Abstract
Research suggests that treatments for depression among individuals with chronic physical disease do not improve disease outcomes significantly, and chronic disease management programs do not necessarily improve mood. For individuals experiencing co-morbid depression and chronic physical disease, demands on the self-regulation system are compounded, leading to a rapid depletion of self-regulatory resources. Because disease and depression management are not integrated, patients lack the understanding needed to prioritize self-regulatory goals in a way that makes disease and depression management synergistic. A framework in which the management of co-morbidity is considered alongside the management of either condition alone offers benefits to researchers and practitioners and may help improve clinical outcomes.
Depression is common among individuals with chronic physical disease (Anderson, Freedland, Clouse, & Lustman, 2001; Aromaa et al., 1994; Ciesla & Roberts, 2001; Dickens, McGowan, Clark-Carter, & Creed, 2002). Independently, both depression (i.e., major depression or elevated depressive symptoms) and chronic physical diseases such as such as asthma, arthritis, cardiovascular disease, diabetes, and HIV/AIDS significantly interfere with life functioning, including decreased work productivity and loss of social functioning (Hays, Wells, Sherbourne, Rogers, & Spritzer, 1995; Hoffman, Rice, & Sung, 1996; Stewart, Ricci, Chee, Hahn, & Morganstein, 2003; Wells & Sherbourne, 1999). Further, just as chronic physical diseases cannot be “cured,” depression is often a chronic disorder with a high likelihood of relapse (Solomon et al., 2000). When occurring together, depression and chronic physical disease may result in particularly poor clinical outcomes as compared to either condition alone, including increased risk for disability, hospitalization, and early mortality (for reviews, see Evans et al., 1999; Krishnan et al., 2002).
In an effort to understand the connection between depression and chronic physical disease, many theorists and researchers have suggested a direct physiological link by which each of these conditions may cause and exacerbate the other. For example, depression is associated with reduced heart rate variability, increased sympathetic nervous system activity, and platelet aggregation, all of which are risk factors for cardiovascular disease (Musselman, Evans, & Nemeroff, 1998); and conversely, cardiovascular disease may be associated with the elevation of pro-inflammatory cytokines (Blum & Miller, 1998) that perpetuate depression (Appels, Bar, Bar, Bruggeman, & De Baets, 2000). Direct causal physiological mechanisms also have been proposed between depression and a range of other chronic diseases (Jacobson, Samson, Weinger, & Ryan, 2002; Krishnan et al., 2002).
Based in part on this proposed causal link, it is logical to assume that improvement of one condition (i.e., depression or chronic physical disease) should improve the other condition through an alteration of physiological mechanisms. Accordingly, there has been mounting interest in the hypotheses that depression treatment programs will improve chronic physical disease outcomes and that chronic disease management will improve depressed mood. In the current paper, we explore whether these hypotheses are supported by the treatment outcome data for individuals with co-morbid1 chronic disease and depression, sampling from empirically supported treatments of depression, psycho-educational disease management programs, and medication-based disease management programs. The theoretical, methodological, and practical issues associated with the mechanisms that link depression and chronic physical disease are explored, with a focus on the management of these co-occurring conditions (e.g., Carney et al., 1999; Frasure-Smith & Lesperance, 2003a; Frasure-Smith & Lesperance, 2003b; Lustman & Clouse, 2002). In light of the findings presented in the treatment outcome literature, a model for integrating patients’ treatment-related cognitive representations, behavioral responses, and social functioning is proposed, with the goal of improving both the major depressive disorder and the symptoms of chronic disease.
The Effects of Depression Management on Disease
Several researchers have suggested that depression causes or exacerbates chronic physical disease. Evidence for the causal link also has been drawn from studies that have identified either major depression or elevated depressive symptoms as a risk factor for the development of chronic diseases such as coronary heart disease (Ruguiles, 2002; Wulsin & Singal, 2003). Further, several studies suggest that depression predicts mortality in samples of patients with myocardial infarction (Bush et al., 2001; Ladwig, Kieser, Konig, Breithardt, & Borggrefe, 1991; Lesperance, Frasure-Smith, Talajic, & Bourassa, 2002; Welin, Lappas, & Wilhelmsen, 2000), coronary artery disease (Barefoot et al., 1996), stroke (Morris, Robinson, Andrzejewski, Samuels, & Price, 1993), congestive heart failure (Jiang et al., 2001; Murberg, Bru, Svebak, Tverteras, & Aarsland, 1999), diabetes (Black & Markides, 1999), cancer (Derogatis, Albeloff, & Melisaratos, 1979), and HIV/AIDS (Cook et al., 2004; Ickovics et al., 2001; Mayne, Vittinghoff, Chesney, Barrett, & Coates, 1996).
Based on the hypothesis that depression causes and exacerbates chronic physical disease, there has been an assumption that modifying depression through anti-depressant medication or psychotherapy should improve chronic disease outcomes. To evaluate this hypothesis, we examined three forms of treatment that have received empirical support for treating depression: tricyclic anti-depressants (e.g., nortriptyline, imipramine), serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine), and cognitive-behavioral therapy (for reviews, see Thase & Kupfer, 1996; DeRubeis & Crits-Cristoph, 1998). The studies reviewed include several disease categories, including cardiovascular disease, diabetes, HIV/AIDS, and arthritis. All studies were randomized controlled trials that examined the effects of depression treatment not only on depressive symptoms as measured by standardized measures such as the Beck Depression Inventory (BDI; Beck et al., 1979) or Modified Hamilton Rating Scale for Depression (MHRSD, Miller, Bishop, Norman, & Maddever, 1985), but also on “hard” disease outcomes such as mortality or biological indicators of disease progression. These 11 studies are described in Table 1.
Table 1.
Randomized controlled trials examining the effects of depression treatment on disease outcomes
| Disease | Authors | # Subjects | Time | Treatment | Control Condition | Outcome Measure for Depression | Outcome Measure for Disease |
|---|---|---|---|---|---|---|---|
| Coronary Heart Disease | Berkman et al. (2003) | 1332 | 24 weeks 2 year follow-up | CBT | Usual Care | + BDI + HRSD |
= Mortality |
| Glassman et al., (2003)/ Serebruary et al., (2003) | 369 | 24 weeks | Sertraline | Placebo | =HRSD | = Mortality + platelet activation |
|
| Strik et al., (2000) | 54 | 9 weeks | Fluoxetine | Placebo | = HRSD | = Cardiac Events | |
| van Melle et al., (2007) | 331 | 18 months | Mirtazapine or Citalopram or psychotherapy | Usual Care | = BDI = ICD-10 |
= Cardiac Events | |
| Diabetes | Katon et al., (2004) | 329 | 12 months | Antidepressant or psychotherapy | Usual Care | + SCL-90 | = Glycemin control |
| Lustman et al., (2000) | 60 | 8 weeks | Fluoxetine | Placebo | + BDI + HRSD |
= Glycemic control | |
| Lustman et al., (1998) | 51 | 10 weeks 6-month follow-up | CBT | Diabetes Education Program | + BDI | = Glycemic control + at follow-up |
|
| Lustman et al., (1997) | 68 | 8 weeks | Nortriptyline | Placebo | + BDI | = Glycemic control | |
| HIV | Elliot et al., (1998) | 75 | 12 weeks | Paroxetine or Imipramine | Placebo | + HRSD | = CD4 cell count |
| Rabkin et al., (1999) | 120 | 8 weeks | Fluoxetine | Placebo | + HRSD count | = CD4 cell | |
| Rabkin et al., (1994) | 97 | 6 weeks | Imipramine | Placebo | + HRSD | = CD4 cell count |
“+” = Depression treatment improved outcome; “=” = no difference; “−“ = Depression treatment worsened outcome
HRSD = Hamilton Rating Scale for Depression; CBT = Cognitive Behavioral Therapy; BDI = Beck Depression Inventory; ICD-10 = International Statistical Classification of Diseases and Related Health Problems, Depressive Disorder; SCL-90 = Hopkins Symptoms Checklist-90 depression score; MI = Myocardial Infarction
Of the 11 studies, several found that the depression treatment significantly improved mood relative to a control; however, there was not a corresponding improvement in chronic physical disease outcome. These findings can be seen in studies of both anti-depressant medication and cognitive-behavioral therapy. For example, Lustman et al. (1997) completed a randomized, placebo controlled, double-blind trial of 68 diabetic patients, 28 of whom had major depression and received either nortriptyline or placebo over 8 weeks of treatment. The reduction in depressive symptoms was significantly greater in depressed patients treated with nortriptyline than with placebo, but patients given nortriptyline were no more likely to have lower glycated hemoglobin levels that patients given placebo. Similarly, Lustman, Freedland, Griffith, and Clouse (2000) conducted a study of sixty patients with either Type I or Type II diabetes and major depression in which individuals were entered into an 8-week trial, randomized to either fluoxetine or placebo. In this trial, reductions in depressive symptoms were significantly better for fluoxetine as measured by both the HRSD and BDI. However, when patients were grouped according to improvement versus lack of improvement in glycemic control, there were no significant differences between fluoxetine and placebo. The Pathways collaborative care study (Katon et al., 2004) found comparable results, with intervention patients (n = 164) reporting improvements in depressive symptoms but not in glycemic control, as compared to usual care patients (n = 165).
Of the large-scale studies in which depression treatment improved mood, there were inconsistent outcomes. For example, the Enhancing Recovery for Coronary Heart Disease (ENRICHD) project was a multi-center, randomized controlled clinical trial that included 1332 depressed patients with coronary heart disease (Berkman et al., 2003). Patients were recruited within 28 days of a heart attack, and diagnosed with either major or minor depression, low social support, or both. Patients were randomized into either a treatment or “usual medical care” group. The “treatment group” received both individual and group CBT for 6 months. Patients in the usual care group received no additional information beyond what they would ordinarily receive from their providers. All patients were referred to a psychiatrist and prescribed anti-depressants as needed. At 6 months, the treatment group had significantly greater reductions in depression, although the effect was small (57% versus 47%). Despite improvements in depression, analyses based on the entire sample of 2,481 patients with either depression or low social support demonstrated that treatment did not result in improved medical outcomes (i.e., lower rates of reinfarction or mortality). After 3 years, 24.4% of patients receiving CBT had died or suffered a second heart attack, as compared to 24.2% of the usual medical care patients. More recent analyses have determined that the intervention had a differential effect on some specific subgroups of the sample, such as white men (Schneiderman et al., 2004), those with refractory depression (Carney et al., 2004), and those taking SSRIs (Taylor et al., 2005). Nonetheless, the overarching conclusion from the ENRICHD study is that treating depression did not consistently improve disease outcomes.
Finally, other studies have found limited efficacy of the depression treatment itself, leading to mixed disease outcome results. For example, the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) examined the efficacy of sertraline as compared to placebo among 369 patients with major depressive disorder and a recent myocardial infarction (MI) over the course of 24 weeks (Glassman et al., 2002). Sertraline did not result in improved depression scores as compared to placebo (as measured by scores on the HRSD), although there was significant improvement on a global improvement scale. Further, the use of sertraline did not improve rates of severe cardiovascular events as compared to placebo, although there was a trend suggesting reduced adverse events (cardiac events and mortality), and re-analysis suggests that the treatment group did show improvements in platelet levels (Serebruany et al., 2003). More recently, the Myocardial Infarction and Depression – Intervention Trial (MIND-IT) randomly assigned 331 patients to an intervention group (who were given mirtazapine or citalopram and/or psychotherapy) or a “care as usual” group (van Melle et al., 2007). At 18-month follow-up, there were no statistically significant differences between the antidepressant treatment groups and usual care groups on depressive symptoms (as measured by the BDI and ICD-10) or on cardiac events (including recurrent MI and mortality).
There are several possible explanations for these mostly null and otherwise mixed findings in the literature. Methodological issues may account for some of the findings. For example, issues of power may limit the ability to detect findings across several studies examining depression treatment effects (see Carney & Freedland, 2007). Alternatively, the time frame for assessing medical outcomes may be too brief; that is, 8 weeks is not long enough to observe meaningful changes in either depression or disease. However, the ENRICHD study showed no effect of depression treatment at a 2-year follow-up. Complexities in defining and assessing depression as well as in conducting and interpreting the results of large scale trials also may account for some of the data discussed here (Joynt & O’Connor, 2005). Nonetheless, the overall findings fail to support the notion that there is a physiological or causal link connecting depression treatment to improved chronic physical disease outcomes.
The Effects of Disease Management on Depression
Prior theory and research suggest that the presence of chronic physical disease causes the onset or exacerbation of depressive symptoms and major depression. This hypothesis is supported by studies demonstrating that the presence of chronic physical disease predicts the onset of major depression or an increase in depressive symptoms over time (Aneshensel, Frerichs, & Huba, 1984; Bruce & Hoff, 1994; Cole & Dendukuri, 2003). In addition, several studies demonstrate that the presence of chronic disease predicts poor outcome among individuals with major depression. In one of the largest studies in the field, Moos and colleagues collected an initial sample of 424 depressed individuals from a range of treatment centers. These individuals were assessed at baseline for a range of factors, including Research Diagnostic Criteria for major or minor depression and “medical conditions such as arthritis and asthma” (Billings & Moos, 1985) and were followed over the course of 4 years. The analyses of these data suggested that the presence of medical conditions predicted non-remission of depression at a one-year follow-up (Krantz & Moos, 1988; Billings & Moos, 1985). Further, the presence of medical conditions predicted a “poor course” of depression, a measure including symptom and functioning outcomes, at a 4-year follow-up period (Swindle, Cronkite, & Moos, 1989). Similarly, in an analysis of the Medical Outcomes Study, Sherbourne, Hays, and Wells (1995) examined predictors of the course of depression among 604 initially depressed patients over one year. Individuals with an increased number of chronic medical conditions reported increased depressive symptoms over the one-year follow-up. Additional studies have confirmed these findings (Katon et al., 1994; Murphy, 1983, Tuma, 2000).
Based on the presumption that chronic physical disease causes the onset or exacerbation of depression, one may speculate that improved management of chronic physical disease should result in improved mood. The ideal type of study to examine this issue would be a randomized controlled trial that involves participants with both depression and chronic physical disease, where both chronic disease and depression outcomes were measured. However, at the time of this writing it appears that no such study has been done. Thus, the current paper reviews randomized controlled trials of the effects of disease management on depression among individuals with chronic disease (who may also have a range of depressive symptoms).
Seven studies of disease management, particularly psycho-education programs (Barlow, Wright, Sheasby, Turner, and Hainsworth, 2002; Weingarten et al., 2002), are included in Table 2. Previous reviews of the literature suggest that psycho-education programs can be efficacious in improving disease management, and thus disease outcomes such as mortality (e.g., Dusseldorp et al., 1999). We define psycho-education as a program providing general education using didactic techniques. Studies that included additional forms of disease management (e.g., behavior therapy, stress management) were not included, as these techniques often are used for depression treatment, and are not a “pure” test of the effects of disease management. Further, two studies of anti-hypertensive medications are highlighted in order to demonstrate the inconsistent effects of medication-based disease management programs. The studies in Table 2 represent randomized controlled trials comparing a disease management program to either a treatment as usual or a placebo control. These studies utilized a standardized measure of depression (e.g., BDI, MHRSD) and cut across a range of chronic physical disease categories (including arthritis, cardiac disease, diabetes, and HIV/AIDS).
