Abstract
Purpose
The aim of this study was to examine the expression of α-crystallins, a small heat shock protein family, and apoptosis in the retinal neoplastic cells.
Methods
Thirteen enucleated globes were included in this study, one with retinocytoma and 12 with retinoblastoma. Formalin-fixed, paraffin-embedded tissue sections were processed for immunohistochemistry with α-crystallins antibodies. In two tumors, proteins were extracted for Western blot analysis to detect αA-crystallin. Apoptotic cells were detected using TUNEL method.
Results
In the retinocytoma, αA-crystallin was expressed in the cytoplasm of all tumor cells, whereas αB-crystallin immunoreactivity was only weakly positive. Western blot analysis supported expression of αA-crystallin in the tumor. Apoptotic cells were rarely noted in retinocytoma cells; the apoptotic index was 0.29. Examination of the retinoblastoma globes revealed 6 cases (50%) that were strongly positive for αA-crystallin. The apoptotic indices in the strongly positive and weakly positive-cases were 3.55 ± 2.61 and 7.50 ± 2.61, respectively. The apoptotic index was significantly higher in those cases weakly positive for αA-crystallin than in those that were strongly positive (P<0.05). No correlation was observed between apoptotic index and αB-crystallin immunoreactivity although 50% of retinoblastomas were strongly positive for αB-crystallin.
Conclusions
αA and αB-crystallins are expressed in retinoblastomas, and αA-crystallin expression may prevent apoptosis of neoplastic cells.
Clinical Relevance
Suppression of αA-crystallin may be useful in controlling the tumor growth.
Keywords: alpha-crystallin, apoptosis, retinoblastoma, retinocytoma
INTRODUCTION
Crystallins, the major structural proteins of the eye lens, are primarily categorized into three distinct families: α, β and γ. The two α-crystallins, αA and αB, are the principal members of the small heat shock protein (Hsp) family acting as molecular chaperones.1 Although αA and αB-crystallins have related amino acid sequences with similar structural properties, they vary significantly in their tissue distribution with different functions: they protect different proteins and are active under different conditions.2-6 Oxidative stress in general is accompanied by upregulation of the hosts of Hsp, including the α-crystallins.7 Rao et al. demonstrated that photoreceptors selectively upregulate αA-crystallin to protect themselves against mitochondrial oxidative stress and stress-mediated apoptosis. 8
The agents that induce oxidative stress include hydrogen peroxide (H2O2), superoxide, hydroxyl radical, and others. The stress can cause cell death, aging, and development of malignancy. 9 H2O2 is a ubiquitous molecule that is able to diffuse freely into membranes due to its nonpolar characteristics. It is recently reported that expression of α-crystallins varies in retinal pigment epithelial cells exposed to H2O2 oxidative stress.10
Recent studies show that αB-crystallin is expressed in various malignant tumors.11-14 One of the major roles of αB-crystallin is to preserve the integrity of mitochondria and restrict the release of cytochrome c, 5, 13 subsequently resulting in tumor growth through escape from apoptosis.15 In addition, αB-crystallin has been suggested as a novel prognostic factor in malignant solid tumors including breast,12, 13 and head and neck cancers,14 since αB-crystallin expression has been correlated with poor patient prognosis.
Retinocytoma is a benign counterpart of retinoblastoma that shows photoreceptor differentiation. Singh et al.16 reported that the proportion of retinocytoma among the population with retinoblastoma is 1.8%, suggesting that retinocytoma develops in rare instances rather than as the usual phenotype of retinoblastoma. From a genetic standpoint, retinocytoma is similar to retinoblastoma, with autosomal dominant inheritance involving a mutation in the RB1 gene.17-20 Moreover, malignant transformation of retinocytoma into retinoblastoma is rare, with a frequency of less than 5%.16 Pineda et al.21 demonstrated that αB-crystallin was expressed in retinoblastoma cells; however, correlation with α-crystallin expression and apoptosis in retinocytoma and retinoblastoma is not elucidated.
The aim of this study was to examine the expression of α-crystallins, both αA and αB, in retinocytoma and retinoblastoma using immunohistochemistry and to correlate the crystallin expression with the tumor cell apoptosis.
