Abstract
AIM: To investigate the protective effects of melatonin on carbon tetrachloride (CCl4)-induced hepatic fibrosis in experimental rats.
METHODS: All rats were randomly divided into normal control group, model control group treated with CCl4 for 12 wk, CCl4 + NAC group treated with CCl4 + NAC (100 mg/kg, i.p.) for 12 wk, CCl4 + MEL-1 group treated with CCl4 + melatonin (2.5 mg/kg) for 12 wk, CCl4 + MEL-2 group treated with CCl4 + melatonin (5.0 mg/kg) for 12 wk, and CCl4 + MEL-3 group treated with CCl4 + melatonin (10 mg/kg). Rats in the treatment groups were injected subcutaneously with sterile CCl4 (3 mL/kg, body weight) in a ratio of 2:3 with olive oil twice a week. Rats in normal control group received hypodermic injection of olive oil at the same dose and frequency as those in treatment groups. At the end of experiment, rats in each group were anesthetized and sacrificed. Hematoxylin and eosin (HE) staining and Van Gieson staining were used to examine changes in liver pathology. Serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and protein concentration were measured with routine laboratory methods using an autoanalyzer. Hydroxyproline (HYP) content in liver and malondialdehyde (MDA) and glutathione peroxidase (GPx) levels in liver homogenates were assayed by spectrophotometry. Serum hyaluronic acid (HA), laminin (LN), and procollagen III N-terminal peptide (PIIINP) were determined by radioimmunoassay.
RESULTS: Pathologic grading showed that the fibrogenesis was much less severe in CCl4 + MEL3 group than in model control group (u = 2.172, P < 0.05), indicating that melatonin (10 mg/kg) can significantly ameliorate CCl4-induced hepatic fibrotic changes. The serum levels of ALT and AST were markedly lower in CCl4 + MEL treatment groups (5, 10 mg/kg) than in model control group (ALT: 286.23 ± 121.91 U/L vs 201.15 ± 101.16 U/L and 178.67 ± 103.14 U/L, P = 0.028, P = 0.007; AST: 431.00 ± 166.35 U/L vs 321.23 ± 162.48 U/L and 292.42 ± 126.23 U/L, P = 0.043, P = 0.013). Similarly, the serum laminin (LN) and hyaluronic acid (HA) levels and hydroxyproline (HYP) contents in liver were significantly lower in CCl4 + MEL-3 group (10 mg/kg) than in model control group (LN: 45.89 ± 11.71 μg/L vs 55.26 ± 12.30 μg/L, P = 0.012; HA: 135.71 ± 76.03 μg/L vs 201.10 ± 68.46 μg/L, P = 0.020; HYP: 0.42 ± 0.08 mg/g tissue vs 0.51 ± 0.07 mg/g tissue, P = 0.012). Moreover, treatment with melatonin (5, 10 mg/kg) significantly reduced the MDA content and increased the GPx activity in liver homogenates compared with model control group (MDA: 7.89 ± 1.49 noml/mg prot vs 6.29 ± 1.42 noml/mg prot and 6.25 ± 2.27 noml/mg prot, respectively, P = 0.015, P = 0.015; GPx: 49.13 ± 8.72 U/mg prot vs 57.38 ± 7.65 U/mg prot and 61.39 ± 13.15 U/mg prot, respectively, P = 0.035, P = 0.003).
CONCLUSION: Melatonin can ameliorate CCl4 -induced hepatic fibrosis in rats. The protective effect of melatonin on hepatic fibrosis may be related to its antioxidant activities.
Keywords: Melatonin, Hepatic fibrosis, Oxidative stress, Hyaluronic acid, Laminin, Malondialdehyde, Glutathione peroxidase
INTRODUCTION
Hepatic fibrosis, a common pathological process of chronic hepatic disease, can lead to irreversible cirrhosis, and involves multiple cellular and molecular events that ultimately result in accumulation of collagen and extra cellular matrix protein in space of Disse. If treated properly at fibrosis stage, cirrhosis can be prevented[1]. However, no effective antifibrosis drugs are available at present. Several lines of evidence suggest that oxidative stress plays an important role in the etiopathogenesis of hepatic fibrosis[2,3].