Table 2.
Randomized controlled trials of the effects of disease management on depression
| Disease | Authors | # Subjects | Time | Treatment | Control Condition | Outcome Measure for Depression |
|---|---|---|---|---|---|---|
| Coronary Heart Disease | Horlick et al., (1984) | 116 | 6 week intervention 24 week follow-up | Education/Group Discussion | Usual Care | = MMPI |
| Congestive Heart Failure | Rogers et al., 1994 | 5,025 | 2 years | Enalapril | Placebo | = POMS |
| Hypertension | Perez-Stable et al., (2000) | 312 | 52 weeks | Propanolol | Placebo | = CESD, BDI |
| Diabetes | ||||||
| Piette et al., (2000) | 240 | 52 weeks | ATDM | Usual Care | + CESD | |
| Wing et al., (1986) | 50 | 12 week intervention follow-up at 48 weeks | Behavioral Weight Control + Glucose Self- | Standard Behavioral Weight Control | + BDI | |
| Arthritis | ||||||
| Lorig et al., (1999) | 331 | 6 week intervention 4 month followup | ASMP | Wait List | = CESD | |
| Barlow et al., (2000) | 240 | 6-weeks | ASMP | Wait List | +HADS | |
| 4 month follow up | ||||||
| Cancer | McQuellon et al., (1998) | 180 | 1 day | Orientation Intervention | Usual Care | + POMS, CESD |
“+” = Depression treatment improved outcome; “=” = no difference
HRSD = Hamilton Rating Scale for Depression; BDI = Beck Depression Inventory; POMS = Profile of Mood States; MMPI = Minnesota Multiphasic Personality Inventory; CESD = Center for Epidemiologic Depression Scale; HADS = Hospital Anxiety and Depression Scale; ASMP = Arthritis Self-Management Program; ATDM = Automated telephone disease management.
The results of this literature are mixed. Several studies of education programs have shown no effect on mood. For example, Lorig, Gonzalez, and Ritter (1999) examined the effect of the Arthritis Self-Management Program (ASMP) on mood among Spanish-speaking patients. A 12-hour educational intervention that consisted of classroom-format discussions of management of arthritis was administered over the course of 6 weeks and compared to a wait-list control. At a 4-month assessment, the patients in the treatment group experienced a significant increase in ratings of self-efficacy to manage their symptoms and in their practice of range of motion exercises. They also experienced a decrease in disability and pain ratings. However, patients in the education program showed no improvement in depression, as measured by the CESD, relative to controls.
Yet some studies do provide evidence that education programs improve levels of depression (Padgett, Mumford, Hynes, & Carter, 1988; Steed, Cooke, & Newman, 2003). For example, a study by Barlow, Turner, and Wright (2000) found that the ASMP program did improve mood in another sample. In this study, 544 people with arthritis were recruited from the community to participate in the ASMP program, delivered for 12 hours over 6 weeks. Three hundred and eleven participants were assigned to the intervention group and 233 were in the control group. At both a 4-month and 12-month follow-up, the ASMP group demonstrated improved depression scores as compared to controls as measured by the Hospital Anxiety and Depression Scale (HADS). Other studies have demonstrated improvements in depressed mood consequent to an education program (see Table 2).
Studies of the effects of medication as compared to placebo on mood have generally reported null findings in reducing depression severity (Beers & Passman, 1990). For example, Rogers et al., (1994) examined the effects of the angiotensin-converting enzyme inhibitor enalapril as compared to placebo on depression among 2,465 patients with symptoms of congestive heart failure enrolled in the Studies of Left Ventricular Dysfunction (SOLVD). Depression was measured by the Profile of Mood States (POMS), and was assessed at baseline, 6 weeks, one-year and two-year follow-ups. Despite evidence that enalapril prolongs life (Riegger, 1993) there were no differences between treatment and control groups on levels of depression. Other studies have found similar results, e.g., Perez-Stable and colleagues (2000) found no differences in depressive symptoms (as measured by the BDI and CESD) at the 12-month follow-up of a placebo-controlled trial of propranolol among 312 patients with diastolic hypertension.
There certainly could be methodological explanations for the mixed findings. Most importantly, as mentioned earlier, a direct test of the effects of disease management on clinically elevated depressive symptoms has not been undertaken. Thus, it is difficult to determine whether differences across studies in the effects of disease management are related to differences in the efficacy of education programs on disease management. Further, most of these studies did not assess for the presence or duration of anti-depressant treatments, and thus we cannot discern whether patients were receiving care for depression that may influence severity of depressive symptoms. Nonetheless, it is clear from the extant research that an integration of chronic disease and depression management is lacking.
Management of Co-Morbid Conditions as Self-Regulation Failure
So why do treatments for depression generally fail to significantly influence disease outcomes and disease management programs generally fail to significantly improve mood? In 1993, the Agency for Health Care Policy and Research (AHCPR, 1993) published and disseminated practice guidelines for integrating depression screening and treatment into primary care settings. Yet there is a mounting literature suggesting that depression treatment is not integrated into medical care, and it is now well established that despite the availability of therapies, depression remains under-diagnosed (Wells et al., 1989), and under-treated (Steffens et al., 2000; Young, Klap, Sherbourne, & Wells, 2001) in medical settings. The lack of integration may be related to a range of factors, including transportability of diagnostic tools and treatments into medical settings and the additional cost of treating depression (Henk, Katzelnick, Kobak, Griest, & Jefferson, 1996; Wells & Sturm, 1996). Although practice guidelines for general medical disorders are numerous, a recent review of 155 guidelines published by the Agency for Healthcare Research and Quality (AHRQ, 2006; www.ahrq.gov) found that 46% failed to include specific recommendations for depression screening or treatment (Kilbourne, Daugherty, & Pincus, 2007). Of the 83 guidelines that addressed depression, the most common recommendation was for depression screening (advocated by 47% of the 83 guidelines). Strikingly, less than 4% of the 83 guidelines addressed any modifications of medical interventions that should be considered when treating a physical health condition in the context of diagnosed depression (Kilbourne, Daugherty, & Pincus, 2007). Indeed, even when contact is made between different members of a treatment team (e.g., psychiatrist and primary care provider), joint treatment planning is rare (Valenstein et al., 1999), recommendations may not be followed (Shah, Odutoye & De, 2001), and the association among chronic illness, depression, and their respective treatments may not be discussed and understood by patient and families.
Inconsistent findings in the treatment outcome literature reflect a lack of integration of disease and depression management. Currently, depression and chronic physical disease are treated as two, distinct conditions that “ought” to interact. However, researchers and practitioners have not yet linked systematically the pathways between depression and specific chronic diseases, leading to less than optimal management of patients’ co-morbid conditions. Efforts to make the management of co-occurring chronic disease and depression more coordinated and efficient should improve self-regulation (e.g., Leventhal, Meyer, & Nerenz, 1980) and treatment outcome.
Self-regulation is a process whereby individuals create a cognitive representation of the health condition, choose a coping response, and then evaluate the outcome (Leventhal, Meyer, & Nerenz, 1980). Self-regulation in response to a health threat is often difficult, but individuals coping with co-morbidity face some unique challenges that may increase the likelihood that their self-regulatory efforts will break down. The ways in which depression and its management may concurrently improve and hinder chronic disease outcomes and the ways in which chronic disease and its management may concurrently improve and hinder depression outcomes may be understood in terms of self-regulatory failure. As attempts are made to regulate one’s behavior, the total pool of self-control resources gradually may be depleted (Baumeister & Heatherton, 1996). Moreover, a decline in the pool of resources with the onset of a chronic illness leads to the adoption of self-management strategies such as selective optimization for efficient use of reduced resources, particularly in the later years of life (Baltes & Baltes, 1990). Selective optimization is seen in studies of behavioral health that find differences in the rate of seeking health care by elderly versus middle aged adults; in comparison to middle aged adults, adults over 65 years of age avoid worry-related depletion of resources by swiftly seeking medical care and reassurance from practitioners in response to symptoms perceived as potentially threatening (Cameron, Leventhal, & Leventhal, 1993; E.Leventhal, Easterling, Leventhal & Cameron, 1995). The desire to conserve resources expressed by elderly individuals also has been observed to slow down the process of seeking replacements for activities they had given up due to severe health events (Duke, Brownlee, Leventhal & Leventhal, 2002). The data suggest, therefore, that the demands of managing multiple, chronic disorders may prove excessive and debilitating, especially for elderly individuals.
Adding the management demands of a psychological diagnosis for individuals with a chronic physical condition, and/or adding the demands of managing a chronic physical condition for someone managing a psychological disorder, may create excessive demands on the resources for self-management. Difficulties in management and increased emotional distress may arise because of conflict between the procedures for managing a psychological condition and the perception of “safe” management for a physical condition, e.g., a prescription of increased activity conflicts with a drive to conserve resources and avoid symptoms of fatigue due to fear of a cardiovascular event (Eifert, Hodson, Tracey, Seville & Gunawardane, 1996). Specifically, the challenges associated with managing co-morbidity can be described in terms of the underregulation and misregulation (e.g., Baumeister & Heatherton, 1996; Carver & Scheier, 1981) of illness responses. For individuals with co-morbid conditions, underregulation may occur when there are characteristics of depression that hamper the regulation of chronic disease and/or there are characteristics of chronic disease that hamper the regulation of depression. Self-regulatory failure also can occur because of misregulation, when the successful management of one condition conflicts with the successful management of the other.
Underregulation of Disease Management as a Result of Depressed Mood
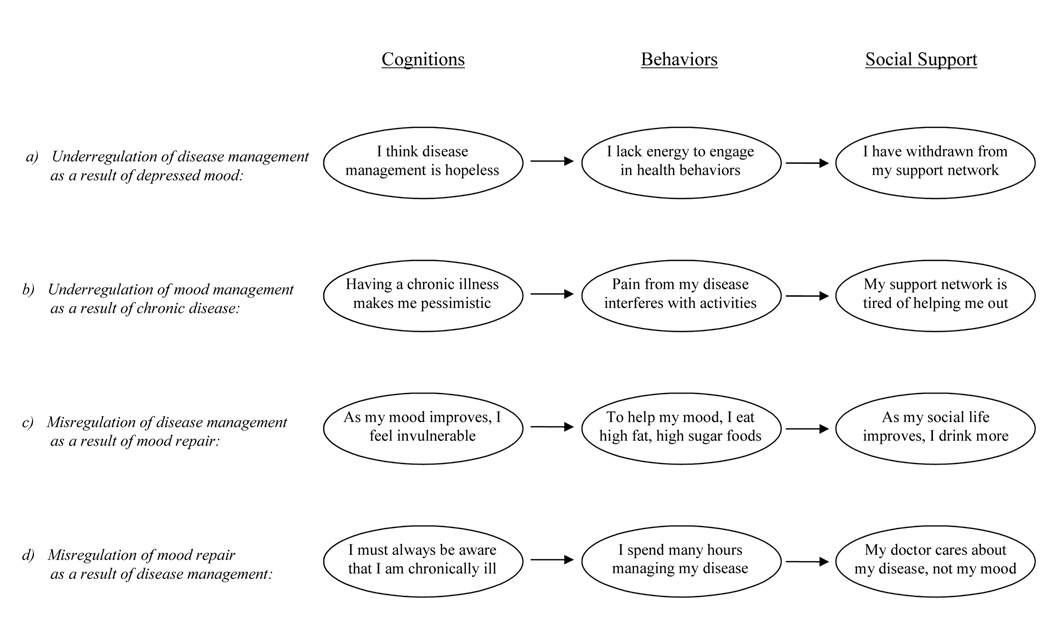
The symptoms of depression, such as low energy, poor concentration and poor sleep, may directly interfere with an individual’s ability to engage in activities that are central to disease management, resulting in underregulation. Taking the example of a woman with co-morbid depression and diabetes, depression-related deficits in optimism may make her feel as if disease management activities are not worthwhile. Similarly, low energy (characteristic of depression) would make it difficult for her to engage in health behaviors such as regular exercise, monitoring of insulin levels and doctor visits, which are critical to diabetes management. Finally, withdrawal from social support networks would make it difficult for her to receive diabetes-related practical or instrumental support (see Figure 1a).
Figure 1.
Pathways for underregulation and misregulation for an individual with co-morbid disease and depression
Depressed individuals experience a range of maladaptive cognitions that may lead to underregulation. Among individuals with chronic physical diseases, depression is associated with lower self-efficacy and lower optimism (Haaga, Dyck, & Ernst, 1991), and each of these may influence behavioral management of chronic disease. For example, both cross-sectional and longitudinal studies have found that low self-efficacy regarding specific disease management behaviors predicts an individual’s adherence to behavioral regimens. Cross-sectional studies suggest that higher self-efficacy regarding diet and exercise is associated with improved adherence to treatment regimens among individuals with chronic diseases such as diabetes (Johnston-Brooks, Lewis, & Garg, 2002; Senecal, Nouwen, & White, 2000). Longitudinal studies also demonstrate that higher self-efficacy predicts improved adherence to behavioral programs. In a study of 110 Type I diabetics, Johnston-Brooks, Lewis, and Garg (2002) found that baseline self-efficacy predicted lower blood glucose levels, and these effects were mediated by the effect of self-efficacy on health behaviors such as diet and exercise. Similarly, Kavanaugh, Gooloey and Wilson (1993) examined the relation among self-efficacy, adherence to diabetic treatment, and glucose levels for 63 diabetic patients over two months. Results showed that increased self-efficacy in the areas of diet, exercise, and glucose testing was a significant predictor of later adherence to diabetes treatment, and that adherence was associated with improved glucose control.
Second, depression may perpetuate lower levels of disease-specific optimism that may result in underregulation through poor adherence to disease management recommendations. For example, Leedham, Meyerowitz, Muirhead and Frist (1995) examined the relation between positive outcome expectations for cardiac transplant patients and adherence to medical regimens (e.g., daily health monitoring, exercise, dietary change) 6 months following transplantation. They found that higher pre-operative expectations predicted improved adherence. Other studies have found that AIDS-specific optimism predicts improved health behaviors among individuals with HIV (Taylor et al., 1992). In a sample of 152 patients prospectively studied for 6 months, Cooper, Lloyd, Weinman, and Jackson (1999) found that non-attendees of cardiac rehabilitation were more likely to believe that they could not control their condition. Overall, these findings suggest that depression may undermine chronic disease management by perpetuating negative cognitions.
Further, several studies suggest that increased depressive symptoms produce fatigue and interfere with activities of daily living (e.g., Stuck et al., 1999). Bruce, Seeman, Merrill, and Blazer (1994) examined a community-based cohort of adults aged 70–79 assessed twice at a 2.5 year interval. Results suggested that high levels of depressive symptoms, as measured by the Hopkins Symptom Checklist at baseline, predicted reduced levels of activities of daily living, adjusting for baseline SES, physical health status, and cognitive functioning. Similarly, in a study of 2,091 male and 3,438 female university students across 16 countries, Allgoewer, Wardle, and Steptoe (2001) found that higher levels of depressive symptoms as measured by the BDI were associated with disruption of activities of daily living, including lack of physical activity and not eating breakfast. These findings imply that symptoms of depression may interfere with activities of daily living, resulting in the underregulation of chronic disease.
