Abstract
Background
Subsequent malignant neoplasms are a major cause of premature death in survivors of hereditary retinoblastoma. Radiotherapy further increases the risk of death. Mortality information is limited among long-term survivors who were irradiated for hereditary retinoblastoma.
Methods
We examined cause-specific mortality among 1854 retinoblastoma survivors who were diagnosed from January 1, 1914, through December 31, 1996, at two US institutions. Standardized mortality ratios (SMRs) were calculated by use of US mortality data to estimate expected numbers of deaths. The relative rates (RRs) of mortality due to subsequent malignant neoplasms associated with multiple risk factors were evaluated with Poisson regression models. Cumulative mortality from subsequent malignant neoplasms was calculated by treating other causes of death as competing risks.
Results
A total of 151 deaths due to subsequent malignant neoplasms occurred among 1092 hereditary retinoblastoma survivors (SMR = 35, 95% confidence interval [CI] = 30 to 41) compared with 12 deaths among 762 nonhereditary retinoblastoma survivors (SMR = 2.5, 95% CI = 1.3 to 4.4). In this extended follow-up of retinoblastoma survivors, we found no evidence of excess mortality from non-neoplastic causes compared with the general population. However, excess mortality from subsequent malignant neoplasms (particularly sarcomas, melanomas, and cancers of the brain and other parts of the nervous system) among hereditary retinoblastoma survivors extended beyond 40 years after retinoblastoma diagnosis. The additional 13 years of follow-up since our last mortality study revealed a previously unreported increased risk of death due to cancers of the corpus uteri (primarily sarcomas) and confirmed the previously reported elevated risk of death from lung cancer among hereditary retinoblastoma survivors. Among hereditary and nonhereditary retinoblastoma survivors, the relative rates of mortality from subsequent malignant neoplasm were higher in those who had been treated with radiotherapy than in those who had not. Cumulative mortality from subsequent malignant neoplasms at 50 years after retinoblastoma diagnosis was 25.5% (95% CI = 20.8% to 30.2%) for hereditary retinoblastoma survivors and 1.0% (95% CI = 0.2% to 1.8%) for nonhereditary retinoblastoma survivors.
Conclusions
The temporal patterns of site-specific excess risks of subsequent malignant neoplasms in retinoblastoma survivors should inform screening programs designed for the early detection and treatment of subsequent malignant neoplasms.
CONTEXT AND CAVEATS
Prior knowledge
Many survivors of hereditary retinoblastoma (ie, survivors who carry a germline mutation in the retinoblastoma [RB1] gene) die prematurely from subsequent malignant neoplasms, and those who were treated with radiotherapy have an even higher risk of premature death from subsequent malignant neoplasms compared with the general population.
Study design
Mortality data for 1854 long-term survivors of hereditary and nonhereditary retinoblastoma were used to quantify cause-specific mortality and to evaluate interactions between hereditary status and treatment with radiotherapy.
Contribution
Compared with the general population, hereditary retinoblastoma survivors had increased risks of death from subsequent malignant neoplasms (particularly sarcomas, melanomas, and cancers of the brain and other parts of the nervous system) that extended beyond 40 years after retinoblastoma diagnosis, as well as for cancers of the corpus uteri (primarily sarcomas), and lung cancer. Among hereditary and nonhereditary retinoblastoma survivors, the relative rates of mortality from subsequent malignant neoplasm were higher in those who had been treated with radiotherapy than in those who had not.
Implications
The temporal patterns of site-specific excess risks of subsequent malignant neoplasms in retinoblastoma survivors should inform screening programs designed for the early detection and treatment of subsequent malignant neoplasms.
Limitations
In some cases, the cause of death listed on death certificates may have been inaccurate and nonfatal cancers could have been missed.
From the Editors
Retinoblastoma is a rare childhood cancer of the eye and the prototypic model for inherited cancers (1,2). The retinoblastoma gene (RB1) was the first tumor suppressor gene cloned; it encodes the p105 retinoblastoma (Rb) protein, which affects cell cycle control, cellular differentiation, and cell survival [reviewed in (2,3)]. Subsequent research has found that several other types of cancer harbor somatic mutations in the RB1 gene, including sarcomas, small cell lung cancer, bladder cancer, and breast cancer [reviewed in (1,2,4)].
Retinoblastoma is now a curable disease. However, compared with the general population, survivors of hereditary retinoblastoma (ie, survivors who carry a germline mutation in the RB1 gene) are at markedly increased risk of subsequent malignant neoplasms, including sarcomas of the bone and soft tissue; melanoma; and cancers of the brain, lung, and, possibly, bladder (5–11). Some survivors even develop three or more subsequent cancers (12).
Radiotherapy, which is currently used much less frequently to treat bilateral retinoblastoma since the mid-1990s due to the recognition of elevated risk for subsequent malignant neoplasms among retinoblastoma patients treated with external beam radiotherapy (13), further increases the risk of subsequent malignant neoplasms, especially those that arise within or near the radiation field (ie, in the skull and face bones, the soft tissues of the head, and the brain). Among our cohort of survivors of hereditary retinoblastoma, a radiation dose–response relationship for sarcomas has been demonstrated (11). In addition, the risk of subsequent malignant neoplasms has been reported to be greater among hereditary retinoblastoma survivors who were irradiated before the age of 12 months than among those irradiated at older ages (14,15).
Although the greatly increased risk of subsequent malignant neoplasms among survivors of hereditary retinoblastoma is well established, data on mortality from subsequent malignant neoplasms among long-term survivors who were treated with radiation are limited. In an earlier mortality analysis through December 31, 1990, we reported an increased risk of death from sarcomas, melanoma, and brain cancer among hereditary retinoblastoma survivors (6). Since that time, we have published two cancer incidence studies in this same population of retinoblastoma survivors through December 31, 1993, and December 31, 2000 (9,11). In this report, we have extended the mortality follow-up for an additional 13 years through December 31, 2003, to investigate mortality from subsequent malignant neoplasms and non-neoplastic outcomes and have expanded the original cohort by including retinoblastoma survivors diagnosed through December 31, 1996. To our knowledge, this is the largest cohort of irradiated long-term survivors of retinoblastoma yet assembled, which allowed us to quantify cause-specific mortality and to evaluate gene–radiotherapy interactions.
Patients and Methods
Study Population
A retrospective cohort of 1729 retinoblastoma patients who were diagnosed from January 1, 1914, through December 31, 1984, was identified from medical records of two medical centers in New York and Boston. Survivors who had died from any cause within 12 months of diagnosis (n = 111), outside the United States (n = 13), or before January 1, 1925 (n = 3), were excluded from the analysis. We also excluded two survivors with no usable tracing data and one patient who we found did not have retinoblastoma. This left 1599 (92.5%) 1-year survivors who were included in the mortality analysis (hereafter referred to as cohort 1).
To study cause-specific mortality among more recently treated retinoblastoma survivors, we assembled a new cohort of 262 consecutive patients who were diagnosed from January 1, 1985, through December 31, 1996, by reviewing medical records at the medical center in New York. We excluded three survivors because of a missing date of retinoblastoma diagnosis, two survivors from other countries who had visited the hospital for consultation only, and two survivors who had died within 12 months of diagnosis, which left 255 (97.3%) 1-year survivors of retinoblastoma eligible for the study (hereafter referred to as cohort 2). Because there were only 14 deaths in cohort 2 and the combined results of cohorts 1 and 2 did not differ materially from those of cohort 1, we present results for the combined cohort unless otherwise specified. For both cohorts combined, a total of 1854 survivors were included in the mortality analysis.
