Abstract
The bone marrow is the site of neutrophil production, a process that is regulated by the cytokine granulocyte colony-stimulating factor (G-CSF). Mature neutrophils are continually released into the circulation, with an estimated 1011 neutrophils exiting the bone marrow daily under basal conditions. These leucocytes have a short half-life in the blood of ∼6·5 hr, and are subsequently destroyed in the spleen, liver and indeed the bone marrow itself. Additionally, mature neutrophils are retained in the bone marrow by the stromal cell-derived factor (SDF-1α)/chemokine (C-X-C motif) receptor 4 (CXCR4) chemokine axis and form the bone marrow reserve. Following infection or inflammatory insult, neutrophil release from the bone marrow reserve is substantially elevated and this process is mediated by the co-ordinated actions of cytokines and chemokines. In this review we discuss the factors and molecular mechanisms regulating the neutrophil mobilization and consider the mechanisms and functional significance of neutrophil clearance via the bone marrow.
Keywords: bone marrow, chemokine, macrophage, neutrophil
Introduction
The innate arm of the immune system provides a critical first line of defence in the immune response against invading pathogens. In particular, neutrophils play an important role in limiting the expansion and dissemination of bacterial and viral infections. However, in a number of inflammatory diseases, such as acute respiratory distress syndrome (ARDS), post ischemic injury and arthritis, neutrophil accumulation and activation in tissues has a detrimental effect. Thus the controlled production, mobilization and subsequent clearance of these cells are a tightly regulated series of events.
Neutrophil mobilization
Neutrophil mobilization during inflammation
There is a large storage pool of mature neutrophils in the bone marrow, termed the bone marrow reserve. These neutrophils may be rapidly mobilized during an inflammatory episode or in response to infection, resulting in a dramatic rise in circulating neutrophil numbers. In mice, the bone marrow reserve is estimated to be 120 million cells, while the total number of neutrophils in the circulation of a naïve mouse is < 2·5 million. As such, the rapid egress of neutrophils from the bone marrow reserve can increase circulating numbers by 10-fold within a matter of hours. Mobilization from the bone marrow therefore represents a critical step in the trafficking of neutrophils to sites of inflammation.
The mature neutrophils that constitute the bone marrow reserve reside in the so-called haematopoietic cords and are separated from the blood by the bone marrow sinusoidal endothelium. Mobilization of neutrophils therefore requires the migration of neutrophils across the sinusoidal endothelium in an ablumenal to lumenal direction. Consistent with this mechanism of egress, we have shown that there is a significant increase in the absolute number of neutrophils present in the bone marrow sinusoids within 15 min of a single intravenous (i.v.) injection of the chemokine macrophage inflammatory protein-2 (MIP-2).1 It has been shown that the adventitial cells and basement membrane underlying these sinusoidal endothelial cells do not form a continuous barrier and thus the blood–bone marrow barrier is composed solely of the bone marrow sinusoidal endothelium. Analysis of the bone marrow by transmission electron microscopy shows that neutrophils traverse the endothelium by the process of transcellular migration, migrating through the endothelial cell body, rather than at cell–cell junctions1 (Fig. 1). Given that in humans 1011 neutrophils are mobilized from the bone marrow per day, it seems logical that cellular egress does not impair the integrity of this endothelial barrier. Indeed, when the endothelial barrier is impaired by administration of cytochalasin D, an agent that disrupts the actin cytoskeleton, leucocytes are released from the bone marrow in the absence of a specific stimulus in a non-selective manner.1 Thus the bone marrow sinusoidal endothelium plays a critical role in regulating leucocyte egress from the bone marrow.
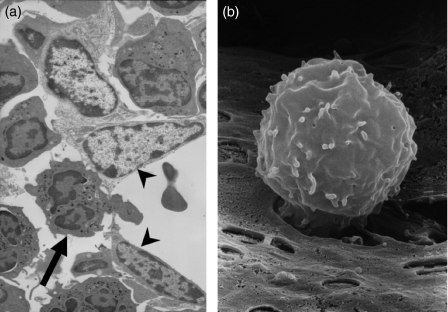
Figure 1.