Finally, individuals with depression may strain and erode vital social support (Coyne, 1976; Keitner & Miller, 1990), which may be necessary for successful self-regulation. Research has suggested that families of depressed patients report severe levels of dysfunction during the depressive episode (Keitner, Miller, Epstein, Bishop, & Fruzzetti, 1987; Miller, Kabacoff, Keitner, Epstein, & Bishop, 1986). This erosion of family functioning and broader social support may interfere with an individual’s ability to engage in behavioral coping with chronic disease. DiMatteo (2004) conducted a meta-analytic review of 122 studies that examined the relation between social support and adherence to medical regimens. Adherence was 1.74 times higher for patients from cohesive families, and 1.53 times lower among patients with high-conflict families. This study further suggested that practical or instrumental support would be particularly helpful in improving disease management. For example, in the case of individuals struggling with heart failure, a family member could remind the individual to take anti-hypertensive medication, prepare low-salt meals, and provide comfort. This suggests that depression’s role in undermining social support among individuals with chronic disease also may contribute to underregulation.
Underregulation of Mood Management as a Result of Chronic Disease
Likewise, the onset or exacerbation of chronic physical disease may contribute to the underregulation of depressive symptoms. First, the presence of a chronic and potentially life-threatening illness is likely to perpetuate negative cognitions that are associated with depression. A patient with diabetes may find that symptoms such as painful neuropathy interfere with enjoyable activities and overall life functioning. She also may rely on her support network to help with self-care behaviors, and she may transfer tasks such as buying insulin, driving to appointments, and selecting healthy foods to her spouse or children. Over time, these demands may lead her family to feel burdened and to the erosion of support (see Figure 1b).
The presence of a chronic physical disease, particularly one that is considered life-threatening, perpetuates negative cognitive processes that may increase depression directly (e.g., Beck, Rush, Shaw, & Emery, 1979) and indirectly. Indeed, depression can be caused by and be a symptom of chronic disease. For example, Leung and Bryant (2000) examined patterns of autobiographical memory among 15 patients with insulin-dependent diabetes mellitus as compared to 15 control participants. Based on the Autobiograhical Memory Test, diabetic patients demonstrated impaired access to specific positive memories as compared to controls. In addition, the presence of chronic disease is associated with poorer self-esteem. Bailis and Chipperfield (2002) examined the relation between collective self-esteem (i.e., an evaluation of one’s social identity) as well as health locus of control and the presence of chronic physical disease in a sample of 1,267 older adults. Patients with the highest number of chronic conditions were marked by the presence of both lower collective self-esteem and a more external locus of control, suggesting the presence of depressogenic cognitions among individuals with more chronic physical diseases.
In addition, there is mounting evidence suggesting that the symptoms associated with chronic physical disease, such as pain, interfere with behavioral functioning and result in depressed mood (Williamson, 1998). Talbot et al. (1999) conducted a cross-sectional study of 237 adults with type 2 diabetes. In this study, the authors conducted a path analysis suggesting that diabetic complications (e.g., retinopathy) were significantly associated with diabetes intrusiveness (i.e., the extent to which diabetes interfered with life functioning), and diabetes intrusiveness predicted depressive symptoms. Further, in a cross-sectional study of 494 patients with diabetic peripheral neuropathy, Vileikyte and colleagues (2005) assessed the association between the objective indicators of neuropathy severity and depressive symptoms. Neuropathic symptoms of unsteadiness, pain and reduced feeling in the feet mediated this association and accounted for most of the explained variance in depression scores. The association between neuropathic symptoms and depression was in part mediated by related restrictions in activities of daily living and changes in social self perception. Similar findings have been demonstrated among individuals with rheumatoid arthritis (Devins et al., 1993; Neugebauer, Katz, & Pasch, 2003) and cancer (Williamson & Schulz, 1995). These results suggest that symptoms of chronic disease may exacerbate and contribute to the subsequent underregulation of depressed mood.
Finally, chronic physical disease also may burden the individual’s support system, undermining the social support required for depression management. There is evidence that families of individuals with chronic physical disease experience significant burden and distress. Over time, these demands may lead to the erosion of support, which in turn may disrupt the patient’s activities of daily living (see Lima & Allen, 2001; Oxman & Hull, 1997; Seeman, Bruce, & McAvay, 1996) and make mood management impossible. Evidence from the expressed emotion literature suggests that relatives who experience an increased burden of care are more likely to harbor critical or hostile attitudes towards the patient (Scazufca & Kuipers, 1996). These critical or hostile attitudes among family members predict relapse, especially among depressed patients (Butzlaff & Hooley, 1998). In two prospective studies of spousal caregivers for individuals with chronic disease, Nieboer et al. (1998) found that increased caregiver burden was associated with increased depression in patients. This basic finding has been replicated in cross-sectional studies (e.g., Lewis, Woods, Hough, & Bensley, 1989), again supporting the contention that characteristics of chronic disease may contribute to the underregulation of depressed mood.
Misregulation of Disease Management as a Result of Mood Repair
Misregulation can be caused by making mistaken assumptions about successful self-regulation, setting unrealistic self-regulatory goals, acting on unimportant or irrelevant parts of the problem (Baumeister & Heatherton, 1996, pp. 9–10), or by misinterpreting physical symptoms and functions. For example, over the past decades, theoretical models of self-regulation of emotion and chronic illness have assumed that patients evaluate the meaning of symptoms and functional changes by comparing these events to underlying prototypes (Leventhal, 1983; Leventhal & Scherer, 1987). The comparison process has been described as involving “if-then” rules (Brownlee, Leventhal, & Leventhal, 2000) or prototype checks (PCs) that compare somatic and functional changes to underlying prototypes (Leventhal, Leventhal & Cameron, 2001). For example, the location, pattern and duration of a symptom are checked against the prototype for everyday acute conditions, such as stress (Bauman, et al, 1989; Cameron, Leventhal & Leventhal, 1995) or a common, acute condition (stomach upset; head cold). If the experienced events do not match these prototypes, they are evaluated against prototypes for a more threatening, and possibly, chronic condition. For example, heart attacks have an expected pattern of severe, sharp pain in chest or arm (pattern and location) that is sudden in onset rather than stable and chronic (time-line). These specific PCs are the units active in matching experience to prototypes (Leventhal, Forster & Leventhal, 2007). As the individual matches symptoms to prototypes of illness based on everyday experience, including prior illness history and observations of illness in others and in the media, the prototypes and matches are frequently inaccurate.
For example, the mismatch to the heart prototype leads to a breakdown in self-management of the symptoms and functional changes of congestive heart failure (i.e., problems breathing while lying down, swollen legs and chronic fatigue mismatch with the expected experiences of chest pain with rapid onset; Horowitz, Rein & Leventhal, 2004). These and other reasons for misregulation may underlie failures in managing co-morbidity. As affective experiences serve as PCs (e.g., feeling good means one is healthy, lacking energy means one is fatigued, aching means one is sick) a depressed patient with co-morbid diabetes may find that as her mood improves, she begins to think that her disease is not so bad after all and that it is more of an acute, rather than chronic, problem. Depression treatment also may lead to behavioral changes such as increased appetite and the subsequent consumption of less healthy foods. Additionally, a patient may try to improve her mood by joining former co-workers for happy hour on Friday nights. Although social interactions may help alleviate depressed mood, exposure to alcohol and unhealthy food choices may directly undermine diabetes management (see Figure 1c).
If depression management is applied successfully, it may interfere with some of the processes that otherwise would have resulted in accurate perceptions of chronic disease symptoms. For example, Ryan et al. (2002) found that diabetic patients with low negative affect had worse detection of hypoglycemic symptoms and less accurate estimates of blood glucose values, as compared to those with high negative affect. Further, Keller, Lipkus, and Rimer (2002) examined the role of depressive symptomatology in perceived risk of getting breast cancer. In this study, participants rated their risk of getting breast cancer before and after receiving either personalized or standard medical risk feedback. Although there were no significant differences in risk estimates at baseline, at follow-up non-depressed individuals did not revise their risks estimates, whereas depressed individuals increased their risk estimates such that they were closer to the estimates provided by the medical feedback.
Individuals who are successful in managing their depression not only may be less likely to perceive the seriousness of the symptoms of chronic disease, but also they may be less likely to perceive the physical disease as chronic and in need of ongoing behavioral management. Non-depressed individuals tend to be less pessimistic and to perceive negative events as less chronic (e.g., Abramson et al., 1978). Individuals who believe that hypertension is an acute or cyclical condition are more likely to minimize the seriousness of high blood pressure, as compared to individuals with a chronic view of hypertension (Croyle, 1990). Indeed, increasing optimism among depressed individuals may inadvertently undermine disease management (Tennen & Affleck, 1987). Holmes and Pace (2002) found that optimistic HIV patients were less likely to be adherent to medication regimens than pessimistic patients, in part because optimists view negative events as transient or acute rather than permanent or chronic (Abramson et al., 1978).
Patients who perceive their chronic disease as acute or cyclical rather than chronic also are likely to doubt the necessity of using preventive behaviors (Horne & Weinman, 2002). Horne and Weinman (1999) examined the relation of illness cognitions to use of preventer inhalers (i.e., use of inhalers regularly, in the absence of symptoms) among 100 community-based asthma patients. Patients who felt that asthma was less chronic perceived less of a necessity for medication. It was shown that perceived treatment necessity mediated the relationship between perceived chronicity and reported adherence. Adams, Pill and Jones (1997) found similar results. Further, this effect appears to maintain over time. Halm, Mora, and Leventhal (2006) interviewed and followed (over a period of six months) 198 adults who had been hospitalized for asthma. The patients were asked whether they thought they had asthma “all of the time” (a chronic view, endorsed by 40% of patients) or “only when having symptoms” (an acute view, endorsed by 53% of patients). The data suggest that an acute view of asthma is associated with unrealistic beliefs about asthma and poor adherence to preventer inhalers across all three time periods (baseline, 1 month, 6 months). Similarly, in a study of 230 patients interviewed about their hypertension, Meyer, Leventhal and Guttmann (1985) carried out a 6-month follow-up interview with newly treated patients. Patients new to treatment were more likely to drop out of treatment if they perceived their condition to be acute. These findings imply that as individuals manage their depression, which includes changing their view that negative events are chronic (e.g., Beck et al., 1979), they may be less likely to engage in healthy behavioral management of chronic disease. In short, treatments for depression may result in the misregulation of chronic disease goals.
Another reason why misregulation of disease management can come about as a result of depression treatment is that the individual may over-prioritize affect regulation (Baumeister & Heatherton, 1996). For example Baumeister and colleagues (e.g., Baumeister, Bratslavsky, Muraven, & Tice, 1998; Muraven, Tice, & Baumeister, 1998) demonstrate in laboratory settings that attempts to regulate affect, including the management of negative moods, can interfere with competing self-regulatory goals, including the performance of physical tasks. Although the emotional and physical challenges experienced in the laboratory may bear little resemblance to health behaviors in the real world, similar hypotheses have been supported in research investigating care seeking among acute coronary syndrome (ACS) and post-MI patients. Specifically, Wong and colleagues (2008) found that ACS patients who reported depression (as measured by the BDI) in their recollection of the 2-week period prior to seeking care were significantly delayed in their presentation for heart attack symptoms. In addition, Bunde and Martin (2006) found that individuals who were coping with depression and its associated fatigue demonstrated slower care seeking following an MI, independent of their cognitive appraisals of the meaning of the MI episode.
The notion that efforts to reduce depression may interfere with disease outcomes also is consistent with the perspective that the emotional system evolved as a mechanism for promoting survival (Cameron, 2003). The behavioral sequelae of depression may serve as a functional system that reduces energy expenditure and prevents harm (e.g., increased sleep, limited activity; Maier & Watkins, 1998; Nesse, 2000). Individuals with high levels of negative affect (i.e., depression and anxiety) may be more likely to amplify existing symptoms of chronic physical disease (Cohen et al., 1995), enhancing detection of existing symptoms and increasing the likelihood that acute and chronic disease management is carried out. Depressed individuals tend to be self-focused (Ingram, 1990), such that attention may be directed toward mood congruent ruminations, experiences of the body, or both. When sad moods produce body-oriented, self-focused attention, symptoms should be more quickly noticed (Safer, Tharps, Jackson, & Leventhal, 1979). Induced negative mood increases reports of symptoms in physically ill participants (Salovey & Birnbaum, 1989) and healthy participants (Croyle & Uretsky, 1987), whereas happier individuals are less likely to notice these somatic cues (Stretton & Salovey, 1998). Similarly, individuals with high trait negative affectivity may be more likely to detect bodily sensations (Mora et al., 2002; Stegen et al., 2001). This increased vigilance may create a more accurate view of both the presence and seriousness of symptoms ultimately resulting in higher levels of disease management behavior (Kohlmann, 2001).
Finally, depression treatment programs may encourage increased contact with social networks that may undermine disease management. There is now considerable evidence that social relationships may be a source of support, but social relationships also may undermine the individual’s self-regulatory efforts (Coyne & DeLongis, 1986). While depression treatment programs may enhance family cohesion and instrumental support, the same support system may inadvertently undermine disease management. For example, several studies have found that individuals in strong social networks are more likely to delay seeking medical intervention (Berkanovic et al., 1981), at least in part due to distrust of the health care delivery system (Suls & Goodkin, 1994). Social networks also may encourage behaviors inconsistent with disease management, e.g., families may expect involvement in holiday feasts, which is inconsistent with the management of chronic illnesses such as diabetes and heart disease. Increases in social contact often associated with depression management may inadvertently increase the maladaptive influences of a support network that discourages help seeking and/or encourages behaviors that undermine disease management.
Misregulation of Mood Repair as a Result of Disease Management
Efforts to manage chronic disease similarly may lead to the misregulation of self-control goals related to depression. A patient with co-morbid depression and diabetes may find that to effectively manage her disease, she must take blood samples throughout the day and avoid appealing foods, leading her to have negative expectations about her self and her future. This patient may report that disease management interferes with life functioning and is depressogenic. Further, she may find that she gets contradictory information from her various health care providers about the importance of her subjective symptoms (i.e., mood) versus her objective symptoms, such as levels of blood hemoglobin (see Figure 1d).
Successful disease management requires an increased awareness of the presence of disease, which may directly perpetuate negative mood, and take focus away from otherwise enjoyable life activities. This is consistent with work by Suls and Fletcher (1985), who suggest that at least in the short-term, more avoidant strategies actually are associated with positive mood. For example, Millar and Millar (1995) conducted a laboratory study in which undergraduate and community-based individuals were asked to think about either a disease detection (e.g., blood pressure checks) or health promoting (e.g., wearing sunscreen) behavior. Individuals asked to think about disease detection reported more negative mood. Previous studies show similar results (Millar & Millar, 1993), and Cioffi (1994) suggests that even disease-focused news of wellness may be associated with negative mood. Additional research suggests knowing disease status for a greater duration of time (e.g., HIV; Kelly et al., 1993) tends to be associated with increased depression. Disease management programs that increase awareness of the presence of disease, its chronicity, and its consequences may worsen depressed mood and interfere with the successful regulation of depressive symptoms.
