Abstract
AIM: To compare the efficacy of pentoxifylline and prednisolone in the treatment of severe alcoholic hepatitis, and to evaluate the role of different liver function scores in predicting prognosis.
METHODS: Sixty-eight patients with severe alcoholic hepatitis (Maddrey score ≥ 32) received pentoxifylline (n = 34, group I) or prednisolone (n = 34, group II) for 28 d in a randomized double-blind controlled study, and subsequently in an open study (with a tapering dose of prednisolone) for a total of 3 mo, and were followed up over a period of 12 mo.
RESULTS: Twelve patients in group II died at the end of 3 mo in contrast to five patients in group I. The probability of dying at the end of 3 mo was higher in group II as compared to group I (35.29% vs 14.71%, P = 0.04; log rank test). Six patients in group II developed hepatorenal syndrome as compared to none in group I. Pentoxifylline was associated with a significantly lower model for end-stage liver disease (MELD) score at the end of 28 d of therapy (15.53 ± 3.63 vs 17.78 ± 4.56, P = 0.04). Higher baseline Maddrey score was associated with increased mortality.
CONCLUSION:Reduced mortality, improved risk-benefit profile and renoprotective effects of pentoxifylline compared with prednisolone suggest that pentoxifylline is superior to prednisolone for treatment of severe alcoholic hepatitis.
Keywords: Alcoholic hepatitis, Pentoxifylline, Prednisolone, Maddrey discriminant function score, Model for end-stage liver disease score, Glasgow alcoholic hepatitis score
INTRODUCTION
Severe alcoholic hepatitis is an acute or acute-on-chronic hepatic inflammatory response syndrome, which is part of the spectrum of diseases that result from alcohol-induced liver injury, ranging from the most common asymptomatic fatty liver to fulminant hepatitis and cirrhosis in the long term. However, it is difficult to predict the clinical response in an individual patient, as only a minority of individuals consuming large amounts of alcohol develop alcoholic hepatitis[1,2]. The importance of acute alcoholic hepatitis lies in its significant morbidity and mortality, with a reported in-hospital mortality as high as 44%[3]. Large amounts of alcohol with binge drinking, malnutrition, and female sex, are some of the factors associated with more severe disease[4]. The presence of coexisting hepatitis C has been found to be associated with worse prognosis[5]. Recent studies have shown that impaired immune response, endoplasmic reticulum stress, mitochondrial dysfunction, and free-radical injury induced by alcohol and its acetaldehyde adduct metabolites, Kupffer cell activation and cytokine production, have an important role in accentuating the hepatocyte injury and disease precipitation[6,7]. Serum level of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6 and IL-8 are elevated in acute alcoholic hepatitis[8]. Studies have shown a linear relationship between TNF-α receptors and mortality from acute alcoholic hepatitis[9].
Maddrey discriminant function (DF) has commonly been used in estimating mortality among patients with acute alcoholic hepatitis, with an elevated DF (> 32) indicating an increased likelihood of death, and conversely, a low DF suggesting a generally favorable prognosis[10,11]. Recently, model for end-stage liver disease (MELD) score and glasgow alcoholic hepatitis score (GAHS) have also gained interest as predictors of disease outcome in patients with severe acute alcoholic hepatitis[12,13].
Although prednisolone is used widely and considered the standard treatment for severe acute alcoholic hepatitis with DF score ≥ 32, it is not free of adverse effects and has had its share of controversies[14]. Recently, pentoxifylline, a non-specific phosphodiesterase inhibitor, with combined anti-inflammatory (TNF-α inhibition) and antifibrogenic properties, has been found to be useful in patients with acute alcoholic hepatitis with DF ≥ 32[15–17]. The beneficial effects are believed to occur through various mechanisms such as inhibition of phosphodiesterases, increased cAMP levels and down-regulation of TNF-α, IL-1, IL-6, transforming growth factor-beta (TGF-β), interferon-gamma (IFN-γ), stellate cell activation and procollagen-I mRNA expression[18].
Although many individual studies are available on the efficacy of pentoxifylline and prednisolone in the treatment of severe alcoholic hepatitis, as far as we are aware, no study has compared the two drugs head to head in a randomized controlled study.
The present study compared the efficacy of pentoxifylline and prednisolone in the management of severe alcoholic hepatitis (DF ≥ 32), and their immediate and short-term outcomes. Also, we evaluated the GAHS and MELD score in patients with severe alcoholic hepatitis and compared them to traditional scores like DF and Child’s score.
