isoforms 1 and 3 do not show the apoptotic defect argues against the involvement of these proteins (Baines et al., 2007), and the other studies suggest that the pores may be lipidic in nature (Basanez et al., 2002; Hardwick and Polster, 2002; Terrones et al., 2004).
The physiological relevance of cardiolipin in MOMP, however, remains controversial. Cardioipin is suggested as a receptor for Bid in mitochondria (Lutter et al., 2000) and is shown to facilitate Bax insertion and oligomerization in liposomes (Lucken-Ardjomande et al., 2008a). In contrast, some studies conclude that cardiolipin is not required for Bax-mediated killing of yeast (Iverson et al., 2004; Polcic et al., 2005), although molecular mechanisms of yeast “death” induced by Bax may not be mediated through MOMP, or the mechanism of yeast MOMP may not be the same as that of mammalian mechanism (Roucou et al., 2002b).
Here, we report on a series of studies investigating the nature of membrane permeabilization induced by cleaved Bid and Bax by using vesicle systems. We have developed proteo-liposome systems that reproduce permeabilization induced by Bax and cleaved Bid with more physiological material. These proteo-liposomes are comprised solely of native MOM components, with cardiolipin present only in trace amounts or absent. It therefore seems that the MOM contains at least one factor other than cardiolipin that helps mediate Bid/Bax-induced membrane permeabilization. Given that this factor was not extracted by organic solvents, it is likely proteinaceous. We also found that cardiolipin dependence was only seen when Bax was activated by cleaved Bid but not by Bid or Bim BH3 peptide or oligomerized Bax. Thus, the proteinaceous factor may be a “receptor” for cleaved Bid. Finally, cryoelectron microscopy (cryo-EM) has revealed pore-like openings in cardiolipin-liposomes after their permeabilization by cleaved Bid and Bax. These data provide long-sought visual evidence consistent with a lipidic pore model, suggested previously based on biochemical observations (Basanez et al., 1999; Kuwana et al., 2002; Terrones et al., 2004). We conclude that MOM proteins assist cleaved Bid in inducing Bax-dependent lipidic pore formation in the MOM.
MATERIALS AND METHODS
Formation of Octylglucoside-Large Unilamellar Vesicles (OG-LUVs), Reformed Outer Membrane Vesicles (ReOMVs), and Reformed Light Membrane Vesicles (ReLMVs)
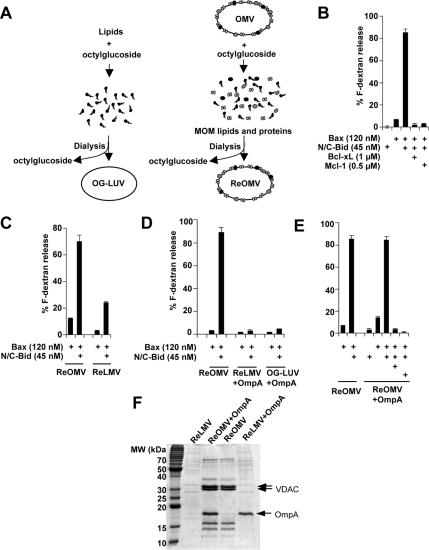
Egg phosphatidylcholine (PC), egg phosphatidylethanolamine (PE), soy phosphatidylinositol (PI), soy phosphatidylserine (PS), and heart cardiolipin (CL) in chloroform were obtained from Avanti Polar Lipids (Alabaster, AL) and were mixed appropriately and dried under argon. The lipid film was resuspended in KKE (10 mM potassium phosphate, pH 7.4, 50 mM KCl, and 1 mM EDTA) buffer containing 2% OG. The suspension was stir-mixed for 1 h at 4°C and dialyzed against KKE to remove detergent. Before dialysis, fluorescein-dextran was added at 5 mg/ml to the samples to be used in the dextran release assay. The liposome suspension was mixed with twice the volume of 70% sucrose in KKE and placed at the bottom of a float-up sucrose step gradient consisting of 32 and 0% sucrose in KKE, and liposomes were collected at the interface. The liposomes were washed and resuspended in KKE buffer. OMVs (∼130 μg of protein) were solubilized in 100 μl of KKE containing 4% OG for 1 h at 4°C with mixing, and ReOMVs were formed following the procedures as described above (Figure 2). For OmpA loading, OmpA in 2% OG-KKE (∼50 μg) was added to lipids (500 μg) resuspended in 2% OG-KKE, detergent-solubilized OMVs (∼130 μg of protein), or LMVs (∼130 μg of protein). The mixture was stirred for 1 h at 4°C, followed by the same procedures described above. OMVs and LMVs were isolated from mitochondria and from endoplasmic reticulum (ER) fractions of Xenopus egg extracts as reported previously (Kuwana et al., 2002).
Figure 2.
OMVs possess components that facilitate N/C-Bid and Bax-induced membrane permeabilization. (A) Schematic representation of the protocol for generating liposomes by detergent-mediated method (left). Dried lipids were resuspended in buffer containing 2–4% OG, and liposomes (OG-LUVs) were formed as detergent was removed by dialysis. ReOMVs were formed based on the same principle (right) from detergent-solubilized OMVs. ReOMVs were made solely from endogenous OMV lipids and proteins. (B) ReOMVs responded to Bcl-2 family proteins in the same way that cardiolipin-containing liposomes do; Bax and N/C-Bid released dextrans and the release was inhibited by antiapoptotic Bcl-xL or Mcl-1. Note that N/C-Bid alone did not permeabilize the vesicles. (C) ReLMVs, generated from detergent-solubilized LMVs, were not permeabilized significantly in the presence of N/C-Bid and Bax. (D) The bacterial outer membrane protein OmpA did not confer responsiveness to liposomes or ReLMVs. Data shown are representative of four independent experiments. (E) OmpA loading did not interfere with the responsiveness of ReOMVs. (F) Coomassie Blue-stained SDS-PAGE gel confirms the loading of membrane proteins in ReLMVs, ReOMVs + OmpA, ReOMVs, and ReLMVs + OmpA. Prominent proteins such as VDAC (a doublet in Xenopus) and OmpA are indicated with arrows.