MATERIALS AND METHODS
The institutional review board of the University of Southern California approved our use of human specimens obtained from the file of Doheny Eye Institute, Pathology Laboratory. All procedures conformed to the Declaration of Helsinki for research involving human subjects. We analyzed one histologically normal appearing retina obtained from a patient with ciliary body melanoma, one enucleated globe with retinocytoma, and 12 enucleated eyes with retinoblastoma. None of patients had received chemo-/radiotherapy before enucleation of the tumor-containing eye. All eyeballs were fixed in 4% paraformaldehyde soon after enucleation.
Immunohistochemistry
The slides were dewaxed, rehydrated, and rinsed in phosphate-buffered saline (PBS) twice for 10 min. As pretreatment, microwave-based antigen retrieval was performed in 10 mM citrate buffer (pH 6.0). These slides were incubated with 3% hydrogen peroxide for 10 min, then with normal goat serum for 30 min. Sections were incubated with anti-rabbit αA-, and αB-crystallin polyclonal antibodies (dilution 1: 100; Stressgen, Ann Arbor, MI) at room temperature for 2 hr. Binding of the primary antibody was localized with the FITC-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min. Negative control consisted of FITC-conjugated mouse IgG incubated without treatment of the primary antibody. Slides were examined using a Zeiss LSM510 (Zeiss,Thornwood, NY) confocal microscope.
In evaluation of immunohistochemistry, necrotic areas in tumor tissues were excluded based on DAPI nuclear staining. In the non-necrotic viable tumor tissues, the number of immunopositive-tumor cells in total tumor cells was evaluated from 3 to 4 fields using microscope (objective lens x20) in same slide for each specimen. The positive rates counted in each field were then averaged, and the positive rate of immunopositive cells in each case was shown as a percentage in viable tumor cells (%). Immunoreactivity for α-crystallins in tumor tissues was scored as strongly positive (>30% of tumor cells positive), weakly positive (<30% of tumor cells positive), or negative (background level staining only) according to the previous report.13
TUNEL assay
Five μm thick serial sections were cut for TUNEL assay to evaluate distribution of TUNEL positive reaction in the same part as immunoreaction with α-crystallins. An in Situ Cell Death Detection Fluorescein Kit (Roche, Indianapolis, IN) was used for TUNEL assay. The slides were dewaxed, rehydrated, and rinsed in PBS twice for 10 min. These slides were incubated with the 3% hydrogen peroxide for 10 min, then permeabilized with proteinase K 20 μg/ml at room temperature for 10 min. Texas red label with enzyme solution was added to each slide and incubated in a humidified chamber at 37°C for 1 hr. DNase-pretreated slides were used as positive controls and slides without added enzyme were negative controls. Apoptotic cells were revealed by confocal microscope (Fig 1). In the non-necrotic viable tumor tissues, at least 300 tumor cells were counted from 3 or 4 fields of same slide for each specimen using high-power field. Apoptotic cells were defined by the presence of perinuclear chromatin condensation and apoptotic bodies. The fraction (%) of the apoptotic cells was considered to be the apoptotic index (AI) as previously described.22
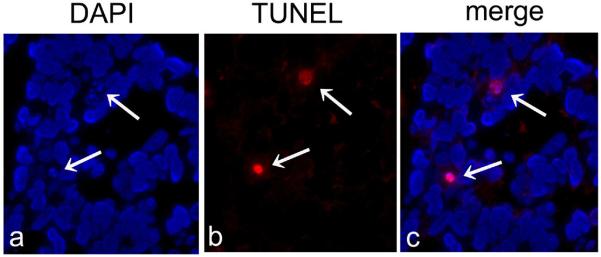
Figure 1.
Detection of apoptotic cells in retinoblastoma tissues using confocal microscopy.
A, The presence of viable tumor cells can be confirmed using DAPI nuclear staining. B, C, In viable tumor tissues, apoptotic cells are clearly detected by a red signal.