MATERIALS AND METHODS
One hundred and fifty-eight chronic alcoholic patients attending the liver clinic, outpatient department or the emergency medical services of the Medical College and Hospitals Calcutta were initially considered. The study was carried out from July 2006 to September 2008. The patients were initially examined clinically, evaluated, and subsequently were admitted for the duration of the study. The study protocol was approved by the institutional ethical committee. All the patients underwent investigations for liver chemistry (liver function tests, prothrombin time), complete hemogram, random blood sugar level, urea, creatinine, electrolytes, viral markers such as hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV) antibody, hepatitis A virus IgM, hepatitis E virus IgM, serum ceruloplasmin, 24-h urinary copper (as and when required), and antinuclear antibody, upper gastrointestinal endoscopy, and Doppler abdominal ultrasound, as and when required. Patients were included who had a history of chronic alcohol intake of more than 50 g/d[19] with clinical and biochemical features of severe alcoholic hepatitis [Maddrey DF ≥ 32 and aspartate aminotransferase: alanine aminotransferase (AST:ALT) > 2:1, with absolute values of AST < 500 IU/L and ALT < 200 IU/L]. Patients with any other potential etiology of liver injury (acute or chronic viral hepatitis, autoimmune liver disease, Wilson’s disease) even in the background of chronic alcohol intake were excluded from the study. Also, patients with a history of abstinence from alcohol in the last month, or who were positive for human immunodeficiency virus antibodies were excluded. Patients with infection, sepsis or spontaneous bacterial peritonitis, gastrointestinal bleeding, hepatorenal syndrome, acute pancreatitis or any other severe associated disease (uncontrolled diabetes, hypertension, heart failure, pulmonary disease or malignancy) at the time of inclusion or in the previous 3 mo were also excluded.
MELD score, GAHS and Child’s score were calculated for all the patients who were included in the study. Only those patients were considered for final study who gave a prior informed written consent for pharmacotherapy.
The included patients were then divided into two groups by a computer-generated randomization table: group I, patients receiving pentoxifylline, and group II, patients receiving prednisolone. The pharmacotherapy (pentoxifylline or prednisolone) was started within a week of admission.
Patients in group I received pentoxifylline (Trental tablets, Sanofi Aventis, Mumbai, India) at a dose of 400 mg thrice daily orally and a placebo tablet in the place of prednisolone for the first 4 wk. Patients in group II received prednisolone tablet (Wysolone, Wreath, Mumbai, India) at a dose of 40 mg once daily for 4 wk and a placebo tablet taken thrice daily in place of pentoxifylline for the same duration. During the study, concomitant treatments with salicylates, nonsteroidal anti-inflammatory drugs, budesonide, anti-TNFα agents, vitamin E, s-adenosyl methionine or ursodeoxycholic acid were not allowed. The investigators who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well the statisticians, were all blinded regarding the nature of the pharmacotherapy. All the patients were admitted in the wards of the Department of Medicine, Medical College and Hospitals, Calcutta for the initial period of 4 wk. All investigations such as liver function tests, prothrombin time, electrolytes, renal profile and abdominal ultrasound were repeated after the initial 4 wk of pharmacotherapy. After the initial 4 wk, the study was opened and the patients allocated to the different groups were revealed. After 4 wk of initial therapy, the dose of prednisolone in group II was tapered by 5 mg/wk over a period of 7 wk and then stopped. Patients in group I (pentoxifylline) who tolerated the drug well, continued to receive the medication at the same dose for the next 8 wk, and then stopped.
Only those patients who were clinically stable at the end of 4 wk were discharged and later followed-up in the liver clinic. All the patients were counseled for strict alcohol abstinence at the time of discharge from the hospital.
The patients were reviewed at least once a month in the liver clinic. During follow-up, all the patients were examined clinically, and asked about drug compliance, intake of alcohol or potential drug adverse effects. Liver function tests, prothrombin time, renal function test, electrolytes, and abdominal ultrasound were performed as and when required. Maddrey DF, MELD, GAHS and Child’s scores were calculated for all the patients during follow-up. Patients who had any alcohol intake in the follow-up period were excluded thereafter from the study.