Second, disease management behaviors are often difficult and time-consuming (e.g., Safford et al., 2005), and while these behaviors may manage disease, they also distract one from other more desirable life functions. For example, diabetic patients report that they dislike blood monitoring because it can take time from work and leisure activities (Rubin & Peyrot, 2001). Research supports this clinical impression. In a study of the relationship between depression and coronary heart disease in participants with Type I diabetes mellitus, Kinder, Karmack, Baum, and Orchard (2002) examined the cross-sectional relation between insulin measurement and depression. Patients who measured their insulin properly had higher levels of depressive symptoms. In another study of 2,855 subjects, the relation between frequency of blood glucose monitoring and depression was examined. Individuals who never monitored blood glucose had significantly lower depressive symptoms than individuals who tested more than once a day (Franciosi et al, 2001). Further, this effect may be due in part to a disruption in life functioning on the part of the patient. Eitel and colleagues (1995) found in a sample of 98 end-stage renal disease patients that those who were self-administering treatment reported increased depression, and this effect was mediated in part by the extent to which the illness disrupted social functioning. Chronic disease management programs are time consuming and disruptive of life functioning, which may generate a sense of unending entrapment in activities disrupting pursuit of desirable life goals.
Contact with health care professionals may contribute to the misregulation of mood. Patients often report receiving contradictory information from different health professionals, resulting in perceptions of wasted time that could otherwise be used for enjoyable life activities, and a perceived lack of understanding on the part of the providers as to the difficulties of managing a chronic disease (Clark & Gong, 2000; Rubin & Peyrot, 2001). For example, while the American Diabetic Association standard of care is to use physician-coordinated teams, including mental health professionals, more than 90% of patients with diabetes receive care from a primary care physician who does not typically use a team approach (Fore, 1996).
Additionally, there is evidence of discrepancies between patients and providers in their perceptions of the disease. Whereas doctors are more likely to focus on the pathophysiology of a given problem (Cohen et al., 1995), patients may emphasize social implications of their disease. Focusing on the specifics of medical disease management and failing to address how the chronic disease impacts interpersonal relationships may keep patients in a pattern of illness management and denial of the joys of living. Effective communication between practitioners and patients can enhance the efficiency of disease management and the development of strategies for engagement in enjoyable patterns of living that will improve both disease outcome and emotional health (Stewart, 1995). Unfortunately, there is no single, agreed-upon method to optimize doctor-patient interactions (Griffin, Kinmonth, Veltman, Gillard, Grant, & Stewart, 2004). When physicians are trained to handle patients’ emotions, patients report significant reductions in emotional distress (Roter, Hall, Kern, Barker, Cole, & Roca, 1995). Such training is not wide-spread, however, and therefore patients may receive incoherent treatment and lack the support they need.
Integrating Depression and Chronic Disease Management
The present research illuminates the central dilemma faced by patients managing co-morbidity: utilizing two separate, self-regulatory goals (i.e., manage disease, manage depression) is problematic. Often, the management of chronic disease and depression overlap or conflict at the level of lower-order actions or behaviors. Patients rely on subjective cues (e.g., mood, energy level, pain) rather than objective indicators of well-being (e.g., blood pressure). Focusing on subjective cues can backfire when actions aimed at improving these cues are inconsistent with actions that improve objective indicators. For example, if a patient with diabetes and depression experiences a loss of energy (a valid, subjective cue for depression but likely an invalid cue for diabetes) she may identify the symptom as being linked either to the goal of managing depression or of managing diabetes, and that link has implications for the behavioral sequences she chooses (e.g., challenge negative cognitions, raise sugar levels).
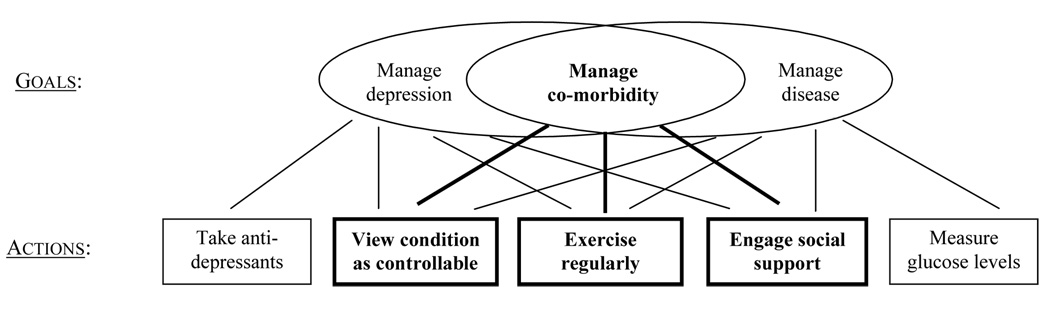
The way to integrate disease and depression management best is to create an additional, pragmatic program: to manage co-morbidity. Specifically, researchers and practitioners should strive to identify a set of specifiable conditions under which the treatment for depression will produce effects that are beneficial to the management of chronic physical disease and vice versa. That is, individuals with co-morbidity have three, rather than two, self-regulatory goals. They must attempt to manage their depressed mood, their physical disease, and the areas of overlap between these conditions (see Figure 2), while maintaining a view of their co-morbid conditions as chronic, but controllable.
Figure 2.
Three self-regulatory goals and associated actions for individuals with co-morbid depression and chronic physical disease
Simply identifying that certain actions are effective in managing both chronic disease and depression is not sufficient. Enhancing a view of the self as active and engaged in goal pursuits related to the ongoing management of co-morbid conditions also is crucial. As Marsh and Craven (2006) describe in their review of the literature on self-concept and performance, “Enhancing skills is not enough; people need to hold positive self-concepts of their abilities in specific areas. …In a wide variety of settings, practitioners who wish to maximize performance are well advised to enhance simultaneously self-concepts and skills in logically related domains (p. 158).” For example, a patient managing co-morbid depression and diabetes must recognize the reasons why she is pursuing the goal of managing her co-morbidity, which may include the fact that she wants to have the energy to keep up with her grandchildren, take care of her house, and volunteer for the community throughout the years to come. Achieving the goal of bi-directional improvement in mood and chronic disease will require caregivers to help patients create frameworks that facilitate adaptive disease management behaviors, and this requires overt links among members of the patient’s support network (e.g., doctor, psychologist, family).
We are not alone in advocating for a more integrated, comprehensive approach to care for chronic illnesses. For example, Wagner et al. (1996) have developed a heuristic model that addresses the need for patient-centered, evidence-based, collaborative care. This approach focuses on redesigning practice settings, emphasizing patient self-management, increasing access to experts, and providing helpful information about care planning. Wagner and colleagues’ approach has influenced the design of programs such as Improving Mood-Promoting Access to Collaborative Treatment (IMPACT; Unützer et al., 2002), a collaborative care management program targeted at people with late-life depression. IMPACT successfully incorporates a multi-dimensional treatment team into depression management in a primary care setting. A randomized controlled trial following 1801 patients over the course of 12 months (Hunkeler et al., 2006) found that IMPACT improves not only mood symptoms, but also indices of physical health, including overall functional impairment and general health ratings (cf. Katon et al., 2004).
Further, we are not alone in promoting the important connections between mood and chronic disease management. Williams and Williams’ (1997) created the LifeSkills program, which emphasizes the importance of enhancing communication, strengthening social relationships, and reducing stress as a means to improve health and well-being, especially among individuals at risk for stress-related diseases such as CHD. A recent study (Bishop et al., 2005) found that, as compared to an information-only control group, patients undergoing coronary artery bypass grafting who participated in LifeSkills not only experienced a significant reduction in depressive symptoms, but also demonstrated significant reductions from baseline to 3-month follow-up in resting heart rate, systolic blood pressure reactivity, and heart rate reactivity.
Despite advances modeled by programs such as IMPACT and LifeSkills, most chronic disease management programs rely on more traditional approaches to care (Wagner et al., 2002), and the field has failed to move from research to widespread implementation of an integrated chronic care model (McEvoy & Barnes, 2007). Additionally, clinically-focused approaches to treating depression among individuals with chronic physical disease fail to focus explicitly on the integration of depression and chronic physical disease management (Franco-Bronson, 1996; Lesperance & Frasure-Smith, 2000; Musselman et al., 1998; Whooley & Simon, 2000). In studies such as those reviewed in the current paper, there was no discussion of how patients perceived the relation between their chronic physical conditions and the negative mood, anhedonia and/or lack of energy that are symptomatic of depression: does the patient see these as separate conditions, as one causing the other, or does s/he see the depressive symptoms as symptoms of the physical condition? Similarly, there was no discussion of whether or how the patient’s physicians were involved in depression treatment: did the physician describe the goals of treatment, attempt to integrate the models of depression with the models of chronic illness and/or integrate the two types of treatment regimens? Finally, were goals related to depression treatment designed to be relevant to disease management and vice versa? As a result of poor integration, standard treatments that provide an intervention for depression (i.e., cognitive behavioral therapy, anti-depressant medication) or chronic physical disease (i.e., standard disease management) appear to create treatment regimens for separate and distinct conditions, creating two separate goals for the patient: one for the management of depression and one for the management of chronic physical disease.
In future investigations involving treatment of disease and depression, the particular approach to managing co-morbidity must be made far more explicit. The social systems at work in shaping self-regulation are complex, and they include the patient, primary social support (usually family), the medical practitioner, and the mental health practitioner. The support network may guide the patient toward subjective cues (by asking, “How do you feel?”) without acknowledging that subjective and objective indicators of well-being may not be aligned. The segments of one’s support network also may disagree on a range of issues, including the nature and consequences of the actions needed to manage the self-regulatory goals. A lack of coherence within the social system may present particular challenges for an individual who is trying to successfully navigate a self-regulatory process that includes more than one illness, and this lack of coherence ultimately may undermine an individual who is struggling with co-morbid conditions.
There are three domains (i.e., cognitive representations, behavioral responses, social functioning) where explicit attention to managing co-morbidity may lead towards productive interventions. Table 3 illustrates how these principles for intervention can be put into effect for a patient with diabetes and depression (see Example 1) as well as for a patient with co-morbid heart disease and depression (see Example 2).
Table 3.
Interventions for co-morbid depression and chronic physical disease
| Principles for Intervention | Example 1: Diabetes and Depression | Example 2: Heart Disease and Depression |
|---|---|---|
| Integrate disease and depression management by recognizing that the physical illness and mood disorder influence one another. | Recognize that depression can exacerbate diabetes; In the winter months, depression may worsen and make diabetes harder to manage. | Recognize that CHD may heighten depression; Heart attack risk may increase stress, which can worsen depression. |
|
Cognitive Representations: Identify and modify maladaptive disease cognitions. |
Reduce self-blame for onset of diabetes; modify time course as chronic but controllable. | Reduce catastrophizing about potential consequences of returning to work after a heart attack; increase sense of personal control. |
|
Behavioral Responses: Link behavioral functioning and engage in behaviors that perpetuate better self-regulation of both conditions. |
Become aware that managing diabetes can be depressing; Incorporate pleasurable activities into one’s daily routine to counterbalance this. | Become aware that depression reduces one’s motivation to engage in activities that control disease; Recognize the multiple benefits of exercise. |
|
Social Functioning: Enlist the aid of one’s social network in adopting coping responses consistent with both conditions. |
Encourage family members to have celebratory dinners, but also to include healthy food choices. | Arrange for a conference call with one’s physician, psychologist, and spouse to discuss reasonable treatment-related goals. |
Cognitive Representations
In order to facilitate management of a co-morbid condition, one’s cognitive representation needs to reflect the coherence of chronic disease and depression management. In the context of either a mental health or medical condition, people are often poor mental accountants of their symptoms (Gonder-Frederick & Cox, 1991), are inaccurate in their reports of symptoms (Barsky, Cleary, Barnett, Christiansen, & Ruskin, 1994; Weinger, Jacobson, Draelos, Finkelstein, & Simonson, 1995), and are likely to under-report their symptoms (Mayne, 1999). When informed of the presence of risk, individuals tend to minimize threats to health status (Croyle, Sun, & Louie, 1993; Jemmott, Ditto, & Croyle, 1986). Ultimately, the lack of perceived seriousness of symptoms may limit engagement in health behavior such as medication adherence (Chambers, Markson, Diamond, Lasch, & Berger, 1999) or exercise (Miller, 1997). This issue is particularly important among individuals with asymptomatic conditions (e.g., hypertension) where patients mistakenly believe they can isolate specific symptoms (Baumann & Leventhal, 1985; Meyer et al., 1985).
Illness identity may facilitate disease management. Durable, central self-identities will affect behavior and shape cognitive processes over the long term (Andrews, 1989; Safran, 1990). For example, individuals who accepted that they were an asthma sufferer were much clearer in respecting their need for prophylactic medication than those individuals who did not accept the identity of an asthma sufferer (Adams, Pill, & Jones, 1997). Similarly, individuals whose cognitive representation includes specific health behaviors may be more likely to engage in these behaviors. Kendzierski & Whitaker (1997) found in a sample of 67 female undergraduates that individuals with a self-schema consistent with dieting were more likely to recover from a lapse in dieting and eventually re-engage in dieting behavior. Comparable findings have been reported among individuals whose self-schema is consistent with exercise (Kendzierski, 1990).
Viewing co-morbidity from the perspective of attainable life goals (i.e., future oriented, controllable) is critical (Czuchta & Johnson, 1998; Davidson and Strauss, 1995). Davidson and Prkachin (1997) examined the interaction of optimism, unrealistic optimism (i.e., the negation of relative risk of health problems), and health behaviors in two studies among undergraduates. In their first study, 72 participants completed measures of optimism and unrealistic optimism on the first day of class. Six weeks later, those participants completed an exercise scale. Individuals high on optimism but low on unrealistic optimism were most likely to engage in self-reported exercise. In a second study, 114 undergraduates were enrolled in a CHD prevention course. Individuals high on optimism but low on unrealistic optimism at the beginning of the course were more likely to report that they gained knowledge. Although these studies utilize undergraduates rather than patients as participants, the findings lend support to the idea that characterizing CHD as a condition that cannot be “cured,” but that can be managed or improved with behavioral regimens such as exercise (i.e., CHD is chronic yet controllable), should facilitate both improved mood and health behaviors.
Behavioral Responses
In reviewing the literature, it appears that the management of chronic disease and depression is linked by behavioral functioning (i.e., behavioral management of these conditions). A mounting body of literature suggests that engagement in behavioral activation, or events that increase pleasure and mastery, is associated with improved mood (Jacobson et al., 1996). Cognitive-behavioral therapies for depression suggest that engagement in enjoyable or mastery-oriented behaviors will improve cognition and mood (Beck et al., 1979). Although positive affect can disrupt control of chronic illness (as discussed earlier), if patients are taught to interpret improvements in mood as a sign that they are mastering their disease-related fears and enhancing their perceived control, they are likely to balance successful disease management with mood-enhancing pleasurable activities.
In working towards the goal of managing co-morbidity, it may be important to focus specifically on behavioral interventions that have demonstrated benefit to both depression and chronic physical disease. As an example, the Center for Disease Control (Pate et al., 1995) suggests that regular physical activity is part of a healthy lifestyle. In particular, exercise has generally been shown to improve disease outcomes (for a review, see Dubbert, 2002) and depression (Boule et al., 2001; Singh, Clements, & Fiatarone, 1997; Singh, Clements, & Fiatarone-Singh, 2001). This effect appears consistent among individuals with chronic physical disease, and among coronary patients, exercise improves not only indicators of disease outcomes, but also depression (Kugler, Seelbach, and Kruskemper, 1994; Milani & Lavie, 2007). However, patients who engage in exercise must be taught to distinguish between frightening subjective cues (e.g., chest pain as a result of post-surgery healing) and objective indicators of distress (e.g., cardiac threat). If exercise leads to frightening symptoms, it can increase anxiety and depressed mood, inhibiting future activity. Only with the help of one’s support network (especially one’s medical doctor) can patients begin to distinguish between threatening and non-threatening symptoms, allowing for the adoption of beneficial behaviors (e.g., exercise). For example, Petrie and colleagues (2002) demonstrated that a three-session, hospital-based intervention that educated patients about the distinction between cardiac and non-cardiac symptoms (including natural but potentially frightening symptoms that may be felt during exercise) led to enhanced functional outcomes post- MI.