Melatonin (N-acetyl-5-metyoxytryptamine), a secretory product of the pineal gland, is a powerful endogenous antioxidant, regulates circadian rhythms, sleep and immune system activity, behaves as a free radical scavenger[4], eliminates oxygen free radicals and reactive intermediates[5–9]. Both in vitro and in vivo experiments have shown that melatonin can protect cells, tissues, and organs against oxidative damage induced by a variety of free-radical-generating agents and processes, such as safrole, lipopolysaccharide (LPS), carbon tetrachloride (CCl4), ischemia-reperfusion, amyloid-protein, and ionizing radiation[10–12]. In addition, melatonin also has an indirect antioxidant effect by enhancing the levels of potential antioxidants such as glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione (GSH)[10–12]. Recent studies showed that melatonin exerts its cytoprotective effects in various experimental models of acute liver injury and reduces fibroblast proliferation and collagen synthesis[12,13], indicating that melatonin may have therapeutic effects on acute and chronic liver injury, through its antioxidant action.
The aim of our present study was to evaluate the possible antifibrotic effect of melatonin on a hepatic fibrosis model of rats. In addition, the antioxidant and anti-inflammatory properties of melatonin were investigated in rats with liver fibrosis.
MATERIALS AND METHODS
Drugs and materials
Crystalline melatonin was purchased from Sigma Chemical Company (St. Louis, MO, USA). The solvent used for melatonin was a mixture of ethanol (1%, v/v) and NaCl (0.9%). N-acetyl-L-cysteine (NAC) was purchased from Shanghai Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Commercial kits used for determining malondialdehyde (MDA), glutathione peroxidase (GPx) and hydroxyproline (HYP) were obtained from Jiancheng Institute of Biotechnology (Nanjing, China). Commercial kits for radioimmunoassay of procollagen III N-terminal peptide (PIIINP), laminin (LN), and hyaluronic acid (HA) were obtained from Beijing North Institute of Biological Technology (Beijing, China). Other commercial chemicals used in experiments were of analytical grade.
Animal experiments and drug treatment
Male Sprague-Dawley rats, weighing 170-240 g at beginning of the study, purchased from Anli Experimental Animal Limited Company (Anhui, China), were kept at a constant temperature (22°C) in a 12-h light and dark cycle, with free access to food and water. All animals were treated humanely according to the National Guidelines for the Care of Animals in China. Rats were randomly divided into normal control group (n = 11), model control group (n = 20) treated with CCl4 for 12 wk, CCl4 + NAC group (n = 20) treated with CCl4 + NAC ( 100 mg/kg, i.p.) for 12 wk, CCl4 + MEL-1 group (n = 20) treated with CCl4 + melatonin (2.5 mg/kg) for 12 wk, CCl4 + MEL-2 group (n = 20) treated with CCl4 + melatonin (5.0 mg/kg) for 12 wk, and CCl4 + MEL-3 group (n = 20) treated with CCl4 + melatonin (10 mg/kg) for 12 wk. Rats in treatment groups were injected subcutaneously with sterile CCl4 (3 mL/kg of body weight) in a ratio of 2:3 with olive oil twice a week. Rats in normal control group received hypodermic injection of olive oil at the same dose and frequency as those in the treatment groups. At the beginning of CCl4 injection, rats received intraperitoneal melatonin daily whereas rats that did not receive melatonin were given the same volume of vehicle (1% ethanol) at the same time point. After 12 wk, a laparotomy was performed and blood was drawn from the abdominal aorta under 3% pentobarbital sodium (1 mL/kg) anesthesia. The animals were then killed with their livers removed. Blood was collected into tubes and centrifuged. Serum was aspirated and stored at -80°C. Liver tissue was fixed in formalin for routine histological examination, or stored at -80°C until required.
Histopathological examination
Liver tissue samples, fixed in 40 g/L paraformaldehyde and embedded in paraffin, were cut into 5-μm thick sections, which were stained with hematoxylin and eosin (HE) and Van Gieson (VG) according to the standard procedure. Van Gieson’s method was used to detect collagen fibers. Hepatic fibrosis was divided into the following stages as previously described[14]: stage 0: no fibrosis; stage 1: expansion of portal tracts without linkage; stage 2: portal expansion with portal to portal linkage; stage 3: expansive portal to portal and focal portal to central linkage; and stage 4: cirrhosis. Two pathologists with no knowledge of liver sources examined the stained sections independently.
Analysis of liver function
Serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and protein concentration were measured with routine laboratory methods using an autoanalyzer (Hitachi Automatic Analyzer, Japan).
Measurement of MDA and GPx levels in liver homogenates
Liver samples were thawed, weighed and homogenized (1:9 w:v) in 0.9% saline. The homogenates were centrifuged at 1000 × g for 10 min at 4°C and supernatant was taken for assay of MDA and GPx with a commercial kit (Jiancheng Institute of Biotechnology, Nanjing, China) following its manufacturer’s instructions. MDA was assayed by measuring the levels of thiobarbituric acid reactive substances (TBARS) at 532 nm and expressed as nmol/mg protein. GPx assay was based on its ability to inhibit oxidation of oxyamine by the xanthine-xanthine oxidase system. Total protein concentration in liver homogenates was determined using the Coomassie blue method with bovine serum albumin as a standard.