RB1 mutation testing data were not available for either cohort. For purposes of this study, survivors with bilateral retinoblastoma and unilateral retinoblastoma survivors with a positive family history of retinoblastoma were classified as having hereditary retinoblastoma (n = 1092 or 58.9%), whereas unilateral retinoblastoma survivors with no family history of retinoblastoma were classified as having nonhereditary retinoblastoma (n = 762 or 41.1%). The proportion of hereditary vs nonhereditary retinoblastoma survivors in this cohort differs from that observed in the general population, in which only 40% of survivors have hereditary disease and 60% have nonhereditary disease (16). This difference between our cohort and the general population is likely attributable to the differential referral of patients to well-known tertiary medical centers.
Follow-up Procedures
Information on demographic variables, medical history, family history of retinoblastoma and other cancers, treatments for retinoblastoma, reports of new cancers, and causes of death was collected from patients'; medical records and radiotherapy files and via telephone interviews conducted in 1987, 1993, and 2000 for cohort 1 and in 1998 for cohort 2. The institutional review board of the National Institutes of Health approved this study. Various tracing sources were also used to update the vital status of the retinoblastoma survivors, including periodic searches of the National Death Index (NDI) and publicly available electronic databases through December 31, 2003. Because there is about a 2-year lag from the date of death until death records appear in the NDI database, the latest date of death for which NDI data were available at the time when this study began in 2005 was December 31, 2003. A trained nosologist coded the causes of deaths that occurred before 1979 and before the NDI was in existence according to the International Classification of Diseases version that was in effect during the year of death. All coded causes of death were subsequently converted to International Classification of Diseases, 8th revision (ICD-8) coding (17), in which tumors are classified according to organ site rather than histology.
Radiation Therapy
Among the irradiated survivors in the combined cohort, 88.6% were treated with external beam radiotherapy, 1.8% with brachytherapy, and 9.6% with a combination of both techniques. Among survivors who were treated with external beam radiotherapy, the most commonly used approaches were a two-field technique that included the nasal and lateral fields and a single-field technique that includes either the lateral or the anterior field. Before 1960, retinoblastoma patients were predominantly treated with orthovoltage x-rays, whereas after 1960, 22- to 23-MV betatron megavoltage photons and cobalt-60 gamma rays were used. The average dose of radiation to the affected eye was 48 Gy (range = 15–115 Gy); the orthovoltage machines delivered the highest average doses. Before 1960, brachytherapy was delivered by plaques that contained radon-222 seeds (average dose = 200 mg-h Ra equivalents; range = 160–400 mg-h Ra equivalents). After 1960, plaques containing cobalt-60 were used (average dose = 400 mg-h Ra equivalents; range = 150–800 mg-h Ra equivalents). Although individual-level data for radiation dose were not available for all survivors, organ dose data for a typical treatment from cohort 1 allowed us to classify organ sites as heavily irradiated (≥1 Gy), moderately irradiated (0.4–1 Gy), or lightly irradiated (<0.4 Gy), as previously described (9); these cut points were defined a priori based on distance from the radiation treatment field. For example, all of the organ sites classified as heavily irradiated were all in, or very close to, the edge of the radiation treatment field.
Statistical Analysis
Follow-up began 1 year after the retinoblastoma diagnosis and ended on the date the patient was last known to be alive, the date of death, or the end of follow-up (December 31, 2003), whichever came first. Survivors who were last known to be alive after January 1, 1979, and who were not found in the NDI, which began recording deaths in the United States in 1979, were presumed to be alive as of December 31, 2003.
External Comparisons.
We estimated the relative risk for each cause of death by calculating the standardized mortality ratio (SMR) and the exact Poisson 95% confidence interval (CI). The expected number of deaths was calculated by applying the US mortality rates (by 5-year age, 5 calendar year, and sex-specific categories) to the appropriate person-time accrued by retinoblastoma survivors in the cohort. The statistical method we used to calculate SMRs uses US mortality rates from 1925 and later (18). Therefore, for the few survivors who were diagnosed before January 1, 1925, the beginning date for follow-up was set to January 1, 1925. To compare our results with those of a British study (7) of retinoblastoma survivors aged 25–84 years, we categorized attained age a priori by using 25 years as a cut point (1–24 and ≥25 years). Comparisons of SMRs were based on the chi-square test of homogeneity (19). To measure the overall burden from each cause of death, we calculated the absolute excess risk as the observed minus the expected number of deaths, divided by the person-years at risk and multiplied by 10 000.
Internal Comparisons.
To simultaneously examine the impact of multiple factors on mortality from subsequent malignant neoplasms, we also estimated the relative rates (RRs) of each factor by fitting multivariable Poisson regression models and using the logarithm of the observed person-years as offsets. The main explanatory variables of interest included hereditary status (hereditary or nonhereditary retinoblastoma), radiotherapy for retinoblastoma (yes or no), attained age (1–24 or ≥25 years), time since retinoblastoma diagnosis [1–9, 10–19, 20–29, 30–39, or ≥40 years, as per our previous mortality analysis (6)], age at retinoblastoma diagnosis (≤12 or >12 months), and year of retinoblastoma diagnosis (1914–1959 or 1960 or later). The selection of the cut point for age at retinoblastoma diagnosis was based on the suggestion by some investigators of an age-related sensitivity to radiation among retinoblastoma survivors who were irradiated before 12 months of age (14). The cut point for year of retinoblastoma diagnosis reflects the timing of major changes in radiotherapy techniques at the collaborating medical centers. The interaction between hereditary status and radiotherapy on subsequent malignant neoplasm mortality was tested by creating a product term. In each Poisson regression model, the statistical significance of each factor was assessed by the likelihood ratio test.
Cumulative Mortality From subsequent malignant neoplasms.
Cause-specific cumulative mortality from subsequent malignant neoplasms was calculated by treating other causes of death as competing risks because deaths from other causes preclude the occurrence of deaths from a specific cause of interest (20). Cumulative mortality was calculated by using the cmprsk package in R statistical software (21).
All P values are two-sided. Statistical significance was defined as P ≤ .05.
Results
Descriptive characteristics of the retinoblastoma survivors are shown in Table 1. The median age of the combined cohort at the end of follow-up was 30 years (range = 1–79 years). At the end of follow-up, 720 (65.9%) hereditary retinoblastoma survivors and 660 (86.6%) nonhereditary retinoblastoma survivors were alive, 346 (31.7%) hereditary retinoblastoma survivors and 77 (10.1%) nonhereditary retinoblastoma survivors were deceased, and 26 (2.4%) hereditary retinoblastoma survivors and 25 (3.3%) nonhereditary retinoblastoma survivors were lost to follow-up (Table 1). The median duration of follow-up since retinoblastoma diagnosis was 28.5 years (range = 1–69 years) for hereditary survivors and 29.6 years (range = 1–77 years) for nonhereditary retinoblastoma survivors. Radiotherapy—either alone or in combination with chemotherapy—was used to treat 87.5% of hereditary retinoblastoma survivors and 17.7% of nonhereditary retinoblastoma survivors (Table 1). The majority (75.2%) of the nonhereditary retinoblastoma survivors were treated with surgery only. The cause of death could not be determined for 16 deceased survivors, all of whom were from cohort 1. Among the 407 retinoblastoma survivors whose cause of death was known, 186 (45.7%) died from retinoblastoma.
Table 1.
