Abstract
Activated T cells, through the production of the receptor activator of NF-κB ligand (RANKL) cytokine, have been implicated in the osteoclast development and bone loss that are associated with autoimmune diseases such as rheumatoid arthritis. However, the cellular pathways that regulate the expression of RANKL and the induction of osteoclasts are still unclear. In this study, we show that, in human effector CD4+ T cells, activation of α1β1 integrin and interleukin (IL)-7 receptor (IL-7R) up-regulates the expression and production of RANKL but has no effect on the production of interferon-γ, an inhibitor of T-cell-mediated osteoclastogenesis. Thus, both α1β1 integrin and IL-7R enhance the ability of these cells to induce the formation of osteoclasts from human monocytes. Furthermore, we found that simultaneous activation of effector CD4+ T cells via α1β1 integrin and IL-7R synergistically increases the production of RANKL and enhances their osteoclastogenic function. We also show that, although α1β1 integrin does not protect human effector CD4+ T cells from IL-2-withdrawal-induced apoptosis, it does enhance the pro-survival effect of IL-7, further emphasizing the importance of the α1β1/IL-7R synergistic effect. Together our results identify a new function of α1β1 integrin in T cells and suggest that activation of effector CD4+ T cells through α1β1 integrin and IL-7R is an important regulatory pathway in T-cell-dependent osteoclastogenesis. Further understanding of the mechanisms by which IL-7R and α1β1 integrin promote T-cell-mediated osteoclastogenesis will lead to new insights into the regulatory pathways of T-cell-dependent bone resorption associated with autoimmune diseases.
Keywords: effector T cells, integrins, osteoclastogenesis, RANKL
Introduction
The homeostasis of the skeletal system depends on a balance between bone-forming osteoblasts and bone-resorbing osteoclasts. The ability of the immune system, through tissue-persisting activated/effector CD4+ T cells, to regulate this balance towards osteoclasts contributes to pathological bone resorption seen in chronic inflammatory diseases such as rheumatoid arthritis (RA), periodontitis and inflammatory bowel disease.1–4 This is in part attributable to the ability of activated T cells to produce the osteoclastogenic cytokine receptor activator of NF-κB ligand (RANKL), which following its binding to its receptor RANK expressed on osteoclast precursors initiates the differentiation programme that leads to the formation of mature osteoclasts.1–5 Activated T cells in vitro or isolated T cells from patients with chronic inflammatory diseases such as RA produce RANKL and directly induce the development of osteoclasts from autologous monocytes used as osteoclast precursors.6,7
The development of osteoclasts is also dependent on additional cytokines such as osteoprotegerin (OPG), which can be produced by stromal cells8 OPG is a decoy receptor of RANKL and interferes with RANKL-induced differentiation of osteoclasts.2 Interferon (IFN)-γ, which can be produced by activated T cells, also blocks the RANKL/RANK signalling pathway and the subsequent formation of osteoclasts.9,10 In this context, activated T cells that produce large amounts of IFN-γ, such as in response to T-cell receptor (TCR) stimulation, are unable to induce the formation of osteoclasts.9–11 In contrast, interleukin (IL)-7, which is an important cytokine in T-cell development and survival,12 has been shown to induce the development of osteoclasts through T-cell production of RANKL,13,14 suggesting that the ability of activated T cells to induce the formation of osteoclasts is probably dependent on how they are activated. It is noteworthy that most of the above studies were conducted with freshly isolated resting T cells, which are unlikely to be representative of the effector T cells found at the sites of tissue injury and which are associated with the inflammatory response. It is therefore still unclear how effector T cells are activated to potently induce the formation of osteoclasts.
Integrins are α/β heterodimeric membrane proteins that mediate cell–cell interactions and cell adhesion to the surrounding extracellular matrix (ECM). In addition, integrins can elicit a wide variety of intracellular signals that modulate cell growth, proliferation and apoptosis.15,16 T cells express several members of the β1 integrin family, which following cellular activation mediate their attachment to the ECM.17,18 In addition, T-cell interactions with the ECM in injured tissues also regulate their effector functions. Recent studies have focused on the function of collagen-binding integrins α1β1 and α2β1, which are expressed on effector/memory T cells infiltrating the tissues.18–20 In this regard, several studies, including ours, have demonstrated that these integrins co-stimulate TCR-dependent proliferation and cytokine production and protect effector T cells from apoptosis.21–24 In addition, α1β1 integrin has been shown to be involved in the development of RA, a chronic inflammatory disease associated with bone resorption. Indeed, infiltrating T cells from the arthritic synovium of patients express high levels of α1β1 integrin25,26 and its blockade with antibodies significantly reduces the inflammatory response and the development of arthritis in animal models.27,28 Whether α1β1 integrin has a direct role in T-cell-mediated osteoclastogenesis has not been explored. To explore this possibility, we have examined whether activation of α1β1 integrin regulates the expression of RANKL and the ability of effector CD4+ T cells to induce the formation of osteoclasts. Furthermore, we have also examined whether IL-7 regulates RANKL expression in these cells, and whether α1β1 integrin enhances the osteoclastogenic function of IL-7, as integrins can act as co-stimulatory molecules.
Our results show that ligation of α1β1 integrin with its ligand collagen IV (Coll IV) or activation of IL-7R with IL-7 up-regulates to the same extent the expression of RANKL in human effector CD4+ T cells. This is correlated with the ability of Coll IV- and IL-7-activated T cells to induce the formation of osteoclasts from monocytes. Importantly, activation of effector CD4+ T cells with both Coll IV and IL-7 led to a synergistic induction of RANKL and development of osteoclasts. Finally, we show that ligation of α1β1 integrin also augments the ability of IL-7 to protect these cells from IL-2-withdrawal-induced apoptosis. Our results indicate that α1β1 integrin and IL-7R may represent two major regulatory pathways that contribute to the osteoclastogenic function of effector CD4+ T cells and may thus be important in T-cell-dependent bone loss associated with the development of autoimmune and chronic inflammatory diseases.