Detection of hydroxyproline content in liver
Total collagen content in fresh liver samples was determined by hydroxyproline assay. Hydroxyproline content was detected with a commercial hydroxyproline detection kit (Jiancheng Institute of Biotechnology, Nanjing, China) following its manufacturer’s instructions.
Measurement of serum HA, LN, and PIIINP levels
Serum HA, LN and PIIINP levels were measured by radioimmunoassay with a commercial kit according to its manufacturer’s instructions (Beijing North Institute of Biological Technology, Beijing, China).
Statistical analysis
Data were analyzed with SPSS software. Quantitative data were presented as mean ± SD and analyzed by one way ANOVA analysis. Frequency data (pathologic grading of hepatic fibrosis) were analyzed by Ridit analysis. P < 0.05 was considered statistically significant.
RESULTS
Pathological changes
Degeneration, necrosis, infiltration of inflammatory cells, and collagen deposition were found in liver tissues of model control group, CCl4 + NAC group and 3 melatonin treatment groups (Figure 1C-F). Liver tissue samples from rats in normal control group showed normal lobular architecture with central veins and radiating hepatic cords (Figure 1A and B). Formation of fibrotic septa encompassing regenerated hepatocytes was observed in liver tissue samples from rats in model group (Figure 1D). A large number of inflammatory cells infiltrated intra- and interlobular regions (Figure 1C). Statistical analysis revealed significant differences in pathologic grading between CCl4 + MEL3 group and model control group (P < 0.05), indicating that fibrogenesis was much less severe in CCl4 + MEL3 group than in model control group (Table 1, Figure 1C-F).
Figure 1.
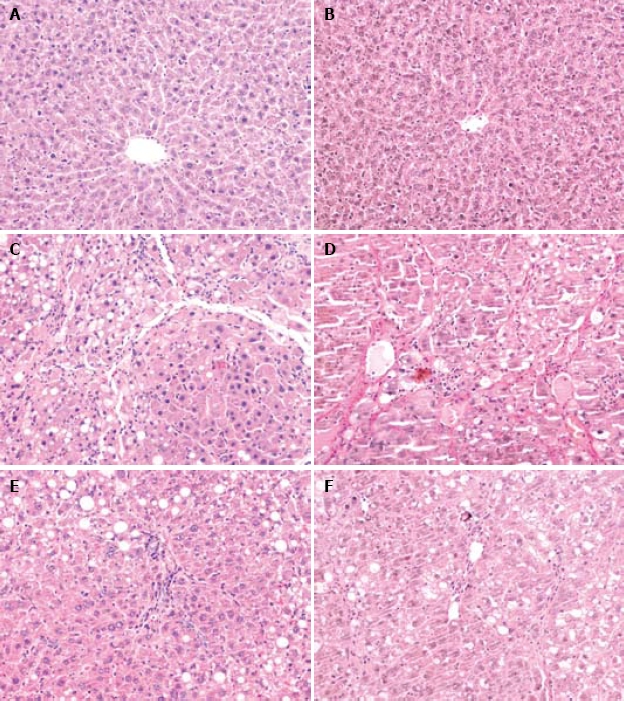
Pathological changes. Light microscopy of liver tissue sections showing normal liver lobular architecture with central veins in the normal control group (HE staining, × 200) (A), no collagen deposition in liver of normal control group (VG staining, × 200) (B), degenerated and necrotic liver cells associated with inflammatory cells in model group (HE staining, × 200) (C), formation of fibrotic septa in model group (VG staining, × 200) (D), and pathological change in liver of CCl4 + melatonin (10 mg/kg) group was rather milder compared with the model group (HE staining, × 200; VG staining, × 200) (E, F).
Table 1.
Pathologic grading of hepatic fibrosis in different groups
| Group | Dose (mg/kg) | n |
Pathologic grading of hepatic fibrosis |
u value | ||||
| 0 | I | II | III | IV | ||||
| Normal | - | 11 | 11 | 0 | 0 | 0 | 0 | 5.5681b |
| Model | - | 13 | 0 | 0 | 1 | 6 | 6 | - |
| NAC | 100 | 12 | 0 | 2 | 5 | 3 | 2 | 1.8838 |
| MEL | 2.5 | 11 | 0 | 2 | 4 | 2 | 3 | 1.5568 |
| 5 | 13 | 0 | 2 | 4 | 3 | 4 | 1.3662 | |
| 10 | 12 | 0 | 4 | 4 | 1 | 3 | 2.1720a | |
u represents the Ridit value of the two groups, P < 0.05 indicates u > 1.96; P < 0.01 indicates u > 2.58;
P < 0.05,
P < 0.01 vs model group.