Formation of ExOMVs
OMVs (780 μg of protein) were mixed with 1 ml of chloroform/methanol (1:1) after which 1 ml of water was added, and the samples were vortexed. The suspension was spun at 500 × g for 10 min in a tabletop centrifuge, and the organic solvent layer was discarded. One milliliter of chloroform was added to the aqueous layer, and the lipids were extracted again. The aqueous solution containing precipitated membrane proteins was mixed with 1.5 ml of pentane and dried under argon. Dried proteins were resuspended in 200 μl of 8 M urea and centrifuged at 150,000 × g for 30 min at 4°C, and the supernatant was collected. The urea solution was diluted threefold with 4% OG-KKE, and the mixture was dialyzed against 2% OG-KKE to completely remove the urea. The dialysate was centrifuged at 150,000 × g for 30 min to remove nonrenatured precipitates in the pellet. A mixture of 100 μg of phospholipids (PC:PE:PI:PS, 59:23:16:2 mol %) was dried and resuspended with the supernatant of the dialysate containing refolded membrane proteins. The lipid–protein–detergent mixture was stir-mixed for 1 h at 4°C, and detergent was removed to form ExOMVs as described above. The final pellet was resuspended in 40–80 μl of KKE and used in the dextran release assay.
OmpA Production
Escherichia coli BL21(DE3) cells were transformed with pET3b-OmpA171 and induced according to the methods described in Pautsch et al. (1999). The bacterial pellet was sonicated in 20 mM Tris, pH 8.5, and the supernatant was discarded. The pellet was resuspended in the same buffer plus 2% NP-40 and spun down to collect the inclusion bodies, which were solubilized in 6 M guanidine-HCl, 20 mM Tris, pH 8.5, in a shaker at 37°C for 2 h. Solubilized inclusion bodies were diluted fourfold with 4% OG-KKE and dialyzed in 2% OG-KKE at 4°C. The purity of the OmpA obtained was >95%, according to a Coomassie-stained gel.
Generation of Recombinant Bcl-2-Family Proteins, BH3 Peptides, Dextran Release Assays, Protein Assay, and Lipid Composition Analysis by Thin Layer Chromatography (TLC)
These experimental procedures were performed as described previously (Kuwana et al., 2002, 2005), except that, in the dextran release assays, at the end of the incubation, vesicles were pelleted at 150,000 × g for 10 min to collect the released dextrans in the supernatant. Data shown for each release assay are representative of >10 independent experiments unless otherwise stated, and all the bar graphs are presented as mean ± SEM.
Protein Identification by Mass Spectrometry
The protein bands were excised from Coomassie Blue-stained SDS-polyacrylamide gel electrophoresis (PAGE) gels, reduced, and alkylated with iodoacetamide and tryptic digest was prepared. Mass spectrometric analysis was performed using a model 4700 Proteomics Analyzer from Applied Biosystems (Foster City, CA). This instrument uses matrix-assisted laser desorption/ionization in conjunction with tandem time-of-flight mass analyzers. The digest was introduced into the instrument in a crystalline matrix of a-cyano-4-hydroxycinnamic acid containing 2 mM ammonium citrate to suppress ionization of matrix clusters. Database searches were performed with Applied Biosystem's GPS explorer software that uses the Mascot search engine. Protein assignments were made on the basis of both mass spectrometry and tandem mass spectrometry spectra. NCBInr was used for protein identification.
Mass Spectrometry for Cardiolipin Quantification
For the internal standards, 5 μg of C14 1,1′,2,2′-tetramyristoyl cardiolipin (Avanti Polar Lipids) was added as an internal standard to 100 μg of OMV preparations before lipid extraction with CHCl3:MeOH:0.15 M NaCl; 4:2:1, per vol (Sorice et al., 2004). Lipids were resuspended in chlorofom:methanol:H2O (10:9:1, per vol) and analyzed in the negative ion mode on a Bruker Esquire HCT ion trap mass spectrometer electrospray ionization at a flow rate of 120 μl/h and a capillary tension of −250 V. Ion fragmentation was induced by argon as collision gas at a pressure of 8 mbar. [M-2H]2-ions of cardiolipin were quantified relative to the internal standard.
Cryo-EM
OG-LUVs were formed as described above but in the absence of fluorescein-dextrans. Typically, 2 mg of phospholipids were dried, and the final pellet was resuspended in 15 μl of KKE to obtain the high concentration that is required for visualization by cryo-EM. Nontreated OG-LUVs were mixed with 4 μM glutathione transferase to control for the presence of proteins. For Bax, tBid, and Bcl-xL treatment, OG-LUVs (6 μl) were incubated with Bax (3.8 μM), N/C-Bid (2.3 μM), or Bcl-xL (26 μM) in 12 μl. The recombinant protein concentrations were increased by 23- to 30-fold, because of the high concentration of vesicles present in the sample (≈100 times more than in the standard dextran release assay). The salt concentration was adjusted by adding KCl to 40–50 mM. For each time point, 3-μl aliquots of each sample were applied to a C-flat holey carbon film (Protochips, Raleigh, NC) supported on a 400 mesh copper grid. These grids were blotted for 2–4 s and plunge-frozen in ethane in a humidity (95%)- and temperature (25°C)-controlled chamber using a Vitrobot automated blotting device (FEI, Eindhoven, The Netherlands). Grids were cleaned before vitrification, using a Solarus Plasma Cleaner (25% O2, 75% argon). The vitreous ice grids were transferred into the electron microscope using a Gatan cryostage, which maintains the grids at a temperature below −170°C. Transmission electron microscopy was performed using low dose methods on a Tecnai F20 microscope (FEI, Hillsboro, OR) operating at 120 KeV, and at a nominal magnification of between 29,000× and 80,000×. Images were acquired using a Tietz slow scan charge-coupled device camera and the Leginon software (Suloway et al., 2005).