Materials and methods
Antibodies and reagents
Anti-CD3 (OKT3), anti-CD28 (CD28·2), anti-CD127 (hIL-7R-M21) and isotype control monoclonal antibodies (mAbs) were purchased from BD Biosciences (San Diego, CA). The anti-α1 integrin (FB12) and poly-l-lysine (PLL) were purchased from Chemicon (Temecula, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-mouse mAbs were purchased from Jackson Immunoresearch (West Grove, PA), Coll IV and IL-2 from Sigma (St Louis, MO), and IL-7, macrophage–colony-stimulating factor (M-CSF) and anti-human RANKL mAb (70525) from R&D Systems (Minneapolis, MN).
Isolation of primary CD4+ T cells and generation of long-term activated lymphoblasts
Peripheral blood mononuclear cells (PBMC) from healthy adult volunteers were isolated on Ficoll gradients. Primary human naïve CD4+ T cells were then purified by negative selection using magnetic beads from StemCell Technologies (Vancouver, BC, Canada) according to the manufacturer’s instructions. Staining with anti-CD3 and anti-CD4 mAbs and flow cytometry [fluorescence-activated cell sorter (FACS)] analysis indicated that more than 97% of the isolated cells were CD3/CD4 double-positive T cells. Freshly isolated blood T cells do not express the collagen-binding integrins α1β1 and α2β1 but do so after in vitro activation.18,19,24 Thus, the cells were activated with immobilized anti-CD3 (2·5 μg/ml) and soluble anti-CD28 (5 μg/ml) mAbs for 24 hr in complete RPMI 1640 medium supplemented with 10% of fetal bovine serum (FBS), 2 mm/l glutamine and 100 units/ml penicillin and streptomycin. The cells were then washed, transferred to flasks and cultured for 10 more days in the presence of 20 U/ml of recombinant IL-2, which was added every 2 days. Because IL-2 down-regulates the expression of IL-7R on activated/effector T cells,29 the cells at day 10 were cultured for one more day in fresh medium in the absence of IL-2 before being used in subsequent experiments. These cells, which will be referred to as effector CD4+ T cells, are responsive to TCR activation, which leads to IL-2 and IFN-γ production.
Cell surface molecule expression
The expression of cell surface receptors on effector CD4+ T cells was determined by immunostaining and flow cytometry analysis. The cells were incubated on ice with 10 μg/ml of anti-α1 integrin or anti-CD127 or with control isotypic mAbs for 45 min in PBS containing 1% FBS. The cells were washed and incubated with FITC-conjugated anti-mouse mAb for another 45 min. Cells were washed in PBS and analysed by flow cytometry using a FACScan instrument (BD Biosciences).
Enzyme-linked immunosorbent assays (ELISAs)
The production of IFN-γ and soluble RANKL was measured using specific ELISAs from R&D Systems (Minneapolis, MN) and Biomedica (Vienna, Austria), respectively. After 24 hr of activation, cell supernatants were harvested and loaded on an ELISA plate according to the manufacturer’s instructions.
Apoptosis assays
Apoptosis was determined using the Annexin V-PE/7AAD detection kit from BD Biosciences. After stimulation, the cells were washed in cold PBS, and 105 cells were incubated in 1× buffer containing 5 μl of Annexin V-PE and 5 μl of 7-aminoactinomycin (7-AAD) for 15 min at room temperature, in the dark. The cells were then washed and analysed by flow cytometry using the FACScan instrument (BD Biosciences) and apoptotic cells were identified as cells that were Annexin V-positive.
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted with Trizol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). First-strand cDNA was prepared from 1 μg of total RNA using the Thermoscript RT-PCR system from Invitrogen. RANKL and β-actin transcripts were amplified by PCR using specific primers as previously described.30 PCR reactions were performed with 1 U Taq polymerase in a total volume of 50 μl, and amplifications were carried out in a Peltier Thermal Cycler (MJ Research, Ramsey, MN). The amplification for each gene was in the linear curve, i.e. under non-saturating conditions during the exponential phase. A volume of 10 μl of the reaction mixture was size-separated on a 2% agarose gel, and specifically amplified products were detected by ethidium bromide staining and UV illumination.
Immunoblot analysis
The cells were resuspended in RPMI 1640 medium and stimulated in six-well plates. At the end of cultures, the cells were harvested and washed in cold PBS, and cell lysates were prepared in radio-immune precipitation assay (RIPA) buffer containing protease and phosphatase inhibitors as previously described.24 Cell lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and analysed by immunoblot using specific anti-RANKL mAb. The blots were stripped and re-probed with control anti-actin mAb (Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equal loading. In all experiments, immunoblots were visualized using a horseradish peroxidase (HRP)-conjugated secondary antibody followed by enhanced chemiluminescence detection (Pierce, Rockford, IL).