Neutrophils which are mobilized from the bone marrow must cross the sinusoidal endothelium. (a) Electron micrograph illustrating the transcellular migration of a neutrophil (arrow) through murine bone marrow sinusoidal endothelium (arrowheads) into the lumen following infusion of 0·1 mm of the chemokine (C-X-C motif) receptor 4 (CXCR4) antagonist AMD3100 (Sigma Aldrich, Poole, UK) for 10 min, followed by 20 min with buffer alone, prior to perfusion fixation and preparation for transmission electron microscopy. (b) Migration of a neutrophil across the sinusoidal endothelium following infusion of macrophage inflammatory protein (MIP)-2. Rat femoral bone marrow was infused with MIP-2 (3 nmol/l) for 10 min, followed by 20 min with buffer alone, prior to perfusion fixation and preparation for scanning electron microscopy.
Neutrophil retention via the stromal cell-derived factor (SDF-1α)/chemokine (C-X-C motif) receptor 4 (CXCR4) chemokine axis
The chemokine SDF-1α (CXCL12) was originally identified in the supernatant of bone marrow stromal cells.2 It has subsequently been shown that this chemokine is expressed constitutively at high levels in the bone marrow. We have shown that mature neutrophils which comprise the bone marrow reserve express low, but detectable levels of CXCR4, the receptor for SDF-1α.3 Interestingly, these cells exhibit high intracellular levels of CXCR4, suggesting that when the receptor is expressed at the cell surface it may be rapidly endocytosed as a result of the high levels of SDF-1α in the bone marrow microenvironment. The level of CXCR4 expression on bone marrow neutrophils is not sufficient to support chemotaxis; however, when a CXCR4 antagonist is administered to mice or humans there is a rapid increase in circulating neutrophils as a result of the egress of neutrophils from the bone marrow, suggesting that SDF-1α acts as a retention factor for neutrophils in the bone marrow.3,4 Other lines of evidence support the hypothesis that endogenous SDF-1α is a retention factor for bone marrow neutrophils. Thus, when irradiated wild-type (WT) mice are re-populated with bone marrow from CXCR4-deficient mice, there are fewer neutrophils in the bone marrow.5 Furthermore, treatment of mice over several days with granulocyte colony-stimulating factor (G-CSF) was shown to reduce levels of SDF-1α and increase the mobilization of neutrophils from the bone marrow.6 Finally, it has been shown that patients with wart, hypogammaglobulinaemia, infection and myelokathexis (WHIM) syndrome have an activating mutation in the C terminal of CXCR4, and leucocytes from these patients which express CXCR4 exhibit enhanced responsiveness to SDF-1α.7 While these patients have normal numbers of neutrophils in their bone marrow they exhibit a blood neutropenia, which may be explained by the increased strength of the SDF-1α/CXCR4 chemokine axis retaining the neutrophils in the bone marrow.
Factors regulating neutrophil mobilization during inflammation
While it has been known for many years that many inflammatory reactions are associated with a rapid and selective mobilization of neutrophils from the bone marrow, the identity of the factors mediating this response was unknown. However, a variety of chemotactic factors, including leukotriene B4, C5a and the chemokine interleukin (IL)-8, have been shown to induce a rapid blood neutrophilia when injected i.v. into rabbits and mice, indicating that such factors can create chemotactic gradients from the blood across the sinusoidal endothelium, thereby driving neutrophil exit from the bone marrow.8–10 We developed an in situ perfusion system of the rat and mouse femoral bone marrow to directly investigate the factors and mechanisms regulating neutrophil mobilization from the bone marrow. In this system the femoral artery is cannulated to allow direct infusion of buffer and reagents into the bone marrow vasculature. Leucocytes exiting the bone marrow are collected via cannulation of the femoral vein. In this way the absolute numbers of leucocytes and their rate of mobilization can be quantified. Using this system we showed that the ELR (Glu-Leu-Arg motif) + CXC chemokines KC (CXCL1) and MIP-2 (CXCL2) infused directly into the femoral artery stimulated the rapid and selective mobilization of neutrophils from the bone marrow.3,11 Interestingly, we also showed that, when the SDF-1α retention signal was blocked with a CXCR4 antagonist, the chemokine-driven mobilization of neutrophils from the bone marrow was enhanced.3
Chemokines are generated locally at sites of inflammation and orchestrate the recruitment of specific subpopulations of leucocytes from the blood into tissues.12 Specifically, they have been shown to stimulate neutrophil adhesion and transmigration across the endothelium of post-capillary venules and to direct the migration of neutrophils within the tissue to the site of inflammation. While numerous studies have reported an elevation in plasma levels of CXC chemokines during inflammatory reactions, the functional significance of this was, for a long time, unclear.