Chemicals and cells
Hydrogen peroxide was obtained from Sigma Aldrich (St. Louis, MO). The human retinoblastoma cell line Y79 was purchased from the American Type Culture Collection (Manassas, VA) and grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA) with 100 U/mL penicillin, 100 μg/mL streptomycin, and 20% fetal bovine serum. Y79 cells were treated with different concentration of H2O2 (0 to 400 μMol) for 24 hr. And then the cells were harvested and protein was extracted.
Protein extraction from formalin-fixed, paraffin embedded retinoblastoma tissues
Total tumor proteins were extracted from two retinoblastoma cases (Case no. 7 and 10) using formalin-fixed, and paraffin-embedded tissue sections, as reported previously 23. Briefly, paraffin sections having five μm thick were cut for hematoxylin & eosin (HE) staining and 30 μm thick serial sections were cut for protein extraction and mounted on plain glass slides. To collect tumor tissues, the target areas were cut macroscopically with a razor blade referring to the microscopical observation of the morphology of serial HE staining. The dissected tissues were then placed in Eppendorf tubes. Three hundred μl of RIPA buffer (Cell Signaling, Danvers, MA), including cocktail of proteinase inhibitors (Sigma, St. Louis, MO), was added to each tube, and the contents were incubated under different conditions as follows: at 0C for 2 hr; at 37C for 2 hr; at 60C for 2 hr; and at 100C for 20 min, followed by incubation at 60C for 2 hr. After incubation, the tissue lysates were centrifuged at 15,000 x g for 20 min at 4C. The supernatants were collected for measurement of protein concentrations and Western blot analysis. 23
Western blot analysis
The protein concentration of extracted proteins from Y79 cultured cells and paraffin-embedded tissues was measured by the Bradford protein assay (Bio-Rad, Richmond, Calif., USA) with bovine serum albumin as the standard protein. 25 μg protein was separated by electrophoresis on SDS polyacrylamide gel electrophoresis (PAGE; 12.5% Ready Gel, Bio-Rad Laboratories, Hercules, CA) at 110V. The protein was then electrotransferred onto polyvinylidene difluoride (PVDF) blotting membrane (Millipore, Bedford, MA). The membranes were blocked in 5% milk and probed with primary antibodies against αA-crystallin (1:500, Stressgen), and GAPDH (1:1000, Millipore, Billerica, MA) for 2 hours at room temperature. Membranes were washed and incubated with a peroxidase-conjugated secondary antibody (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Images were developed by adding enhanced chemiluminescence (ECL) detection solution (GE Healthcare, Cleveland, OH).
Statistical analysis
The AI data is presented as mean ± standard deviation. Statistical evaluations were performed using the student’s t-test. Accepted level of significance for all tests was P<0.05.
RESULTS
In the normal appearing retina, αA-crystallin immunoreactivity was predominantly detected in the cytoplasm of photoreceptors (Fig 2A-D). Negative control, in which the respective antibody was omitted, showed no staining in the retina (Fig 2E). Both crystallins were immunopositive in the cytoplasm of lens epithelial cells in all sections examined in this study. αB-crystallin was also expressed in the corneal epithelium and endothelium and in the optic nerve head (data not shown).
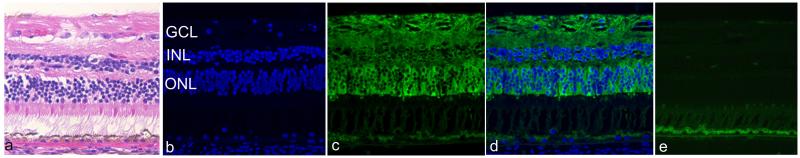
Figure 2.
A, Hematoxylin & eosin staining, B, D, DAPI nuclear staining (blue), and C, D, immunodetection of αA-crystallin (green) in the normal retina.
A,B, Three nuclear layers consisting of ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) are clearly confirmed.
C,D, αA-crystallin immunoreactivity is predominantly detected in the cytoplasm of photoreceptors, which is weakly noted in GCL and INL D. E, Negative control shows no specific immunoreaction in the retina.