Detection of liver function
The serum ALT and AST levels were significantly higher in experimental and model groups than in normal control group (P < 0.01). The ALT and AST levels were significantly higher in model group than in CCl4 + NAC and CCl4 + MEL groups (5, 10 mg/kg) (P < 0.05, P < 0.01). Melatonin (5, 10 mg/kg) significantly decreased the elevated serum transaminase levels (Table 2), whereas no significant difference in the ratio of A/G was observed between model control group, CCl4 + NAC and CCl4 + MEL groups (Table 2).
Table 2.
Effect of melatonin on serum ALT, AST levels and A/G value in different groups (mean ± SD)
| Group | Dose (mg/kg) | n | ALT (U/L) | AST (U/L) | A/G |
| Normal | - | 11 | 70.00 ± 35.27 | 139.82 ± 72.83 | 0.94 ± 0.40 |
| Model | - | 13 | 286.23 ± 121.91b | 431.00 ± 166.35b | 0.74 ± 0.09 |
| NAC | 100 | 12 | 194.42 ± 90.83bc | 293.33 ± 94.60bc | 0.78 ± 0.11 |
| MEL | 2.5 | 11 | 211.09 ± 97.03b | 357.09 ± 153.26b | 0.68 ± 0.15 |
| 5 | 13 | 201.15 ± 101.16bc | 321.23 ± 162.48bc | 0.77 ± 0.15 | |
| 10 | 12 | 178.67 ± 103.14bd | 292.42 ± 126.23bc | 0.73 ± 0.07 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; A: Albumin; G: Globumin.
P < 0.01 vs normal control group;
P < 0.05 vs model control group;
P < 0.01 vs model control group.
MDA content and GPx activity in liver homogenates
The MDA level was significantly higher while GPx activity was significantly lower in liver homogenates of CCl4 + NAC and CCl4 + MEL groups than in those of normal control group (P < 0.01). The MDA level was significantly higher in model control group than in CCl4 + NAC and CCl4 + MEL groups (5, 10 mg/kg) (P < 0.05). Melatonin (5, 10 mg/kg) significantly blocked the elevated MDA level. GPx activity was significantly lower in the model control group than in CCl4 + NAC and CCl4 + MEL groups (5, 10 mg/kg) (P < 0.05, P < 0.01, Table 3).
Table 3.
MDA content and GPx activity in liver homogenates of different groups (mean ± SD)
| Group | Dose (mg/kg) | n | MDA (noml/mg prot) | GPx (U/mg prot) |
| Normal | - | 11 | 4.3 ± 1.87 | 80.68 ± 10.76 |
| Model | - | 13 | 7.89 ± 1.49b | 49.13 ± 8.72b |
| NAC | 100 | 12 | 6.29 ± 1.36bc | 64.68 ± 8.22bd |
| MEL | 2.5 | 11 | 6.84 ± 1.10b | 53.44 ± 9.35b |
| 5 | 13 | 6.29 ± 1.42bc | 57.38 ± 7.65bc | |
| 10 | 12 | 6.25 ± 2.27bc | 61.39 ± 13.15bd |
MDA: Malondialdehyde; GPx: Glutathione peroxidase.
P < 0.01 vs normal control group;
P < 0.05 vs model control group;
P < 0.01 vs model control group.
HYP contents in liver tissue
Hepatic fibrosis was quantified by measuring hepatic hydroxyproline. The hydroxyproline content was significantly higher in model control, CCl4 + NAC and CCl4 + MEL groups than in normal control group
(P < 0.01), and significantly higher in model group than in CCl4 + NAC and CCl4 + MEL groups (10 mg/kg) (P < 0.05). Treatment with melatonin (10 mg/kg) or NAC reduced the hydroxyproline content in liver homogenates, and therefore prevented hepatic fibrosis induced by CCL4 (Figure 2).
Figure 2.
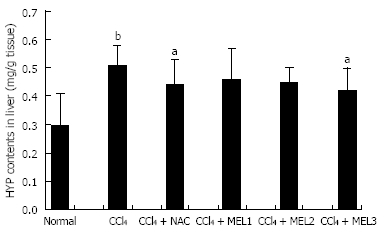
Effect of melatonin on hydroxyproline content in liver of rats with fibrosis fibrotic induced by CCl4. aP < 0.05 vs model control group; bP < 0.01 vs normal control group.