Selected characteristics of 1-year survivors of retinoblastoma*
| Characteristic | Total, No. (%) | Hereditary, No. (%) | Nonhereditary, No. (%) |
| Total no. of subjects | 1854 (100) | 1092 (100) | 762 (100) |
| Laterality | |||
| Unilateral | 817 (44.1) | 55 (5.0) | 762 (100.0) |
| Bilateral | 1037 (55.9) | 1037 (95.0) | 0 (0.0) |
| Sex | |||
| Male | 956 (51.6) | 568 (52.0) | 388 (50.9) |
| Female | 898 (48.4) | 524 (48.0) | 374 (49.1) |
| Age at retinoblastoma diagnosis, y | |||
| <1 | 769 (41.5) | 612 (56.0) | 157 (20.6) |
| 1 | 519 (28.0) | 306 (28.0) | 213 (28.0) |
| 2 | 341 (18.4) | 126 (11.5) | 215 (28.2) |
| ≥3 | 225 (12.1) | 48 (4.4) | 177 (23.2) |
| Vital status | |||
| Alive | 1380 (74.4) | 720 (65.9) | 660 (86.6) |
| Deceased | 423 (22.8) | 346 (31.7) | 77 (10.1) |
| Lost to follow-up | 51 (2.8) | 26 (2.4) | 25 (3.3) |
| Calendar year of retinoblastoma diagnosis | |||
| 1914–1949 | 181 (9.8) | 106 (9.7) | 75 (9.8) |
| 1950–1959 | 301 (16.2) | 201 (18.4) | 100 (13.1) |
| 1960–1969 | 508 (27.4) | 311 (28.5) | 197 (25.9) |
| 1970–1979 | 448 (24.2) | 254 (23.3) | 194 (25.5) |
| 1980–1989 | 300 (16.2) | 171 (15.7) | 129 (16.9) |
| 1990–1996 | 116 (6.3) | 49 (4.5) | 67 (8.8) |
| Age at last observation, y | |||
| 1–9 | 258 (13.9) | 184 (16.9) | 74 (9.7) |
| 10–19 | 291 (15.7) | 188 (17.2) | 103 (13.5) |
| 20–29 | 355 (19.2) | 185 (16.9) | 170 (22.3) |
| 30–39 | 449 (24.2) | 278 (25.5) | 171 (22.4) |
| 40–79 | 501 (27.0) | 257 (23.5) | 244 (32.0) |
| Family history of retinoblastoma | |||
| Yes | 333 (18.0) | 333 (30.5) | 0 (0.0) |
| No | 1196 (64.5) | 575 (52.7) | 621 (81.5) |
| Uncertain | 325 (17.5) | 184 (16.9) | 141 (18.5) |
| Treatment | |||
| Surgery only | 681 (36.7) | 108 (9.9) | 573 (75.2) |
| Radiation only | 613 (33.1) | 534 (48.9) | 79 (10.4) |
| Radiation and chemotherapy | 462 (24.9) | 408 (37.4) | 54 (7.1) |
| Radiation and unknown chemotherapy | 16 (0.9) | 14 (1.3) | 2 (0.3) |
| Chemotherapy only | 64 (3.5) | 21 (1.9) | 43 (5.6) |
| Uncertain | 18 (1.0) | 7 (0.6) | 11 (1.4) |
| Any radiotherapy | |||
| Yes | 1091 (58.8) | 956 (87.5) | 135 (17.7) |
| No | 752 (40.6) | 132 (12.1) | 620 (81.4) |
| Uncertain | 11 (0.6) | 4 (0.4) | 7 (0.9) |
Percentages for some categories do not total 100 because of rounding.
In cohort 1, there were 393 deaths from a known cause (322 among hereditary retinoblastoma survivors and 71 among nonhereditary retinoblastoma survivors). During the 13 years since the last mortality report for this cohort (6), there were 70 additional deaths due to subsequent malignant neoplasms in cohort 1, of which 33 (47.1%) were cancers of the bone and connective tissue. We have previously reported five incident cases of pineoblastoma in our earlier incidence study of cohort 1 survivors (9). However, we noted that the cause of death of these pineoblastoma patients was attributable to other causes, including three deaths that were attributed to retinoblastoma, one to malignant brain tumor, and one to an external cause. In cohort 2, there were 14 deaths from a known cause (11 among hereditary retinoblastoma survivors and three among nonhereditary retinoblastoma survivors), including three deaths from bone cancers, eight from retinoblastoma, one from pineoblastoma, one from sequelae of chronic liver disease, and one from non–drug-induced interstitial lung disease. In the combined cohort, 151 deaths due to subsequent malignant neoplasms occurred among 1092 hereditary retinoblastoma survivors (SMR = 35, 95% CI = 30 to 41) compared with 12 such deaths among 762 nonhereditary retinoblastoma survivors (SMR = 2.5, 95% CI = 1.3 to 4.4) (Table 2). In hereditary retinoblastoma survivors, the most common subsequent malignant neoplasms included sarcomas of the bone and connective tissue, melanoma, and cancers of the brain and other parts of the nervous system (including four cancers of the sympathetic nervous system, one of which was an esthesioneuroblastoma in the ethmoid sinus and three were unspecified neuroblastoma). Among the hereditary retinoblastoma survivors, substantial elevations in SMRs (ie, SMRs > 10) were also observed for cancers of the lung, corpus uteri, nasal cavities, and buccal cavity and pharynx (due to nasopharyngeal cancer in two cases). Among the nonhereditary retinoblastoma survivors, statistically significantly elevated mortality from subsequent malignant neoplasms was found for deaths due to cancers of the breast (n = 3 deaths; SMR = 5.7, 95% CI = 1.2 to 17). In addition, among the nonhereditary retinoblastoma survivors, the SMR for subsequent malignant neoplasms was statistically significantly elevated for those who received radiotherapy (n = 6 deaths; SMR = 7.3, 95% CI = 2.7 to 15.8) but not for those who did not or whose radiotherapy status was uncertain (n = 6 deaths; SMR = 1.5, 95% CI = 0.6 to 3.3).
Table 2.
