Abstract
B-cell expression of certain Toll-like receptors (TLRs) is important in linking innate and adaptive immune responses in normal and pathological conditions. The expression of TLR9 plays a role in the recognition of conserved pathogen motifs in a manner that is dependent on B-cell localization, deduced from B-cell phenotype. The nature of TLR9 function is unclear. A first step in unravelling the function of this pattern recognition receptor is to discover the precise nature of the cell types that express TLR9. This study used three-colour flow cytometry to characterize the B lymphocytes from human peripheral blood mononuclear cells (PBMCs) that express TLR9 on the surface. We sorted TLR9-positive B and non-B cells from the PBMC population and detected TLR9 expression on naïve and memory B cells. Moreover, we identified two discrete subpopulations of B cells: CD19+ CD27− CD23+ cells and CD19+ CD27high CD80+ cells. These subpopulations expressed high levels of membrane TLR9 and exhibited a strong in vitro response to binding a relevant CpG motif by secreting high levels of interleukin-6 (compared to controls). Our finding that this pattern recognition receptor is expressed on a variety of cell subsets adds to the current understanding of the functional complexity of B-cell membrane TLR9.
Keywords: B cells, CpG DNA, Toll-like receptors
Introduction
The innate immune system uses a restricted number of receptors to discriminate among a large number of pathogenic agents and self-elements. This ability to discriminate is critical in mediating the body’s reaction to danger signals,1 and requires complexity for a successful response to pathogen mutations. Discrimination properties evolved from a variety of receptors that recognized conserved prokaryotic pathogen motifs not found in higher eukaryotes. These motifs play essential roles in prokaryotic pathogen biology and are thus immutable. They are termed ‘pathogen-associated molecular patterns’ (PAMPs), and they primarily bind to receptors on phagocytic cells termed ‘pattern recognition receptors’ (PRRs). There are two principal classes of PRRs: those that mediate phagocytosis and those that drive the activation of the proinflammatory cascade; some PRRs possess properties of both classes.2
Among factors that principally drive inflammation, Toll-like receptors (TLRs) are expressed by multiple types of immune cells and recognize a large and diverse range of viral, bacterial and parasitic agents.3 TLR9 specifically binds to the cytosine–phosphate–guanosine (CpG) motifs that are prevalent in bacterial, viral and parasitic DNA. Ligand binding triggers a biochemical cascade that begins at the cytoplasmic domain of TLR9 and terminates with the activation of nuclear factor-κB.
Several studies have shown that TLR9 is highly expressed on B cells.4,5 When several human and murine cell types, including human B lymphocytes, were exposed to CpG motifs the expression of class II major histocompatibility complexes, CD40, CD80 and CD86 were significantly augmented, and the secretion of interleukin-12 (IL-12) was stimulated.6 After reevaluating TLR9 expression on human B lymphocytes obtained from blood buffy coats,7,8 we demonstrated that various B-type CpG oligodeoxynucleotides (CpG-ODNs) were also reactive with human lymph node B cells. We observed that one CpG-ODN, DSP30, upregulated TLR9 messenger RNA (mRNA) expression in activated B cells from blood and lymph nodes.7 We also observed the induction of CD69 expression, followed by the sequential expression of CD80, CD86, and activation of the nuclear factor-κB pathway.7 Exposure to DSP30 also influenced the mRNA expression of the B-cell-derived cytokines IL-1β and IL-6. Interestingly, when these B cells were costimulated in vitro with IL-2, IL-10 and soluble CD40 ligand (sCD40L), they had markedly different responses to different CpG-ODNs in terms of their terminal differentiation into immunoglobulin-secreting cells. Among the six currently available B-type CpG-ODNs tested, only one (K101) did not significantly augment the production of at least one immunoglobulin (Ig) class (IgA, IgG or IgM). Bohle-CpG and CpG-K16 significantly augmented the production of IgA and IgM, while CpG-1686 and CpG-K19 affected the production of IgM only. Only CpG-DSP30 proved capable of significantly augmenting the production of all three immunoglobulin classes compared to the culture medium control (IL-2, IL-10 and CD40L) and compared to its own CpG-ODN control, DSP30K.7 In human B cells, TLR9 stimulation by CpG-DNA in association with IL-10 initiated gene transcription of germline immunoglobulin heavy chain constant regions Cγ1, Cγ2 and Cγ3.9 In addition, in response to CpG in vitro human memory B cells proliferated and produced antibodies.10 Moreover, Capolunghi et al. showed that the most immature B-cell type in peripheral blood responded to TLR9 stimulation; first they acquired the phenotype of IgM memory B cells and then they terminally differentiated into plasma cells that produced antibacterial antibodies.11
Based on those findings, the present study investigated the distribution of TLR9 on blood CD19+ B cells that were either positive or negative for the cell marker, CD27. This allowed us to identify subsets of TLR9+ B cells within the naïve and memory blood B-cell population, and to investigate the capacity of these cells to produce IL-6 upon stimulation.