Social Functioning
Managing co-morbidity by enhancing social functioning and engaging the social network will facilitate more effective disease and depression management. Several theorists have suggested that social role functioning is important to long-term management of mental illness (Davidson & Strauss, 1995) as well as aging (Lemon, Bengtson, & Peterson, 1972). Disruption of valued life activities increases risk of a more general disruption of the self (Mechanic, 1995). Consistent with findings that social roles are disrupted by chronic physical conditions, e.g., diabetic neuropathy (Vileikyte et al., 2005), there is some evidence that increased focus on social role functioning improves mood. Katz and Yelin (1995) examined the relation of social role functioning and depression in a four-year longitudinal study of individuals with rheumatoid arthritis. Whereas overall functional decline was not a risk factor for the development of depressive symptoms in this sample, loss of valued activities predicted higher levels of depression at follow-up.
Further, enhanced social functioning may provide reinforcement for engaging in difficult self-management behaviors, thus limiting the associated negative affect (Keough & Markus, 1998). For example, not participating in a holiday feast will not be depressogenic if it is perceived to be compatible with another higher-order, rewarding principle (e.g., being a responsible parent and spouse). The short-term sacrifice of participation in a given social event ensures the longer-term survival of the role and functions that are required for a valued member of the family. Success in defining the self in terms of higher-order principles will require sharing goal-relevant information with family members.
Evidence also suggests that interventions that integrate spouses into diabetic care for elderly individuals are associated with improvement in metabolic control of diabetes (Gilden, Hendryx, Casia, & Singh, 1989). Others have examined an integrated social network approach for depression alone. Miller and colleagues (2005) examined whether delivering family therapy to depressed patients with poor family functioning would improve outcomes. In this study, 121 hospitalized depressed patients were grouped on the basis of their level of cognitive impairment (high, low) and family dysfunction (high, low), and then were either “matched” or “mismatched” to a treatment designed to directly address this deficit. The four treatment conditions were: a) pharmacotherapy and clinical management, b) cognitive therapy and pharmacotherapy, c) family therapy and pharmacotherapy, and d) cognitive therapy plus family therapy and pharmacotherapy. Randomized treatment began at discharge from the hospital and continued for 24 weeks on an outpatient basis. Results indicated that while treatment matching only improved short-term outcome for patients who were symptomatic at discharge from the hospital, patients who received additional family therapy had significantly better outcomes than those who did not, as indicated by a faster decrease in depressive symptoms and significantly lower levels of symptoms at a Week 12 assessment. These advantages for patients receiving family therapy diminished slightly over time, with fewer significant differences obtained at the Week 24 assessment. However, the overall pattern of results strongly suggests that an integrated approach that adds family therapy to medical and individual treatment substantially improves the outcome for severely depressed patients, and may be useful when managing co-morbid depression and chronic physical disease.
Clinical Implications
The management of co-morbidity requires the patient to utilize a multi-level process of attending to symptoms moment-by-moment, conceptualizing effectual actions for the present and the future, and incorporating a broad, positive view of one’s self (e.g., “I can see a longer term goal for me to be active and involved in family occasions, even though I have diabetes”). In order to illustrate the clinical implications of our model, consider again an individual who struggles with both depression and type 2 diabetes and who is invited regularly to social events by family, friends, and co-workers. This individual is using “existing care”: two separate treatment providers with two separate goals of managing depression and diabetes. Depressed individuals often experience symptoms such as sadness, lack of pleasure in previously enjoyable activities, and reduced appetite, weight, and energy (American Psychiatric Association, 1994). Perhaps with the assistance of a mental health professional, this individual may determine that depression could be managed by actively engaging in social events, particularly those that may be festive and enjoyable (Beck et al., 1979; Jacobson et al., 1996). Attending happy hour after work, going out to dinner with friends, and joining family gatherings on the weekend potentially improve mood and facilitate specific positive goals (e.g., viewing oneself as important to others, consuming enjoyable food and thus improving appetite).
Now consider that individual from the perspective of diabetes management. If the individual is preparing to attend the same social events while attempting to manage diabetes, there is likely to be exposure to unhealthy, yet desirable, high sugar/high fat foods. Upon engaging in disease management behavior (American Diabetes Association, 2002), the individual needs to keep her glucose levels and weight stable. Therefore she will need to significantly restrict her eating, eat differently (e.g., bring her own food), or avoid the event. As a result, she may feel that disease management will deprive her of food and enjoyable life activities (Rubin & Peyrot, 2001). Further, because elaborate food preparation and consumption is central to the daily lives of many cultures, and diabetic diets often are inconsistent with culturally accepted diet patterns (Griffin, Gilliland, Perez, Upson, & Carter, 2000), she may feel that disease management will require disengagement and create feelings of social isolation, a stimulus to depressed mood.
How does an integrated view of disease and depression management influence the actions of an individual trying to cope with social events such as these? Current practice, which separates the two conditions (e.g., I have to manage both depression and diabetes), leads to actions that overlap in content (that is, managing social interaction and food intake), yet may conflict (e.g., attend dinner vs. not attend dinner, eat enjoyable food vs. restrict). Although a compromise, such as attending the event but eating less (or not at all), may seem ideal and easily attainable if limited in scope, these types of compromises are difficult as they recur and are likely to lead to loss of pleasure and loss of support from family and friends (Rubin & Peyrot, 2001; Sorkin, 2002). Whereas engaging in healthy behaviors may be difficult for most people (Bellg, 2003), for those with co-morbid chronic physical disease and depression, mismanagement of these self-regulation goals can be devastating. Limiting pleasurable activities, restricting food intake, and avoiding social events may exacerbate major depression, whereas increasing relaxation and enjoyment, but increasing sugar and fat intake could worsen diabetes; both outcomes increase risk for loss of functioning and for mortality.
Through the addition of a self-regulatory goal of managing co-morbidity, one’s likelihood of resolving competing or conflicting actions is increased. Cognitive representations of disease and depression need to be integrated, and performance of behaviors that are synergistic with chronic disease and depression management should be encouraged. Patients need to be prepared for and aware of the possibility that management of one condition may conflict with management of the other, and they need to create an action plan for success. The plan would likely integrate elements of self-management with behaviors designed to manage the social environment, i.e., to prepare others to accept one’s needs, to prepare the foods needed for disease management, and to encourage those “in the know” to act as informers and/or protective agents. Care needs to be integrated within the patient’s existing social system. It is critical that patients and practitioners work together to develop a goal related to the overlap between the two conditions, focusing on representations of patient experiences and behavioral strategies (Clark & Gong, 2000). Ideally, the patient, family, medical doctor and mental health provider could meet to discuss issues related to how to best navigate challenging events such as dinners at friends’ houses and gatherings with family members, thus minimizing contradictory messages from the various members of the treatment team.
Finally, the patient must work to foster a positive self-view as related to the management of the co-morbid conditions by looking beyond the particular actions (e.g., food choices) or events (e.g., a family celebration). That is, the patient, her practitioners, and her support system may want to identify long-term goals, such as being healthy enough to be an active spouse, parent and grandparent. Therefore, when the patient chooses a strategy to help manage the situation (e.g., bringing her own food to an event, saying she is a vegetarian, taking a stroll after eating) the forethought and energy she expends can be reconstrued as time invested in living a long, healthy life; and rather than being offended that their food is not good enough, family and friends can recognize that their loved one’s food choices are wise and that positive regard for and encouragement of the patient’s healthy behavior will help to enhance her positive mood.
Discussion
This review highlights the inconsistent findings from studies examining whether treating depression improves disease outcomes and whether chronic disease management improves depressed mood. We propose that disease management may contribute to the underregulation and misregulation of depression management and vice versa. When individuals attempt to manage one aspect of their co-morbid condition, they may assign too much priority to these particular self-regulatory goals, thus interfering with or undermining goals related to the other condition. Given the lack of integration of disease and depression management, it is not surprising that patients lack the understanding needed to prioritize self-regulatory goals in a way that makes disease and depression management synergistic. The theoretical framework presented in the current paper describes how depression treatments and chronic disease management programs may be conceptually integrated. To resolve failures in self-regulation, patients should consider management of co-morbidity as an additional self-regulatory goal, allowing for open discussion (among patients, practitioners and researchers) of actions that are likely to improve both depression and chronic physical disease. Clinical outcomes will likely improve once depression treatment and chronic disease management are better integrated.
The roles of cognition, behavior, and social functioning in understanding and managing co-morbid depression and chronic physical disease must receive close attention. Managing either condition involves behavioral processes, including medication adherence, diet, exercise, and self-monitoring. For the purpose of the current paper, studies investigating the effect of psychosocial interventions such as behavior therapy or stress management on disease outcome were not included, because such programs are highly overlapping with empirically supported treatments for depression. Nonetheless, it is important to acknowledge that others have demonstrated the utility of such interventions in managing risk factors and symptoms related to cancer (Penedo, 2006), CHD (Blumenthal et al., 2002), HIV/AIDS (Antoni et al., 2006), and diabetes (the Diabetes Prevention Program Research Group, 2005), for example. The importance of managing stress and negative mood among individuals with chronic illnesses is evidenced by clinical interventions such as these, yet a consensus from randomized controlled trials of psychosocial programs’ overall effect on morbidity and mortality is lacking (Fekete, Antoni, & Schneiderman, 2007; Goldston & Baillie, 2008). Perhaps due to the numerous biological mechanisms linking disease and depression, most models of co-morbidity have focused on biological factors, and medication treatment trials far exceed psychosocial intervention trials. A coordinated approach to managing behavioral processes in the context of co-morbid depression is essential, and improved treatment of co-morbid conditions will fail to be effective without a direct focus on these processes.
Although the current paper has focused on major depressive disorder, some of the issues related to managing co-morbidity may not be specific to depression. For example, researchers have explored the challenges associated with managing schizophrenia in the context of co-morbid medical illnesses (e.g., Lambert, Velakoulis, & Pantelis, 2003), giving particular attention to the management of important factors such as cigarette smoking, diet and exercise, unsafe sexual behavior, and medication side effects (Green et al., 2003). Anxiety disorders also frequently co-occur with medical illnesses, and a recent review by Roy-Byrne and colleagues (2008) argues that anxiety disorders may play as important of a role in morbidity and mortality as depression. Indeed, it is possible that one of the ways that disease and depression influence each other is through their mutual effect on anxiety. For example, many of the effects of negative affect on awareness of symptoms may be the result of anxiety, rather than depression (Leventhal, Hansell, Diefenbach, Leventhal, & Glass, 1996). Similarly, increased health care consumption may be the result of anxiety rather than depressed mood (Strik et al., 2004). And as Barlow (1991) has suggested, anxiety specific to health threats may be a critical precursor of depression in response to the occurrence, recurrence and/or worsening of physical disease.
As this is a broad, multi-faceted field, several important issues were beyond the scope of the current review. First, chronic physical disease and depression were conceptualized throughout the paper as separate entities, and the term “co-morbid” was used to describe individuals suffering from both conditions. This is in contrast to previous illness cognition models that assume emotional reactions (e.g., depressed mood) are directly linked to the presence of disease (Leventhal et al., 1992). The possible causes for depression are too numerous to assume etiology, and regardless of etiology, the presence of major depression may result in its own set of biological, cognitive and behavioral processes that perpetuate disease. Resolution of these issues ultimately will have important implications for how chronic disease and depression are conceptualized and treated. Second, some otherwise complex processes have been simplified, and the set of self-regulatory goals we propose is incomplete. For example, the potential benefit of depression treatment on adherence is highlighted, as though strict adherence is always associated with improved outcome. However, this may not always be the case (Hays et al., 1994; Petrie et al., 2002).
Third, the current review focuses primarily on cognitive, behavioral, and social processes. The complex biological processes that may underlie the link between co-morbid conditions and the issues related to the pharmacologic management of disease and depression were beyond the scope of this paper. For example, it is possible that some of the effects of medications aiming to improve the depressive disorder may exacerbate disease symptoms and vice versa (e.g., Norra, Skobel, Arndt, & Schauerte, 2008). Reviews and ongoing research focusing on the biological bases of the relationships between depression and disease are crucial (Krishnan et al., 2002; Skala, Freedland, & Carney, 2006), and our limited discussion of these issues in no way reflects our estimation of its importance.
Finally, we do not intend to suggest that treating both depression and physical disease as separate entities is not worthwhile. On the contrary, managing depression is an important avenue to overall disease management and vice versa. Assuming that the cumulative effects of treating one condition in isolation are not harmful, treating both conditions as unique and separable is not only important to do, but inevitable. Medical doctors need not become experts in depression management, and therapists need not become experts in disease management. Instead, practitioners and researchers should communicate with specialists in different fields, with the goal of identifying areas of overlap. Providers of care must be unambiguous in describing their own and other practitioners’ goals in order to help patients identify the ways in which their self-regulatory goals are interrelated. Integration of the treatment system will bolster patients’ abilities to identify and manage their own self-regulatory goals, ultimately leading to better physical and mental health outcomes.
Acknowledgments
Order of authorship between the first and second authors was determined by a coin-flip. We thank Melanie Cohen for her assistance in reviewing the literature and Brian Detweiler-Bedell for his counsel throughout the writing process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Throughout this paper, when we refer to “co-morbid conditions” or “co-morbidity” we are referring to co-morbid depression and chronic physical disease.
Contributor Information
Jerusha B. Detweiler-Bedell, Lewis and Clark College
Michael A. Friedman, Rutgers, the State University of New Jersey
Howard Leventhal, Rutgers, the State University of New Jersey.
Ivan W. Miller, Butler Hospital and Brown University
Elaine A. Leventhal, University of Medicine and Dentistry of New Jersey and Robert Wood Johnson Medical School
References
- Abramson LY, Seligman MEP, Teasdale J. Learned helplessness in humans: Critique and reformulation. Journal of Abnormal Psychology. 1978;87:49–74. [PubMed] [Google Scholar]
- Adams S, Pill R, Jones A. Medication, chronic illness and identity: The perspective of people with asthma. Social Science and Medicine. 1997;45:189–201. doi: 10.1016/s0277-9536(96)00333-4. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Policy and Research. Depression in Primary Care, 1: Detection and Diagnosis. Rockville, MD: Agency for Healthcare Policy and Research, US Dept of Health and Human Services, Public Health Services; 1993. AHCPR publication 93–0550. [Google Scholar]
- Agency for Healthcare Research and Quality. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Preventive Services Task Force; Preventive Services Resource Links. 2006 http://www.ahrq.gov/clinic/uspstf/resource.htm.