Causes of death other than retinoblastoma in 1-year survivors of retinoblastoma, by hereditary status*
| Cause of death† (ICD-8 code) | Hereditary |
Nonhereditary |
||||
| O | SMR (95% CI) | AER | O | SMR (95% CI) | AER | |
| Malignant and benign neoplasms other than retinoblastoma | 160 | 37 (31 to 43) | 55.1 | 12 | 2.5 (1.3 to 4.3) | 3.3 |
| Malignant neoplasms other than retinoblastoma‡ | 151 | 35 (30 to 41) | 51.9 | 12 | 2.5 (1.3 to 4.4) | 3.3 |
| Bone (170) | 56 | 595 (449 to 773) | 19.8 | 0 | 0 (0 to 49) | 0.0 |
| Connective tissue (171) | 31 | 329 (223 to 467) | 10.9 | 0 | 0 (0 to 44) | 0.0 |
| Melanoma (172) | 13 | 89 (47 to 151) | 4.5 | 0 | 0 (0 to 25) | −0.1 |
| Brain and other parts of nervous system (191–192) | 10 | 25 (12 to 46) | 3.4 | 2 | 5.9 (0.7 to 21) | 0.8 |
| Brain (191) | 6 | 18 (6.5 to 39) | 2.0 | 2 | 6.7 (0.8 to 24) | 0.8 |
| Other parts of nervous system (192)§ | 4 | 61 (16 to 155) | 1.4 | 0 | 0 (0 to 80) | 0.0 |
| Lung and trachea (162) | 8 | 12 (5.3 to 24) | 2.6 | 0 | 0 (0 to 3.8) | −0.5 |
| Corpus uteri (182)‖ | 5 | 154 (50 to 359) | 1.8 | 1 | 24 (0.3 to 134) | 0.4 |
| Nasal cavities (160) | 5 | 790 (254 to 1843) | 1.8 | 0 | 0 (0 to 570) | 0.0 |
| Breast (174) | 2 | 4.4 (0.5 to 16) | 0.5 | 3 | 5.7 (1.2 to 17) | 1.1 |
| Buccal cavity and pharynx (140–149)¶ | 2 | 34 (3.8 to 123) | 0.7 | 0 | 0 (0 to 51) | −0.03 |
| Leukemia (204–207)# | 1 | 1.7 (0.02 to 9.5) | 0.1 | 1 | 2.2 (0.03 to 12) | 0.2 |
| Thyroid (193) | 0 | 0 (0 to 436) | 0.0 | 1 | 102 (1.3 to 568) | 0.5 |
| Bladder (188) | 1 | 40 (0.5 to 224) | 0.3 | 0 | 0 (0 to 90) | 0.0 |
| Benign neoplasms (210–239)** | 9 | 72 (33 to 137) | 3.1 | 0 | 0 (0 to 33) | −0.1 |
| Other known causes of death | 19 | 0.9 (0.5 to 1.4) | −0.9 | 20 | 1.0 (0.6 to 1.6) | 0.2 |
| Infections (000–139) | 1 | 0.7 (0.01 to 3.9) | −0.1 | 2 | 1.7 (0.2 to 6.0) | 0.4 |
| Endocrine and metabolic diseases (240–279) | 1 | 1.5 (0.02 to 8.4) | 0.1 | 1 | 1.5 (0.02 to 8.3) | 0.2 |
| Mental disorders (290–315) | 0 | 0 (0 to 11) | −0.1 | 3 | 9.7 (2.0 to 28) | 1.2 |
| Neurological diseases (320–389) | 2 | 2.9 (0.3 to 10) | 0.5 | 0 | 0 (0 to 6.0) | −0.3 |
| Circulatory diseases (390–458) | 4 | 1.3 (0.3 to 3.2) | 0.3 | 3 | 0.7 (0.2 to 2.2) | −0.5 |
| Arteriosclerotic heart disease (410–414) | 3 | 2.0 (0.4 to 5.7) | 0.5 | 1 | 0.5 (0.01 to 2.6) | −0.5 |
| Cerebrovascular accidents (430–438) | 1 | 2.1 (0.03 to 11) | 0.2 | 0 | 0 (0 to 6.5) | −0.3 |
| Respiratory diseases (460–519) | 2 | 2.3 (0.3 to 8.2) | 0.4 | 0 | 0 (0 to 3.7) | −0.5 |
| Digestive system diseases (520–579) | 3 | 3.3 (0.7 to 9.5) | 0.7 | 2 | 2.1 (0.2 to 7.6) | 0.5 |
| External causes (800–998) | 6 | 0.5 (0.2 to 1.0) | 2.4 | 9 | 0.9 (0.4 to 1.7) | 0.5 |
| Ill-defined conditions (796) | 6 | 13 (4.7 to 28) | 2.0 | 4 | 9.9 (2.7 to 25) | 1.7 |
Number of persons followed up = 1092 for hereditary and 762 for nonhereditary; person-years of follow-up = 28 250 for hereditary and 21 674 for nonhereditary. List of malignant neoplasms is based on observed numbers of deaths in the combined cohort (in descending order). ICD-8 = International Classification of Diseases, 8th revision; O = observed number of deaths; SMR = standardized mortality ratio; CI = confidence interval; AER = absolute excess risk of death per 10 000 person-years.
Death certificate was not available for 13 hereditary retinoblastoma survivors and three nonhereditary retinoblastoma survivors.
Cancer sites not listed for hereditary retinoblastoma survivors include two of retroperitoneal tissue (ICD-8 158.0); one each of colon (ICD-8 153.0), nonmelanoma skin cancer of the scalp and neck (ICD-8 173.4), nonmelanoma skin cancer, site unspecified (ICD-8 173.9), ovary (ICD-8 183.0), kidney (ICD-8 189.0), pineal gland (ICD-8 194.4), lymphoid tissue (ICD-8 202.2), and abdomen, ill-defined (ICD-8 195.0), and seven of cancer, not otherwise specified (ICD-8 199). Cancer sites not listed for nonhereditary retinoblastoma survivors include four of cancer, not otherwise specified (ICD-8 199).
Includes four neuroblastoma (ICD-8 192.5).
Includes three leiomyosarcomas, one carcinoma, and one Müllerian mixed tumor among hereditary retinoblastoma survivors. No additional information was available for the nonhereditary retinoblastoma survivor who died of cancer of the corpus uteri.
Both nasopharynx (ICD-8 147).
Includes one acute lymphoid leukemia (ICD-8 204.0) in a hereditary retinoblastoma survivor and one acute myeloid leukemia (ICD-8 205.0) in a nonhereditary retinoblastoma survivor.
Benign tumors included one meningioma of the spine (ICD-8 225.4), one benign neoplasm of the pituitary gland and craniopharyngeal duct (ICD-8 226.2), and seven brain neoplasms with unspecified nature (ICD-8 238.1).
We found an apparent excess mortality from benign tumors in hereditary retinoblastoma survivors (n = 9; SMR = 72, 95% CI = 33 to 137); these deaths included one from spinal meningioma (ICD-8 225.4), one from benign neoplasm of the pituitary gland and caniopharyngeal duct (ICD-8 226.2), and seven from unspecified brain tumors (ICD-8 238.1).
Compared with the general population, neither hereditary nor nonhereditary retinoblastoma survivors experienced elevated mortality from non-neoplastic causes (Table 2). The only exception was an excess mortality from mental disorders among three nonhereditary retinoblastoma survivors (SMR = 9.7, 95% CI = 2.0 to 28), including one death from alcohol addiction and two from drug dependence (Table 2).
Among hereditary retinoblastoma survivors, mortality from subsequent malignant neoplasms was statistically significantly elevated compared with that in the general population in all time intervals investigated (Table 3). However, among hereditary retinoblastoma survivors, compared with those younger than 25 years, those aged 25 years or older had a statistically significantly lower SMR for subsequent malignant neoplasms but a higher absolute excess risk of death (Table 3). The extended follow-up revealed that excess mortality from sarcomas, melanoma, and cancers of the brain and other parts of the nervous system among the hereditary retinoblastoma survivors extended beyond 40 years after retinoblastoma diagnosis. Among the hereditary retinoblastoma survivors, cancers of the bone and connective tissue accounted for 76.5% of all deaths from subsequent malignant neoplasms before an attained age of 25 years (48 deaths from bone cancer, 14 deaths from connective tissue cancer), whereas they accounted for 35.7% of all deaths from subsequent malignant neoplasms at age 25 years or older (eight deaths from bone, 17 deaths from connective tissue cancer). At age 25 years or older, excess mortality from cancers of the following sites began to emerge: lung (n = 7 deaths; SMR = 11, 95% CI = 4.3 to 22), corpus uteri (n = 5 deaths, including three from leiomyosarcoma, one from carcinoma, and one from mixed Müllerian tumor; SMR = 162, 95% CI = 52 to 378), and digestive organs and peritoneum (n = 3 deaths, including one from colon cancer and two from retroperitoneal tissue cancer; SMR = 5.2, 95% CI = 1.04 to 15). The majority of deaths due to melanoma occurred at age 25 years or older (n = 10 deaths; SMR = 75, 95% CI = 36 to 139), although the SMR was also elevated among those younger than 25 years at death (n = 3; SMR = 212, 95% CI = 43 to 618).