Expression of α-Crystallins in Retinocytoma
Retinocytoma cells represented a relatively uniform nuclear shape without marked nuclear atypia. Mitotic figures were not observed (Fig 3A). Calcification was partially present in the tumor tissue. αA-crystallin was expressed in the cytoplasm of all tumor cells (Fig 3B). In contrast, αB-crystallin immunoreactivity was weakly detected in less than 30% of tumor cells (Fig 3C).
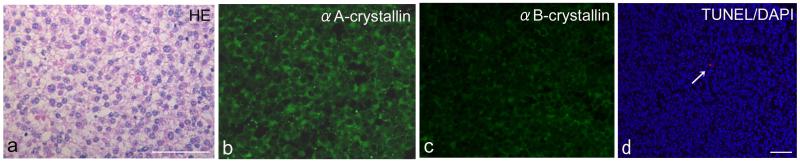
Figure 3.
A, Hematoxylin & eosin staining, B, immunodetection of αA-crystallin and C, αB-crystallin, and D, TUNEL (red)/DAPI nuclear staining (blue) in human retinocytoma.
A, Retinocytoma cells represent a relatively uniform nuclear shape with no apparent nuclear atypia. Mitotic figures are not observed in the tumor tissue. B, αA-crystallin is strongly expressed in the cytoplasm of all tumor cells. C, αB-crystallin immunoreactivity is weakly detected in tumor cells. D, Apoptotic cell is rarely detected in retinocytoma tissue (arrow).
Bar indicates 50μm.
Expression of α-Crystallins in Retinoblastoma
Retinoblastoma cells demonstrated diffuse, high cellular population with atypical cells with prominent and hyperchromatic nuclei (Fig 4A). As summarized in Table 1, six retinoblastoma cases (50 %) were strongly positive for αA-crystallin. The positive reaction was restricted to the cytoplasm of tumor cells (Fig 4A-D, green). Six cases (50 %) were also strongly positive for αB-crystallin. In these tumors, αB-crystallin was homogeneously expressed in the cytoplasm of the neoplastic cells (Fig 4F-I; green).
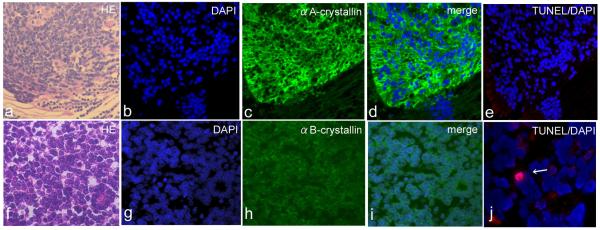
Figure 4.
A, F, Hematoxylin & eosin staining, B, D, F, E, G, I, J, DAPI nuclear staining (blue) and C,D, immunodetection of αA-crystallin (green) and H,I, αB-crystallin (h, i; green), and E,J, TUNEL-positive tumor cells (red) in human retinoblastoma.
A-D, αA-crystallin expression is prominent in the cytoplasm of retinoblastoma cells compared to the normal retina adjacent to the tumor (green) in the representative case. E, TUNEL-positive tumor cells are not detected in the αA-crystallin-positive area. F, Undifferentiated retinoblastoma cells show high proliferation with atypical nuclei. H,I, αB-crystallin is homogeneously expressed in the cytoplasm of retinoblastoma cells (green). J, Apoptotic tumor cell is observed in αB-crystallin-positive area (arrow).
Table 1.
Clinicopathological profiles in retinoblastoma cases examined in this study
| I mmunoreactivity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| no. | Age (years) | Gender | Side | Diff | Choroidal invasion | ON head invasion | αA (%) | αB (%) | Apoptotic index (%) |
| 1 | 2 | F | L | un | + | - | 30 | 70 | 4 |
| 2 | 1 | F | L | well | - | + | 70 | 60 | 0.5 |
| 3 | 1 | M | L | un | + | + | 20 | 20 | 10.1 |
| 4 | 1 | F | R | un | - | - | 80 | 80 | 3.5 |
| 5 | 3 | M | R | un | - | - | 10 | 10 | 6.6 |
| 6 | 2 | M | L | un | + | + | 20 | 50 | 9.4 |
| 7 | 2 | F | L | un | - | - | 90 | 30 | 2.3 |
| 8 | 3 | M | L | un | - | + | 40 | <10 | 4.1 |
| 9 | 1 | F | L | well | + | - | 30 | 30 | 5.2 |
| 10 | 2 | M | R | well | - | - | 60 | 30 | 2.6 |
| 11 | 2 | F | L | un | - | + | 40 | 50 | 8.4 |
| 12 | 1 | F | L | un | - | - | 20 | 60 | 9.8 |
F, female; M, male; Diff, differentiation; un, undifferentiated; ON, optic nerve; αA, αA-crystallin, αB, αB-crystallin.