To assess whether chemokines contributed to neutrophil mobilization during an inflammatory response, we used a murine model of acute peritonitis. Two hours following a single intraperitoneal (i.p.) injection of thioglycollate, we noted a significant increase in the circulating numbers of neutrophils. This was inhibited by 84% when mice were pretreated with neutralizing monoclonal antibodies (mAbs) to the CXC chemokines KC and MIP-2, indicating that these chemokines, generated in the peritoneum, acted remotely to promote neutrophil mobilization from the bone marrow.13 Indeed, when chemokine alone was administered i.p., after 2 hr we observed an increase in both circulating numbers of neutrophils and the numbers of neutrophils in the peritoneum, consistent with the concept that chemokines have a dual action, acting locally to stimulate recruitment and systemically to promote mobilization.
Mice with genetic deletion of either G-CSF or the G-CSF receptor (G-CSFR) have very few neutrophils in their blood and bone marrow, and evidence suggests that under homeostatic conditions G-CSF regulates both granulopoiesis and neutrophil mobilization from the bone marrow.6,14 The latter effect is thought to be attributable to an indirect effect of G-CSF reducing the production of SDF-1α by stromal cells and down-regulating CXCR4 expression on neutrophils.6,15,16 While these are chronic effects of G-CSF, it has also been shown that a single intravenous injection of G-CSF leads to a rapid increase in circulating neutrophil numbers in both mice and humans. We have shown that direct infusion of G-CSF into the bone marrow vasculature using the in situ perfusion system of the femoral bone marrow leads to the selective mobilization of neutrophils.13 Increased serum levels of G-CSF have been associated with inflammation in animal models and in humans.17–20 In the acute peritonitis model, blockade of G-CSF caused a significant reduction in both the blood and tissue neutrophils. However, interestingly, an i.p. injection of G-CSF alone resulted in a rapid increase in circulating neutrophil numbers, but critically did not stimulate neutrophil recruitment into the peritoneum. Thus, in contrast to the chemokines, G-CSF generated at the site of inflammation acts solely to stimulate neutrophil mobilization from the bone marrow (Fig. 2). These findings suggest that the mechanisms underlying neutrophil mobilization by chemokines and G-CSF are distinct. Indeed, in contrast to ELR + CXC chemokines, G-CSF was neither chemotactic or chemokinetic for murine neutrophils in vitro. Further, G-CSF did not prime the migratory responses of neutrophils to chemokines.
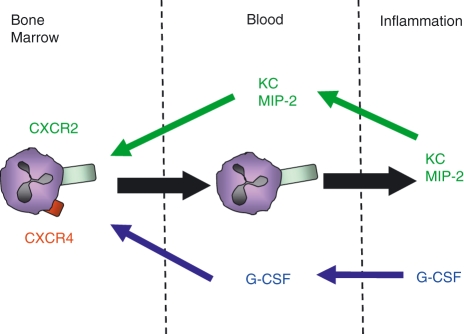
Figure 2.
Granulocyte colony-stimulating factor (G-CSF) and ELR (Glu-Leu-Arg motif) + CXC chemokines act in a co-ordinated manner to mobilize and recruit neutrophils to the site of inflammation. G-CSF (blue arrows) and the ELR + CXC chemokines macrophage inflammatory protein (MIP)-2 and KC (green arrows) act to mobilize neutrophils from the bone marrow into the circulation; however, only MIP-2 and KC recruit neutrophils from the blood to the site of inflammation. KC and MIP-2 act via chemokine CXC receptor 2 (CXCR2) on neutrophils, whereas G-CSF down-regulates the expression of CXCR4 on neutrophils, thus reducing retention via the CXCR4/stromal cell-derived factor (SDF-1α) axis and mobilizing neutrophils into the circulation.
As described above, we have previously shown that the chemokine SDF-1α delivers a negative signal to bone marrow neutrophils, reducing their migration in response to the CXC chemokines. We believe that this negative signal acts in vivo to retain neutrophils in the bone marrow, where SDF-1α is produced constitutively. Intriguingly, we found that in vitro the inhibitory effect of SDF-1α on neutrophil chemotaxis could be abrogated by addition of G-CSF, thereby reconstituting chemotaxis towards the CXC chemokine.13 It has previously been reported that, when mice are treated over several days with G-CSF, levels of SDF-1α are reduced in the bone marrow.6 Furthermore, G-CSF can reduce the expression of CXCR4 on neutrophils.15,16 Our results suggest that G-CSF can also rapidly inhibit the retention signal delivered by SDF-1α acting via CXCR4, thereby stimulating the acute mobilization of neutrophils from the bone marrow. The molecular mechanisms underlying this response are currently unclear.