Statistical analysis
Student’s t test was used for analysis of continuous variables, Fisher’s exact test for binary variables, and the χ2 test was used for categorical variables. All results of continuous variables are expressed as mean ± SD. Survival curves were estimated according to the Kaplan-Meier method and were compared using the log-rank test. Survival comparisons between groups were performed on an intent-to-treat basis. Results were considered statistically significant at P < 0.05.
RESULTS
Of the 158 patients initially evaluated, 74 who fulfilled the inclusion criteria without any other potential etiology of liver injury or severe co-morbid states were considered. Two patients refused consent for the study and another two patients refused to be admitted for the duration of the study. Seventy patients who fulfilled the inclusion and exclusion criteria and who gave informed written consent were randomized and divided into two groups: group I (pentoxifylline) had 34 patients, and group II (prednisolone) had 36 patients. The total duration of follow-up was 12 mo, with the patients being examined and evaluated in the liver clinic on a monthly basis. Two patients in group II withdrew voluntarily from the study and were excluded.
A total of 68 patients, 34 in each group, were considered for the final analysis. The baseline clinical and biochemical parameters of the patients receiving pentoxifylline or prednisolone are elaborated in Table 1, and were found to be comparable.
Table 1.
Comparison of baseline parameters of patients receiving pentoxifylline (group I) vs those receiving prednisolone (group II) in the treatment of severe alcoholic hepatitis (mean ± SD)
| Parameter | Group I (pentoxifylline) (n = 34) | Group II (prednisolone) (n = 34) | P value |
| Age (yr) | 47.53 ± 11.16 | 46.47 ± 9.67 | 0.68 |
| Male:female | 34:0 | 33:1 | - |
| Ascites | 31 | 33 | 0.37 |
| Encephalopathy | 20 | 23 | 0.61 |
| Varices | 23 | 22 | 0.80 |
| Maddrey DF score | 54.25 ± 16.24 | 57.78 ± 17.08 | 0.39 |
| MELD score | 23.14 ± 3.97 | 22.65 ± 3.33 | 0.58 |
| GAHS | 8.23 ± 1.07 | 7.94 ± 0.95 | 0.24 |
| Child’s score | 11.85 ± 1.62 | 12.15 ± 1.28 | 0.41 |
| Mean TLC (/cm3) | 13926.47 ± 3068.15 | 15225 ± 11836.18 | 0.5379 |
| Serum Na (mEq/L) | 135.26 ± 8.26 | 132.80 ± 6.90 | 0.1908 |
| Serum K (mEq/L) | 4.18 ± 0.72 | 4.293 ± 0.98 | 0.6207 |
| Urea (mg/dL) | 31.68 ± 27.63 | 25.74 ± 16.92 | 0.2889 |
| Creatinine (mg/dL) | 1.42 ± 0.61 | 1.19 ± 0.32 | 0.057 |
| Bilirubin (mg/dL) | 5.40 ± 2.50 | 6.604 ± 3.90 | 0.1345 |
| Albumin (gm/dL) | 3.19 ± 0.67 | 3.040 ± 0.75 | 0.3870 |
| ALT (IU/L) | 54.88 ± 23.25 | 57.38 ± 20.50 | 0.6397 |
| INR | 1.97 ± 0.34 | 2.04 ± 0.31 | 0.3493 |
P < 0.05 considered statistically significant. TLC: Total leucocyte count.
In group I, pentoxifylline therapy had to be stopped prematurely (within 3 mo) in five patients because of the development of life-threatening complications, all of whom unfortunately succumbed to the disease. Two patients expired following massive gastrointestinal bleeding. Two patients were lost to progressive hepatic encephalopathy and one patient died of sepsis, not responding to conservative management. Out of the five patients lost, two patients succumbed in the first 4 wk and three expired between 4 wk and 3 mo of therapy.
In group II, prednisolone therapy was stopped prematurely (within 3 mo) in 13 patients because of development of life-threatening complications. Two patients developed sepsis and both of them died of septic shock. Two patients had upper gastrointestinal bleed and succumbed to hemodynamic failure. One patient developed acute pancreatitis 26 d after inclusion; prednisolone was stopped and the patient responded to conservative management who has been doing well till the end of this study. Six patients died of hepatorenal Syndrome, not responding to conservative management. This is in sharp contrast to Group-I where none of the included patients developed hepatorenal Syndrome. One patient died of progressive hepatic encephalopathy and the cause of death could not be determined in one of the patients. Out of the total of 12 patients who expired in group II, seven succumbed in the first 4 wk and five more were lost between 4 wk and 3 mo of therapy. The cause of death and the complication profile are shown in Tables 2 and 3. The mortality was significantly higher among patients receiving prednisolone (35.29%) as compared to 14.71% among those receiving pentoxifylline, as elaborated by Kaplan-Meier analysis shown in Figure 1 (P = 0.04).