RESULTS
Extracted OMV Lipids Do Not Support Membrane Permeabilization by Bid/Bax
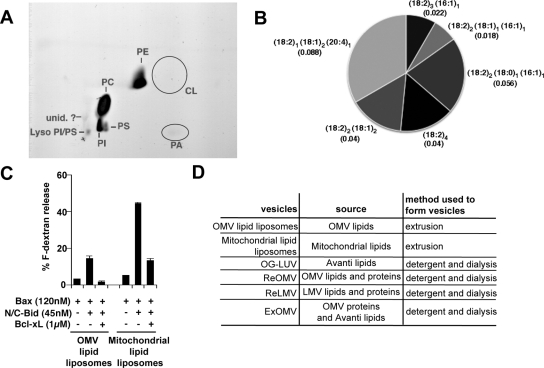
N/C-Bid and Bax-mediated permeabilization of liposomes was earlier shown to be cardiolipin dependent (Kuwana et al., 2002; Yethon et al., 2003; Terrones et al., 2004). We therefore examined the possibility that cardiolipin is required for the response of native MOMs to N/C-Bid. Cardiolipin amounts in Xenopus egg OMVs, however, were below the limits of detection for TLC (Figure 1A), and quantitative lipid mass spectrometry for cardiolipin revealed the presence of this lipid at only 0.26 ± 0.14 mol % (n = 5; Figure 1B). This low level could represent the real amount of cardiolipin, or it could theoretically result from minor contamination of our OMV preparations with inner membranes that are enriched with cardiolipin.
Figure 1.
OMVs do not contain 7 mol % cardiolipin in their total lipids and the extracted lipids from OMVs do not support Bax-mediated membrane permeabilization. (A) Lipids were extracted from OMVs and subjected to two-dimensional TLC analysis. Cardiolipin was not visible in the expected location (circled). (B) Relative abundance of cardiolipin species in Xenopus OMV preparations detected by mass spectrometry quantification. OMVs were prepared and each cardiolipin species (based on differences in their fatty acyl chains) was quantified against the internal standard, cardiolipin C14 (see Materials and Methods). Four independently prepared OMV batches and one duplicate sample were analyzed. Numbers in parenthesis show the abundance of the respective cardiolipin species as the average mole percent of total phospholipids in the sample. The total cardiolipin content in our Xenopus OMVs was 0.26 ± 0.14 mol % (n = 5). Data shown are representative of four independent analyses. (C) OMV lipid-liposomes did not show significant levels of dextran release. Lipids were extracted from OMVs (trace amount of cardiolipin) or whole mitochondria (7 mol % cardiolipin), and liposomes were formed by the extrusion method. A dextran release assay was performed as described in Kuwana et al. (2002). Data shown are representative of two independent experiments. (D) A list of vesicles used in this study.
Next, we determined whether native OMV lipids could support Bid/Bax-mediated permeabilization. Native OMVs responded to N/C-Bid without the addition of exogenous Bax protein, presumably because the MOM already harbors a Bax-like molecule in the membrane. In contrast, liposomes formed by the extrusion method from lipids extracted from OMVs responded poorly to the addition of N/C-Bid and Bax. As shown previously, the same preparations of recombinant N/C-Bid and Bax were able to permeabilize liposomes formed from lipids extracted from whole mitochondria, which contain ∼7 mol % cardiolipin (Kuwana et al., 2002) (Figure 1C). That the OMVs responded readily to N/C-Bid, despite their low content of cardiolipin, suggests that other OMV components may compensate for the reduced amounts of cardiolipin or that the cardiolipin present in the MOM is concentrated into small functional domains, as has been suggested for contact sites between the outer and inner membranes (Ardail et al., 1990; Lutter et al., 2001). All the reconstituted vesicles reported in this study are listed in Figure 1D.
ReOMVs with Greatly Reduced Amounts of Cardiolipin Respond Well to Bid/Bax
To determine biochemically whether MOM components facilitate Bid/Bax-mediated pore formation, it was necessary to disassemble the OMV components by using detergent. To this end, we first solubilized OMVs by using OG, removed OG by dialysis, and then allowed vesicles to form (Figure 2A). We observed that both endogenous membrane proteins and lipids assembled into proteo-liposomes, as the detergent was gradually removed. The resulting proteo-liposomes, termed ReOMVs, were permeabilized after the addition of a combination of N/C-Bid and Bax, and this permeabilization was inhibited by the presence of antiapoptotic Bcl-xL or Mcl-1, as in cardiolipin-liposomes (Kuwana et al., 2002). Notably, although the OMVs became permeabilized in the presence of N/C-Bid alone, the ReOMVs did not, but rather required both N/C-Bid and Bax. Presumably, this was because the ReOMVs lacked a Bax-like molecule that is present in the OMVs but is lost during the solubilization and reconstitution process.
To rule out the possibility that nonspecific membrane protein loading makes liposomes responsive to Bid/Bax-mediated permeabilization, we loaded a well-characterized bacterial outer membrane protein, OmpA (Pautsch and Schulz, 1998; Pautsch et al., 1999), into liposomes lacking cardiolipin, and also into detergent-solubilized LMVs, which consist mainly of ER membranes. As shown in Figure 2, C and E, OmpA loading did not confer Bid/Bax responsiveness to noncardiolipin-bearing liposomes generated by the use of detergent (OG-LUVs; Figure 2A) or ReLMVs. Instead, OmpA loading was inhibitory in those vesicles at higher doses. In ReOMVs, however, OmpA loading did not inhibit dextran release, even though more OmpA was present with respect to the estimated amount of lipid in the ReOMVs (Figure 2, D and E). Thus, OmpA does not interfere with the function of N/C-Bid and Bax. We also found that exogenous addition of nonpermeabilizing levels of OG (0.001–0.1%) did not affect Bid/Bax-dependent permeabilization of cardiolipin-liposomes generated by extrusion method that does not involve detergent (data not shown). This experiment was to mimic the situation in which a small amount of OG is present in liposome membranes after dialysis.