Measurement of serum HA, LN, and PIIINP levels
The serum LN and HA levels were significantly higher in model control, CCl4 + NAC, and CCl4 + MEL groups than in normal control group (P < 0.05, P < 0.01), and significantly decreased after treatment with melatonin (10 mg/kg) (P < 0.05). Treatment with NAC significantly reduced the serum HA level (P < 0.05). The serum PIIINP level was significantly higher in model control, CCl4 + NAC and CCl4 + MEL groups than in normal control group (P < 0.05). However, no significant difference was observed among the five groups (Table 4).
Table 4.
Serum HA, LN and PIIINP levels in different groups (mean ± SD)
| Group | Dose (mg/kg) | n | HA (μg/L) | LN (μg/L) | PIIINP (μg/L) |
| Normal | - | 11 | 71.65 ± 27.64 | 37.65 ± 6.09 | 26.41 ± 7.28 |
| Model | - | 13 | 201.10 ± 68.46b | 55.26 ± 12.30b | 35.88 ± 5.92b |
| NAC | 100 | 12 | 131.31 ± 58.58ac | 48.28 ± 4.93b | 32.89 ± 3.90b |
| MEL | 2.5 | 11 | 174.41 ± 72.99b | 48.15 ± 6.40b | 34.21 ± 5.76b |
| 5 | 13 | 153.54 ± 86.19b | 48.22 ± 9.51b | 33.12 ± 4.71b | |
| 10 | 12 | 135.71 ± 76.03ac | 45.89 ± 11.71ac | 31.99 ± 6.09a |
HA: Hyaluronic acid; LN: Laminin; PIIINP: Procollagen III N-terminal peptide.
P < 0.05 vs normal control group;
P < 0.01 vs normal control group;
P < 0.05 vs model control group.
DISCUSSION
CCl4 is widely used to induce hepatic fibrosis and cirrhosis in animal models. In this study, hepatic fibrosis was successfully induced by subcutaneous injection of sterile CCl4 twice weekly for 12 wk. Through this hepatic fibrosis model, the effects of melatonin on hepatic fibrosis induced by CCl4 in rats were examined.
N-acetylcysteine (NAC), a free radical scavenger, is a glutathione precursor which increases glutathione levels in hepatocytes[15,16]. Increased glutathione levels limit the production of reactive oxygen species (ROS) which can cause hepatocellular injury. NAC can also inhibit the proliferation of hepatic stellate cells[17]. Therefore, it was used as a positive control in this study.
It is well known that oxidative damage can induce hepatic fibrogenesis. ROS, such as H2O2, O.2_, and .OH, are implicated in the development and pathological progress of hepatic fibrosis[18,19]. Free radicals and biomolecular reaction products promote phagocytic and myofibroblastic activities. Lipid peroxidation accelerates collagen synthesis by stimulating stellate cells[20]. It has been shown that melatonin is an effective antioxidant and a free radical scavenger. Due to its small size and high lipophilicity, melatonin can cross biological membranes easily and reach all compartments within the cell[21], thus protecting DNA, proteins, and biological membrane lipids from the deleterious effects of free radicals[22]. It has been found that melatonin has a higher antioxidant efficiency than vitamin E and GSH, which are known as powerful antioxidants[10]. The antioxidant properties of melatonin prevent acute liver injury induced by ischemia-reperfusion[23], irradiation[24], bile duct ligation[25–27], and toxins[18,28,29]. Several lines of evidence suggest that melatonin plays an important role in regulation of collagen levels and inhibition of collagen accumulation. Ostrowska et al[30] showed that melatonin is negatively related with urine hydroxyproline levels in fasting rats. Cunnane et al[31] demonstrated that primary biliary cirrhosis is related with melatonin deficiency in pinealectomized rats. Tahan et al[12] reported that daily melatonin injection at pharmacological doses is effective against liver damage in a rat liver fibrosis model induced by a 14-d dimethylnitrosamine regimen. In the present study, liver injury was assessed with histological and biochemical parameters. The results suggest that melatonin could decrease the scores of hepatic fibrosis and serum ALT and AST levels in rats with hepatic injury caused by CCl4. Melatonin at a dose of 10 mg/kg was as effective as 100 mg/kg NAC in reducing serum ALT and AST levels, indicating that melatonin can protect liver and alleviate the progression of hepatic fibrosis. However, further study is needed on the liver function protective effect of melatonin in cirrhotic patients.