In the peritonitis model, blockade of either G-CSF or CXC chemokines alone resulted in > 70% inhibition of neutrophil mobilization, suggesting to us that during an inflammatory response optimal mobilization requires the combined actions of these two factors. Indeed, using the in situ perfusion system of the murine femoral bone marrow, we showed that infusion of either chemokine or G-CSF alone led to the acute mobilization of neutrophils; however, when G-CSF was co-infused with the chemokine, the number of neutrophils mobilized was more than additive.13
We propose a new paradigm for the acute mobilization of neutrophils from the bone marrow in response to inflammation that is dependent on the co-ordinated actions of both G-CSF and CXC chemokines acting via distinct mechanisms. Thus we believe that G-CSF disrupts the retention signal delivered by SDF-1α in the bone marrow and thereby facilitates the migration of neutrophils across the bone marrow sinusoidal endothelium in response to the chemotactic gradient created by blood-borne chemokines.
Molecular mechanisms regulating neutrophil mobilization
Integrins
Neutrophils in the blood adhere to and migrate across the vascular endothelium of postcapillary venules to gain access to sites of inflammation. The role of specific adhesion molecules in this process has been studied extensively (reviewed in Ley et al.21). In contrast, very little is known about the role of adhesion molecules in the egress of leucocytes across the bone marrow sinusoidal endothelium during their mobilization. Of note, prior studies examining the bone marrow by immunohistochemistry revealed that the bone marrow sinusoidal endothelium is unique in that it constitutively expresses vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), P-selectin and E-selectin, molecules normally up-regulated at sites of inflammation.22,23 Using our in situ perfusion system and specific blocking mAbs, we therefore investigated the role of specific adhesion molecules in the mobilization process.
The expression of L-selectin (CD62L) on neutrophils plays a role in tethering and rolling along postcapillary venules, prior to their transmigration across the vascular endothelium to sites of inflammation.24 When activated with chemokines, neutrophils rapidly shed L-selectin within a matter of minutes.25 While shedding is not required for leucocyte transmigration per se, it regulates the velocity with which neutrophils roll along the endothelium.26,27 In terms of neutrophil egress out of the bone marrow, neutrophils do not roll along the endothelium; hence the relevance of L-selectin shedding to this process is questionable. However, analysis of phenotypic changes in neutrophils during inflammatory responses in humans and in animal models consistently reveals that neutrophils rapidly mobilized from the bone marrow exhibit very low levels of L-selectin.28,29 Furthermore, it has been proposed that L-selectin is a retention factor for neutrophils in the bone marrow and that shedding is a prerequisite for mobilization.29 Similarly, using the in situ perfusion system of the femoral bone marrow, we have shown that neutrophils shed L-selectin as they are mobilized from the bone marrow in response to chemokines.11 However, when a sheddase inhibitor was infused into the bone marrow vasculature, chemokine-driven neutrophil mobilization was unaffected, suggesting that shedding is not required for this process.11
Neutrophils express high levels of the CD18 integrins, in particular CD11a:CD18 [lymphocyte function-associated antigen (LFA)-1] and CD11b:CD18 (Mac-1). These integrins mediate firm adhesion to ICAM-1, an adhesion molecule expressed by the endothelium during inflammation, and are invariably required for the recruitment of neutrophils into tissue during inflammatory reactions.21 However, there is a report that shows that blood neutrophilia stimulated by an intravenous injection of lipopolysaccharide (LPS), C5a or tumour necrosis factor (TNF)-α is not reduced by neutralization of CD18.30 Further, our studies show that blockade of CD18 with a neutralizing mAb does not affect the rate or total number of neutrophils mobilized from the bone marrow in response to a CXC chemokine.11 Taken together, these results indicate that CD18 is not required for neutrophil mobilization during inflammation.