Table 3.
Mortality from subsequent malignant neoplasms in 1-year survivors of retinoblastoma, by selected characteristics*
| Characteristic | Hereditary |
Nonhereditary |
||||
| O | SMR (95% CI) | AER | O | SMR (95% CI) | AER | |
| Age at retinoblastoma diagnosis, mo | ||||||
| 0–12 | 95 | 51 (41 to 62) | 58.6 | 3 | 3.8 (0.8 to 11) | 4.5 |
| >12 | 56 | 23 (18 to 30) | 43.4 | 9 | 2.3 (1.0 to 4.3) | 3.0 |
| Calendar year of retinoblastoma diagnosis | ||||||
| 1914–1959 | 73 | 25 (19 to 31) | 73.0 | 9 | 2.5 (1.1 to 4.8) | 7.7 |
| 1960 or later | 78 | 59 (46 to 73) | 41.1 | 3 | 2.5 (0.5 to 7.3) | 1.2 |
| Latency, y | ||||||
| 1–9 | 19 | 38 (23 to 59) | 21.2 | 1 | 3.0 (0.04 to 17) | 1.1 |
| 10–19 | 52 | 132 (98 to 173) | 64.3 | 3 | 9.4 (1.9 to 28) | 4.4 |
| 20–29 | 25 | 49 (32 to 73) | 40.4 | 2 | 4.4 (0.5 to 16) | 3.3 |
| 30–39 | 30 | 36 (24 to 51) | 78.6 | 0 | 0 (0 to 4.7) | −2.8 |
| ≥40 | 25 | 12 (8.0 to 18) | 132.8 | 6 | 2.1 (0.8 to 4.5) | 18.2 |
| Attained age, y | ||||||
| 1–24 | 81 | 76 (61 to 95) | 41.5 | 4 | 5.4 (1.5 to 14) | 2.4 |
| ≥25 | 70 | 22 (17 to 28) | 74.3 | 8 | 2.0 (0.9 to 3.9) | 5.0 |
O = observed number of deaths; SMR = standardized mortality ratio; CI = confidence interval; AER = absolute excess risk per 10 000 person-years.
Among hereditary retinoblastoma survivors, those who were irradiated had an SMR for all malignant neoplasms other than retinoblastoma that was 3.4 times that of nonirradiated survivors (Table 4). Among the hereditary retinoblastoma survivors who died of a subsequent malignant neoplasm, the median age at death among the 140 irradiated survivors was less (20.5 years, range = 1–67 years) than the median age at death for the 11 nonirradiated survivors (44 years, range = 10–64 years). With the exception of lung cancer, SMRs for specific sites were consistently higher among hereditary retinoblastoma survivors who were irradiated than among those who were not (Table 4). The highest SMRs (ie, SMR > 100) were observed for cancers in heavily irradiated organs (≥1 Gy) near or in the radiation treatment field, with the exception of cancer of the corpus uteri, a lightly irradiated organ (<0.4 Gy).
Table 4.
Mortality from subsequent malignant neoplasms in 1-year survivors of hereditary retinoblastoma, by radiotherapy status*
| Cancer site (ICD-8 code) by radiation dose category | Radiotherapy |
No or uncertain radiotherapy |
||
| O | SMR (95% CI) | O | SMR (95% CI) | |
| Heavily irradiated sites (≥1 Gy) | ||||
| Bone (170)† | 54 | 673 (506 to 879) | 2 | 143 (16 to 519) |
| Connective tissue (171)‡ | 31 | 395 (268 to 560) | 0 | 0 (0 to 234) |
| Brain and other parts of nervous system (191–192) | 10 | 30 (14 to 55) | 0 | 0 (0 to 55) |
| Nasal cavities (160) | 5 | 981 (316 to 2290) | 0 | 0 (0 to 2963) |
| Buccal cavity and pharynx (140–149) | 2 | 44 (4.9 to 158) | 0 | 0 (0 to 282) |
| Moderately irradiated sites (0.4–1.0 Gy) | ||||
| Cutaneous melanoma (172) | 11 | 94 (47 to 168) | 2 | 68 (7.6 to 245) |
| Lung and trachea (162) | 4 | 8.2 (2.2 to 21) | 4 | 24 (6.4 to 61) |
| Breast (174) | 2 | 5.8 (0.7 to 21) | 0 | 0 (0 to 32) |
| Leukemia (204–207) | 1 | 2.0 (0.03 to 11) | 0 | 0 (0 to 42) |
| Lightly irradiated sites (<0.4 Gy) | ||||
| Corpus uteri (182)§ | 4 | 164 (44 to 421) | 1 | 122 (1.6 to 676) |
| Large intestine (153) | 1 | 6.2 (0.08 to 35) | 0 | 0 (0 to 75) |
| Bladder (188) | 1 | 53 (0.7 to 297) | 0 | 0 (0 to 602) |
| All malignant neoplasms other than retinoblastoma‖ | 140 | 41 (35 to 49) | 11 | 12 (6.1 to 22) |
| Absolute excess risk per 10 000 person-years | 56.6 | 24.6 | ||
Number of persons followed up = 956 for radiotherapy and 136 for no or uncertain radiotherapy; person-years of follow-up = 24 146 for radiotherapy and 4104 for no or uncertain radiotherapy. List of malignant neoplasms under each radiation dose category is based on observed numbers of deaths in the combined cohort (in descending order). ICD-8 = International Classification of Diseases, 8th revision; O = observed number of deaths; SMR = standardized mortality ratio; CI = confidence interval.
Locations of bone cancers in irradiated survivors include 17 of the skull and face (ICD-8 170.0), four of the long bones of lower limb (ICD-8 170.7), and 33 of unspecified site (ICD-8 170.9). Locations of bone cancers in nonirradiated survivors include one of the ribs (ICD-8 170.3) and one of unspecified site (ICD-8 170.9).
Locations of connective tissue cancers in irradiated survivors include five of the head, face, and neck (ICD-8 171.0), and 26 of unspecified site (ICD-8 171.9).
Includes three leiomyosarcomas, one carcinoma, and one Müllerian mixed tumor.
Cancer sites not listed for irradiated survivors include two each of retroperitoneal tissue (ICD-8 158.0), and nonmelanoma skin cancer (ICD-8 173.4, 173.9); one each of ovary (ICD-8 183.0), kidney (ICD-8 189.0), pineal gland (ICD-8 194.4), and abdomen, ill-defined (ICD-8 195.0), and six of cancer, not otherwise specified (ICD-8 199). Cancer sites not listed for nonirradiated survivors include one each of lymphoid tissue (ICD-8 202.2), and cancer, not otherwise specified (ICD-8 199).
Among irradiated hereditary retinoblastoma survivors, those who were aged 12 months or younger at irradiation were 2.2 times more likely to die of subsequent malignant neoplasms than those who were older than 12 months at irradiation (mean = 23.15 months, range = 12.01–102 months) (SMR = 59 [95% CI = 48 to 73] vs 27 [95% CI = 20 to 35]; P < .001). The absolute excess risk for subsequent malignant neoplasm mortality was 1.3 times higher among survivors irradiated at age 12 months or younger than among those older than 12 months at irradiation (62.9 per 10 000 person-years vs 47.5 per 10 000 person-years).