HA and LN are known to be good serum markers of hepatic fibrogenesis[32–34]. HYP in liver is an important index reflecting the degree of hepatic fibrosis and hepatic fibrosis can be quantified by measuring hepatic hydroxyproline[33,35]. In the present study, treatment with melatonin (10 mg/kg) could significantly reduce HA and LN in serum and HYP in liver. The decreased of hepatic hydroxyproline and serum LN and HA levels indicate that melatonin can inhibit collagen deposition in liver.
Oxidative stress plays an important role in the formation of hepatic fibrosis via increasing stellate cell activation and collagen synthesis. MDA is the main product of lipid peroxidation and its concentration is generally presented as the total level of lipid peroxidation products[36]. As an end product of lipid peroxidation, MDA can produce ozone, which reacts rapidly with cellular structures, generates hydrogen peroxide and other reactive oxygen species, leading to peroxidation and denaturation of membranes[37]. It has been shown that MDA can activate stellate cells that produce collagen. The results of this study suggest that treatment with melatonin (5, 10 mg/kg) could significantly block increased MDA, suggesting that melatonin decreases lipid peroxidation and plays an anti-oxidative role in hepatic fibrosis induced by CCl4 in rats.
Melatonin is not only a direct antioxidant but also an indirect antioxidant through enhancement of antioxidant enzyme activities in liver[38]. It was reported that melatonin can reduce free radical damage by elevating GPx activation[11,39]. Montilla et al[25] reported that acute ligation of the bile duct is accompanied with decreased GSH levels both in plasma and in liver, as well as significantly reduced antioxidant enzyme activities. Treatment with melatonin is associated with a significant recovery of anti-oxidative enzymes such as GPx[25]. Tahan et al[12] found that melatonin can restore GPx activity in a rat liver fibrosis model induced by a 14-d dimethylnitrosamine regimen. In this study, the GPx activity was significantly lower in model control group than in CCl4 + NAC and CCl4 + MEL groups (5, 10 mg/kg), indicating that melatonin can protect liver against CCl4-induced hepatic fibrosis in rats, possibly through its direct and indirect antioxidant effects.
In conclusion, melatonin may have beneficial effects on hepatic fibrosis induced by CCl4 in rats. The protective effect of melatonin on hepatic fibrosis may be related to its antioxidant activities.
COMMENTS
Background
In China, the incidence of liver cirrhosis is still high. Liver cirrhosis results from fibrosis. If treated properly at fibrosis stage, cirrhosis can be prevented. However, no effective antifibrosis drugs are available at present. Several lines of evidences suggest that oxidative stress plays an important role in the etiopathogenesis of hepatic fibrosis. Melatonin can protect cells, tissues, and organs against oxidative damage induced by a variety of free-radical-generating agents and processes. The possible fibrosuppressant effect of melatonin on hepatic fibrosis was evaluated in this study. In addition, the antioxidant and anti-inflammatory properties of melatonin were investigated in rats with fibrosis.
Research frontiers
Although the exact pathogenesis of hepatic fibrosis is still obscure, the role of free radicals and lipid peroxides in the development of hepatic fibrosis has attracted considerable attention. If treated properly at this stage, cirrhosis can be successfully prevented. However, it remains a problem to prevent hepatic fibrosis or to control its progression. Great efforts have been made to find safe and effective drugs. Recent experiments demonstrate that melatonin may have therapeutic effects on acute and chronic liver injury, possibly through its antioxidant activities.
Innovations and breakthroughs
Melatonin may have beneficial effects on hepatic fibrosis induced by carbon tetrachloride in rats. The protective effect of melatonin may be related to its antioxidant activities.
Applications
Melatonin can be used as an antifibrotic drug, protect liver cells against fibrosis and inhibits collagen fiber deposition in liver, thus providing a basis for further studies on its therapeutic effect on hepatic fibrosis.
Terminology
Melatonin (N-acetyl-5-metyoxytryptamine), a secretory product of the pineal gland, is a powerful endogenous antioxidant. It regulates circadian rhythms, sleep and immune system activity, behaves as a free radical scavenger, and eliminates oxygen free radicals and reactive intermediates.
Peer review
This is a well-designed study describing the protective effect of melatonin on fibrosis induced by carbon tetrachloride. Methods are appropriate and results are consistent with the conclusion. The study is very interesting with a great amount of data, which corroborate the major conclusion.
Acknowledgments
The authors thank Dr. Shu-Jun Xia for his help in preparing this manuscript.