αA-Crystallin expression in protein extracted from formalin-fixed, paraffin-embedded retinoblastoma tissues
The electrophoresed gel of the extracted protein with Coomassie blue staining of electrophoresed gel showed the proteins especially ranging from 10 kDa to 75 kDa, and they appeared well preserved (data not shown). The western blot analysis clearly revealed expression of αA-crystallin in both two cases. The expression was stronger in case no. 7 than in no. 10 (Fig 5).
Figure 5.
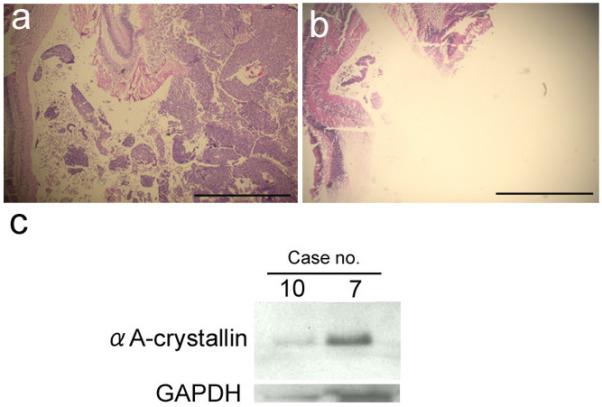
A, B, Hematoxylin & eosin staining, and C, Western blot analysis using retinoblastoma proteins from formalin-fixed, and paraffin-embedded tissues.
A, 5-μm-thick section shows retinoblastoma before dissection. B, 30-μm-thick serial section reveals dissection of retinoblastoma tissue. Tumor tissue is excised, where non-cancerous retina is remained. Bars equal 2mm. C, Expression of αA-crystallin is clearly detected in retinoblastoma tissues.
Increased expression of αA-Crystallin in Y79 cells treated with H2O2
αA-crystallin was expressed in Y79 cells treated with 100 μMol, which reached maximum at 150 μMol (Fig 6). Expression of αA-crystallin was low in Y79 cells exposed to high concentration of H2O2, but was sustained up to 400 μMol addition (data not shown). In untreated Y79, αA-crystallin protein expression was hardly detected.
Figure 6.
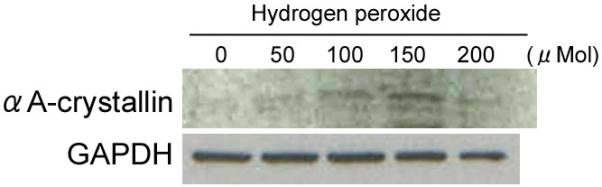
Expression of αA-Crystallin protein in human cultured retinoblastoma cells (Y79) treated with different concentration of hydrogen peroxide (H2O2). In untreated Y79, αA-crystallin protein expression is hardly detected. αA-crystallin is expressed in Y79 cells treated with 100 μMol, which reaches maximum at 150 μMol. Expression of αA-crystallin is low in Y79 cells exposed to high concentration of H2O2.
Detection of Apoptotic Cells and Correlation with α-Crystallin Immunoreactivity
Apoptotic cells were rarely detected in retinocytoma cells; the AI was 0.29 % (Fig 3D). On the other hand, the mean AI in retinoblastomas was 5.53. The AIs in cases of strongly positive and weakly positive for αA-crystallin were 3.55 ± 2.61 and 7.50 ± 2.61, respectively (Table 2). In tumors expressing αB-crystallin, AIs for strongly positive and weak/negative-cases were 5.94 ± 3.80 and 5.12 ± 2.92, respectively (Table 2). The AI was significantly higher in cases weakly positive for αA-crystallin than in those that were strongly positive (P<0.05); no correlation was observed between αB-crystallin immunoreactivity and AI (Table 2). Indeed, as shown in Figure 4, no TUNEL-positive tumor cells were noted in the αA-crystallin-positive area (Fig 4E), but TUNEL-positive tumor cells were found in the αB-crystallin-positive area (Fig 4J).