Among survivors with hereditary retinoblastoma who were irradiated, there was no statistically significant difference by sex in the SMRs for all subsequent malignant neoplasms combined (Table 5). An analysis of mortality by years since retinoblastoma diagnosis revealed that females generally had higher SMRs and absolute excess risks from all subsequent malignant neoplasms combined than males up to 39 years after retinoblastoma diagnosis. At 40 years or more of follow-up, both the SMR and the absolute excess risk for subsequent malignant neoplasms were higher in males than in females, but the differences were not statistically significant. For all latency intervals combined, females had statistically significantly higher SMRs compared with males for cancers of certain heavily irradiated sites such as the brain and other parts of the nervous system and the buccal cavity and pharynx (Table 5). In addition, females had higher SMRs than males for melanoma (n = 7, SMR = 158 [95% CI = 63 to 325] vs n = 4, SMR = 55 [95% CI = 15 to 140]; P = .07) and for cancer of the nasal cavities (n = 4, SMR = 2012 [95% CI = 542 to 5153] vs n = 1, SMR = 322 [95% CI = 4.2 to 1790]; P = .07), but those differences were not statistically significant.
Table 5.
Mortality from subsequent malignant neoplasms following radiotherapy for hereditary retinoblastoma, by sex*
| Cancer site | Males |
Females |
P† | ||||
| O | SMR (95% CI) | AER | O | SMR (95% CI) | AER | ||
| All subsequent malignant neoplasms combined by time since retinoblastoma diagnosis | |||||||
| 1–9 y | 7 | 27 (11 to 55) | 16.9 | 12 | 67 (34 to 117) | 32.7 | .048 |
| 10–19 y | 26 | 124 (81 to 181) | 68.7 | 25 | 197 (127 to 291) | 78.6 | .095 |
| 20–29 y | 10 | 40 (19 to 74) | 34.5 | 12 | 67 (35 to 117) | 50.1 | .226 |
| 30–39 y | 9 | 27 (12 to 51) | 51.0 | 20 | 56 (34 to 86) | 138.5 | .060 |
| ≥40 y | 12 | 19 (9.6 to 32) | 173.0 | 7 | 8.5 (3.4 to 17) | 94.9 | .092 |
| All intervals | 64 | 38 (29 to 48) | 48.2 | 76 | 46 (36 to 57) | 66.3 | .254 |
| Subsequent malignant neoplasms at heavily irradiated sites for all latency intervals combined‡ | |||||||
| Bone (170)§ | 34 | 684 (473 to 956) | 26.2 | 20 | 656 (400 to 1013) | 17.8 | .852 |
| Connective tissue (171)‖ | 15 | 333 (186 to 549) | 11.6 | 16 | 478 (273 to 777) | 14.2 | .294 |
| Brain and other parts of nervous system (191–192) | 1 | 5.1 (0.07 to 28) | 0.6 | 9 | 67 (30 to 127) | 7.9 | .001 |
| Nasal cavities (160) | 1 | 322 (4.2 to 1790) | 0.8 | 4 | 2012 (542 to 5153) | 3.6 | .068 |
| Buccal cavity and pharynx (140–149) | 0 | 0 (0 to 115) | −0.02 | 2 | 146 (16 to 526) | 1.8 | .033 |
O = observed number of deaths; SMR = standardized mortality ratio; CI = confidence interval; AER = absolute excess risk per 10 000 person-years; ICD-8 = International Classification of Diseases, 8th revision.
P value from chi--square test of homogeneity comparing two SMRs (two-sided).
Values in parentheses indicate cause of death according to International Classification of Diseases, 8th revision.
Locations of bone cancers in male irradiated hereditary survivors include nine of the skull and face (ICD-8 170.0), one of the long bones of lower limb (ICD-8 170.7), and 24 of unspecified site (ICD-8 170.9). Locations of bone cancers in female irradiated hereditary patients include eight of the skull and face (ICD-8 170.0), three of the long bones of lower limb (ICD-8 170.7), and nine of unspecified site (ICD-8 170.9).
Locations of connective tissue cancers in male irradiated hereditary survivors include five of the head, face, and neck (ICD-8 171.0) and 10 of unspecified site (ICD-8 171.9). Locations of connective tissue cancers in female irradiated hereditary survivors include 16 of unspecified site (ICD-8 171.9).
Radiotherapy for retinoblastoma was associated with increased subsequent malignant neoplasm mortality for both hereditary and nonhereditary retinoblastoma survivors (Table 6). The lower relative rate of death associated with radiation among hereditary survivors (RR = 2.46, 95% CI = 1.39 to 4.84) than among nonhereditary survivors (RR = 7.19, 95% CI = 2.21 to 23.37) may reflect a higher background rate for subsequent malignant neoplasms among hereditary survivors due to their genetic susceptibility to subsequent malignant neoplasms (5–7,9,10). Among hereditary retinoblastoma survivors, other statistically significant risk factors for subsequent malignant neoplasm mortality included female sex, retinoblastoma diagnosis before 1960, and longer latency since retinoblastoma diagnosis (Table 6). A retinoblastoma diagnosis at age 12 months or younger marginally increased the risk of subsequent malignant neoplasm mortality but not statistically significantly (Table 6).
Table 6.
Multivariable Poisson regression model of relative rate of mortality from subsequent malignant neoplasms in 1-year survivors of retinoblastoma, by hereditary status*
| Characteristic | Hereditary |
Nonhereditary |
||||
| O | RR (95% CI) | P† | O | RR (95% CI) | P† | |
| Radiation | .001 | .002 | ||||
| No or uncertain | 11 | 1.0 (referent) | 6 | 1.0 (referent) | ||
| Yes | 140 | 2.46 (1.39 to 4.84) | 6 | 7.19 (2.21 to 23.37) | ||
| Sex | .03 | .42 | ||||
| Male | 67 | 1.0 (referent) | 4 | 1.0 (referent) | ||
| Female | 84 | 1.41 (1.03 to 1.95) | 8 | 1.63 (0.51 to 6.21) | ||
| Age at retinoblastoma diagnosis, mo | .06 | .63 | ||||
| 0–12 | 95 | 1.37 (0.99 to 1.93) | 3 | 1.40 (0.31 to 4.79) | ||
| >12 | 56 | 1.0 (referent) | 9 | 1.0 (referent) | ||
| Calendar year of retinoblastoma diagnosis | .05 | .10 | ||||
| 1914–1959 | 78 | 1.44 (1.00 to 2.05) | 9 | 3.85 (0.77 to 21.02) | ||
| 1960 or later | 73 | 1.0 (referent) | 3 | 1.0 (referent) | ||
| Latency, y | <.001 | .05 | ||||
| 1–9 | 19 | 1.0 (referent) | 1 | 1.0 (referent) | ||
| 10–19 | 52 | 3.40 (2.05 to 5.90) | 3 | 3.28 (0.41 to 66.62) | ||
| 20–29 | 25 | 2.01 (0.91 to 4.22) | 2 | 0.94 (0.02 to 40.50) | ||
| 30–39 | 30 | 3.59 (1.33 to 9.56) | 0 | NA | ||
| ≥40 | 25 | 5.82 (2.09 to 15.95) | 6 | 2.79 (0.07 to 170.38) | ||
| Attained age, y | .76 | .37 | ||||
| 1–24 | 81 | 1.0 (referent) | 4 | 1.0 (referent) | ||
| ≥25 | 70 | 1.13 (0.52 to 2.56) | 8 | 4.16 (0.22 to 74.39) | ||
O = observed number of deaths; RR = relative rate; CI = confidence interval; NA = not applicable.
P value from likelihood ratio test (two-sided).