Supported by The Natural Science Foundation of Anhui Province No. 01043904 and the Natural Science Research Project of Colleges and Universities of Anhui Province No.KJ2007B146
Peer reviewers: Wendy M Mars, PhD, Department of Pathology, University of Pittsburgh, S-411B South Biomedical Science Tower Pittsburgh, PA 15261, United States; Ana Cristina Simões e Silva, MD, PhD, Professor, Faculdade de Medicina UFMG, Departamento de Pediatria, sala 267, Avenida Professor Alfredo Balena, 190, Bairro Santa Efigênia, Belo Horizonte, Minas Gerais 30130-100, Brazil
S- Editor Tian L L- Editor Wang XL E- Editor Yin DH
References
- 1.Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis. 2004;36:231–242. doi: 10.1016/j.dld.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu I. Antifibrogenic therapies in chronic HCV infection. Curr Drug Targets Infect Disord. 2001;1:227–240. doi: 10.2174/1568005014606053. [DOI] [PubMed] [Google Scholar]
- 4.Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin's interaction with reactive species. J Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079x.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan DX, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST, Vijayalaxmi , Shepherd AM. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- 6.Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic Biol Med. 2000;29:1177–1185. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 7.Longoni B, Salgo MG, Pryor WA, Marchiafava PL. Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 1998;62:853–859. doi: 10.1016/s0024-3205(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 8.Stasica P, Ulanski P, Rosiak JM. Melatonin as a hydroxyl radical scavenger. J Pineal Res. 1998;25:65–66. doi: 10.1111/j.1600-079x.1998.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 9.Zang LY, Cosma G, Gardner H, Vallyathan V. Scavenging of reactive oxygen species by melatonin. Biochim Biophys Acta. 1998;1425:469–477. doi: 10.1016/s0304-4165(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 10.Rozov SV, Filatova EV, Orlov AA, Volkova AV, Zhloba AR, Blashko EL, Pozdeyev NV. N1-acetyl-N2-formyl-5-methoxykynuramine is a product of melatonin oxidation in rats. J Pineal Res. 2003;35:245–250. doi: 10.1034/j.1600-079x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Sener-Muratoğlu G, Paskaloğlu K, Arbak S, Hürdağ C, Ayanoğlu-Dülger G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci. 2001;46:318–330. doi: 10.1023/a:1005652815921. [DOI] [PubMed] [Google Scholar]
- 12.Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S, Yildirim B, Aytekin H, Ozbay G, Mert A, Senturk H. Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J Pineal Res. 2004;37:78–84. doi: 10.1111/j.1600-079X.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 13.Cruz A, Padillo FJ, Torres E, Navarrete CM, Muñoz-Castañeda JR, Caballero FJ, Briceño J, Marchal T, Túnez I, Montilla P, et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J Pineal Res. 2005;39:143–150. doi: 10.1111/j.1600-079X.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 15.Doğru-Abbasoğlu S, Balkan J, Kanbağli O, Cevikbaş U, Aykaç-Toker G, Uysal M. Aminoguanidine, an inducible nitric oxide synthase inhibitor, plus N-acetylcysteine treatment reduce the lipopolysaccharide-augmented hepatotoxicity in rats with cirrhosis. Hum Exp Toxicol. 2002;21:359–364. doi: 10.1191/0960327102ht256oa. [DOI] [PubMed] [Google Scholar]
- 16.Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, Altomare E. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol. 2001;175:130–139. doi: 10.1006/taap.2001.9234. [DOI] [PubMed] [Google Scholar]
- 17.Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- 18.Serviddio G, Pereda J, Pallardó FV, Carretero J, Borras C, Cutrin J, Vendemiale G, Poli G, Viña J, Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711–720. doi: 10.1002/hep.20101. [DOI] [PubMed] [Google Scholar]
- 19.Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, Inoue H, Honda H, Ito S. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004;74:897–907. doi: 10.1016/j.lfs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Geesin JC, Hendricks LJ, Falkenstein PA, Gordon JS, Berg RA. Regulation of collagen synthesis by ascorbic acid: characterization of the role of ascorbate-stimulated lipid peroxidation. Arch Biochem Biophys. 1991;290:127–132. doi: 10.1016/0003-9861(91)90598-d. [DOI] [PubMed] [Google Scholar]