Osteoclastogenesis experiments
The ability of RANKL-expressing effector CD4+ T cells to induce the development of osteoclasts was tested in a co-culture assay with human monocytes used as osteoclasts precursors. Human CD16-negative monocytes, which have the ability to differentiate into osteoclasts,31 were isolated from the peripheral blood of healthy volunteers by immunomagnetic negative selection (StemCell Technologies, Vancouver, BC, Canada). Monocytes (1 × 105 cells) were seeded in 48-well plates and were activated with M-CSF (100 ng/ml) in alpha minimum eagle medium (αMEM) supplemented with serum and antibiotics. Shortly thereafter, effector T cells (1 × 105 cells) that had been activated or not for 12 hr with IL-7, Coll IV, anti-CD3 mAb, IL-7 + Coll IV or anti-CD3 + Coll IV were added to the monocytic cultures. Culture media and activated T cells were replaced every 2 days with fresh media and freshly activated effector T cells, respectively, as above. At the end of the experiment (day 8), osteoclast development was visualized by tartrate-resistant acid phosphatase (TRAP) staining (Sigma) according to the manufacturer’s instructions. After staining, multinucleated (≥4 nuclei) TRAP-positive cells were counted as osteoclasts.
Results
Expression of α1β1 integrin and IL-7R on effector CD4+ T cells
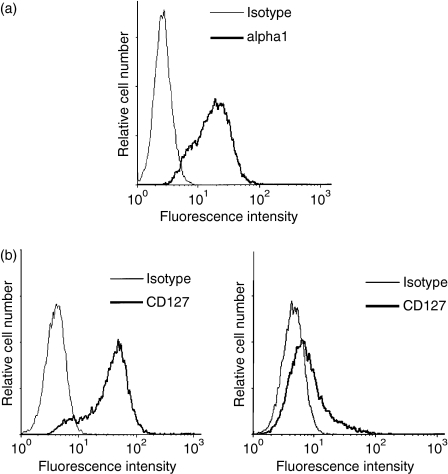
To determine the involvement of α1β1 in T-cell-mediated osteoclastogenesis, we used effector CD4+ T cells generated after in vitro activation. These cells express high levels of α1 integrin, as we and others have previously shown.19,24,32 After 12 days of activation, almost 78% of the total cell population stained positive for α1 integrin, with an average mean fluorescence intensity (MFI) of 25·71 (Fig. 1a). These cells were also found to express high levels of IL-7R, as 90% of the total cell population stained positive for the α chain of IL-7R (CD127) with an MFI of 50 (Fig. 1b; left panel). The expression of IL-7R detected on human effector CD4+ T cells is in agreement with reports showing that effector T cells can maintain significant levels of IL-7R.33–35 The cells were also responsive to IL-7 stimulation, as this led to a significant reduction in the levels of CD127 on the cell surface (Fig. 1b, right panel) as previously observed.36
Figure 1.
Human effector CD4+ T cells express α1β1 integrin and the interleukin-7 receptor (IL-7R). The expression of these cell surface receptors was determined by fluorescence-activated cell sorting (FACS) analysis as described in the Materials and methods. (a) Expression of α1β1 integrin. (b) Expression of IL-7R on effector CD4+ T cells without stimulation (left panel) and upon stimulation with IL-7 for 8 hr (right panel). The results are representative of three independent experiments performed with T cells from different blood donors.
α1β1 integrin and IL-7R up-regulate the expression and production of RANKL in effector CD4+ T cells
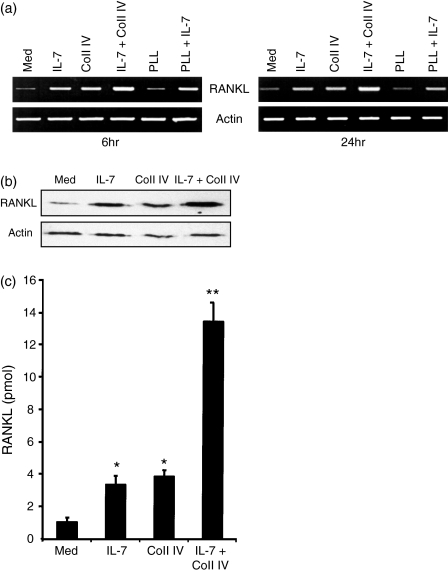
To examine whether α1β1 integrin regulates RANKL gene expression, we stimulated effector CD4+ T cells with Coll IV, the ligand of α1β1 integrin, IL-7 or IL-7 + Coll IV. As shown in Fig. 2a, effector CD4+ T cells expressed low levels of RANKL mRNA, which were significantly increased upon stimulation for 6 hr with IL-7 or with Coll IV. Importantly, the combination of IL-7 and Coll IV led to a stronger up-regulation of RANKL mRNA levels. As a control, PLL, a non-integrin-binding ligand, had no effect on RANKL gene expression and did not affect the IL-7 response. Similar results were also obtained when the cells were activated for 24 hr (Fig. 2a). These results indicate that α1β1 integrin and IL-7R are important regulatory pathways of RANKL gene expression in human effector CD4+ T cells. To determine whether the up-regulation of RANKL mRNA could also be observed at the protein level, we measured the levels of RANKL protein by immunoblot analysis. As shown in Fig. 2(b), IL-7 and Coll IV increased the protein levels of RANKL and their combination led to enhanced production of RANKL.
Figure 2.