To evaluate the interaction between hereditary status and treatment with radiotherapy, a multivariable Poisson regression model was fitted for hereditary and nonhereditary survivors combined. With nonirradiated nonhereditary retinoblastoma survivors as the referent group, the relative rates of mortality from subsequent malignant neoplasms were 7.12 (95% CI = 2.70 to 20.7), 7.20 (95% CI = 2.25 to 23.0), and 17.9 (95% CI = 8.55 to 45.8) for nonirradiated hereditary retinoblastoma survivors, irradiated nonhereditary retinoblastoma survivors, and irradiated hereditary retinoblastoma survivors, respectively. However, the interaction term did not reach statistical significance (P = .12, likelihood ratio test).
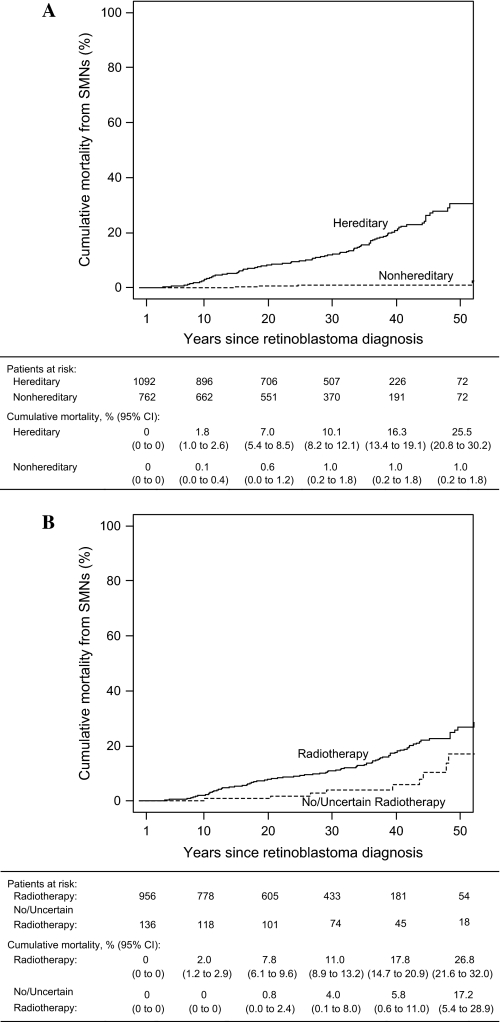
The cumulative mortality from subsequent malignant neoplasms at 50 years after retinoblastoma diagnosis after adjusting for competing risks of death from other causes was 25.5% (95% CI = 20.8% to 30.2%) for hereditary retinoblastoma survivors and 1.0% (95% CI = 0.2% to 1.8%) for nonhereditary retinoblastoma survivors (Figure 1, A). Among hereditary retinoblastoma survivors, the cumulative mortality from subsequent malignant neoplasms at 50 years after retinoblastoma diagnosis was 26.8% (95% CI = 21.6% to 32.0%) for those who received radiotherapy and 17.2% (95% CI = 5.4% to 28.9%) for those who did not (Figure 1, B).
Figure 1.
Cumulative mortality due to SMNs following diagnosis of retinoblastoma. A) Cumulative mortality by hereditary status. B) Cumulative mortality among hereditary retinoblastoma survivors by radiotherapy status. SMN = subsequent malignant neoplasm; CI = confidence interval.
Discussion
To our knowledge, this is the largest mortality study to date among survivors of retinoblastoma (1092 hereditary retinoblastoma survivors and 762 nonhereditary retinoblastoma survivors), with the longest follow-up for irradiated retinoblastoma survivors. Among hereditary retinoblastoma survivors, the high mortality risks persisted for cancers of the bone, connective tissue, and brain and other parts of the nervous system, and for melanoma (Table 2). The additional 13 years of follow-up since our last mortality study revealed a previously unreported increased risk of death due to cancer of the corpus uteri (primarily sarcomas), and confirmed the elevated lung cancer risk that we previously reported (8). This study also revealed that after 50 years of follow-up, hereditary retinoblastoma survivors, most of whom were treated with radiotherapy, had a 25.5% cumulative risk of dying from a subsequent malignant neoplasm compared with a 1.0% cumulative risk for nonhereditary retinoblastoma survivors, most of whom were treated surgically.
We have previously demonstrated a radiation dose–response effect for soft tissue and bone sarcomas among hereditary retinoblastoma survivors (11). In this analysis, many of the bone and some of the connective tissue sarcomas developed in irradiated sites in a manner that suggested an interactive effect between genetic susceptibility and radiation exposure. However, we did not detect a statistically significant interaction between hereditary status and treatment with radiotherapy. Such an interaction is difficult to detect when the proportions of survivors treated with radiotherapy differ so greatly by hereditary status, as was the case in our cohort (Table 1). The other difficulty in detecting such an interaction is because of the very small numbers of cancers that resulted in death among the nonhereditary survivors, only 12 overall and only six among those treated with radiotherapy.
The proportion of deaths due to epithelial cancers in this retinoblastoma cohort differed from that in a British cohort that consisted largely of nonirradiated survivors aged 25–84 years (144 hereditary and 307 nonhereditary) (7). The British study reported excess mortality from cancers of the lung, bladder, and other epithelial sites among the hereditary retinoblastoma survivors. We also noted an excess of lung cancer deaths, which was unrelated to radiotherapy and possibly due to increased susceptibility to cigarette smoking (8), but no excess of bladder cancer was seen. The difference in the findings related to bladder cancer in the two cohorts may be related to the older age distribution in the British cohort (median ages of hereditary and nonhereditary retinoblastoma survivors: 61 and 60 years, respectively) compared with that in our cohort (median ages of hereditary and nonhereditary retinoblastoma survivors: 29 and 32 years, respectively). However, the SMR for all subsequent malignant neoplasms for hereditary retinoblastoma survivors older than 25 years in our cohort (SMR = 22, 95% CI = 17 to 28) (Table 3) greatly exceeded that for hereditary retinoblastoma survivors older than 25 years in the British cohort (SMR = 5.4, 95% CI = 3.9 to 7.3). An updated analysis on incidence of subsequent malignant neoplasms from the Dutch retinoblastoma registry of 668 retinoblastoma survivors was published (10) after we completed our analysis. In that study, Marees et al. (10) reported statistically significantly elevated incidence from both cancers of the lung and bladder among their 298 hereditary retinoblastoma survivors compared with the general population (standardized incidence ratio [SIR] = 16.8, 95% CI = 3.47 to 49.2, and SIR = 124, 95% CI = 34.0 to 319, respectively) but not in the 370 nonhereditary retinoblastoma survivors.
Among irradiated hereditary retinoblastoma survivors, the SMR for subsequent malignant neoplasms was 2.2 times higher for those who were irradiated at 12 months of age or younger than for those irradiated at older ages. The reason for the increased susceptibility to subsequent malignant neoplasms among survivors diagnosed at very young ages is unclear but may be due to age-related sensitivity to radiation (14), particularly because dosimetry data from cohort 1 suggest that the average organ doses were comparable in the two age groups (M. Stovall, PhD, unpublished observations). By contrast, some investigators suggest that the reason for the increased risk for subsequent malignant neoplasms among those irradiated before 12 months compared with those irradiated at older ages is not due to age-related sensitivity; rather, irradiation before 12 months may be a marker of other risk factors for subsequent malignant neoplasms (15).