The adhesion molecule VCAM-1 is not constitutively expressed on endothelium in the majority of tissues, but up-regulated during inflammatory reactions. However, this adhesion molecule is expressed constitutively by bone marrow stromal cells and the bone marrow sinusoidal endothelium.22,23 The alpha 4 integrin CD49d, which binds to VCAM-1, is expressed at low levels on rodent neutrophils and has been shown to play a role in neutrophil recruitment into tissues during specific inflammatory reactions.31,32 We found that the expression of CD49d is elevated as neutrophils are mobilized from the bone marrow. Further, both neutralizing mAbs and a specific CD49d antagonist inhibited neutrophil mobilization stimulated by the CXC chemokine MIP-2.11 This suggests that the transendothelial migration of neutrophils across the bone marrow endothelium is facilitated by an interaction of CD49d on neutrophils with VCAM-1 expressed by the sinusoidal endothelium. Whether such interactions also operate in humans is currently unknown.
Signalling molecules
We propose that chemokines stimulate the egress of neutrophils from the bone marrow by effectively creating a chemotactic gradient across the sinusoidal endothelium and thereby stimulating neutrophil chemotaxis and transmigration. The recruitment of neutrophils to sites of inflammation in vivo and their chemokine-driven migration in vitro can be severely reduced by application of specific inhibitors of p38MAP kinase.33–35 We showed that an inhibitor of p38MAP kinase caused a dose-dependent inhibition of the chemotaxis of rat bone marrow-derived neutrophils towards MIP-2 in vitro and caused a marked inhibition of the chemokine-stimulated mobilization of neutrophils in situ.1 Inhibition of neutrophil mobilization may therefore represent another relevant target for the anti-inflammatory actions of p38 inhibitors.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) have been shown to play a role in the recruitment of neutrophils to sites of inflammation, where neutrophils are required to migrate extravascularly through the extracellular matrix. In contrast, using a broad-spectrum MMP inhibitor, we have found no direct involvement of MMPs in neutrophil mobilization stimulated by the CXC chemokines.1
Clearance of neutrophils via the bone marrow
Trafficking to the bone marrow
Following their recruitment to sites of infection and injury, there is a subsequent need for the removal and clearance of infiltrating leucocytes, including neutrophils, in order to promote the resolution of inflammation.36 Neutrophil clearance in such situations has been well characterized;37 however, neutrophil clearance under homeostatic conditions has been less well documented. Under normal conditions apoptotic neutrophils are not detected in the circulation and when we consider that the half-life of a neutrophil in the circulation is ∼6·5 hr38 and that 1011 of these cytotoxic cells are released into the blood per day, the need for an efficient removal system is evident.
Neutrophils are programmed to respond rapidly to inflammatory insults and so the expression of their cell surface receptors is extremely dynamic. Additionally, alterations in receptor expression patterns occur as cells age; thus, as neutrophils become senescent, there is a progressive decrease in CXCR2 expression and a concomitant increase in CXCR4.3,39 This corresponds functionally to a decline in migration towards ELR + CXC chemokines, such as KC and MIP-2, and an increased responsiveness to SDF-1α. These events occur prior to the onset of apoptosis, and thus senescent neutrophils are functionally and phenotypically distinct from apoptotic neutrophils. The bone marrow is known to constitutively express high levels of SDF-1α, and we and others have shown that the migration of senescent neutrophils to this tissue is dependent upon the CXCR4/SDF-1α chemokine axis. However, the fate of neutrophils upon reaching the bone marrow has previously been unclear. We have shown that CXCR4hi-expressing cells preferentially home to the bone marrow and thus we hypothesized that the bone marrow is a site of neutrophil clearance. Consistent with this theory, in both human and animal models, following the i.v. injection of radiolabelled neutrophils under basal conditions, high levels of radioactivity were detected in the bone marrow, liver and spleen.40–43 We have quantified this in a murine model and have confirmed that each of these organs is responsible for the clearance of ∼30% of neutrophils from the circulation under homeostatic conditions.44
The role of stromal macrophages