- 21.Sener G, Jahovic N, Tosun O, Atasoy BM, Yeğen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003;74:563–572. doi: 10.1016/j.lfs.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Reiter RJ, Poeggeler B, Tan DX, Chen LD, Manchester LC, Guerrero JM. Antioxidant capacity of melatonin: A novel action not requiring a receptor. Neuroendocrinol Lett. 1993;15:103–116. [Google Scholar]
- 23.Zavodnik LB, Zavodnik IB, Lapshina EA, Belonovskaya EB, Martinchik DI, Kravchuk RI, Bryszewska M, Reiter RJ. Protective effects of melatonin against carbon tetrachloride hepatotoxicity in rats. Cell Biochem Funct. 2005;23:353–359. doi: 10.1002/cbf.1160. [DOI] [PubMed] [Google Scholar]
- 24.El-Missiry MA, Fayed TA, El-Sawy MR, El-Sayed AA. Ameliorative effect of melatonin against gamma-irradiation-induced oxidative stress and tissue injury. Ecotoxicol Environ Saf. 2007;66:278–286. doi: 10.1016/j.ecoenv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Montilla P, Cruz A, Padillo FJ, Túnez I, Gascon F, Muñoz MC, Gómez M, Pera C. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res. 2001;31:138–144. doi: 10.1034/j.1600-079x.2001.310207.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohta Y, Kongo M, Kishikawa T. Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J Pineal Res. 2003;34:119–126. doi: 10.1034/j.1600-079x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 27.Padillo FJ, Cruz A, Navarrete C, Bujalance I, Briceño J, Gallardo JI, Marchal T, Caballero R, Túnez I, Muntané J, et al. Melatonin prevents oxidative stress and hepatocyte cell death induced by experimental cholestasis. Free Radic Res. 2004;38:697–704. doi: 10.1080/10715760410001705131. [DOI] [PubMed] [Google Scholar]
- 28.El-Sokkary GH, Abdel-Rahman GH, Kamel ES. Melatonin protects against lead-induced hepatic and renal toxicity in male rats. Toxicology. 2005;213:25–33. doi: 10.1016/j.tox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Sigala F, Theocharis S, Sigalas K, Markantonis-Kyroudis S, Papalabros E, Triantafyllou A, Kostopanagiotou G, Andreadou I. Therapeutic value of melatonin in an experimental model of liver injury and regeneration. J Pineal Res. 2006;40:270–279. doi: 10.1111/j.1600-079X.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowska Z, Swientochowska E, Buntner B, Marek B, Zwirska-Korczala K, Spyra Z, Banas I I. Assessment of relationship between secretion of melatonin, activity of thyroid, adrenal cortex as well as testes and chosen markers of collagen metabolism in starved rats. Endocr Regul. 1996;30:83–92. [PubMed] [Google Scholar]
- 31.Cunnane SC, Manku MS, Horrobin DF. The pineal and regulation of fibrosis: pinealectomy as a model of primary biliary cirrhosis: roles of melatonin and prostaglandins in fibrosis and regulation of T lymphocytes. Med Hypotheses. 1979;5:403–414. doi: 10.1016/0306-9877(79)90107-5. [DOI] [PubMed] [Google Scholar]
- 32.Kaneda H, Hashimoto E, Yatsuji S, Tokushige K, Shiratori K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2006;21:1459–1465. doi: 10.1111/j.1440-1746.2006.04447.x. [DOI] [PubMed] [Google Scholar]
- 33.Dang SS, Wang BF, Cheng YA, Song P, Liu ZG, Li ZF. Inhibitory effects of saikosaponin-d on CCl4-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2007;13:557–563. doi: 10.3748/wjg.v13.i4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CH, Pan LH, Yang ZW, Li CY, Xu WX. Preventive effect of Qianggan-Rongxian Decoction on rat liver fibrosis. World J Gastroenterol. 2008;14:3569–3573. doi: 10.3748/wjg.14.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayasaka A, Saisho H. Serum markers as tools to monitor liver fibrosis. Digestion. 1998;59:381–384. doi: 10.1159/000007493. [DOI] [PubMed] [Google Scholar]
- 36.Drewa G, Krzyzyńska-Malinowska E, Woźniak A, Protas-Drozd F, Mila-Kierzenkowska C, Rozwodowska M, Kowaliszyn B, Czajkowski R. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med Sci Monit. 2002;8:BR338–BR343. [PubMed] [Google Scholar]
- 37.Ajamieh HH, Menéndez S, Martínez-Sánchez G, Candelario-Jalil E, Re L, Giuliani A, Fernández OS. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia-reperfusion. Liver Int. 2004;24:55–62. doi: 10.1111/j.1478-3231.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol. 2000;78:447–453. doi: 10.1139/o00-018. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Xu DX, Lv JW, Ning H, Wei W. Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic liver damage in D-galactosamine-sensitized mice. Toxicology. 2007;237:49–57. doi: 10.1016/j.tox.2007.04.021. [DOI] [PubMed] [Google Scholar]