Interleukin (IL)-7 and collagen IV (Coll IV) induce the expression of receptor activator of NF-κB ligand (RANKL). (a) Effector CD4+ T cells were activated with IL-7 (2.5 ng/ml), Coll IV (20 μg/ml), poly-l-lysine (PLL; 20 μg/ml), IL-7 + Coll IV, and IL-7 + PLL for 6 or 24 hr. The cells were harvested, total RNA was prepared and the expression of RANKL was determined by reverse transcriptase–polymerase chain reaction (RT-PCR) analysis. As a control, the levels of actin mRNA were also determined. The results are representative of three independent experiments performed with T cells from different blood donors. (b) Cells were activated as indicated for 24 hr and cell lysates were prepared. The expression of RANKL was then determined by immunoblot analysis. The blot was stripped and re-probed with anti-actin monoclonal antibody to ensure equal loading. The results are representative of two independent experiments performed with T cells from different blood donors. (c) Cells were activated as indicated for 24 hr, and then cell supernatants were harvested and the production of soluble RANKL was determined by enzyme-linked immunosorbent assay (ELISA). The results are presented as mean values from three independent experiments performed in triplicate with T cells from different blood donors, with standard errors as indicated. Statistical analysis was carried out using Student’s t-test: *P < 0·05 between IL-7-treated and untreated samples (med), and between Coll IV-treated and untreated samples (med). **P < 0·05 between IL-7 + Coll IV- and IL-7-treated samples, and between IL-7 + Coll IV- and Coll IV-treated samples.
Similar to other tumour necrosis factor (TNF) ligands, RANKL also undergoes molecular shedding, leading to the production of a soluble form that is measured in cell supernatants.37 We therefore measured the levels of soluble RANKL and, as shown in Fig. 2(c), effector CD4+ T cells produced detectable levels of soluble RANKL, which were significantly increased by IL-7 and Coll IV. The combination of IL-7 + Coll IV also led to a synergistic production of soluble RANKL. The up-regulatory effect of IL-7, Coll IV and IL-7 + Coll IV on RANKL gene expression and production was not a result of augmented cell proliferation as no changes in cell numbers were detected in any of the tested conditions (data not shown). Together these results indicate that ligation of α1β1 with Coll IV up-regulates the levels of RANKL in human effector CD4+ T cells and synergizes with IL-7R in this process.
Activation of effector CD4+ T cells through α1β1 and IL-7R does not modulate IFN-γ production
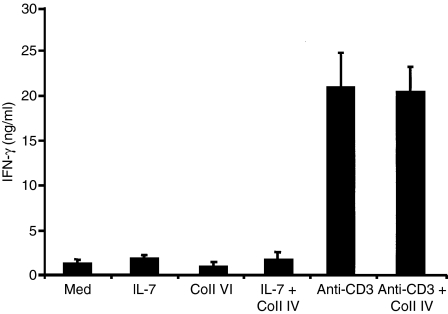
As activated T cells are known to produce IFN-γ, which is an inhibitor of RANKL-induced osteoclastogenesis, we verified the expression of IFN-γ in effector CD4+ T cells. As shown in Fig. 3, these cells expressed low levels of IFN-γ, which were not modulated by IL-7, Coll IV or IL-7 + Coll IV. In contrast, and in agreement with our recent report,23 cells activated via TCR (anti-CD3 mAb) produced high levels of IFN-γ, and Coll IV did not modulate these levels (Fig. 3). Furthermore, and in agreement with previous findings,11,30,38,39 we found that effector CD4+ T cells activated or not with IL-7, Coll IV or IL-7 +Coll IV did not express osteoprotegerin (OPG) (data not shown).
Figure 3.
Interleukin (IL)-7 and collagen IV (Coll IV) co-stimulation does not increase the production of interferon (IFN)-γ. Cells were activated as indicated for 24 hr, and then cell supernatants were harvested and the production of IFN-γ was determined by enzyme-linked immunosorbent assay (ELISA). The results are presented as mean values from four independent experiments performed in triplicate with T cells from different blood donors, with standard errors as indicated.
α1β1 integrin and IL-7R up-regulate the osteoclastogenic function of effector CD4+ T cells
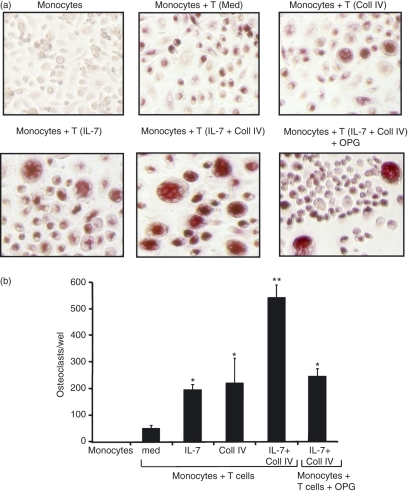
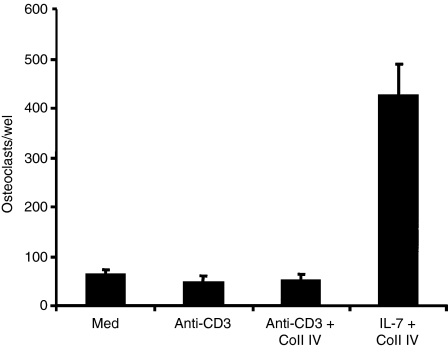
To examine the functional role of IL-7R and α1β1 integrin in T-cell-dependent osteoclastogenesis, we sought to determine the ability of effector CD4+ T cells activated with IL-7, Coll IV or both to induce the formation of osteoclasts as measured by the number of TRAP-positive multinucleated cells. As shown in Fig. 4, IL-7-activated effector CD4+ T cells significantly increased the formation of osteoclasts from monocytes compared with non-activated cells. Furthermore, Coll IV-activated T cells also increased the formation of osteoclasts (Fig. 4a,b). Importantly, IL-7 + Coll IV-activated T cells were the most potent in inducing the formation of osteoclasts (Fig. 4a,b), indicating that the synergistic effect of IL-7 and Coll IV on RANKL expression also occurs at the functional level. The formation of osteoclasts was attributable to RANKL as it was significantly reduced by recombinant OPG, and monocytes cultured without effector T cells did not form osteoclasts (Fig. 4a,b).