We also analyzed the cardiolipin content in ReOMVs by two-dimensional TLC to rule out the possibility that cardiolipin is enriched in these vesicles and responsible for their permeabilization. Cardiolipin was under the detection limit (data not shown), confirming that the content of this lipid in ReOMVs is minimal, like that of OMVs. Taken together, our data suggest that native MOMs have components that substitute for cardiolipin, or possibly rearrange small amounts of this lipid into local domains, to promote Bid/Bax-induced membrane permeabilization.
MOM Proteins Are Sufficient to Confer Bid/Bax-Responsiveness to Proteo-Liposomes
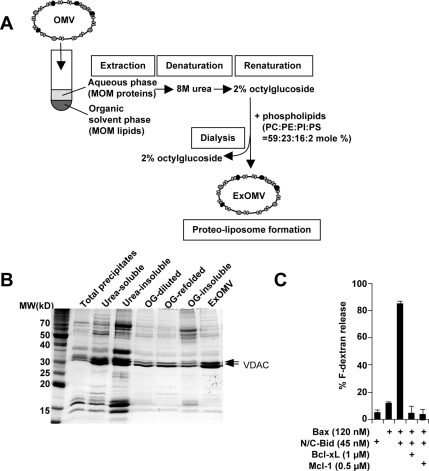
We next asked whether proteins or lipids are responsible for Bid/Bax-induced permeabilization. Experiments using protease treatment were inconclusive, because for unknown reasons, this treatment led to the loss of both MOM proteins and endogenous lipids. Thus, we instead used organic solvents to separate MOM proteins from lipids. Endogenous lipids were extracted from the OMVs as in the experiment shown in Figure 1C, except that the aqueous phase containing the precipitated membrane proteins was collected. These proteins were lyophilized under a stream of argon and then resuspended in 8 M urea. The urea-denatured membrane proteins were refolded in 2% OG and then loaded onto liposomes with a lipid composition that mimicked that of the OMVs (PC:PE:PI:PS, 59:23:16:2 mol %; Figure 3A); note that cardiolipin was absent. We found that this strategy led to the loading of some proteins into the final liposomes (Figure 3B) and that these proteo-liposomes, termed extraction OMVs (ExOMVs), responded to N/C-Bid and Bax to the same extent as do ReOMVs and cardiolipin-bearing liposomes (Figure 3C). This indicates that the factors relevant to permeabilization are not organic-solvent extractable, and likely proteinaceous. Thus, we conclude that cardiolipin is not required for Bid/Bax-mediated permeabilization of the native MOM and that under physiological conditions, unidentified MOM proteins alone can facilitate this process without cardiolipin.
Figure 3.
MOM proteins facilitate Bax-mediated membrane permeabilization. (A) Schematic diagram for the generation of ExOMVs. OMVs were mixed with organic solvents to extract the lipids. The membrane proteins, which had partitioned into the aqueous phase, were denatured in 8 M urea, and then allowed to refold in detergent by dilution and dialysis. A mixture of purified phospholipids (Avanti Polar Lipids) was mixed with the refolded proteins in detergent. OG was then removed by dialysis to allow the formation of proteo-liposomes composed of MOM proteins and exogenous phospholipids (ExOMVs). The lipid composition of PC:PE:PI:PS, 59:23:16:2 mol %, mimics that of OMVs. (B) MOM proteins were loaded in ExOMVs. An aliquot of the sample from each step was loaded and stained by Coomassie Blue. (C) ExOMVs were permeabilized by N/C-Bid and Bax, and this release was inhibited by Bcl-xL or Mcl-1. ExOMVs exhibited the same characteristics as cardiolipin-containing liposomes and ReOMVs. Data shown are representative of five experiments.
Cardiolipin Dependence Is Specific for N/C-Bid
We investigated whether cardiolipin dependence of liposome permeabilization induced by N/C-Bid and Bax was because of N/C-Bid or Bax. We made liposomes with no or 7 mol % cardiolipin and analyzed the release of dextrans. Bid or Bim BH3 peptide can activate Bax directly in our liposome system; however, unlike N/C-Bid, this activation did not depend on the presence of cardiolipin (Figure 4A). Furthermore, octylglucoside-oligomerized Bax (OG-Bax) showed only a slight dependence on cardiolipin (Figure 4B). Therefore, we conclude that what is dependent on cardiolipin for membrane permeabilization is N/C-Bid but not Bax. It seems that if Bax is activated, it can permeabilize membranes containing no cardiolipin. This finding suggests that the proteinaceous factor in the ExOMVs likely cooperates with N/C-Bid, rather than Bax, and that Bax does not require specific components in the MOM to permeabilize once it is activated.
Figure 4.
Cardiolipin dependence is mainly conferred by N/C-Bid. (A) Liposomes containing 0 or 7% mol cardiolipin were generated and dextran release assays were performed with different activators of Bax, namely, N/C-Bid, Bid BH3 peptide, or Bim BH3 peptide. Among those, only N/C-Bid showed cardiolipin dependence. The peptides alone did not permeabilize liposomes (data not shown). Data shown are representative of four experiments. (B) OG-oligomerized Bax (OG-Bax) permeabilized liposomes in the absence of cardiolipin to a significant degree. Data shown are representative of two experiments. (C) Mtch2, a reported Bid-binding protein in the MOM (Grinberg et al., 2005), was detected in ReOMVs but not in ExOMVs. ReOMVs and ExOMVs were loaded on to a SDS-PAGE gel, and proteins were stained by Coomassie Blue. Predominant bands ∼30-kDa were subjected to trypsin digestion, and the proteins were identified by mass spectrometry. Note that Xenopus VDAC (xVDAC) variants xVDAC1 and 2 do not correspond to the mammalian VDAC isoforms.