Table 2.
Causes of death in patients receiving pentoxifylline or prednisolone in the treatment of severe alcoholic hepatitis (n = 34)
| Cause of death | Group I (pentoxifylline) | Group II (prednisolone) |
| Hepatorenal syndrome | 0 | 6 |
| Sepsis | 1 | 2 |
| Gastrointestinal bleed | 2 | 2 |
| Encephalopathy | 2 | 1 |
| Unknown | 0 | 1 |
| Total | 5 | 12 |
Table 3.
Morbidity/complication profile of patients receiving pentoxifylline (group I) or prednisolone (group II) in the treatment of severe alcoholic hepatitis
| Complications |
Duration of follow-up |
|||
|
0-3 mo |
3 mo to 1 yr |
|||
| Group I (n = 34) | Group II (n = 34) | Group I (n = 29) | Group II (n = 22) | |
| Nausea | 24 | 19 | 14 | 4 |
| Vomiting | 12 | 8 | 4 | 1 |
| Dyspepsia | 3 | 7 | 1 | 1 |
| GI bleed | - | 2 | 2 | 4 |
| Oral thrush | - | 6 | - | - |
| Sepsis | 2 | 2 | 3 | |
| Recurrent encephalopathy | 2 | - | 5 | - |
| Worsening ascites | - | 2 | - | 2 |
| Impaired glucose tolerance | - | 2 | - | 2 |
| Delayed wound healing | - | 2 | - | 1 |
| Deep vein thrombosis | - | 1 | - | - |
| Pancreatitis | - | 1 | - | - |
| Hepatorenal syndrome | - | 6 | - | - |
Figure 1.
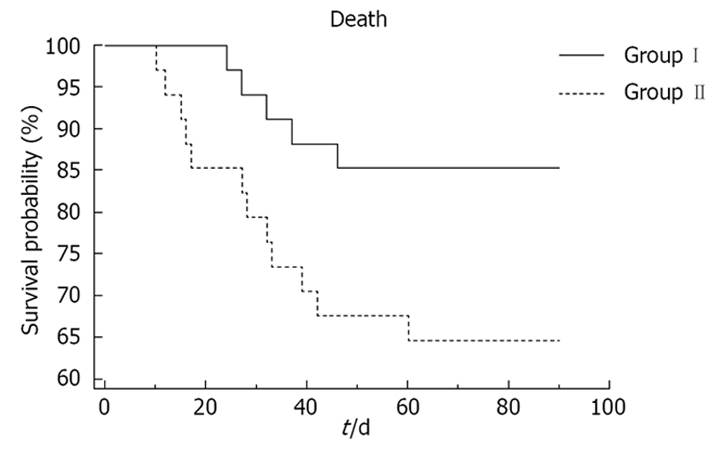
Survival curves (Kaplan-Meier life table analysis) of patients receiving pentoxifylline (group I) as compared to patients receiving prednisolone (group II), at the end of 3 mo of therapy.
Thirty-two patients in group I and 27 in group II were evaluated in the liver clinic at the end of 4 wk. The study was opened at this point in time and the allotment of patients to the different groups was revealed. The investigations done at the baseline were repeated, and the patients were re-admitted if deemed necessary. The patients were followed-up on a monthly basis and the investigations were repeated at the end of 3 mo, 6 mo and 1 year. The patients did relatively well beyond 3 mo of follow-up, and no more patients succumbed to the disease. In group I (pentoxifylline), one patient resumed alcohol consumption and another was lost to follow-up after 5 mo, and they were excluded from further analysis. In group II (prednisolone), two patients resumed alcohol consumption, after 8 and 10 m of follow-up, respectively, and both were excluded from further analysis.