Figure 4.
Interleukin (IL)-7- and collagen IV (Coll IV)-activated effector CD4+ T cells induce the formation of osteoclasts. Effector CD4+ T cells (T) were activated with IL-7, Coll IV or IL-7 + Coll IV or left untreated (med). They were washed and co-cultured with monocytes in the presence of macrophage colony-stimulating factor (M-CSF). Monocytes cultured alone and monocytes cultured with T cells activated with IL-7 + Coll IV in the presence of recombinant osteoprotegerin (OPG) are also shown. After 8 days, the cultures were washed and the adherent cells stained for tartrate-resistant acid phosphatase (TRAP) activity as described in the Materials and methods. (a) Representative images showing the formation of TRAP-positive multinucleated osteoclast cells. (b) Quantification of osteoclast formation induced by activated T cells. After TRAP staining, the total numbers of TRAP-positive cells with at least four nuclei were counted. The results are presented as mean values from three independent experiments performed in duplicate with T cells from different blood donors, with standard errors as indicated. Statistical analysis was carried out using Student’s t-test: *P < 0·05 between IL-7-treated and untreated samples (med), between Coll IV-treated and untreated samples (med), and between IL-7 + Coll IV + OPG-treated samples and untreated samples. **P < 0·05 between IL-7 + Coll IV- and IL-7-treated samples, between IL-7 + Coll IV- and Coll IV-treated samples, and between IL-7 + Coll IV- and IL-7 + Coll IV + OPG-treated samples.
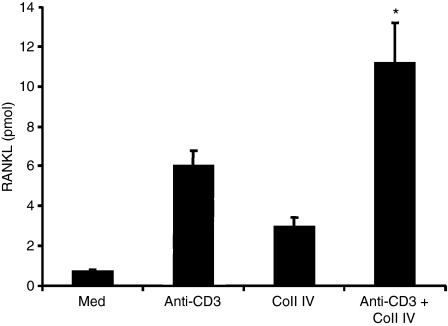
As TCR-activated effector T cells produce large amounts of IFN-γ, we investigated whether these cells have the ability to induce the formation of osteoclasts, and found that this is not the case (Fig. 5). Interestingly, activation of effector CD4+ T cells via TCR also induced RANKL expression, which was also up-regulated by Coll IV (Fig. 6). Together, these results indicate that activation of IFN-γ-producing effector T cells has the ability to induce the development of osteoclasts when cells are activated through IL-7R and α1β1 integrin.
Figure 5.
T-cell receptor (TCR)-activated effector CD4+ T cells do not induce the formation of osteoclasts. The cells were activated with anti-CD3 monoclonal antibody in the presence or absence of collagen IV (Coll IV). The cells were washed and co-cultured with monocytes in the presence of macrophage colony-stimulating factor (M-CSF). After 8 days, the cultures were washed and stained for tartrate-resistant acid phosphatase (TRAP) activity as described in the Materials and methods. The results are presented as in Fig. 4(b).
Figure 6.
Activation of effector CD4+ T cells via the T-cell receptor (TCR) and α1β1 integrin also augments the production of receptor activator of NF-κB ligand (RANKL). The cells were activated or not with anti-CD3 monoclonal antibody, collagen IV (Coll IV) or both stimuli. After 24 hr, cell supernatants were harvested and the production of soluble RANKL assessed by enzyme-linked immunosorbent assay (ELISA). The results are presented as in Fig. 2(c). Statistical analysis was carried out using Student’s t-test: *P < 0·05 between anti-CD3 + Coll IV- and anti-CD3-treated samples.
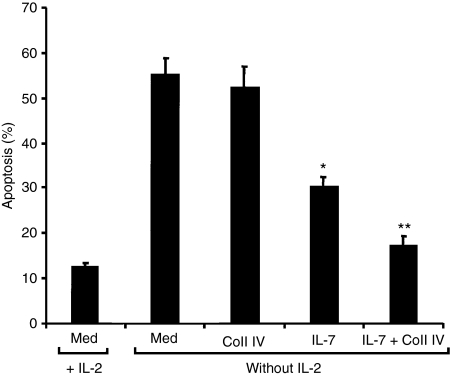
Coll IV potentiates the survival effect of IL-7
Protection of effector CD4+ T cells from IL-2-withdrawal-induced apoptosis can contribute to their accumulation in inflamed tissues40,41 and thus to T-cell-mediated tissue injury, and IL-7 is known to protect T cells from this form of apoptosis.12,42–44 We therefore investigated whether ligation of α1β1 integrin with Coll IV also regulates T-cell survival or potentiates the anti-apoptotic function of IL-7. Effector CD4+ T cells were activated or not in the absence of IL-2 with IL-7, Coll IV or IL-7 + Coll IV and their apoptosis was determined. As shown in Fig. 7, IL-2-starved effector T cells showed significant cell death after 72 hr, as nearly 60% of the cells were Annexin V-positive. The presence of IL-7 reduced apoptosis by 45%; Coll IV by itself had no effect but potentiated the IL-7 effect. Indeed, IL-7 + Coll IV reduced apoptosis by 73% compared with the 45% inhibition of apoptosis elicited by IL-7 alone. Together these results suggest that Coll IV enhances not only IL-7-induced production of RANKL but also IL-7-induced survival, suggesting that Coll IV + IL-7 may represent an important signalling pathway in the immunopathogenic function of effector CD4+ T cells.
Figure 7.