Because a MOM protein called mitochondrial carrier homologue 2 (Mtch2) has been reported to interact with cleaved Bid and implicated in MOMP (Grinberg et al., 2005; Gross, 2005), we identified MOM proteins around the size of Mtch2 (33 kDa) present in ReOMVs and ExOMVs. As shown in Figure 4C, mass spectroscopy detected Mtch2 in ReOMVs, but not in ExOMVs, suggesting that the proteinaceous factor in ExOMVs is not Mtch2.
Cryo-EM Reveals Features of Bid/Bax-mediated Permeabilization of the Lipid Membrane
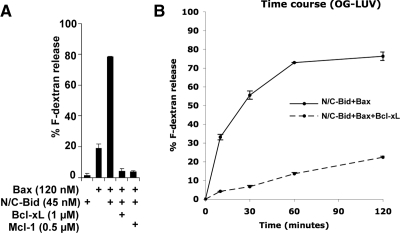
To investigate the nature of the “openings” in the membrane, we chose to visualize the membrane by cryo-EM. In contrast to conventional transmission electron microscopy (TEM), cryo-EM allows the sample to be captured instantaneously in a hydrated state; thus, it is widely applied to visualize liposomes (Almgren, 2000; Johnsson and Edwards, 2003; Frederik and Hubert, 2005). The drawback of this technique, however, is that it is not amenable to the study of larger and more physiologically relevant objects, such as OMVs or mitochondria (∼500 nm), as the specimens must be contained in a thin vitrified ice slab that is penetrable by the electron beam. We found that OG-LUVs (Figure 2A) are well suited for cryo-EM because of their small diameter (50–100 nm). OG-LUVs containing 7 mol % cardiolipin responded to Bcl-2 family proteins in a manner similar to LUVs generated by the extrusion method (Kuwana et al., 2002) (Figure 5A). An earlier study showed that in individual apoptotic cells, the release of green fluorescent protein-cytochrome c from mitochondria is complete within 6 min (Goldstein et al., 2000), but we found the release from liposomes slower, reaching its peak after 1 h (Figure 5B). We speculate that this is because the liposome system lacks factors that facilitate Bax translocation and that the association of Bax with the liposome membrane is thus limited by diffusion.
Figure 5.
OG-LUVs respond to recombinant Bcl-2 family proteins. (A) Dextrans were released from OG-LUVs containing 7 mol % cardiolipin. N/C-Bid and Bax permeabilized the vesicles, and the release was inhibited by the addition of the anti-apoptotic protein Bcl-xL or Mcl-1. N/C-Bid and Bax-mediated release from OG-LUVs was cardiolipin dependent (data not shown). (B) Release from OG-LUVs was monitored at 10, 30, 60, and 120 min. In the presence of Bax (120 nM) and N/C-Bid (45 nM), the dextran release from a population of vesicles reached a plateau at 1 h. This release was inhibited when Bcl-xL (1 μM) was also present. Data shown are representative of three independent experiments.
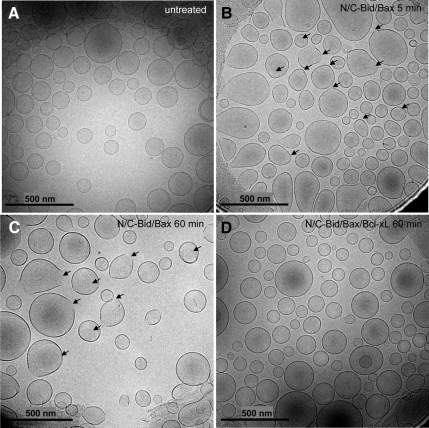
For cryo-EM, OG-LUVs were treated with recombinant N/C-Bid and Bax (and Bcl-xL) at room temperature and instantaneously frozen using a Vitrobot, which provides a temperature- and humidity-controlled environment; it is crucial to keep the vesicles in an environment close to 100% humidity to prevent the induction of an osmotic effect by evaporation during the millisecond between blotting and plunge-freezing (Frederik and Hubert, 2005). Untreated vesicles seemed perfectly round and the membrane was smooth and continuous (Figure 6A). In contrast, vesicles treated with N/C-Bid and Bax exhibited discontinuity in the membrane, and pore-like structures with diameters of 25–100 nm became visible over time (Figures 6, B and C, and 7, A and B). It seems from the images that the pores start to form from one or more foci in a vesicle and then dilate (Figure 7A). The presence of Bcl-xL prevented pore formation (Figure 6D), and the percentage of pored vesicles correlated well with the dextran release curves (Figures 5B and 7C), arguing strongly that the pores are a result of the permeabilizing effect of activated Bax on the membrane. The edges of the pores seemed smooth or rough, suggestive of a mainly lipidic composition. Based on the variation in size and shape, the pores are unlikely to be proteinaceous channels.
Figure 6.
N/C-Bid and Bax induce pore-like structures in the OG-LUV membrane. (A) Nontreated OG-LUVs seemed perfectly round and had smooth and continuous membranes. Vesicles were instantaneously frozen in vitrified ice and visualized by cryo-EM. (B) After a 5-min incubation with N/C-Bid and Bax, the liposome membranes exhibited regions of irregularity and some pore-like structures were visible (arrows). (C) After 60 min of incubation with N/C-Bid and Bax, almost all the vesicles exhibited membrane abnormalities (arrows). (D) In the presence of Bcl-xL, the presence of the pores and abnormal membranes were greatly diminished.
Figure 7.
(A) A gallery of pore-bearing vesicles and a cartoon illustration of how the pores may be formed (bottom). Bar, 100 nm. (B) Views of two permeabilized vesicles (liposome 1 and 2) with tilted angles: −45°, 0°, and +45°. (C) Summary of the number of porous vesicles over time. The percentages of vesicles with pores correlated well with dextran-release shown in Figure 5B. the total number of counted vesicles is shown in parentheses.