The morbidity/complication profiles among the two groups were comparable (Table 3). Nausea followed by vomiting and dyspepsia were the most common adverse effects encountered in both groups. Patients receiving pentoxifylline more frequently complained of nausea and vomiting, whereas dyspepsia was more common among those receiving prednisolone. Recurrent hepatic encephalopathy was only seen in group I (pentoxifylline), while oral thrush, worsening of ascites, impaired glucose tolerance, delayed wound healing and deep vein thrombosis were seen only in group II (Table 3). On follow-up, recurrent encephalopathy was observed among five patients in group-I (pentoxifylline) in contrast to none in group II. The summary of the trial and its design is shown in Figure 2.
Figure 2.
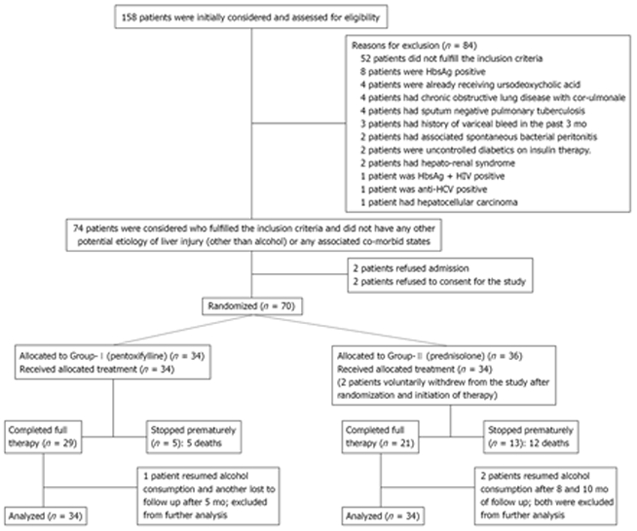
Summary of trial design and follow-up.
Table 4 shows the baseline profile of patients who succumbed to various complications as compared to those surviving at the end of the study. It shows that baseline Maddrey DF score and international normalized ratio (INR) was significantly higher among patients who succumbed to the disease as compared to those who survived (P = 0.038 and 0.049 respectively; Table 4). The baseline MELD score, GAHS and Child’s score was not significantly different among the patients who expired as compared to those who survived (Table 4).
Table 4.
Comparison of baseline parameters of patients succumbing to various complications to those surviving at the end of the study (12 mo)
| Parameter | Patients succumbing to complications (n = 17) | Surviving patients (n = 51) | P value |
| Age (yr) | 44.53 ± 11.19 | 47.82 ± 10.07 | 0.26 |
| Male:female | 17:0 | 50:1 | - |
| Ascites | 17 | 47 | 0.23 |
| Encephalopathy | 8 | 35 | 0.19 |
| Maddrey DF score | 63.22 ± 18.58 | 53.61 ± 15.39 | 0.038 |
| MELD score | 23 ± 4.15 | 22.86 ± 3.50 | 0.89 |
| GAHS | 8.35 ± 0.99 | 8 ± 1.02 | 0.21 |
| Child’s score | 12 ± 1.06 | 12 ± 1.57 | 1.00 |
| Mean TLC (/cm3) | 14008.82 ± 2804.14 | 14764.71 ± 9827.88 | 0.77 |
| Serum Na (mEq/L) | 131.76 ± 4.51 | 134.80 ± 8.35 | 0.16 |
| Serum K (mEq/L) | 4.22 ± 1.12 | 4.25 ± 0.76 | 0.90 |
| Urea (mg/dL) | 31.94 ± 27.95 | 27.63 ± 21.22 | 0.51 |
| Creatinine (mg/dL) | 1.19 ± 0.32 | 1.33 ± 0.55 | 0.34 |
| Bilirubin (mg/dL) | 6.88 ± 4.92 | 5.71 ± 2.56 | 0.21 |
| Albumin (gm/dL) | 2.97 ± 0.74 | 3.16 ± 0.70 | 0.32 |
| ALT (IU/L) | 52 ± 20.66 | 57.51 ± 22.18 | 0.37 |
| INR | 2.14 ± 0.32 | 1.96 ± 0.31 | 0.049 |
All values are expressed as mean ± SD. P < 0.05 considered statistically significant.
Table 5 shows the progression of GAHS, MELD, Child’s and Maddrey DF score in the patients over 12 mo. The fall in Maddrey DF score and GAHS was comparable among the patients receiving pentoxifylline or prednisolone. MELD score was observed to be significantly lower among the patients receiving pentoxifylline at the end of 4 wk, as compared to those receiving prednisolone.