The role of macrophages in the removal of cellular debris, including apoptotic neutrophils, from sites of inflammation has been well described.37 Furthermore, reticular endothelial macrophages in the spleen and liver are responsible for the removal of pre-apoptotic neutrophils from the circulation under homeostatic and inflammatory conditions.41,44,45 Thus we hypothesized that resident bone marrow macrophages present in the haematopoietic cords may have a similar function. A role for stromal macrophages in removal of extraneous cellular material in bone marrow is not without precedent as they are known to be essential for the removal of non-productive B cells during lymphopoiesis46,47 and removal of nuclei expelled by developing erythroblasts.48 Additionally, early electron microscope (EM) studies revealed that the bone marrow is an active site of neutrophil degradation during embyrogenesis. In one study 75% of leucocytes in the fetal bone marrow were shown to be granulocytes in varying stages of cell death, and apoptotic neutrophils within macrophages were observed.49 The conclusion was drawn that prior to the establishment of haematopoiesis the bone marrow was the site of neutrophil destruction. Further to this, electromicrographs from human adult bone marrow revealed apoptotic neutrophils within stromal macrophages.50 However, in both cases it was unclear if these represented cells that had returned to the marrow or that were being destroyed prior to release into the circulation. We have recently demonstrated that myeloperoxidase-positive macrophages containing apoptotic neutrophils can be readily observed in normal bone marrow under homeostatic conditions. Furthermore, using a model system where senescent neutrophils were stably labelled with fluorescent microspheres and injected intravenously into mice, we were able to demonstrate that, upon return to the bone marrow, senescent neutrophils are specifically cleared by resident stromal macrophages.44
The role of G-CSF and IL-17
Whilst macrophages of the reticular endothelial system in the spleen and the liver are ideally situated for the removal of circulating cells, neutrophils must actively cross the bone marrow sinusoidal endothelium to enter the hematopoietic compartment of the bone marrow where stromal macrophages are located. This demonstrates that neutrophil migration to the bone marrow is an active rather than passive event, as apoptotic neutrophils are unable to migrate. Furthermore, such an expenditure of energy implies a functional purpose for this process. This may be explained by considering the dual function of the bone marrow in both the production and clearance of neutrophils.
In addition to its role in mobilization of the neutrophil reserve during inflammation, G-CSF plays a key role in granulopoiesis under both inflammatory and homeostatic conditions; however, the cellular source of G-CSF in the bone marrow and the factors regulating its production under homeostatic conditions has not been identified.51 Interestingly, we have shown that the uptake of apoptotic neutrophils by bone marrow macrophages stimulates the production of G-CSF.44 This is in contrast to inflammatory macrophages, for example peritoneal macrophages, which down-regulate cytokine (including G-CSF) and chemokine production following phagocytosis of apoptotic cells and thus dampen the immune response and promote resolution and clearance of inflammation.36 It is perhaps not surprising that stromal macrophages respond in a different manner to activated, inflammatory macrophages in terms of cytokine and chemokine expression and this is consistent with the observation that these different subsets of macrophages rely on differential receptor expression for the phagocytosis of apoptotic cells.52,53 We propose that the clearance of neutrophils in the bone marrow represents a potential mechanism for the local regulation of G-CSF, and hence neutrophil, production. Our model suggests that under homeostatic conditions senescent neutrophils return to the bone marrow via the CXCR4/SDF-1α chemokine axis where they are cleared by resident stromal macrophages. This stimulates the production of G-CSF, which may act as a positive mechanism for maintaining neutrophil homeostasis (Fig. 3).
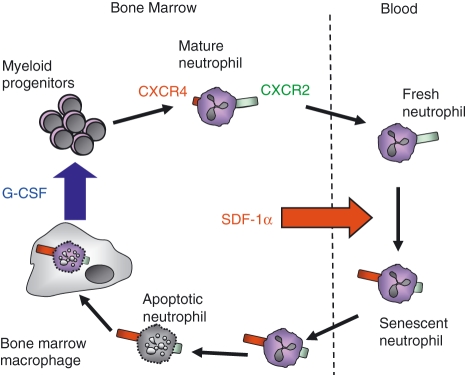
Figure 3.
Proposed mechanism for neutrophil production and clearance in the bone marrow under homeostatic conditions. Granulocyte colony-stimulating factor (G-CSF) promotes the differentiation of mature neutrophils from myeloid precursors in the bone marrow. Stromal cell-derived factor (SDF-1α) is expressed constitutively in the bone marrow, and mature neutrophils, which express low levels of chemokine (C-X-C motif) receptor 4 (CXCR4), are retained via the CXCR4/SDF-1α axis. In the absence of inflammation, neutrophils become senescent and express high levels of CXCR4, directing them back to the bone marrow. Once in the haematopoietic compartment of the marrow, neutrophils become apoptotic and are engulfed by resident stromal macrophages, which subsequently induces the production of G-CSF by the bone marrow macrophages.