Collagen IV (Coll IV) enhances interleukin (IL)-7-induced cell survival. Effector CD4+ T cells were stimulated or not in the absence of IL-2 with IL-7, Coll IV or IL-7 + Coll IV. As a control, the cells were also cultured in the presence of IL-2. After 72 hr, cell apoptosis was determined by Annexin V/7AAD staining and fluorescence-activated cell sorting (FACS) analysis. The results are presented as mean values from three independent experiments performed in triplicate with T cells from different blood donors, with standard errors as indicated. Statistical analysis was carried out using Student’s t-test: *P < 0·05 between IL-7-treated and untreated (med) samples. **P < 0·05 between IL-7 + Coll IV- and IL-7-treated samples.
Discussion
The mechanisms by which effector T cells are activated to induce the formation of osteoclasts are not well understood. In the present study, we have demonstrated that α1β1 integrin, which is expressed on effector CD4+ T cells found in inflamed tissues, induces the expression of RANKL and enhances the formation of osteoclasts, thus identifying a novel role of α1β1 integrin in T-cell functions. In addition, our results extend the previously reported osteoclastogenic function of IL-7 in naïve T cells14 to human effector CD4+ T cells. More importantly, our results show that IL-7R and α1β1 integrin on these cells synergize to augment their expression of RANKL, their ability to induce the formation of osteoclasts and their survival. Together these results indicate that IL-7R and α1β1 integrin represent major regulatory pathways involved in T-cell-mediated osteoclastogenesis.
Our results show that stimulation of IFN-γ-producing effector CD4+ T cells with Coll IV and IL-7 augments the levels of RANKL but does not affect the expression of IFN-γ; an inhibitor of osteoclast development. This is correlated with the ability of these activated cells to induce the formation of osteoclasts. Although they express basal levels of IFN-γ, it is likely that these levels are insufficient to prevent the formation of osteoclasts. This has also been reported with naïve T cells stimulated with phytohaemagglutinin,45 suggesting that IFN-γ-producing effector CD4+ T cells can induce the formation of osteoclasts when they are activated with stimuli that limit the production of IFN-γ. This possibility is also supported by our finding that effector CD4+ T cells activated through the TCR were unable to induce the formation of osteoclasts, despite the fact that these cells also produce RANKL. These results are in agreement with previous studies showing that activation of T cells via the TCR, which leads to the production of high levels of IFN-γ, interferes with their ability to induce osteoclastogenesis.9–11
We also examined whether activation of effector T cells through the TCR and IL-7R and α1β1 integrin enhances their osteoclastogenic function. We found that simultaneous activation of effector T cells via IL-7R and TCR led to slightly increased production of both IFN-γ and RANKL, and Coll IV/α1β1 slightly enhanced IL-7R + TCR-mediated production of RANKL but not IFN-γ. However, these cells were still unable to induce the formation of osteoclasts because of the high levels of IFN-γ produced by these T cells (data not shown). These results further indicate the importance of the IL-7R/α1β1 integrin pathway in T-cell-mediated osteoclastogenesis.
Although Th1 cells have been associated with the pathogenesis of RA, an autoimmune disease associated with bone resorption, it was not possible to measure significant levels of IFN-γ in the synovial tissue.46 This suggests that, in the synovial tissue, these T cells can be activated with pro-inflammatory signals that do not lead to the production of high amounts of IFN-γ, such as through α1β1 integrin and IL-7R, as proposed herein. In this context, α1β1 integrin is expressed on effector CD4+ T cells infiltrating the synovium and has been implicated in the development of RA.25–28 Furthermore, synovial T cells from patients were also shown to express IL-7R.47 IL-7 is an important cytokine in T-cell development and survival and is produced by stromal cells found in the extracellular compartment of many tissues.12 Importantly, IL-7 levels are augmented in chronic inflammatory diseases such as RA, and IL-7 has been implicated in the activation of synovial T cells48 and in the induction of bone loss in vivo.13 Therefore, our study strongly suggests that α1β1 integrin and IL-7R could represent two major signalling pathways implicated in the regulation of T-cell-mediated bone loss associated with RA. Our results further support the notion that activation of effector T cells can occur independently of the TCR,49–51 especially in chronic inflammatory diseases such as RA, where cytokines and, as shown herein, ECM proteins can be critical in driving the activation of effector T cells.
In addition to T cells, osteoblasts also produce RANKL and induce the formation of osteoclasts. In this regard, it was recently demonstrated that adhesion of human osteoblasts to collagen type I (Coll I) also up-regulates RANKL and the induction of osteoclasts.52 Together with ours, these studies identify cell–ECM interactions as important not only in cell adhesion but also in the regulation of bone resorption. We previously demonstrated that ligation of α2β1 integrin with Coll I reduces TCR- and doxorubicin-dependent expression of RANKL in Jurkat T cells.30 We have also shown that α2β1 integrin enhances the production of TCR-dependent production of IFN-γ23 and, similar to Coll IV, we found that anti-CD3 + Coll I-activated effector T cells also do not induce the formation of osteoclasts (data not shown). However, although Coll I enhanced IL-7-dependent RANKL expression in human effector CD4+ T cells, it did not enhance their ability to induce the development of osteoclasts (data not shown). The mechanisms underlying the effect of Coll I in the context of IL-7 co-stimulation are currently unknown but are not due to the up-regulation of IFN-γ. Additional experiments are required to determine how Coll I modulates T-cell-mediated osteoclastogenesis. Although α2β1 integrin is expressed along with α1β1 integrin on effector/memory T cells, its blockade had only a minor effect on the development of arthritis compared with the blockade of α1β1 integrin.27 Thus, these studies suggest that α1β1 integrin may be more important than α2β1 integrin in the regulation of T-cell-dependent bone loss.