- Allgoewer A, Wardle J, Steptoe A. Depressive symptoms, social support, and personal health behaviors in young men and women. Health Psychology. 2001;20:223–227. [PubMed] [Google Scholar]
- American Diabetes Association. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:202–212. doi: 10.2337/diacare.25.1.202. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fourth edition. Washington, D.C.: American Psychiatric Association. Analysis; 1994. [Google Scholar]
- Anderson RJ, Freedland KK, Clouse RE, Lustman PJ. The prevalence of co-morbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Andrews JD. Psychotherapy of depression: A self-confirmation model. Psychological Review. 1989;96:576–607. doi: 10.1037/0033-295x.96.4.576. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Frerichs RR, Huba GJ. Depression and physical illness: A multiwave, nonrecursive causal model. Journal of Health and Social Behavior. 1984;25:350–371. [PubMed] [Google Scholar]
- Antoni MH, Carrico AW, Duran RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosomatic Medicine. 2006;68:143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomology, and coronary artery disease. Psychosomatic Medicine. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Aromaa A, Reunanen A, Impivaara O, Heliovaara M, Knekt P, Lehtinen V, et al. Depression and cardiovascular diseases. Acta Psychiatrica Scandinavica. 1994;377:77–82. doi: 10.1111/j.1600-0447.1994.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Bailis DS, Chipperfield JG. Compensating for losses in perceived personal control over health: A role for collective self-esteem in health aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P531–P539. doi: 10.1093/geronb/57.6.p531. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MM, editors. Successful aging: Perspectives from the behavioral sciences. New York: Cambridge University Press; 1990. pp. 1–34. [Google Scholar]
- Barefoot JC, Helms MJ, Mark DB, Blumenthal JA, Califf RM, Haney TL, et al. Depression and long-term mortality risk in patients with coronary artery disease. American Journal of Cardiology. 1996;78:613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- Barlow DH. The nature of anxiety: Anxiety, depression, and emotional disorders. In: Rapee RM, Barlow DH, editors. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. New York: Guilford Press; 1991. pp. 1–28. [Google Scholar]
- Barlow JH, Turner AP, Wright CC. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Education Research. 2000;15:665–680. doi: 10.1093/her/15.6.665. [DOI] [PubMed] [Google Scholar]
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: A review. Patient Education and Counseling. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Cleary PD, Barnett MC, Christiansen CL, Ruskin JN. The accuracy of symptom reporting by patients complaining of palpitations. The American Journal of Medicine. 1994;97:214–221. doi: 10.1016/0002-9343(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Baumann L, Cameron LD, Zimmerman R, Leventhal H. Illness representations and matching labels with symptoms. Health Psychology. 1989;8:449–469. doi: 10.1037//0278-6133.8.4.449. [DOI] [PubMed] [Google Scholar]
- Baumann LJ, Leventhal H. I can tell when my blood pressure is up, can’t I? Health Psychology. 1985;4:203–218. doi: 10.1037//0278-6133.4.3.203. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7:1–15. [Google Scholar]
- Baumeister RF, Bratlavsky E, Muraven M, Tice DM. Ego-depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beers MH, Passman LJ. Antihypertensive medications and depression. Drugs. 1990;40:792–799. doi: 10.2165/00003495-199040060-00003. [DOI] [PubMed] [Google Scholar]
- Bellg AJ. Maintenance of health behavior change in preventive cardiology: Internalization and self-regulation of new behaviors. Behavior Modification. 2003;27:103–131. doi: 10.1177/0145445502238696. [DOI] [PubMed] [Google Scholar]
- Berkanovic E, Telesky C, Reeder S. Structural and social psychological factors in the decision to seek medical care for symptoms. Medical Care. 1981;19:693–709. doi: 10.1097/00005650-198107000-00001. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. Journal of the American Medical Association. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Billings AG, Moos RH. Psychosocial processes of remission in unipolar depression: Comparing depressed patients with matched community controls. Journal of Consulting and Clinical Psychology. 1985;53:314–325. doi: 10.1037//0022-006x.53.3.314. [DOI] [PubMed] [Google Scholar]
- Bishop GD, Kaur M, Tan VLM, Chua Y-L, Liew S-M, Mak K-H. Effects of a psychosocial skills training workshop on psychophysiological and psychosocial risk in patients undergoing coronary artery bypass grafting. American Heart Journal. 2005;150:602–609. doi: 10.1016/j.ahj.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Black SA, Markides KS. Depressive symptoms and mortality in older Mexican Americans. Annals of Epidemiology. 1999;9:45–52. doi: 10.1016/s1047-2797(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Blum A, Miller H. Role of cytokines in heart failure. American Heart Journal. 1998;135:181–186. doi: 10.1016/s0002-8703(98)70080-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak M, Wei J, O’Connor C, Waugh R, Eisenstein E, Mark D, Sherwood A, Woodley PS, Irwin RJ, Reed G. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. American Journal of Cardiology. 2002;89:164–168. doi: 10.1016/s0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Journal of the American Medical Association. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- Brownlee S, Leventhal EA, Leventhal H. Regulation, self-regulation and regulation of the self in maintaining physical health. In: Boekartz M, Pintrich PR, Zeidner M, editors. Handbook of Self-Regulation. San Diego, CA: Academic Press; 2000. pp. 369–416. [Google Scholar]
- Bruce ML, Hoff RA. Social and physical health risk factors for first-onset major depressive disorder in a community sample. Social Psychiatry and Psychiatric Epidemiology. 1994;29:165–171. doi: 10.1007/BF00802013. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur studies of successful aging. American Journal of Public Health. 1994;84:1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunde J, Martin R. Depression and prehospital delay in the context of myocardial infarction. Psychosomatic Medicine. 2006;68:51–57. doi: 10.1097/01.psy.0000195724.58085.f0. [DOI] [PubMed] [Google Scholar]
- Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. American Journal of Cardiology. 2001;88:337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse. Archives of General Psychiatry. 1998;55:547–552. doi: 10.1001/archpsyc.55.6.547. [DOI] [PubMed] [Google Scholar]
- Cameron L, Leventhal EA, Leventhal H. Symptom representations and affect as determinants of care seeking in a community dwelling adult sample population. Health Psychology. 1993;12:171–179. doi: 10.1037//0278-6133.12.3.171. [DOI] [PubMed] [Google Scholar]
- Cameron LC, Leventhal EA, Leventhal H. Seeking medical care in response to symptoms and life stress. Psychosomatic Medicine. 1995;57:37–47. doi: 10.1097/00006842-199501000-00006. [DOI] [PubMed] [Google Scholar]
- Cameron LD. Anxiety, cognition, and responses to health threats. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. New York: Routledge; 2003. pp. 157–183. [Google Scholar]
- Carney RM, Freedland KE. Does treating depression improve survival after acute coronary syndrome? British Journal of Psychiatry. 2007;190:467–468. doi: 10.1192/bjp.bp.107.035360. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC, Jaffe AS. Can treating depression reduce mortality after an acute myocardial infarction? Psychosomatic Medicine. 1999;61:666–675. doi: 10.1097/00006842-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosomatic Medicine. 2004;66:466–474. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Attention and self-regulation: A control-theory approach to human behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- Chambers CV, Markson L, Diamond JJ, Lasch L, Berger M. Health beliefs and compliance with inhaled corticosteroids by asthmatic patients in primary care practices. Respiratory Medicine. 1999;93:88–94. doi: 10.1016/s0954-6111(99)90296-2. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cioffi D. When good news is bad news: Medical wellness as a nonevent in undergraduates. Health Psychology. 1994;13:63–72. doi: 10.1037//0278-6133.13.1.63. [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong M. Management of chronic disease by practitioners and patients: Are we teaching the wrong things? British Medical Journal. 2000;320:572–575. doi: 10.1136/bmj.320.7234.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gwaltney JM, Jr, Doyle WJ, Skoner DP, Fireman P, Newsom JT. State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. Journal of Personality and Social Psychology. 1995;68:159–169. doi: 10.1037//0022-3514.68.1.159. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. American Journal of Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. American Journal of Public Health. 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Lloyd G, Weinman J, Jackson G. Why patients do not attend cardiac rehabilitation: Role of intentions and illness beliefs. Heart. 1999;82:234–236. doi: 10.1136/hrt.82.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC. Depression and the response of others. Journal of Abnormal Psychology. 1976;85:186–193. doi: 10.1037//0021-843x.85.2.186. [DOI] [PubMed] [Google Scholar]
- Coyne JC, DeLongis A. Going beyond social support: The role of social relationships in adaptation. Journal of Consulting & Clinical Psychology. 1986;54:454–460. doi: 10.1037//0022-006x.54.4.454. [DOI] [PubMed] [Google Scholar]
- Croyle RT. Biased appraisal of high blood pressure. Preventive Medicine. 1990;19:40–44. doi: 10.1016/0091-7435(90)90005-5. [DOI] [PubMed] [Google Scholar]
- Croyle RT, Sun Y, Louie DH. Psychological minimization of cholesterol test results: Moderators of appraisal in college students and community residents. Health Psychology. 1993;12:503–507. doi: 10.1037//0278-6133.12.6.503. [DOI] [PubMed] [Google Scholar]
- Croyle RT, Uretsky MB. Effects of mood on self-appraisal of health status. Health Psychology. 1987;6:239–253. doi: 10.1037//0278-6133.6.3.239. [DOI] [PubMed] [Google Scholar]
- Czuchta DM, Johnson BA. Reconstructing a sense of self in patients with chronic mental illness. Perspectives in Psychiatric Care. 1998;34:31–36. doi: 10.1111/j.1744-6163.1998.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Davidson L, Strauss JS. Beyond the biopsychosocial model: Integrating disorder,health, and recovery. Psychiatry. 1995;58:44–55. doi: 10.1080/00332747.1995.11024710. [DOI] [PubMed] [Google Scholar]
- Davidson K, Prkachin K. Optimism and unrealistic optimism have an interacting impact on health-promoting behavior and knowledge changes. Personality and Social Psychology Bulletin. 1997;23:617–625. [Google Scholar]
- Derogatis LR, Abeloff MD, Melisaratos N. Psychological coping mechanisms and survival time in metastatic breast cancer. Journal of the American Medical Association. 1979;242:1504–1508. [PubMed] [Google Scholar]
- DeRubeis RJ, Crits-Christoph P. Empirically supported individual and group psychological treatments for adult mental disorders. Journal of Consulting and Clinical Psychology. 1998;66:37–52. doi: 10.1037//0022-006x.66.1.37. [DOI] [PubMed] [Google Scholar]
- Devins GM, Edworthy SM, Paul LC, Mandin H, Seland P, Klein, et al. Restless sleep, illness intrusiveness, and depressive symptoms in three chronic illness conditions: Rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. Journal of Psychosomatic Research. 1993;37:163–170. doi: 10.1016/0022-3999(93)90083-r. [DOI] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosomatic Medicine. 2002;64:52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- DiMatteo RM. Social support and patient adherence to medical treatment: A meta-analysis. Health Psychology. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- Dubbert PM. Physical activity and exercise: Recent advances and current challenges. Journal of Consulting and Clinical Psychology. 2002;70:526–536. [PubMed] [Google Scholar]
- Duke J, Brownlee S, Leventhal EA, Leventhal H. Giving up and replacing activities in response to illness. Journal of Gerontology: Psychological Sciences. 2002;57B:367–376. doi: 10.1093/geronb/57.4.p367. [DOI] [PubMed] [Google Scholar]
- Dusseldorp E, van Elderen T, Maes S, Meulman J, Kraaij V. A meta-analysis of psychoeducational programs for coronary heart disease patients. Health Psychology. 1999;18:506–519. doi: 10.1037//0278-6133.18.5.506. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Hodson SE, Tracey DR, Seville JL, Gunawardane K. Heart-focused anxiety, illness beliefs, and behavioral impairment: comparing healthy heart-anxious patients with cardiac and surgical inpatients. Journal of Behavioral Medicine. 1996;19:385–399. doi: 10.1007/BF01904764. [DOI] [PubMed] [Google Scholar]
- Eitel P, Hatchett L, Friend R, Griffin KW, Wadhwa NK. Burden of self-care in seriously ill patients: Impact on adjustment. Health Psychology. 1995;14:457–463. doi: 10.1037//0278-6133.14.5.457. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Uldall KK, Bergam K, Russo J, Claypoole K, Roy-Byrne PP. Randomized, placebo-controlled trial of paroxetine versus imipramine in depressed HIV-positve outpatients. American Journal of Psychiatry. 1998;155:367–372. doi: 10.1176/ajp.155.3.367. [DOI] [PubMed] [Google Scholar]
- Evans DL, Staab JP, Petitto JM, Morrison MF, Szuba MP, Ward, et al. Depression in the medical setting: Biopsychological interactions and treatment considerations. Journal of Clinical Psychiatry. 1999;60:40–55. [PubMed] [Google Scholar]
- Fekete EM, Antoni MH, Schneiderman N. Psychosocial and behavioral interventions for chronic medical conditions. Current Opinion in Psychiatry. 2007;20:152–157. doi: 10.1097/YCO.0b013e3280147724. [DOI] [PubMed] [Google Scholar]
- Fore WW. Managed care approaches to diabetes mellitus. Hospital Practice. 1996;31:115–117. doi: 10.1080/21548331.1996.11443315. [DOI] [PubMed] [Google Scholar]
- Franciosi M, Pellegrini F, De Barardis G, Belfiglio M, Cavaliere D, Di Nardo B, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients. Diabetes Care. 2001;24:1870–1877. doi: 10.2337/diacare.24.11.1870. [DOI] [PubMed] [Google Scholar]
- Franco-Bronson K. The management of treatment-resistant depression in the medically ill. The Psychiatric Clinics of North America. 1996;19:329–348. doi: 10.1016/s0193-953x(05)70291-4. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression: A cardiac risk factor in search of a treatment. Journal of the American Medical Association. 2003a;289:3171–3173. doi: 10.1001/jama.289.23.3171. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry. 2003b;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- Gilden L, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. Journal of the American Geriatrics Society. 1989;37:1023–1030. doi: 10.1111/j.1532-5415.1989.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. Journal of the American Medical Association. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- Goldston K, Baillie AJ. Depression and coronary heart disease: A review of the epidemiological evidence, explanatory mechanisms and management approaches. Clinical Psychology Review. 2008;28:288–306. doi: 10.1016/j.cpr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Gonder-Frederick LA, Cox DJ. Symptom perception, symptom beliefs, and blood glucose discrimination in the self-treatment of insulin-dependent diabetes. In: Skelton JA, Croyle RT, editors. Mental representation in health and illness. New York: Springer-Verlag; 1991. pp. 220–246. [Google Scholar]
- Green AI, Canuso CM, Brenner MJ, Wojcik JD. Detection and management of comorbidity in patients with schizophrenia. Psychiatric Clinics of North America. 2003;26(1):115–139. doi: 10.1016/s0193-953x(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Griffin JA, Gilliland SS, Perez G, Upson D, Carter JS. Challenges to participating in a lifestyle intervention program: The Native American diabetes project. Diabetes Education. 2000;26:681–689. doi: 10.1177/014572170002600416. [DOI] [PubMed] [Google Scholar]
- Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: A systematic review of trials. Annals of Family Medicine. 2004;2:595–608. doi: 10.1370/afm.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaga DA, Dyck MJ, Ernst D. Empirical status of cognitive theory of depression. Psychological Bulletin. 1991;110:215–236. doi: 10.1037/0033-2909.110.2.215. [DOI] [PubMed] [Google Scholar]