Table 5.
Progression of scores evaluating the severity of liver disease of patients receiving pentoxifylline (group I) as compared to those receiving prednisolone (group II) in the treatment of severe alcoholic hepatitis (mean ± SD)
| Liver disease score | Baseline |
Duration of follow-up |
|||
| 4 wk | 3 mo | 6 mo | 1 yr | ||
| Maddrey DF score | |||||
| Group I1 | 54.25 ± 16.24 | 23.29 ± 12.07 | 14.3 ± 4.53 | 10.24 ± 4.27 | 7.79 ± 3.2 |
| Group II2 | 57.78 ± 17.08 | 27.82 ± 11.73 | 15.60 ± 6.21 | 11.16 ± 3.70 | 7.27 ± 2.67 |
| P value | 0.39 | 0.15 | 0.39 | 0.43 | 0.94 |
| MELD Score | |||||
| Group I1 | 23.14 ± 3.97 | 15.53 ± 3.63 | 12.41 ± 2.88 | 10.37 ± 2.32 | 9.18 ± 1.59 |
| Group II2 | 22.65 ± 3.33 | 17.78 ± 4.56 | 13.45 ± 2.77 | 11.14 ± 1.83 | 9.4 ± 1.88 |
| P value | 0.58 | 0.04 | 0.20 | 0.21 | 0.67 |
| GAHS | |||||
| Group I1 | 8.23 ± 1.07 | 6.37 ± 0.79 | 6.10 ± 0.77 | 5.96 ± 0.90 | 5.74 ± 0.66 |
| Group II2 | 7.94 ± 0.95 | 6.52 ± 1.09 | 5.91 ± 0.61 | 5.91 ± 0.61 | 5.7 ± 0.57 |
| P value | 0.24 | 0.56 | 0.34 | 0.81 | 0.83 |
| Child’s score | |||||
| Group I1 | 11.85 ± 1.62 | 9.69 ± 2.57 | 7.14 ± 1.60 | 5.96 ± 1.09 | 5.78 ± 0.89 |
| Group II2 | 12.15 ± 1.28 | 9.81 ± 2.08 | 7.59 ± 1.68 | 6.23 ± 0.97 | 5.9 ± 0.79 |
| P value | 0.41 | 0.84 | 0.33 | 0.38 | 0.63 |
P < 0.05 considered statistically significant.
In group I: n = 34 at baseline, n = 32 at 4 wk and n = 29 at 3 mo, n = 27 at 6 mo and 1 year.
In group II: n = 34 at baseline, n = 27 at 4 wk and n = 22 at 3 mo, 6 mo and n = 20 at 1 year.
DISCUSSION
The pathogenesis of alcohol-induced liver injury has not yet been clearly elucidated. Oxidative and nitrosative stress are believed to have a key role in the pathogenesis of alcoholic liver disease, and greater emphasis has been given to the role of cytochrome P450 2E1 in mitochondrial stress and disruption[20]. Altered signaling pathways and involvement of extrahepatic mediators such as adiponectin may also have a key role[20]. Augmented TNF-α production by macrophages and Kupffer cells and signaling via the p55 TNF receptor have been shown to be critical in the development of steatosis and hepatitis following chronic alcohol intake[21]. Pentoxyfilline, a non-specific phosphodiesterase inhibitor, with combined anti-inflammatory and antifibrogenic properties, has been shown to block the activation of hepatic stellate cells in culture[22]. It also has inhibitory effects on basic mechanisms of fibrogenesis such as cell proliferation and extracellular matrix synthesis[23]. Pentoxifylline has an added advantage of fewer adverse effects, such as gastrointestinal bleeding and renal shutdown, as compared to steroids. In the present study, none of the patients developed hepatorenal syndrome in the pentoxifylline group as compared to six in the prednisolone group. The MELD score in the pentoxifylline group was found to be significantly lower at the end of 4 wk of therapy (Table 5), as compared to the prednisolone group, confirming the renoprotective effects of pentoxifylline (as serum creatinine is a component of MELD score). Also gastrointestinal bleeding occurred more frequently in the prednisolone group as compared to the pentoxifylline group (Table 3).