Table 2.
Correlation between α-crystallins expression and apoptotic index (AI) inretinoblastomas
| αA-crystallin | AI | αB-crystallin | AI |
|---|---|---|---|
| Strongly positive (n=6) | 3.55±2.61 | Strongly positive (n=6) | 5.94±3.80 |
| Weakly positive (n=6) | 7.50±2.61 | Weakly/negative (n=6) | 5.12±2.92 |
| P value | 0.0273 | P value | 0.68 |
DISCUSSION
αB-crystallin is frequently expressed in tumor tissues and is known both to preserve the integrity of mitochondria and to restrict release of cytochrome c, 5, 11-14 which in turn, results in resistance to tumor cell apoptosis. In contrast, Rao et al. have proposed that αA-crystallin, rather than αB, has the more important role in protecting photoreceptor cells from apoptosis in some pathologic conditions. 8 In the current study, all retinocytoma cells were positive for αA-crystallin where the number of apoptotic cells was low. Furthermore, immunoreactivity for αA-crystallin, but not for αB-crystallin, correlated with a low number of apoptotic retinoblastoma cells. These results suggest that αA-crystallin may protect retinal tumor cells from the apoptotic process.
The present study demonstrated that αA-crystallin was expressed in photoreceptors of the normal retina and in retinocytoma cells which show clear photoreceptor differentiation. Rao et al. recently demonstrated a selective αA-crystallin upregulation in experimental uveitis retina, and this crystallin was localized primarily in the photoreceptor inner segments, the site of mitochondrial oxidative stress. They also showed that upregulation of αA-crystallin was associated with photoreceptor protection against apoptosis resulting from oxidative stress. 8 In the retina, αB-crystallin was not upregulated in this animal model of uveitis, suggesting that retina may use αA-crystallin selectively for protection against stress.
It was previously reported that oxidative stress was high in retinoblastoma.24 Such stress might contribute to the high expression of αA-crystallin in retinal tumors. This is supported in part by our current in vitro study that αA-crystallin was upregulated in cultured human retinoblastoma cells exposed to H2O2 related oxidative stress (Fig. 6). Together with immunohistopathological results and oxidative stress known to induce apoptosis and to counteract such cell death, it appears that αA-crystallin is overexpressed.
Since it is likely that immunofluorescent signals from sections are highly variable depending on uncontrolled subtlety of specimen preparation and fixation, semi-quantitative immunohistochemistry may be tricky. In order to support the data evaluated by immunohistochemistry, we extracted total protein from formalin-fixed, paraffin-embedded tissue sections and performed Western blot analysis. As shown in Fig. 5, αA-crystallin overexpression was clearly detected supporting the findings of immunohistochemistry. Demonstrated absence of correlation between αB-crystallin levels with apoptotic index may complement and support validity of the data noted with αA-crystallin.
Based on the inverse correlation with apoptosis in the current study, suppression of αA-crystallin expression, rather than αB-crystallin, may be useful in controlling tumor growth. This novel observation reveals the necessity of future studies including upregulation of αA-crystallin in a retinoblastoma animal model as the next step. Anti-sense or nucleotide-based anti-αA-crystallin therapies may be tried to inhibit αA-crystallin expression in retinoblastoma, similar to the reported molecular approach of αB-crystallin suppression for treatment of various systemic cancers.25
Acknowledgments
Supported by NIH grants EY015714 and EY03040 and by an unrestricted grant from Research to Prevent Blindness.