The cytokine IL-17 has been shown to be involved in neutrophil recruitment in several inflammatory models via the induction of G-CSF and ELR + CXC chemokines.54–57 Studies using adhesion molecule-deficient mice have implicated IL-17 in homeostatic regulation, but the high plasma levels of both G-CSF and IL-17, and thus inherent neutrophilia, in these mice complicate interpretation of these data.58 However, IL-17-deficient animals have normal numbers of circulating neutrophils compared with wild type59 and the blockade of G-CSF but not IL-17 under basal conditions decreases the number of circulating neutrophils.44 Together, these data indicate that IL-17 plays no role in the trafficking of neutrophils to the bone marrow under basal conditions, but may be an important factor under inflammatory conditions.
Concluding remarks
Neutrophils are one of the first cells to be recruited in order to defend the body against invading pathogens, yet also are a major cause of pathology when uncontrolled. A more complete understanding of the mechanisms that control their mobilization, recruitment and clearance will help elucidate the processes by which the body responds to and resolves inflammation and may thus reveal new therapeutic strategies for the treatment of inflammatory disorders.
Acknowledgments
The authors thank C. A. Dewer for help with the EM and S. C. Pitchford and P. C. Burdon (Imperial College) for supplying the murine TEM and rat SEM pictures. This work was supported by The Wellcome Trust.
References
- 1.Burdon PC, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br J Haematol. 2008;142:100–8. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- 2.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin C, Burdon PCE, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–93. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 4.Hendrix CW, Collier AC, Lederman MM, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37:1253–62. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 5.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 6.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–23. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–4. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 8.Jagels MA, Hugli TE. Neutrophil chemotactic factors promote leukocytosis. A common mechanism for cellular recruitment from bone marrow. J Immunol. 1992;148:1119–28. [PubMed] [Google Scholar]
- 9.Terashima T, English D, Hogg JC, van Eeden SF. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood. 1998;92:1062–9. [PubMed] [Google Scholar]
- 10.Jagels MA, Chambers JD, Arfors KE, Hugli TE. C5a- and tumor necrosis factor-alpha-induced leukocytosis occurs independently of beta 2 integrins and L-selectin: differential effects on neutrophil adhesion molecule expression in vivo. Blood. 1995;85:2900–9. [PubMed] [Google Scholar]
- 11.Burdon PC, Martin C, Rankin SM. The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood. 2005;105:2543–8. doi: 10.1182/blood-2004-08-3193. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–16. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 13.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–9. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–46. [PubMed] [Google Scholar]
- 15.De La Luz SM, Gasperini P, McCormick PJ, Zhu J, Tosato G. Transcription factor Gfi-1 induced by G-CSF is a negative regulator of CXCR4 in myeloid cells. Blood. 2007;110:2276–85. doi: 10.1182/blood-2007-03-081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HK, De La Luz SM, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–20. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf D, Robb L, Dunn AR, Mifsud S, Di RL. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755–64. [PubMed] [Google Scholar]
- 18.Shahbazian LM, Quinton LJ, Bagby GJ, Nelson S, Wang G, Zhang P. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med. 2004;32:1740–6. doi: 10.1097/01.ccm.0000132900.84627.90. [DOI] [PubMed] [Google Scholar]
- 19.Cataisson C, Pearson AJ, Tsien MZ, et al. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J Clin Invest. 2006;116:2757–66. doi: 10.1172/JCI27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noursadeghi M, Bickerstaff MC, Herbert J, Moyes D, Cohen J, Pepys MB. Production of granulocyte colony-stimulating factor in the nonspecific acute phase response enhances host resistance to bacterial infection. J Immunol. 2002;169:913–9. doi: 10.4049/jimmunol.169.2.913. [DOI] [PubMed] [Google Scholar]
- 21.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 22.Simmons PJ, Masinovsky B, Longenecker BM, Berenson R, Torok-Storb B, Gallatin WM. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992;80:388–95. [PubMed] [Google Scholar]