Theoretically, the openings we observed might have been formed by vesicle rupture because of a hydrostatic force generated upon the formation of thin ice within regions “weakened” by activated Bax. We therefore carefully chose fields in which the vesicles maintained their original morphology and were not under pressure. Also, it is unlikely that deformation takes place when the specimen freezes rapidly without ice crystal formation. The large apparent diameter of the pores was consistent with the observation that different-sized macromolecules are released, and it is entirely reasonable to suppose that fluorescein-dextrans escape from these pores. Given that the same pore-like structures were also seen in cardiolipin-liposomes generated by the extrusion method (data not shown), we can conclude that the membrane changes were not caused by residual detergent.
DISCUSSION
MOMP is a critical step in apoptosis and a logical target for therapeutic intervention for diseases such as cancer, given that once MOMP occurs, cells usually cannot recover sufficiently to proliferate, even when caspases are inactivated (Xiang et al., 1996; McCarthy et al., 1997; Chipuk and Green, 2005). How Bcl-2 family proteins interact with one another to promote or inhibit apoptosis during MOMP has been studied extensively, but the final membrane permeabilization step remains a mystery. Bax and Bak are effector proteins in MOMP and are activated by certain BH3-only proteins. Thus, a synergy between Bax and BH3-only proteins is observed in in vitro systems (Kuwana et al., 2002; Roucou et al., 2002a; Roucou et al., 2002b; Yethon et al., 2003; Terrones et al., 2004). As a result of MOMP, several intermembrane space proteins are released into the cytoplasm.
In our previous studies, we found that the presence of 7 mol % cardiolipin optimizes the permeabilization of pure lipid vesicles by N/C-Bid and Bax, but we did not address the physiological relevance of cardiolipin (Kuwana et al., 2002). Cardiolipin facilitates Bax insertion, oligomerization, and permeabilization of the membrane in liposomes (Kuwana et al., 2002; Terrones et al., 2004; Lucken-Ardjomande et al., 2008b) and truncated Bid (a carboxy-terminal portion after the cleavage) targeting to mitochondria (Lutter et al., 2000, 2001). In contrast, the physiological relevance of cardiolipin in Bax-mediated cell death has been questioned by the studies that use yeast systems (Iverson et al., 2004; Polcic et al., 2005; Ott et al., 2007). Here, we addressed this question biochemically. Because OMVs contain little cardiolipin, and because extracted OMV lipids did not by themselves support permeabilization, we hypothesized that MOM proteins may act either by substituting for cardiolipin or by arranging the membrane in such a way that the local concentration of this lipid is sufficiently high to facilitate permeabilization. Our discovery that proteo-liposomes formed solely from OMV lipids and proteins (ReOMVs) did respond efficiently to N/C-Bid and Bax supports this idea. However, we could not rule out the possibility that trace amounts of cardiolipin were present in ReOMVs and contributed to permeabilization, or that some other endogenous lipid(s) mediated this effect.
Subsequently, we separated OMV proteins and lipids through organic solvent-based extraction. After the lipids were extracted, we followed a standard method for purifying and incorporating membrane proteins into a lipid bilayer. This approach has been particularly successful in the study of membrane proteins with a β-barrel structure (Surrey and Jahnig, 1992; Pautsch and Schulz, 1998; Pautsch et al., 1999; Tamm et al., 2004; Arnold et al., 2007; Shanmugavadivu et al., 2007). Our finding that the resulting ExOMVs respond to N/C-Bid- and Bax-mediated permeabilization strongly supports the notion that the responsible factor is proteinaceous. Moreover, as these vesicles possess no cardiolipin, we can conclude that one or more MOM proteins substitute functionally for cardiolipin rather than rearranging it to increase the local concentration of the lipid. As controls, we tested ReLMVs and OmpA-loaded liposomes, and the inability of these proteo-liposomes to be permeabilized by Bid/Bax argues that the permeabilization we observed in ExOMVs is dependent on specific proteins present rather than proteo-liposome formation per se.
Bid or Bim BH3 peptide and Bax together permeabilized noncardiolipin containing liposomes equally well as cardiolipin-containing liposomes. Also, detergent-oligomerized Bax displayed only slight cardiolipin dependence. These findings suggest that N/C-Bid is the component that is dependent on cardiolipin in our liposome system. Therefore, the proteinaceous factor that confers responsiveness to N/C-Bid and Bax might be a “Bid receptor.” A report on an in vitro liposome system has shown that cleaved Bid induces binding of Bax and Bcl-xL to the membrane (Billen et al., 2008). Our putative Bid receptor hypothesis fits with this scenario. Billen et al. (2008) used tBid, the C-fragment portion of cleaved Bid, which might have mitigated the strict requirement for cardiolipin like that for N/C-Bid. Still, the idea of Bid association with the membrane preceding Bax binding to the membrane agrees with our finding. Recently, Gross and colleagues have reported that Mtch2, a MOM protein, binds to cleaved Bid and regulates MOMP (Grinberg et al., 2005; Gross, 2005). This protein might be a good candidate for our proteinaceous factor; however, we did not detect Mtch2 in our ExOMVs. Although it might play some role in ReOMVs, we suspect that Mtch2 is not essential in our ExOMV permeabilization. The identity of the factor together with its physiological relevance and the detailed molecular mechanisms through which this factor helps Bid activate Bax will be addressed in the future studies. Our results also suggest that activated Bax may not require other specific components in the lipid bilayer to permeabilize it, so the critical apoptotic regulations may be at the point of Bax activation (Leber et al., 2007).