The most important observation was the significantly reduced mortality among patients in the pentoxifylline group (14.71%) as compared to those receiving prednisolone (35.29%, P = 0.04, Figure 1). This reduced mortality in the pentoxifylline group was observed in spite of the increased occurrence of recurrent encephalopathy among patients in the pentoxifylline group. The patients with recurrent attacks of encephalopathy responded well to conservative management. This reduced mortality among patients in the pentoxifylline group can at least in part be explained by the renoprotective effects of pentoxifylline and the lower occurrence of gastrointestinal bleeding. In spite of the increased occurrence of nausea, and to a lesser extent vomiting, among patients in the pentoxifylline group, they were not severe enough to warrant stoppage of therapy. Also, with time, the occurrence of these complications was reduced (Table 3). Oral thrush, impaired glucose tolerance, poor wound healing, deep venous thrombosis and pancreatitis were some of the significant problems faced by the patients in the prednisolone group (Table 3).
Retrospectively, on analyzing the different liver function scores at the time of inclusion, only a higher Maddrey DF score was associated with the occurrence of increased mortality among patients with severe alcoholic hepatitis (Table 4). Thus Maddrey DF score remains the score of choice in determining prognosis of patients with severe alcoholic hepatitis, even after the advent of newer scores like MELD and GAHS.
One of the limitations of this study is the absence of evidence of histological improvement and survival among patients receiving pentoxifylline or prednisolone, because of the lack of availability of transjugular liver biopsy. Also the assessment of immunological and inflammatory status (e.g. TNF-α) of the patients was not possible. Nevertheless, a reduced mortality and more advantageous risk-benefit profile of pentoxifylline compared with prednisolone in patients with severe alcoholic hepatitis suggest that pentoxifylline is at least equivalent to prednisolone in the treatment of severe alcoholic hepatitis. However, further studies with a larger cohort of patients is warranted to decide if pentoxifylline is actually superior to the traditional drug prednisolone in the treatment of severe alcoholic hepatitis.
COMMENTS
Background
Severe alcoholic hepatitis is an acute, potential life-threatening manifestation of alcohol-induced liver injury, and forms part of the spectrum of liver disease, ranging from asymptomatic fatty liver to cirrhosis. The importance of severe alcoholic hepatitis lies in its significant morbidity and mortality, with a reported in-hospital mortality as high as 44%. Prednisolone has been used widely and is considered the standard treatment for severe acute alcoholic hepatitis with maddrey discriminant function (DF) score ≥ 32. However, it is not free of adverse effects and has had its share of controversies.
Research frontiers
Various other drugs have been tried in the treatment of alcoholic hepatitis, such as antioxidants, colchicines, calcium channel inhibitors, propylthiouracil and d-penicillamine, without much success. Augmented tumor necrosis factor (TNF)-α production by macrophages and Kupffer cells plays an important role in the pathogenesis of severe alcoholic hepatitis. However, infliximab, a human-mouse chimeric antibody to TNF-α, when used with prednisolone, has been found to be associated with severe infections, and is thus potentially harmful. The challenge is to find a drug whose efficacy is not only comparable to that of the standard drug prednisolone, but also safe and easy to administer over long periods of time.
Innovations and breakthroughs
Recently, pentoxifylline, a non-specific phosphodiesterase inhibitor, with anti-inflammatory (TNF-α inhibition) and antifibrogenic properties has been found to be useful in patients with severe alcoholic hepatitis. The idea was to evaluate the efficacy of pentoxifylline and compare it to the standard drug prednisolone in the treatment of severe alcoholic hepatitis in a randomized controlled study, and to study the immediate and short term outcomes. Significantly reduced mortality, a more advantageous risk-benefit profile, and renoprotective effects of pentoxifylline compared with prednisolone in patients with severe alcoholic hepatitis may be considered as a breakthrough.
Applications
The authors found that pentoxifylline was tolerated well in the treatment of severe alcoholic hepatitis, and was associated with significantly lower mortality, significantly lower model for end-stage liver disease (MELD) score at the end of 4 wk, and absence of hepatorenal syndrome. This should encourage the use of pentoxifylline in the treatment of severe alcoholic hepatitis. However, long-term prospective studies with a larger cohort of patients are needed to decide if pentoxifylline is actually superior to the traditional drug prednisolone in the treatment of severe alcoholic hepatitis.
Terminology
MELD score is a measure of the severity of liver dysfunction and has been recently used in the assessment of patients with severe alcoholic hepatitis. However Maddrey DF score remains the standard for the assessment of patients with severe alcoholic hepatitis.