REFERENCES
- 1.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Voorter CE, Mulders JW, Bloemendal H, de Jong WW. Some aspects of the phosphorylation of alpha-crystallin A. Eur J Biochem. 1986;160:203–210. doi: 10.1111/j.1432-1033.1986.tb09958.x. [DOI] [PubMed] [Google Scholar]
- 3.Kantorow M, Piatigorsky J. Phosphorylations of alpha A- and alpha B-crystallin. Int J Biol Macromol. 1998;22:307–314. doi: 10.1016/s0141-8130(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 4.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 5.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 6.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Liu L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. doi: 10.1016/j.exer.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto RI. Stress, aging, and neurodegenerative disease. N Engl J Med. 2006;355:2254–2255. doi: 10.1056/NEJMcibr065573. [DOI] [PubMed] [Google Scholar]
- 8.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated Retina-Specific Expression of the Small Heat Shock Protein, {alpha}A-crystallin, Is Associated with Photoreceptor Protection in Experimental Uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 9.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis. 2007;13:566–577. [PMC free article] [PubMed] [Google Scholar]
- 11.Hitotsumatsu T, Iwaki T, Fukui M, Tateishi J. Distinctive immunohistochemical profiles of small heat shock proteins (heat shock protein 27 and alpha B-crystallin) in human brain tumors. Cancer. 1996;77:352–361. doi: 10.1002/(SICI)1097-0142(19960115)77:2<352::AID-CNCR19>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, Rademaker A, Gradishar WJ, Morrow M, Khan SA, Cryns VL. alphaB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 13.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. Alpha B-crystallin, a new independent marker for poor prognosis in head and neck cancer. Laryngoscope. 2005;115:1239–1242. doi: 10.1097/01.MLG.0000164715.86240.55. [DOI] [PubMed] [Google Scholar]
- 15.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 16.Singh AD, Santos CM, Shields CL, Shields JA, Eagle RC., Jr. Observations on 17 patients with retinocytoma. Arch Ophthalmol. 2000;118:199–205. doi: 10.1001/archopht.118.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Gallie BL, Ellsworth RM, Abramson DH, Phillips RA. Retinoma: spontaneous regression of retinoblastoma or benign manifestation of the mutation? Br J Cancer. 1982;45:513–521. doi: 10.1038/bjc.1982.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallie BL, Phillips RA, Ellsworth RM, Abramson DH. Significance of retinoma and phthisis bulbi for retinoblastoma. Ophthalmology. 1982;89:1393–1399. doi: 10.1016/s0161-6420(82)34622-9. [DOI] [PubMed] [Google Scholar]
- 19.Abramson DH. Retinoma, retinocytoma, and the retinoblastoma gene. Arch Ophthalmol. 1983;101:1517–1518. doi: 10.1001/archopht.1983.01040020519002. [DOI] [PubMed] [Google Scholar]
- 20.Balmer A, Munier F, Gailloud C. Retinoma. Case studies. Ophthalmic Paediatr Genet. 1991;12:131–137. doi: 10.3109/13816819109029394. [DOI] [PubMed] [Google Scholar]
- 21.Pineda R, 2nd, Chan CC, Ni M, Hayden BJ, Johnson MA, Nickerson J, Chader GJ. Human retinoblastoma cells express alpha B-crystallin in vivo and in vitro. Curr Eye Res. 1993;12:239–245. doi: 10.3109/02713689308999469. [DOI] [PubMed] [Google Scholar]
- 22.Kase S, Osaki M, Honjo S, Adachi H, Tsujitani S, Kaibara N, Ito H. Expression of cyclo-oxygenase-2 is correlated with high intratumoral microvessel density and low apoptotic index in human esophageal squamous cell carcinomas. Virchows Arch. 2003;442:129–135. doi: 10.1007/s00428-002-0706-x. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Monden T, Kanoh T, Tsujie M, Izawa H, Haba A, Ohnishi T, Sekimoto M, Tomita N, Shiozaki H, Monden M. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J Histochem Cytochem. 1998;46:397–403. doi: 10.1177/002215549804600314. [DOI] [PubMed] [Google Scholar]
- 24.Adithi M, Nalini V, Krishnakumar S. The role of nitric oxide synthases and nitrotyrosine in retinoblastoma. Cancer. 2005;103:1701–1711. doi: 10.1002/cncr.20961. [DOI] [PubMed] [Google Scholar]
- 25.Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]