- Halm EA, Mora P, Leventhal H. No symptoms, no asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129:573–580. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- Hays RD, Wells KBB, Sherbourne CD, Rogers W, Spritzer K. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Archives of General Psychiatry. 1995;52:11–19. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- Hays RD, Kravitz RL, Mazel RM, Sherbourne CD, DiMatteo MR, Rogers WH, et al. The impact of patient adherence on health outcomes for patients with chronic disease in the Medical Outcomes Study. Journal of Behavioral Medicine. 1994;17:347–360. doi: 10.1007/BF01858007. [DOI] [PubMed] [Google Scholar]
- Henk H, Katzelnick D, Kobak K, Griest J, Jefferson J. Medical costs attributed to depression among patients with high medical expenses in a health maintenance organization. Archives of General Psychiatry. 1996;53:899–904. doi: 10.1001/archpsyc.1996.01830100045006. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. Journal of the American Medical Association. 1996;276:1473–1479. [PubMed] [Google Scholar]
- Holmes WC, Pace JL. HIV-seropositive individuals’ optimistic beliefs about prognosis and relation to medication and safe sex adherence. Journal of General Internal Medicine. 2002;17:677–683. doi: 10.1046/j.1525-1497.2002.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlick L, Cameron R, Firor W, Bhalerao U, Baltzan R. The effects of education and group discussion in the post myocardial infarction patient. Journal of Psychosomatic Research. 1984;28:484–492. doi: 10.1016/0022-3999(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. Journal of Psychosomatic Research. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychology and Health. 2002;17:17–32. [Google Scholar]
- Horowitz CR, Rein SB, Leventhal H. A story of maladies, misconceptions and mishaps: effective management of heart failure. Social Science & Medicine. 2004;58:631–643. doi: 10.1016/s0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkeler E, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomized trial for depressed elderly patients in primary care. British Medical Journal. 2006;332:259–263. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. Journal of the American Medical Association. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Self-focused attention in clinical disorders: Review and a conceptual model. Psychological Bulletin. 1990;107:156–176. doi: 10.1037/0033-2909.107.2.156. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Samson JA, Weinger K, Ryan CM. Diabetes, the brain, and behavior: Is there a biological mechanism underlying the association between diabetes and depression? International Review of Neurobiology. 2002;51:455–479. doi: 10.1016/s0074-7742(02)51013-8. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64:295–304. doi: 10.1037//0022-006x.64.2.295. [DOI] [PubMed] [Google Scholar]
- Jemmott JB, III, Ditto PH, Croyle RT. Judging health status: Effects of perceived prevalence and personal relevance. Journal of Personality and Social Psychology. 1986;50:899–905. doi: 10.1037//0022-3514.50.5.899. [DOI] [PubMed] [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- Johnston-Brooks CH, Lewis MA, Garg S. Self-efficacy impacts self-care and HbA1c in young adults with type 1 diabetes. Psychosomatic Medicine. 2002;64:43–51. doi: 10.1097/00006842-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Joynt KE, O’Connor CM. Lessons from SADHART, ENRICHD, and other trials. Psychosomatic Medicine. 2005;67 Suppl. 1:S63–S66. doi: 10.1097/01.psy.0000163454.25036.fc. [DOI] [PubMed] [Google Scholar]
- Katon W, Lin E, Von Korff M, Bush T, Walker E, Simon G, Robinson P. The predictors of persistence of depression in primary care. Journal of Affective Disorders. 1994;31:81–90. doi: 10.1016/0165-0327(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Katon WJ, Von Korff M, Lin EHB, Simon G, Ludman E, Russo J, et al. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Archives of General Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Katz PP, Yelin EH. The development of depressive symptoms among women with rheumatoid arthritis. Arthritis and Rheumatism. 1995;38:49–56. doi: 10.1002/art.1780380108. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Gooley S, Wilson PH. Prediction of adherence and control in diabetes. Journal of Behavioral Medicine. 1993;16:509–522. doi: 10.1007/BF00844820. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Miller IW. Family functioning and major depression: An overview. American Journal of Psychiatry. 1990;147:1128–1137. doi: 10.1176/ajp.147.9.1128. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Miller IW, Epstein NB, Bishop DS. Family functioning and the course of major depression. Comprehensive Psychiatry. 1987;28:54–64. doi: 10.1016/0010-440x(87)90044-7. [DOI] [PubMed] [Google Scholar]
- Keller PA, Lipkus IM, Rimer BK. Depressive realism and health risk accuracy: The negative consequences of positive mood. Journal of Consumer Research. 2002;29:57–69. [Google Scholar]
- Kelly JA, Murphy DA, Bahr GR, Koob JJ, Morgan MG, Kalichman SC, et al. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with Human Immunodeficiency Virus (HIV) infection. Health Psychology. 1993;12:215–219. doi: 10.1037//0278-6133.12.3.215. [DOI] [PubMed] [Google Scholar]
- Kendzierski D. Exercise self-schemata: Cognitive and behavioral correlates. Health Psychology. 1990;9:69–82. doi: 10.1037//0278-6133.9.1.69. [DOI] [PubMed] [Google Scholar]
- Kendzierski D, Whitaker DJ. The role of self-schema in linking intentions with behavior. Personality and Social Psychology Bulletin. 1997;23:139–147. [Google Scholar]
- Keough K, Markus H. On being well: The role of the self in building the bridge from philosophy to biology. Psychological Inquiry. 1998;9:49–53. [Google Scholar]
- Kilbourne AM, Daugherty B, Pincus HA. What do general medical guidelines have to say about depression care? Depression treatment recommendations in general medical practice guidelines. Current Opinion in Psychiatry. 2007;20:626–631. doi: 10.1097/YCO.0b013e3282f0c4d3. [DOI] [PubMed] [Google Scholar]
- Kinder LS, Karmarck TW, Baum A, Orchard TJ. Depressive symptomatology and coronary heart disease in type 1 diabetes mellitus: A study of possible mechanisms. Health Psychology. 2002;21:542–552. doi: 10.1037//0278-6133.21.6.542. [DOI] [PubMed] [Google Scholar]
- Kohlmann C, Ring C, Carroll D, Mohiyeddini C, Bennett P. Cardiac coping style, heartbeat detection, and the interpretation of cardiac events. British Journal of Health Psychology. 2001;6:285–301. doi: 10.1348/135910701169214. [DOI] [PubMed] [Google Scholar]
- Krantz SE, Moos RH. Risk factors at intake predict nonremission among depressed patients. Journal of Consluting and Clinical Psychology. 1988;56:863–869. doi: 10.1037//0022-006x.56.6.863. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Delong M, Kraemer H, et al. Comorbidity of depression with other medical diseases in the elderly. Biological Psychiatry. 2002;52:559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Delong M, Kraemer H, Carney R, Spiegel D, Gordon C, et al. Co-morbidity of depression with other medical diseases in the elderly. Biological Psychiatry. 2002;52:559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- Kugler J, Seelbach H, Kruskemper GM. Effects of rehabilitation exercise programmes on anxiety and depression in coronary patients: A meta-analysis. British Journal of Clinical Psychology. 1994;33:401–410. doi: 10.1111/j.2044-8260.1994.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Ladwig KH, Kieser M, König J, Breithardt G, Borggrefe M. Affective disorders and survival after acute myocardial infarction: Results from the post-infarction late potential study. European Heart Journal. 1991;12:959–964. [PubMed] [Google Scholar]
- Lambert TJ, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Medical Journal of Australia. 2003;178 suppl.:S67–S70. doi: 10.5694/j.1326-5377.2003.tb05311.x. [DOI] [PubMed] [Google Scholar]
- Leedham B, Meyerowitz BE, Muirhead J, Frist WH. Positive expectations predict health after heart transplantation. Health Psychology. 1995;14:74–79. doi: 10.1037//0278-6133.14.1.74. [DOI] [PubMed] [Google Scholar]
- Lemon BW, Bengtson VL, Peterson JA. An exploration of the activity theory of aging: Activity types and life satisfaction among in-movers to a retirement community. Journal of Gerontology. 1972;27:511–523. doi: 10.1093/geronj/27.4.511. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N. Depression in patients with cardiac disease: A practical review. Journal of Psychosomatic Research. 2000;48:379–391. doi: 10.1016/s0022-3999(99)00102-6. [DOI] [PubMed] [Google Scholar]
- Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- Leung P, Bryant RA. Autobiographical memory in diabetes mellitus patients. Journal of Psychosomatic Research. 2000;49:435–438. doi: 10.1016/s0022-3999(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Leventhal E, Hansell S, Diefenbach M, Leventhal H, Glass D. Negative affect and self-report of physical symptoms: Two longitudinal studies of older adults. Health Psychology. 1996;15:193–199. doi: 10.1037//0278-6133.15.3.193. [DOI] [PubMed] [Google Scholar]
- Leventhal EA, Easterling D, Leventhal H, Cameron L. Conservation of energy, uncertainty reduction and swift utilization of medical care among the elderly: Study II. Medical Care. 1995;33:988–1000. doi: 10.1097/00005650-199510000-00002. [DOI] [PubMed] [Google Scholar]
- Leventhal H. Behavioral medicine: Psychology in health care. In: Mechanic D, editor. Handbook of Health, Health Care, and the Health Professions. New York: Free Press; 1983. pp. 709–743. [Google Scholar]
- Leventhal H. Symptom reporting: A focus on process. In: McHugh S, Vallis TM, editors. Illness Behavior: A Multi-Disciplinary Model. New York: Plenum Press; 1986. pp. 219–237. [Google Scholar]
- Leventhal H, Scherer KR. The relationship of emotion to cognition: A functional approach to semantic controversy. Cognition and Emotion. 1987;1:3–28. [Google Scholar]
- Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Cognitive Therapy and Research. 1992;16:143–163. [Google Scholar]
- Leventhal H, Forster R, Leventhal EA. Self-regulation of health threats, affect, and the self: Lessons from older adults. In: Aldwin CM, Park CL, Spiro A III, editors. Handbook of Health Psychology and Aging. New York: The Guilford Press; 2007. pp. 341–366. [Google Scholar]
- Leventhal H, Leventhal EA, Cameron L. Representations, procedures and affect in illness self regulation: A perceptual-cognitive model. In: Baum A, Revenson T, Singer J, editors. Handbook of Health Psychology. New York: Earlbaum; 2001. pp. 19–47. [Google Scholar]
- Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. In: Rachman S, editor. Contributions to medical psychology. Vol. 2. Oxford: Pergamon; 1980. pp. 7–30. [Google Scholar]
- Lewis FM, Woods NF, Hough EE, Bensley LS. The family’s functioning with chronic illness in the mother: The spouse’s perspective. Social Science & Medicine. 1989;29:1261–1269. doi: 10.1016/0277-9536(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Lima JC, Allen SM. Targeting risk for unmet needs: Not enough help versus no help at all. Journals of Gerontology: Series B: Psychological Sciences & Social Sciences. 2001;56B:302–310. doi: 10.1093/geronb/56.5.s302. [DOI] [PubMed] [Google Scholar]
- Lorig K, Gonzalez VM, Ritter P. Community-based Spanish language arthritis education program: A randomized trial. Medical Care. 1999;37:957–963. doi: 10.1097/00005650-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Treatment of depression in diabetes: Impact on mood and medical outcome. Journal of Psychosomatic Research. 2002;53:917–924. doi: 10.1016/s0022-3999(02)00416-6. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: A randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al. Effects of nortriptyline on depression and glycemic control in diabetes: Results of a double-blind, placebo-controlled trial. Psychosomatic Medicine. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus: A randomized, controlled trial. Annals of Internal Medicine. 1998;129:613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bi-directional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marsh HW, Craven RG. Reciprocal effects of self-concept and performance from a multidimensional perspective: Beyond seductive pleasure and unidimensional perspectives. Perspectives on Psychological Science. 2006;1:133–163. doi: 10.1111/j.1745-6916.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- Mayne TJ. Negative affect and health: The importance of being earnest. Cognition and Emotion. 1999;13:601–635. [Google Scholar]
- Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Archives of Internal Medicine. 1996;156:2233–2238. [PubMed] [Google Scholar]
- McEvoy P, Barnes P. Using the chronic care model to tackle depression among older adults who have long-term physical conditions. Journal of Psychiatric and Mental Health Nursing. 2007;14:233–238. doi: 10.1111/j.1365-2850.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- McQuellon RP, Wells M, Hoffman S, Craven B, Russell G, Cruz J, et al. Reducing distress in cancer patients with an orientation program. Psycho-Oncology. 1997;7:207–217. doi: 10.1002/(SICI)1099-1611(199805/06)7:3<207::AID-PON304>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Mechanic D. Sociological dimensions of illness behavior. Social Science and Medicine. 1995;41:1207–1216. doi: 10.1016/0277-9536(95)00025-3. [DOI] [PubMed] [Google Scholar]
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: The example of hypertension. Health Psychology. 1985;4:115–135. doi: 10.1037//0278-6133.4.2.115. [DOI] [PubMed] [Google Scholar]
- Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. The American Journal of Medicine. 2007;120:799–806. doi: 10.1016/j.amjmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Millar MG, Millar K. Negative affective consequences of thinking about disease detection beheaviors. Health Psychology. 1995;14:141–146. doi: 10.1037//0278-6133.14.2.141. [DOI] [PubMed] [Google Scholar]
- Millar MG, Millar KU. Affective and cognitive responses to disease detection and health promotion behaviors. Journal of Behavioral Medicine. 1993;16:1–23. doi: 10.1007/BF00844752. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop SB, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: Reliability and validity. Psychiatry Research. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Miller IW, Kabacoff RI, Keitner GI, Epstein NB, Bishop DS. Family functioning in the families of psychiatric patients. Comprehensive Psychiatry. 1986;27:302–312. doi: 10.1016/0010-440x(86)90006-4. [DOI] [PubMed] [Google Scholar]
- Miller IW, Keitner G, Ryan C, Solomon D, Cardemil E, Beevers CG. Treatment matching in the posthospital care of depressed patients. American Journal of Psychiatry. 2005;162:2131–2138. doi: 10.1176/appi.ajp.162.11.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. American Journal of Medicine. 1997;102:43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- Mora PA, Robitaille C, Leventhal H, Swigar M, Leventhal EA. Trait negative affect relates to prior-week symptoms, but not to reports of illness episodes, illness symptoms, and care seeking among older persons. Psychosomatic Medicine. 2002;64:436–449. doi: 10.1097/00006842-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Morris PLP, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. American Journal of Psychiatry. 1993;150:124–129. doi: 10.1176/ajp.150.1.124. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as a limited resource: Regulatory depletion patterns. Journal of Personality & Social Psychology. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Murberg TA, Bru E, Svebak S, Tveterås R, Aarsland T. Depressed mood and subjective health symptoms as predictors or mortality in patients with congestive heart failure: A two-years follow-up study. International Journal of Psychiatry in Medicine. 1999;29:311–326. doi: 10.2190/0C1C-A63U-V5XQ-1DAL. [DOI] [PubMed] [Google Scholar]
- Murphy E. The prognosis of depression in old age. British Journal of Psychiatry. 1983;142:111–119. doi: 10.1192/bjp.142.2.111. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Archives of General Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Neugebauer A, Katz PP, Pasch LA. Effect of valued activity disability, social comparisons, and satisfaction with ability on depressive symptoms in rheumatoid arthritis. Health Psychology. 2003;22:253–262. doi: 10.1037/0278-6133.22.3.253. [DOI] [PubMed] [Google Scholar]