Both Coll IV and IL-7 are present in the bone tissue,48,53 and IL-7 has previously been shown to increase the adhesion of T cells to ECM proteins including Coll IV.54 Thus, during bone resorption, IL-7 can increase the adhesion of effector T cells to Coll IV, which will result in their retention in the bone tissue and to the synergistic production of RANKL. Subsequently, this will lead to the development of osteoclasts either from monocytes recruited from the periphery or from myeloid cells of the bone marrow, which will ultimately lead to bone erosion.
In addition to the up-regulation of RANKL, our results also show that α1β1 integrin enhances the pro-survival function of IL-7. While Coll IV had no effect on IL-2-withdrawal-induced apoptosis of effector CD4+ T cells, it did enhance the protective effect of IL-7. This form of apoptosis occurs during the contraction phase of the immune response and protection from it has been suggested to be a critical step in the development of memory T cells as well as in the persistence of activated T cells during chronic inflammation.40,41 Our results may help to explain why synovial T cells, which were shown to be resistant to apoptosis, manage to survive in the inflamed synovium, where only minor amounts of IL-2 were detected.55,56 The role of IL-7 in T-cell survival and in the development of T-cell memory has been well studied.12,43,44 However, recent studies indicate that IL-7R alone is not sufficient for the efficient survival of effector T cells and their differentiation towards memory T cells.35,57 In contrast, α1β1 integrin has been implicated in the protection of effector T cells from apoptosis during influenza infection.58 The results of the present study suggest that it is highly possible that the functional interaction between these two receptors may be the key for long-term survival of effector/memory T cells. Thus, our results suggest that, during chronic inflammation associated with bone loss, IL-7R and α1β1 integrin signalling pathways can rescue effector/memory T cells and promote bone resorption through the up-regulation of RANKL expression.
Our findings strongly suggest that β1 integrins can also provide signals that are co-stimulatory to cytokine signalling. A recent study showed that α1β1 integrin synergizes with the TNF II receptor in protecting effector CD8+ T cells from Fas-induced apoptosis.21 Thus, in addition to TCR signalling, integrins can be important modulators of cytokine signalling in T cells.
In summary, we have identified a new signalling pathway composed of α1β1 integrin and IL-7R that contributes to T-cell survival and to T-cell-mediated osteoclastogenesis. Further investigation of the signalling mechanisms underlying the effects of IL-7 + Coll IV is likely to provide new insights into how T cells contribute to the T-cell-mediated bone erosion seen in autoimmune diseases.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and from the Canadian Arthritis Network Centre of Excellence to FA who is a recipient of a CIHR New Investigator award. SG is a recipient of a scholarship from the Canadian Arthritis Network Centre of Excellence.
Abbreviations
- 7-AAD
7-aminoactinomycin
- Coll I
collagen type I
- Coll IV
collagen type IV
- ECM
extracellular matrix
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- IFN-γ
interferon-γ
- IL-7
interleukin-7
- IL-7R
interleukin-7 receptor
- mAb
monoclonal antibody
- M-CSF
macrophage–colony-stimulating factor
- MFI
mean fluorescence intensity
- OPG
osteoprotegerin
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PE
phycoerythrine
- PLL
poly-l-lysine
- RA
rheumatoid arthritis
- RANKL
receptor activator of NF-κB ligand
- RT-PCR
reverse transcriptase–polymerase chain reaction
- TCR
T-cell receptor
- TNF
tumour necrosis factor
- TRAP
tartrate-resistant acid phosphatase
References
- 1.Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology (Oxford) 2001;40:623–30. doi: 10.1093/rheumatology/40.6.623. [DOI] [PubMed] [Google Scholar]
- 2.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 3.Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne FR, Morony S, Warmington K, et al. CD4 + CD45RBHi T cell transfer induced colitis in mice is accompanied by osteopenia which is treatable with recombinant human osteoprotegerin. Gut. 2005;54:78–86. doi: 10.1136/gut.2003.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 6.Miranda-Carus ME, Benito-Miguel M, Balsa A, Cobo-Ibanez T, Perez de Ayala C, Pascual-Salcedo D, Martin-Mola E. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–64. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]
- 7.Kotake S, Udagawa N, Hakoda M, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003–12. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Brandstrom H, Bjorkman T, Ljunggren O. Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem Biophys Res Commun. 2001;280:831–5. doi: 10.1006/bbrc.2000.4223. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 10.Fox SW, Chambers TJ. Interferon-gamma directly inhibits TRANCE-induced osteoclastogenesis. Biochem Biophys Res Commun. 2000;276:868–72. doi: 10.1006/bbrc.2000.3577. [DOI] [PubMed] [Google Scholar]
- 11.Wyzga N, Varghese S, Wikel S, Canalis E, Sylvester FA. Effects of activated T cells on osteoclastogenesis depend on how they are activated. Bone. 2004;35:614–20. doi: 10.1016/j.bone.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–33. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci USA. 2003;100:125–30. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–8. [PubMed] [Google Scholar]
- 15.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–99. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 17.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–80. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 18.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 19.Krivacic KA, Levine AD. Extracellular matrix conditions T cells for adhesion to tissue interstitium. J Immunol. 2003;170:5034–44. doi: 10.4049/jimmunol.170.10.5034. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin Immunol. 2004;113:119–29. doi: 10.1016/j.clim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Richter MV, Topham DJ. The alpha1beta1 integrin and TNF receptor II protect airway CD8 + effector T cells from apoptosis during influenza infection. J Immunol. 2007;179:5054–63. doi: 10.4049/jimmunol.179.8.5054. [DOI] [PubMed] [Google Scholar]