Earlier studies addressing the nature of the Bax pore have generated conflicting evidence, some suggesting that it is proteinaceous (Shimizu et al., 1999; Saito et al., 2000) and others that it is lipidic (Basanez et al., 1999, 2002; Terrones et al., 2004). Although our previous study failed to detect gross morphological changes in the OMV membranes after they were treated with N/C-Bid (Kuwana et al., 2002), it was inconclusive because it relied on conventional TEM, which requires chemical fixation and dehydration that could destroy subtle membrane changes. We avoided such potential artifacts in the current study by applying cryo-EM, which does not require harsh treatment of the vesicles and is able to capture transient changes. When permeabilized by N/C-Bid and Bax, the liposomes exhibited pore-like openings at one or more foci. The finding that the diameter of these pores was ∼25–100 nm accounted for our previous observation that different-sized dextrans are released from permeabilized vesicles (Kuwana et al., 2002). Such pores might also explain the large channel activity recorded in mitochondria during MOMP (Pavlov et al., 2001; Guihard et al., 2004; Dejean et al., 2005). Given that we observed a good correlation between the number of vesicles with pores and the amount of dextran release, we conclude that dextrans escape through these pores. Based on our images, which reveal variability in size and relatively smooth but irregular edges, we propose that the openings induced by activated Bax are mostly lipidic, consistent with the earlier proposal by Hardwick and Polster (2002). The lipidic-pore (or toroidal) model (Shai, 1999; Sobko et al., 2004) for Bax-dependent membrane permeabilization has been suggested by the studies using a Bax α5 peptide (a 34 mer taken from helix 5 of Bax) (Garcia-Saez et al., 2007; Qian et al., 2008). Indeed, our Bax-pores seem different from proteinaceous pores formed by a bacterial toxin pneumolysin, which is visualized by cryo-EM (Tilley et al., 2005). Pneumolysin pore formation is supposed to follow the barrel stave model because of its β-barrel structure (Tilley and Saibil, 2006). These pores are uniform in size and the toxin molecules are visible all around the edges of the pore. We did not see any protein structures in the vicinity of the Bax pore in our liposomes. Unfortunately, OMVs cannot be visualized at all by cryo-EM, because of their large size. ReOMVs are smaller than OMVs and our preliminary results suggest that the ReOMVs undergo a membrane destabilization similar to that observed with OG-LUVs (data not shown). Visualization of pores in such objects using a more suited EM technique should answer whether the pores are also present in vivo.
ACKNOWLEDGMENTS
We thank Donald Newmeyer (La Jolla Institute for Allergy and Immunology, La Jolla, CA); and Shahram Khademi, Mike Knudson, and Michael Cohen (University of Iowa, Iowa City, IA) for stimulating discussion and valuable comments throughout the study. We also thank Georg Schulz (Albert-Ludwigs-Universitat, Freiburg, Germany) for the OmpA plasmid and advice on the production of recombinant OmpA and Olivier Aebischer (University of Fribourg, Switzerland) for help with lipid mass spectrometry. We are grateful to Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children's Research Hospital (Memphis, TN) for identification of Xenopus proteins by mass spectrometry. This work was supported by National Institutes of Health grants R21AG024157 and R21AG024478 (to T. K.) and The Swiss National Science Foundation grants PP00A3-110450 and 3100A0-120650/1 (to R. S.). The National Resource for Automated Molecular Microscopy is supported by the National Institutes of Health through the National Center for Research Resources' P41 program (RR17573).
Abbreviations used:
- ExOMV
proteo-liposomes composed of lipid-extracted outer membrane vesicle
- LMV
light membrane vesicle
- LUV
large unilamellar vesicle
- MOM
mitochondrial outer membrane
- MOMP
mitochondrial outer membrane permeabilization
- OG
octylglucoside
- OMV
outer membrane vesicle
- ReOMV
reformed outer membrane vesicle.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1056) on February 25, 2009.
REFERENCES
- Almgren M. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim. Biophys. Acta. 2000;1508:146–163. doi: 10.1016/s0005-2736(00)00309-6. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit S., Sanchez B., Martinou J. C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Ardail D., Privat J. P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- Arnold T., Poynor M., Nussberger S., Lupas A. N., Linke D. Gene duplication of the eight-stranded beta-barrel OmpX produces a functional pore: a scenario for the evolution of transmembrane beta-barrels. J. Mol. Biol. 2007;366:1174–1184. doi: 10.1016/j.jmb.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Baines C. P., Kaiser R. A., Sheiko T., Craigen W. J., Molkentin J. D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G., Nechushtan A., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K. A., Hsu Y., Zimmerberg J., Youle R. J. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G., Sharpe J. C., Galanis J., Brandt T. B., Hardwick J. M., Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- Billen L. P., Kokoski C. L., Lovell J. F., Leber B., Andrews D. W. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6:e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Attardi L. D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- Brown N. M., Martin S. M., Maurice N., Kuwana T., Knudson C. M. Caspase inhibition blocks cell death and results in cell cycle arrest in cytokine-deprived hematopoietic cells. J. Biol. Chem. 2007;282:2144–2155. doi: 10.1074/jbc.M607961200. [DOI] [PubMed] [Google Scholar]
- Chipuk J. E., Green D. R. Do inducers of apoptosis trigger caspase-independent cell death? Nat. Rev. Mol. Cell Biol. 2005;6:268–275. doi: 10.1038/nrm1573. [DOI] [PubMed] [Google Scholar]
- Colell A., et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Dejean L. M., et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol. Biol. Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Fesik S. W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Frederik P. M., Hubert D. H. Cryoelectron microscopy of liposomes. Methods Enzymol. 2005;391:431–448. doi: 10.1016/S0076-6879(05)91024-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez A. J., Chiantia S., Salgado J., Schwille P. Pore formation by a Bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophys. J. 2007;93:103–112. doi: 10.1529/biophysj.106.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. C., Waterhouse N. J., Juin P., Evan G. I., Green D. R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Green D. R., Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J. Clin. Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg M., Schwarz M., Zaltsman Y., Eini T., Niv H., Pietrokovski S., Gross A. Mitochondrial carrier homolog 2 is a target of tBID in cells signaled to die by tumor necrosis factor alpha. Mol. Cell Biol. 2005;25:4579–4590. doi: 10.1128/MCB.25.11.4579-4590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A. Mitochondrial carrier homolog 2, a clue to cracking the BCL-2 family riddle? J. Bioenerg. Biomembr. 2005;37:113–119. doi: 10.1007/s10863-005-6222-3. [DOI] [PubMed] [Google Scholar]