In our previous mortality analysis of cohort 1, we reported a statistically significantly higher SMR for subsequent malignant neoplasms among female survivors than among male survivors (6), although a subsequent analysis of cancer incidence did not observe such a difference in SIRs by sex (11). In this study, we found that among hereditary retinoblastoma survivors who were treated with radiotherapy, females had a strikingly elevated SMR compared with males for cancers of the brain and other parts of the nervous system. High-dose radiotherapy in childhood is an established risk factor for brain tumors (22); however, the reason for this statistically significant difference in SMRs by sex in our cohort is unclear. In a cohort of 20 483 5-year survivors of common childhood cancers, Mertens et al. (23) reported that females had a nonstatistically significantly higher SMR for subsequent malignant neoplasms than males.
Among the nonhereditary retinoblastoma survivors, we observed a statistically significant increase in overall subsequent malignant neoplasm mortality compared with the general population. Excess mortality from subsequent malignant neoplasms among nonhereditary retinoblastoma survivors was also reported by Acquaviva et al. (5) (four observed deaths compared with 0.97 expected). Fletcher et al. (7) did not find excess mortality for subsequent malignant neoplasms among all nonhereditary retinoblastoma survivors (29 observed death compared with 23.59 expected), although the authors did report a small excess mortality among nonhereditary retinoblastoma survivors younger than 45 years (six observed deaths compared with 1.90 expected). Radiotherapy may explain part of the elevated SMR for subsequent malignant neoplasms among nonhereditary retinoblastoma survivors in our cohort in cancers sites that are sensitive to radiation, such as thyroid and breast. Alternatively, the excess mortality may also reflect some potential misclassification in hereditary status resulting from our study definition, which classified all unilateral retinoblastoma patients without a family history as nonhereditary retinoblastoma patients. A small percentage of unilateral retinoblastoma survivors without a family history of retinoblastoma may have had a germline mutation in RB1 (24). Therefore, there could be some misclassification using our definition of nonhereditary retinoblastoma, although the extent of misclassification is not likely to be large.
It should be noted that deaths from non-neoplastic causes were generally not increased compared with the general population. For example, there was no increase in mortality from circulatory diseases among retinoblastoma survivors, which is in contrast to the mortality increases observed for atomic bomb survivors (25) and survivors who were irradiated for other childhood cancers (26–30). However, at the end of follow-up, only 179 (9.4%) of the survivors in the combined cohort were older than 50 years, the age at which mortality from circulatory diseases rises sharply in the general population (31). In addition, the radiation dose to the heart in this cohort was estimated to be about 0.4 Gy (9), which is much lower than the organ doses reported in other childhood cancer survivor studies (26–30). A longer follow-up of our study population will clarify the risks of death from circulatory diseases among retinoblastoma survivors who received low-scatter radiation doses to the heart at very young ages.
Our study has some limitations. First, information on cause of death listed on death certificates may have been inaccurate in some cases. In particular, a search of hospital and/or pathology records that were available for seven of the nine hereditary retinoblastoma survivors whose cause of death was coded to benign tumors revealed that four actually had soft tissue sarcomas of the cranial or facial area, one had malignant glioma, and another had sebaceous carcinoma of the eyelid; the remaining patient had meningioma of the cervical spine. Second, although to our knowledge this is the largest retinoblastoma cohort with long-term follow-up, a sizable proportion of the survivors are still younger than 50 years, when the incidence of epithelial cancers and circulatory diseases starts to rise sharply in the general population. Therefore, the numbers of these outcomes may not be large enough to be evaluated reliably.
In conclusion, our extended follow-up of retinoblastoma survivors found no evidence that retinoblastoma survivors are at greater risk of death from non-neoplastic causes compared with the general population. However, excess mortality from subsequent malignant neoplasms among hereditary retinoblastoma survivors extends beyond 40 years after retinoblastoma diagnosis, especially among irradiated survivors. This study revealed a previously unreported increased risk of death from cancer of the corpus uteri (mainly sarcomas) in survivors older than 25 years and confirmed the increased risk of lung cancer among hereditary retinoblastoma survivors. The temporal patterns of site-specific excess risks of subsequent malignant neoplasms in retinoblastoma survivors should inform screening programs designed for the early detection and treatment of subsequent malignant neoplasms.
Funding
Intramural Research Program of the National Institutes of Health and the National Cancer Institute.
Footnotes
The authors take full responsibility for the study design, data collection, analysis and interpretation of data, the decision to submit the manuscript for publication, and the writing of the manuscript.
We thank Lisa Newman and Susan Wilson (RTI International) for field work support, and Henry Chen (IMS, Inc.) for computer support.
References
- 1.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2(12):910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 2.Goodrich DW. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene. 2006;25(38):5233–5243. doi: 10.1038/sj.onc.1209616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg RA. The Biology of Cancer. New York, NY: Garland Science; 2007. [Google Scholar]
- 5.Acquaviva A, Ciccolallo L, Rondelli R, et al. Mortality from second tumour among long-term survivors of retinoblastoma: a retrospective analysis of the Italian retinoblastoma registry. Oncogene. 2006;25(38):5350–5357. doi: 10.1038/sj.onc.1209786. [DOI] [PubMed] [Google Scholar]
- 6.Eng C, Li FP, Abramson DH, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85(14):1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher O, Easton D, Anderson K, et al. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96(5):357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 8.Kleinerman RA, Tarone RE, Abramson DH, et al. Hereditary retinoblastoma and risk of lung cancer. J Natl Cancer Inst. 2000;92(24):2037–2039. doi: 10.1093/jnci/92.24.2037. [DOI] [PubMed] [Google Scholar]
- 9.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Marees T, Moll AC, Imhof SM, et al. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100(24):1771–1779. doi: 10.1093/jnci/djn394. [DOI] [PubMed] [Google Scholar]
- 11.Wong FL, Boice JD, Jr, Abramson DH, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278(15):1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 12.Abramson DH, Melson MR, Dunkel IJ, Frank CM. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001;108(10):1868–1876. doi: 10.1016/s0161-6420(01)00713-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007;67(15):2173–2185. doi: 10.2165/00003495-200767150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105(4):573–579. doi: 10.1016/S0161-6420(98)94006-4. discussion 579–580. [DOI] [PubMed] [Google Scholar]
- 15.Moll AC, Imhof SM, Schouten-Van Meeteren AY, et al. Second primary tumors in hereditary retinoblastoma: a register-based study, 1945-1997: is there an age effect on radiation-related risk? Ophthalmology. 2001;108(6):1109–1114. doi: 10.1016/s0161-6420(01)00562-0. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz RL, Shields CL, Shields JA, et al. Retinoblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 825–846. [Google Scholar]
- 17.US Department of Health, Education, and Welfare, Public Health Service, National Center for Health Statistics. Eighth Revision of International Classification of Diseases, Adapted for use in the United States. Washington, DC:: US Government Printing Office; 1968. [Google Scholar]
- 18.Monson RR. Analysis of relative survival and proportional mortality. Comput Biomed Res. 1974;7(4):325–332. doi: 10.1016/0010-4809(74)90010-x. [DOI] [PubMed] [Google Scholar]
- 19.Breslow NE, Day NE. Statistical Methods in Cancer Research: Volume II—The Design and Analysis of Cohort studies. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16(3):1141–1154. [Google Scholar]
- 22.Boice JD., Jr . Ionizing radiation. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press; 2006. pp. 259–293. [Google Scholar]
- 23.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317–327. doi: 10.1177/107327480401100506. [DOI] [PubMed] [Google Scholar]
- 25.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160(4):381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 26.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 27.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin's disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 28.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol. 1993;11(7):1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 29.Heikens J, Ubbink MC, van der Pal HP, et al. Long term survivors of childhood brain cancer have an increased risk for cardiovascular disease. Cancer. 2000;88(9):2116–2121. [PubMed] [Google Scholar]
- 30.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]