- 23.Schweitzer KM, Drager AM, van der Valk P, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–66. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 26.Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–48. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–72. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogowski O, Sasson Y, Kassirer M, et al. Down-regulation of the CD62L antigen as a possible mechanism for neutrophilia during inflammation. Br J Haematol. 1998;101:666–9. doi: 10.1046/j.1365-2141.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 29.Kassirer M, Zeltser D, Gluzman B, et al. The appearance of L-selectin (low) polymorphonuclear leukocytes in the circulating pool of peripheral blood during myocardial infarction correlates with neutrophilia and with the size of the infarct. Clin Cardiol. 1999;22:721–6. doi: 10.1002/clc.4960221109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larangeira AP, Silva AR, Gomes RN, et al. Mechanisms of allergen- and LPS-induced bone marrow eosinophil mobilization and eosinophil accumulation into the pleural cavity: a role for CD11b/CD18 complex. Inflamm Res. 2001;50:309–16. doi: 10.1007/PL00000249. [DOI] [PubMed] [Google Scholar]
- 31.Issekutz AC, Ayer L, Miyasaka M, Issekutz TB. Treatment of established adjuvant arthritis in rats with monoclonal antibody to CD18 and very late activation antigen-4 integrins suppresses neutrophil and T-lymphocyte migration to the joints and improves clinical disease. Immunology. 1996;88:569–76. doi: 10.1046/j.1365-2567.1996.d01-695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issekutz TB, Miyasaka M, Issekutz AC. Rat blood neutrophils express very late antigen 4 and it mediates migration to arthritic joint and dermal inflammation. J Exp Med. 1996;183:2175–84. doi: 10.1084/jem.183.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nick JA, Young SK, Brown KK, et al. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol. 2000;164:2151–9. doi: 10.4049/jimmunol.164.4.2151. [DOI] [PubMed] [Google Scholar]
- 34.Cara DC, Kaur J, Forster M, McCafferty DM, Kubes P. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J Immunol. 2001;167:6552–8. doi: 10.4049/jimmunol.167.11.6552. [DOI] [PubMed] [Google Scholar]
- 35.Branger J, van den BB, Weijer S, et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–7. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- 36.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–6. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 37.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM. Leukokinetic studies II. A method for labelling granulocytes in vitro with radioactive diidopropylfluorophosphate (DFP) J Clin Invest. 1960;39:1481–6. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagase H, Miyamasu M, Yamaguchi M, et al. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol. 2002;71:711–7. [PubMed] [Google Scholar]
- 40.Thakur ML, Lavender JP, Arnot RN, Silvester DJ, Segal AW. Indium-111-labeled autologous leukocytes in man. J Nucl Med. 1977;18:1014–21. [PubMed] [Google Scholar]
- 41.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L913–21. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- 42.Lovas K, Knudsen E, Iversen PO, Benestad HB. Sequestration patterns of transfused rat neutrophilic granulocytes under normal and inflammatory conditions. Eur J Haematol. 1996;56:221–9. doi: 10.1111/j.1600-0609.1996.tb01933.x. [DOI] [PubMed] [Google Scholar]
- 43.Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. Br J Haematol. 1985;61:675–85. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 44.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–9. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–30. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 46.Osmond DG, Rico-Vargas S, Valenzona H, et al. Apoptosis and macrophage-mediated cell deletion in the regulation of B lymphopoiesis in mouse bone marrow. Immunol Rev. 1994;142:209–30. doi: 10.1111/j.1600-065x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 47.Dogusan Z, Montecino-Rodriguez E, Dorshkind K. Macrophages and stromal cells phagocytose apoptotic bone marrow-derived B lineage cells. J Immunol. 2004;172:4717–23. doi: 10.4049/jimmunol.172.8.4717. [DOI] [PubMed] [Google Scholar]
- 48.Sadahira Y, Mori M. Role of the macrophage in erythropoiesis. Pathol Int. 1999;49:841–8. doi: 10.1046/j.1440-1827.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki K, Iwatsuki H, Suda M, Itano C. Accumulation and massive cell death of polymorphonuclear neutrophils in the developing bone marrow of the mouse: a histological study. Acta Anat (Basel) 1995;153:111–8. doi: 10.1159/000147721. [DOI] [PubMed] [Google Scholar]
- 50.Dresch C, Flandrin G, Breton-Gorius J. Phagocytosis of neutrophil polymorphonuclears by macrophages in human bone marrow: importance in granulopoiesis. J Clin Pathol. 1980;33:1110–3. doi: 10.1136/jcp.33.11.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biol Chem. 2002;383:1501–17. doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- 52.Fadok VA, Savill JS, Haslett C, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–35. [PubMed] [Google Scholar]
- 53.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 54.Witowski J, Pawlaczyk K, Breborowicz A, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–21. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 55.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 56.Laan M, Cui ZH, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 57.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–72. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 58.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]