- Guihard G., Bellot G., Moreau C., Pradal G., Ferry N., Thomy R., Fichet P., Meflah K., Vallette F. M. The mitochondrial apoptosis-induced channel (MAC) corresponds to a late apoptotic event. J. Biol. Chem. 2004;279:46542–46550. doi: 10.1074/jbc.M405153200. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Polster B. M. Bax, along with lipid conspirators, allows cytochrome c to escape mitochondria. Mol. Cell. 2002;10:963–965. doi: 10.1016/s1097-2765(02)00751-7. [DOI] [PubMed] [Google Scholar]
- Iverson S. L., Enoksson M., Gogvadze V., Ott M., Orrenius S. Cardiolipin is not required for Bax-mediated cytochrome c release from yeast mitochondria. J. Biol. Chem. 2004;279:1100–1107. doi: 10.1074/jbc.M305020200. [DOI] [PubMed] [Google Scholar]
- Johnsson M., Edwards K. Liposomes, disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly(ethylene glycol)-phospholipids. Biophys. J. 2003;85:3839–3847. doi: 10.1016/S0006-3495(03)74798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. M., Brown N. M. Mitochondria potential, bax “activation,” and programmed cell death. Methods Mol. Biol. 2008;414:95–108. doi: 10.1007/978-1-59745-339-4_9. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M. R., Perkins G., Ellisman M. H., Latterich M., Schneiter R., Green D. R., Newmeyer D. D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Newmeyer D. D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Leber B., Lin J., Andrews D. W. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T., et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Cepero E., Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S., Montessuit S., Martinou J. C. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ. 2008a;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S., Montessuit S., Martinou J. C. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008b;5:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- Lutter M., Fang M., Luo X., Nishijima M., Xie X.-S., Wang X. Cardiolipin provides specificity for targeting of tBID to mitochondria. Nat. Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- Lutter M., Perkins G. A., Wang X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001;2:22. doi: 10.1186/1471-2121-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N. J., Whyte M. K., Gilbert C. S., Evan G. I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J. Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A., Smith C. L., Hsu Y. T., Youle R. J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A., Smith C. L., Lamensdorf I., Yoon S. H., Youle R. J. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Zhivotovsky B., Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- Pautsch A., Schulz G. E. Structure of the outer membrane protein A transmembrane domain. Nat. Struct. Biol. 1998;5:1013–1017. doi: 10.1038/2983. [DOI] [PubMed] [Google Scholar]
- Pautsch A., Vogt J., Model K., Siebold C., Schulz G. E. Strategy for membrane protein crystallization exemplified with OmpA and OmpX. Proteins. 1999;34:167–172. [PubMed] [Google Scholar]
- Pavlov E. V., Priault M., Pietkiewicz D., Cheng E. H., Antonsson B., Manon S., Korsmeyer S. J., Mannella C. A., Kinnally K. W. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcic P., Su X., Fowlkes J., Blachly-Dyson E., Dowhan W., Forte M. Cardiolipin and phosphatidylglycerol are not required for the in vivo action of Bcl-2 family proteins. Cell Death Differ. 2005;12:310–312. doi: 10.1038/sj.cdd.4401566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Wang W., Yang L., Huang H. W. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc. Natl. Acad. Sci. USA. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucou X., Montessuit S., Antonsson B., Martinou J. C. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 2002a;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucou X., Rostovtseva T., Montessuit S., Martinou J. C., Antonsson B. Bid induces cytochrome c-impermeable Bax channels in liposomes. Biochem. J. 2002b;363:547–552. doi: 10.1042/0264-6021:3630547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Korsmeyer S. J., Schlesinger P. H. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- Shanmugavadivu B., Apell H. J., Meins T., Zeth K., Kleinschmidt J. H. Correct folding of the beta-barrel of the human membrane protein VDAC requires a lipid bilayer. J. Mol. Biol. 2007;368:66–78. doi: 10.1016/j.jmb.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Ide T., Yanagida T., Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Narita M., Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Sobko A. A., Kotova E. A., Antonenko Y. N., Zakharov S. D., Cramer W. A. Effect of lipids with different spontaneous curvature on the channel activity of colicin E 1, evidence in favor of a toroidal pore. FEBS Lett. 2004;576:205–210. doi: 10.1016/j.febslet.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Sorice M., Circella A., Cristea I. M., Garofalo T., Di Renzo L., Alessandri C., Valesini G., Esposti M. D. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- Suloway C., Pulokas J., Fellmann D., Cheng A., Guerra F., Quispe J., Stagg S., Potter C. S., Carragher B. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Surrey T., Jahnig F. Refolding and oriented insertion of a membrane protein into a lipid bilayer. Proc. Natl. Acad. Sci. USA. 1992;89:7457–7461. doi: 10.1073/pnas.89.16.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Imai Y., Nakayama H., Takahashi K., Takio K., Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., Hong H., Liang B. Folding and assembly of beta-barrel membrane proteins. Biochim. Biophys. Acta. 2004;1666:250–263. doi: 10.1016/j.bbamem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Terrones O., Antonsson B., Yamaguchi H., Wang H. G., Liu Y., Lee R. M., Herrmann A., Basanez G. Lipidic pore formation by the concerted action of pro-apoptotic BAX and tBID. J. Biol. Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- Tilley S. J., Orlova E. V., Gilbert R. J., Andrew P. W., Saibil H. R. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Tilley S. J., Saibil H. R. The mechanism of pore formation by bacterial toxins. Curr. Opin. Struct. Biol. 2006;16:230–236. doi: 10.1016/j.sbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Chao D. T., Korsmeyer S. J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yethon J. A., Epand R. F., Leber B., Epand R. M., Andrews D. W. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]