- Nieboer AP, Schulz R, Matthews KA, Scheier MF, Ormel J, Lindenberg SM. Spousal caregivers’ activity restriction and depression: A model for changes over time. Social Science & Medicine. 1998;47:1361–1371. doi: 10.1016/s0277-9536(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Norra C, Skobel EC, Arndt M, Schauerte P. High impact of depression in heart failure: Early diagnosis and treatment options. International Journal of Cardiology. 2008;125:220–231. doi: 10.1016/j.ijcard.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Oxman TE, Hull JG. Social support, depression, and activities of daily living in older heart surgery patients. Journals of Gerontology: Series B: Psychological Sciences & Social Sciences. 1997;52B:P1–P14. doi: 10.1093/geronb/52b.1.p1. [DOI] [PubMed] [Google Scholar]
- Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. Journal of Clinical Epidemiology. 1988;41:1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health: A recommendation from the centers for disease control and prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive behavioral stress management in localized prostate cancer: Development of stress management skills improves quality of life and benefit finding. Annals of Behavioral Medicine. 2006;31:261–270. doi: 10.1207/s15324796abm3103_8. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Halliday R, Gardiner PS, Baron RB, Hauck WW, Acree M, et al. The effects of propranolol on cognitive function and quality of life: A randomized trial among patients with diastolic hypertension. The American Journal of Medicine. 2000;108:359–365. doi: 10.1016/s0002-9343(00)00304-1. [DOI] [PubMed] [Google Scholar]
- Petrie KJ, Cameron L, Ellis CJ, Buick D, Weinman J. Changing illness perceptions after myocardial infarction: An early intervention randomized controlled trial. Psychosomatic Medicine. 2002;64:580–586. doi: 10.1097/00006842-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Piette JD, Weinberger M, McPhee SJ. The effect of automated calls with telephone nurse follow-up on patient-centered outcomes of diabetes care. Medical Care. 2000;38:218–230. doi: 10.1097/00005650-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Rabkin R, Harrison W, Wagner G. Effect of imipramine on mood and enumerative measures of immune status in depressed patients with HIV illness. American Journal of Psychiatry. 1994;151:516–523. doi: 10.1176/ajp.151.4.516. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Wagner GJ, Rabkin R. Fluoxetine treatment for depression in patients with HIV and AIDS: A randomized, placebo-controlled trial. American Journal of Psychiatry. 1999;156:101–107. doi: 10.1176/ajp.156.1.101. [DOI] [PubMed] [Google Scholar]
- Riegger GA. Lessons from recent randomized controlled trials for the management of congestive heart failure. American Journal of Cardiology. 1993;71:38E–40E. doi: 10.1016/0002-9149(93)90951-8. [DOI] [PubMed] [Google Scholar]
- Rogers WJ, Johnstone DE, Yusuf S, Weiner DH, Gallagher P, Bittner VA, et al. Quality of life among 5,025 patients with left ventricular dysfunction randomized between placebo and enalapril: The studies of left ventricular dysfunction. Journal of the American College of Cardiology. 1994;23:393–400. doi: 10.1016/0735-1097(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians' interviewing skills and reducing patients' emotional distress. A randomized clinical trial. Archives of Internal Medicine. 1995;155:1877–1884. [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJG, Goodwin RD, Kubzansky L, et al. Anxiety disorders and comorbid medical illness. General Hospital Psychiatry. 2008;30:208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Peyrot M. Psychological issues and treatments for people with diabetes. Journal of Clinical Psychology. 2001;57:457–478. doi: 10.1002/jclp.1041. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Suprasongsin C, Dulay D, Becker DJ. Detection of symptoms by adolescents and young adults with type 1 diabetes during experimental induction of mild hypoglycemia. Diabetes Care. 2002;25:852–858. doi: 10.2337/diacare.25.5.852. [DOI] [PubMed] [Google Scholar]
- Safer MA, Tharps QJ, Jackson TC, Leventhal H. Determinants of three stages of delay in seeking care at a medical clinic. Medical Care. 1979;17:11–29. doi: 10.1097/00005650-197901000-00002. [DOI] [PubMed] [Google Scholar]
- Safford MM, Russell L, Suh DC, Roman S, Pogach L. How much time do patients with diabetes spend on self-care? The Journal of the American Board of Family Practice. 2005;18:262–270. doi: 10.3122/jabfm.18.4.262. [DOI] [PubMed] [Google Scholar]
- Safran JD. Towards a refinement of cognitive therapy in light of interpersonal theory: I. Theory. Clinical Psychology Review. 1990;10:87–105. [Google Scholar]
- Salovey P, Birnbaum D. Influence of mood on health-relevant cognitions. Journal of Personality and Social Psychology. 1989;57:539–551. doi: 10.1037//0022-3514.57.3.539. [DOI] [PubMed] [Google Scholar]
- Scazufca M, Kuipers E. Links between expressed emotion and burden of care in relatives of patients with schizophrenia. British Journal of Psychiatry. 1996;168:580–587. doi: 10.1192/bjp.168.5.580. [DOI] [PubMed] [Google Scholar]
- Skala JA, Freedland KE, Carney RM. Coronary heart disease and depression: A review of recent mechanistic research. Canadian Journal of Psychiatry. 2006;51:738–745. doi: 10.1177/070674370605101203. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Saab PG, Catellier DJ, et al. Psychosocial treatment within sex by ethnicity subgroups in the Enhancing Recovery in Coronary Heart Disease clinical trial. Psychosomatic Medicine. 2004;66:475–483. doi: 10.1097/01.psy.0000133217.96180.e8. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Saab PG, Catellier DJ, Powell LH, DeBusk RF, Williams RB, et al. Psychosocial treatment within sex by ethnicity subgroups in the Enhancing Recovery in Coronary Heart Disease clinical trial. Psychosomatic Medicine. 2004;66:475–483. doi: 10.1097/01.psy.0000133217.96180.e8. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Bruce ML, McAvay GJ. Social network characteristics and onset of ADL disability: MacArthur studies of successful aging. Journals of Gerontology: Series B: Psychological Sciences & Social Sciences. 1996;51B:S191–S200. doi: 10.1093/geronb/51b.4.s191. [DOI] [PubMed] [Google Scholar]
- Senecal C, Nouwen A, White D. Motivation and dietary self-care in adults with diabtetes: Are self-efficacy and autonomous self-regulation complementary or competing constructs? Health Psychology. 2000;19:452–457. doi: 10.1037//0278-6133.19.5.452. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, Van Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) platelet substudy. Circulation. 2003;108:939–944. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- Shah A, Odutoye K, De T. Depression in acutely medically ill elderly inpatients: A pilot study of early identification and intervention by formal psychogeriatric consultation. Journal of Affective Disorders. 2001;62:233–240. doi: 10.1016/s0165-0327(99)00182-2. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Hays RD, Wells KB. Personal and psychosocial risk factors for physical and mental health outcomes and course of depression among depressed patients. Journal of Consulting and Clinical Psychology. 1995;63:345–355. doi: 10.1037//0022-006x.63.3.345. [DOI] [PubMed] [Google Scholar]
- Singh NA, Clements KM, Fiatarone Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: A randomized, controlled trial. Journals of Gerontology. Series A, Biological Sciences & Medical Sciences. 2001;56:M497–M504. doi: 10.1093/gerona/56.8.m497. [DOI] [PubMed] [Google Scholar]
- Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. Journals of Gerontology. Series A, Biological Sciences & Medical Sciences. 1997;52:M27–M35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea T, Coryell W, Warshaw M, Turvey C, Maser JD, Endicott J. Multiple recurrences of major depressive disorder. American Journal of Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- Sorkin KP. For parents. Parties and holidays. A map through the mindfields. Diabetes Self-Management. 2002;19:111–112. 114–115, 117. [PubMed] [Google Scholar]
- Steed L, Cooke D, Newman S. A systematic review of psychosocial outcomes following education, self-management, and psychological interventions in diabetes mellitus. Patient Education and Counseling. 2003;51:5–15. doi: 10.1016/s0738-3991(02)00213-6. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, et al. Prevalence of depression and its treatment in an elderly population: The Cache County study. Archives of General Psychiatry. 2000;57:601–607. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- Stegen K, Van Diest I, Van de Woestijne KP, Van den Bergh O. Do persons with negative affect have an attentional bias to bodily sensations? Cognition and Emotion. 2001;15:813–829. [Google Scholar]
- Stewart MA. Effective physician-patient communication and health outcomes: A review. Canadian Medical Association Journal. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among U.S. workers with depression. Journal of the American Medical Association. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Stretton MS, Salovey P. Cognitive and affective components of hypochondriacal concerns. In: Flack WF, Laird JD, editors. Emotions in psychopathology: Theory and research. Series in affective science. London: Oxford; 1998. pp. 265–279. [Google Scholar]
- Strik J, Honig A, Lousberg R, Lousberg AHP, Cheriex EC, Tuynman-Qua HG, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: Findings from a double-blind, placebo-controlled trial. Psychosomatic Medicine. 2000;62:783–789. doi: 10.1097/00006842-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Strik J, Lousberg R, Cheriex EC, Honig A. One year cumulative incidence of depression following myocardial infarction and impact on cardiac outcome. Journal of Psychosomatic Research. 2004;56:59–66. doi: 10.1016/S0022-3999(03)00380-5. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, Buela CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Suls J, Fletcher B. The relative efficacy of avoidant and nonavoidant coping strategies: A meta-analysis. Health Psychology. 1985;4:249–288. doi: 10.1037//0278-6133.4.3.249. [DOI] [PubMed] [Google Scholar]
- Suls JM, Goodkin F. Medical gossip and rumor: Their role in the lay referral system. In: Goodman RF, Ben-Ze’ev A, editors. Good gossip. Lawrence, KS: University Press of Kansas; 1994. pp. 169–179. [Google Scholar]
- Swindle RW, Jr, Cronkite RC, Moos RH. Life stressors, social resources, coping, and the 4-year course of unipolar depression. Journal of Abnormal Psychology. 1989;98:468–477. doi: 10.1037//0021-843x.98.4.468. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A, Gingras J, Belanger A, Audet J. Relations of diabetes intrusiveness and personal control to symptoms of depression among adults with diabetes. Health Psychology. 1999;18:537–542. doi: 10.1037//0278-6133.18.5.537. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Archives of General Psychiatry. 2005;62:792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Kemeny ME, Aspinwall LG, Schneider SG, Rodriguez R, Herbert M. Optimism, coping, psychological distress, and high-risk sexual behavior among men at risk for acquired immunodeficiency syndrome (AIDS) Journal of Personality and Social Psychology. 1992;63:460–473. doi: 10.1037//0022-3514.63.3.460. [DOI] [PubMed] [Google Scholar]
- Tennen H, Affleck G. The costs and benefits of optimistic explanations and dispositional optimism. Journal of Personality. 1987;55:377–393. doi: 10.1111/j.1467-6494.1987.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Thase ME, Kupfer DJ. Recent developments in the pharmacotherapy of mood disorders. Journal of Consulting and Clinical Psychology. 1996;64:646–659. doi: 10.1037//0022-006x.64.4.646. [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma TA. Outcome of hospital-treated depression at 4.5 years: An elderly and a younger adult cohort compared. British Journal of Psychiatry. 2000;176:224–228. doi: 10.1192/bjp.176.3.224. [DOI] [PubMed] [Google Scholar]
- Unützer J, Katon W, Callahan C, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Journal of the American Medical Association. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Valenstein M, Klinkman M, Becker S, Blow FC, Barry KL, Sattar A, et al. Concurrent treatment of patients with depression in the community: Provider practices, attitudes, and barriers to collaboration. Journal of Family Practice. 1999;48:180–187. [PubMed] [Google Scholar]
- van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AMG, Crijns H, Schins A, Tulner D, van den Berg MP, Ormel J. Effects of antidepressant treatment following myocardial infarction. British Journal of Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: The association revisited. Diabetes Care. 2005;28:2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- Wagner E, Austin B, Von Korff M. Organizing the care for patients with chronic illness. Milbank Quarterly. 1996;74:511–544. [PubMed] [Google Scholar]
- Wagner E, Davis C, Schaefer J, Von Korff M, Austin B. A survey of leading chronic disease management programs: Are they consistent with the literature? Journal of Nursing Care Quality. 2002;16:67–80. [PubMed] [Google Scholar]
- Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Jr, et al. Interventions used in disease management programmes for patients with chronic illness–which ones work? Meta-analysis of published reports. British Medical Journal. 2002;325:913–914. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger K, Jacobson AM, Draelos MT, Finkelstein DM, Simonson DC. Blood glucose estimation and symptoms during hyperglycemia and hypoglycemia in patients with insulin-dependent diabetes mellitus. The American Journal of Medicine. 1995;98:22–31. doi: 10.1016/S0002-9343(99)80077-1. [DOI] [PubMed] [Google Scholar]
- Welin C, Lappas G, Wilhelmsen L. Independent importance of psychosocial factors for prognosis after myocardial infarction. Journal of Internal Medicine. 2000;247:629–639. doi: 10.1046/j.1365-2796.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- Wells KB, Sherbourne C. Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Archives of General Psychiatry. 1999;56:897–904. doi: 10.1001/archpsyc.56.10.897. [DOI] [PubMed] [Google Scholar]
- Wells KB, Sturm R. Informing the policy process: From efficacy to effectiveness data on pharmacotherapy. Journal of Consulting and Clinical Psychology. 1996;64:638–645. doi: 10.1037//0022-006x.64.4.638. [DOI] [PubMed] [Google Scholar]
- Wells KB, Hays RD, Burnam MA, Rogers W, Greenfield S, Ware JE. Detection of depressive disorder for patients receiving prepaid or fee-for-service care: Results from the medical outcomes study. Journal of the American Medical Association. 1989;262:3298–3302. [PubMed] [Google Scholar]
- Whooley MA, Simon GE. Managing depression in medical outpatients. The New England Journal of Medicine. 2000;343:1942–1950. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- Williams V, Williams RB. LifeSkills: Eight Simple Ways to Build Stronger Relationships, Communicate More Clearly, and Improve Your Health and Even the Health of Those Around You. New York: Times Books; 1997. [Google Scholar]
- Williamson GM. The central role of restricted normal activities in adjustment to illness and disability: A model of depressed affect. Rehabilitation Psychology. 1998;43:327–347. [Google Scholar]
- Williamson GM, Schulz R. Activity restriction mediates the association between pain and depressed affect: A study of younger and older adult cancer patients. Psychology and Aging. 1995;10:369–378. doi: 10.1037//0882-7974.10.3.369. [DOI] [PubMed] [Google Scholar]
- Wing RR, Epstein LH, Nowalk MP, Scott N, Koeske R, Hagg S. Does self-monitoring of blood glucose levels improve dietary compliance for obese patients with type 2 diabetes? The American Journal of Medicine. 1986;81:830–836. doi: 10.1016/0002-9343(86)90354-2. [DOI] [PubMed] [Google Scholar]
- Wong C-K, Tang EW, Herbison P, Birmingham B, Barclay L, Fu SYF. Pre-existent depression in the 2 weeks before an acute coronary syndrome can be associated with delayed presentation of the heart attack. Q J Med. 2008;101:137–144. doi: 10.1093/qjmed/hcm153. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Archives of General Psychiatry. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]