Materials and methods
Cell preparation
Human blood was obtained from healthy donors at the Auvergne-Loire Regional Blood Bank. Peripheral blood mononuclear cells (PBMCs) were prepared from buffy coats as described previously7 using density gradient centrifugation (Lymphoprep™; Nycomed, Oslo, Norway). Purified blood B cells were subjected to anti-CD2, anti-CD4, anti-CD8, anti-CD14 and anti-CD16 monoclonal antibodies (mAbs) for negative selection using magnetic beads coated with anti-mouse IgG according to the manufacturer’s instructions (Dynal Biotech, Invitrogen, Compiègne, France).12–14 In the isolated population, > 98% of the cells expressed CD19 and CD20 and < 1% expressed CD2 or CD14. In our laboratory, the B-cell purity after flow cytometry was routinely 97–98%, and these B cells were previously shown to be non-activated.7,13
Detection of nuclear factors by Western blot
Polyacrylamide gel electrophoreses was performed with 25 μg human PBMC lysate or 10 μg positive control human small intestine tissue lysate (Imgenex, San Diego, CA) per well on a 10% acrylamide gel (Sigma-Aldrich, Saint Quentin Fallavier, France). After electrophoresis, the separated proteins were transferred to a 0·45-μm nitrocellulose membrane (Amersham Pharmacia, Orsay, France) by electroblotting. The transfer buffer was supplemented with 20% methanol (Sigma-Aldrich). Blots were blocked overnight at 4° in phosphate-buffered saline, 0·1% Tween-20 and 1% bovine serum albumin (ID Bio, Limoges, France). The blots were then incubated with a primary mAb to TLR9 (clone 26C593; Imgenex) for 90 min at room temperature. Next, the blots were washed three times, for 10 min each time, with blocking buffer, then incubated for another 90 min with the secondary antibody, horseradish peroxidase-linked goat anti-mouse antibody, diluted at 1:5000 (Santa Cruz Biotechnology, Santa Cruz, CA) as determined previously. Then, blots were incubated with a chemiluminescent substrate according to the manufacturer’s instructions (ECL; Amersham Pharmacia) and finally exposed to radiographic film (Sigma-Aldrich).
Flow cytometry
The PBMCs were incubated with the indicated antibodies for 45 min at 4° in the dark, washed twice in endotoxin-free phosphate-buffered saline–fetal calf serum 10% and resuspended in 1% paraformaldehyde. The PBMCs were stained with the following: phycoerythrin Cy-7 fluorochrome-conjugated mAb to CD19 (clone SJ25C1; BD Biosciences, Le Pont de Claix, France); allophycocyanin-conjugated anti-human mAb to CD27 (clone O323; eBioscience, San Diego, CA); -associated mAb to TLR9 (clone 26C593; Imgenex); fluorescein fluorochrome-phycoerythrin-conjugated mAb to CD21 (clone 1F8; DAKO, Trappes, France); mAb to CD22 (clone HIB22; BD Biosciences); mAb to CD23 (clone MHM6; DAKO); mAb to CD38 (clone AT13/5; DAKO); mAb to CD40 (clone 5C3; BD Biosciences); mAb to CD80 (clone 2D10·4; DAKO); mAb to CD95 (clone DX2; DAKO); IgD (clone IA6-2; BD Biosciences); and IgM (clone G20-127; BD Biosciences). Notably, it has been shown that CD19 is expressed on all B cells throughout ontogeny.15–17 Labelled cells were incubated and stained with fluorochrome-conjugated mAbs or with control isotype-matched mouse IgGs at the appropriate concentrations. To avoid non-specific antibody binding, staining experiments were performed in the presence of an FcR blocking reagent (BD Biosciences). Events were recorded with a FACSCalibur (BD Biosciences) and analysed with CellQuestPro software from BD Biosciences.