Peer review
The experiments were planned and executed well and the manuscript is well written.
Peer reviewer: Cheng Ji, Professor of Research, Department of Medicine, University of Southern California, 2011 Zonal Ave., HMR-101, Los Angeles, CA 90033, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Lin YP
References
- 1.Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis. 2005;9:37–53. doi: 10.1016/j.cld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 3.Perrot S, Beaugrand M. [Treatment of alcoholic hepatitis. A review of randomized trials] Gastroenterol Clin Biol. 1988;12:521–531. [PubMed] [Google Scholar]
- 4.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Li Y, Lai JP, Douglas SD, Metzger DS, O'Brien CP, Ho WZ. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57–65. doi: 10.1053/jhep.2003.50295. [DOI] [PubMed] [Google Scholar]
- 6.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23 Suppl 1:S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degoul F, Sutton A, Mansouri A, Cepanec C, Degott C, Fromenty B, Beaugrand M, Valla D, Pessayre D. Homozygosity for alanine in the mitochondrial targeting sequence of superoxide dismutase and risk for severe alcoholic liver disease. Gastroenterology. 2001;120:1468–1474. doi: 10.1053/gast.2001.24051. [DOI] [PubMed] [Google Scholar]
- 8.Latvala J, Hietala J, Koivisto H, Järvi K, Anttila P, Niemelä O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am J Gastroenterol. 2005;100:1303–1310. doi: 10.1111/j.1572-0241.2005.41509.x. [DOI] [PubMed] [Google Scholar]
- 9.Spahr L, Giostra E, Frossard JL, Bresson-Hadni S, Rubbia-Brandt L, Hadengue A. Soluble TNF-R1, but not tumor necrosis factor alpha, predicts the 3-month mortality in patients with alcoholic hepatitis. J Hepatol. 2004;41:229–234. doi: 10.1016/j.jhep.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 10.McCullough AJ, O'Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 11.Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 12.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 13.Forrest EH, Morris AJ, Stewart S, Phillips M, Oo YH, Fisher NC, Haydon G, O'Grady J, Day CP. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut. 2007;56:1743–1746. doi: 10.1136/gut.2006.099226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen E. Alcoholic hepatitis--glucocorticosteroids or not? J Hepatol. 2002;36:547–548. doi: 10.1016/s0168-8278(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal K, Kontorinis N, Kontorinis N, Dieterich DT, Dieterich DT. Alcoholic Hepatitis. Curr Treat Options Gastroenterol. 2004;7:451–458. doi: 10.1007/s11938-004-0004-6. [DOI] [PubMed] [Google Scholar]
- 16.Levitsky J, Mailliard ME. Diagnosis and therapy of alcoholic liver disease. Semin Liver Dis. 2004;24:233–247. doi: 10.1055/s-2004-832937. [DOI] [PubMed] [Google Scholar]
- 17.Haber PS, Warner R, Seth D, Gorrell MD, McCaughan GW. Pathogenesis and management of alcoholic hepatitis. J Gastroenterol Hepatol. 2003;18:1332–1344. doi: 10.1046/j.1440-1746.2003.03217.x. [DOI] [PubMed] [Google Scholar]
- 18.Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, Riecken EO, Schuppan D. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut. 2002;50:241–247. doi: 10.1136/gut.50.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savolainen VT, Liesto K, Männikkö A, Penttilä A, Karhunen PJ. Alcohol Consumption and Alcoholic Liver Disease: Evidence of a Threshold Level of Effects of Ethanol. Alcohol Clin Exp Res. 2007;17:1112–1117. doi: 10.1111/j.1530-0277.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- 20.Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328–338. doi: 10.1097/MOG.0b013e3282fbceca. [DOI] [PubMed] [Google Scholar]
- 21.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KS, Cottam HB, Houglum K, Wasson DB, Carson D, Chojkier M. Pentoxifylline blocks hepatic stellate cell activation independently of phosphodiesterase inhibitory activity. Am J Physiol. 1997;273:G1094–G1100. doi: 10.1152/ajpgi.1997.273.5.G1094. [DOI] [PubMed] [Google Scholar]
- 23.Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:181–196. doi: 10.1016/s0306-3623(96)00314-x. [DOI] [PubMed] [Google Scholar]