- 22.Rao WH, Hales JM, Camp RD. Potent costimulation of effector T lymphocytes by human collagen type I. J Immunol. 2000;165:4935–40. doi: 10.4049/jimmunol.165.9.4935. [DOI] [PubMed] [Google Scholar]
- 23.Boisvert M, Gendron S, Chetoui N, Aoudjit F. Alpha2 beta1 integrin signaling augments T cell receptor-dependent production of interferon-gamma in human T cells. Mol Immunol. 2007;44:3732–40. doi: 10.1016/j.molimm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Gendron S, Couture J, Aoudjit F. Integrin alpha2beta1 inhibits Fas-mediated apoptosis in T lymphocytes by protein phosphatase 2A-dependent activation of the MAPK/ERK pathway. J Biol Chem. 2003;278:48633–43. doi: 10.1074/jbc.M305169200. [DOI] [PubMed] [Google Scholar]
- 25.Bank I, Roth D, Book M, et al. Expression and functions of very late antigen 1 in inflammatory joint diseases. J Clin Immunol. 1991;11:29–38. doi: 10.1007/BF00918792. [DOI] [PubMed] [Google Scholar]
- 26.Hemler ME, Glass D, Coblyn JS, Jacobson JG. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J Clin Invest. 1986;78:696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, et al. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–9. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ianaro A, Cicala C, Calignano A, et al. Anti-very late antigen-1 monoclonal antibody modulates the development of secondary lesion and T-cell response in experimental arthritis. Lab Invest. 2000;80:73–80. doi: 10.1038/labinvest.3780010. [DOI] [PubMed] [Google Scholar]
- 29.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13759–64. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gendron S, Couture J, Aoudjit F. Collagen type I signaling reduces the expression and the function of human receptor activator of nuclear factor -kappaB ligand (RANKL) in T lymphocytes. Eur J Immunol. 2005;35:3673–82. doi: 10.1002/eji.200535065. [DOI] [PubMed] [Google Scholar]
- 31.Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther. 2006;8:R152. doi: 10.1186/ar2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein I, Ben-Horin S, Li J, Bank I, Jiang H, Chess L. Expression of the alpha1beta1 integrin, VLA-1, marks a distinct subset of human CD4 + memory T cells. J Clin Invest. 2003;112:1444–54. doi: 10.1172/JCI19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8 + memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–7. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 36.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Kanamaru F, Iwai H, Ikeda T, Nakajima A, Ishikawa I, Azuma M. Expression of membrane-bound and soluble receptor activator of NF-kappaB ligand (RANKL) in human T cells. Immunol Lett. 2004;94:239–46. doi: 10.1016/j.imlet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004;63:1379–86. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yun S, Gustafsson K, Fabre JW. Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the amino terminal domain. Transplantation. 1998;66:103–11. doi: 10.1097/00007890-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 40.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–5. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–87. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 42.Pajusto M, Ihalainen N, Pelkonen J, Tarkkanen J, Mattila PS. Human in vivo-activated CD45R0(+) CD4(+) T cells are susceptible to spontaneous apoptosis that can be inhibited by the chemokine CXCL12 and IL-2, -6, -7, and -15. Eur J Immunol. 2004;34:2771–80. doi: 10.1002/eji.200324761. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–6. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 45.Kotake S, Nanke Y, Mogi M, et al. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35:3353–63. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 46.Kotake S, Schumacher HR, Jr, Yarboro CH, et al. In vivo gene expression of type 1 and type 2 cytokines in synovial tissues from patients in early stages of rheumatoid, reactive, and undifferentiated arthritis. Proc Assoc Am Physicians. 1997;109:286–301. [PubMed] [Google Scholar]
- 47.Van Roon JA, Glaudemans KA, Bijlsma JW, Lafeber FP. Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:113–9. doi: 10.1136/ard.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52:1700–10. doi: 10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
- 49.Cope AP. Studies of T-cell activation in chronic inflammation. Arthritis Res. 2002;4(Suppl. 3):S197–211. doi: 10.1186/ar557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cool SM, Nurcombe V. Substrate induction of osteogenesis from marrow-derived mesenchymal precursors. Stem Cells Dev. 2005;14:632–42. doi: 10.1089/scd.2005.14.632. [DOI] [PubMed] [Google Scholar]
- 51.Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest. 2004;114:471–4. doi: 10.1172/JCI22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirai F, Nakayamada S, Okada Y, Saito K, Kurose H, Mogami A, Tanaka Y. Small GTPase Rho signaling is involved in beta1 integrin-mediated up-regulation of intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. Biochem Biophys Res Commun. 2007;356:279–85. doi: 10.1016/j.bbrc.2007.02.121. [DOI] [PubMed] [Google Scholar]
- 53.Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–7. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 54.Ariel A, Hershkoviz R, Cahalon L, et al. Induction of T cell adhesion to extracellular matrix or endothelial cell ligands by soluble or matrix-bound interleukin-7. Eur J Immunol. 1997;27:2562–70. doi: 10.1002/eji.1830271015. [DOI] [PubMed] [Google Scholar]
- 55.Firestein GS, Xu WD, Townsend K, Broide D, Alvaro-Gracia J, Glasebrook A, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988;168:1573–86. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Combe B, Pope RM, Fischbach M, Darnell B, Baron S, Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985;59:520–8. [PMC free article] [PubMed] [Google Scholar]
- 57.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: limiting amounts of IL-7 do not control contraction of CD4 + T cell responses. J Immunol. 2007;178:4027–31. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–79. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]