To detect B-lymphocyte cell surface and intracellular TLR9 expression by flow cytometry, purified B cells originating from PBMCs were fixed, or not, in paraformaldehyde (3·6%, Sigma-Aldrich) and permeabilized with TRITON (0·4X, Sigma-Aldrich).
The sorting of CD19+ CD27− CD23+ TLR9+ and CD19+ CD27high CD80+ TLR9+ B cells was performed by three-colour staining on Ficoll-isopaque-purified cell suspensions enriched in B cells to 95–98% by magnetic cell separation (see above). One of the following was used for sorting: anti-human mAb to TLR9 (clone 26C593; Imgenex) conjugated to phycoerythrin; anti-human mAb to CD27 (clone O323; eBioscience); or purified anti-human mAb to CD80 (clone 2D10·4; DAKO) and anti-human mAb to CD23 (clone MHM6; DAKO). Cell sorting was performed with FACSVantage (Becton Dickinson, San Diego, CA). CD27 is a useful marker in assessing the number of circulating B cells and B-cell subsets because it permits one-step identification of the major B-cell compartments (CD27− are naïve and CD27+ are memory B cells) and classification of CD27high plasma cells.18,19 We used three different concentrations of anti-CD27 for staining to enable designations of B-cell subsets as CD27− (CD27low), CD27+, or CD27++ (CD27high).
B-cell subset stimulation and IL-6 production assays
Sorted CD19+ CD27− CD23+ TLR9+ and CD19+ CD27high CD80+ TLR9+ B cells were cultured in Iscove modified Dulbecco’s medium (Bio-Whittaker Europe, Verviers, Belgium) supplemented as described previously.7 The cells were exposed to IL-2 (10 ng/ml) and IL-10 (50 ng/ml; Peprotech, Rocky Hill, NJ) and to the recombinant CD40L-FLAG-tag fusion protein as described by the manufacturer’s protocols (sCD40L; Alexis Biochemicals, Paris, France); we also included 1 μg/ml CD40L enhancer (Alexis Biochemicals), according to the manufacturer’s recommendation. All experiments were performed in 96-well, flat-bottom microtitre plates (Falcon, Oxnard, CA).
To investigate the capacity of B-cell subsets to be activated by non-phosphorothioate-modified (nonPTO-modified) CpG-ODNs, CD19+ CD27− CD23+ TLR9+, CD19+ CD27high CD80+ TLR9+, CD19+ CD27− CD23+ TLR9− and CD19+ CD27high CD80+ TLR9−, cultured at 8000 cells/well, were exposed to 2 μm concentrations of well-characterized human stimulatory CpG-ODNs: ODN 2006: (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) or ODN 2006-G5: (5′-TCGTCGTTTTGTCGTTTTGTCGTTGGGGG-3′). The addition of a 3′ poly-G string (ODN 2006-G5) was reported to improve ODN internalization and was correlated with increased IL-6 secretion and PBMC proliferation.20 To improve internalization of ODN 2006, therefore, we used ODN 2006-G5 instead of adding PTO modifications. The control ODN (ODN 2006 control) had the same sequence except the CpG dinucleotides were replaced by GpC dinucleotides: 5′-TGCTGCTTTTGTGCTTTTGTGCTT-3′. The stimulatory and control ODNs were obtained from Cayla-InvivoGen Europe (Toulouse, France).
To evaluate the functional capacity of sorted B cells, especially B-cell subsets that were unresponsive to CpG, we stimulated with a 0·01% concentration of Staphylococcus aureus, Cowan strain (SAC; Sigma Chemicals, Saint Quentin Fallavier, France) and 50 ng/ml of human recombinant IL-2 (R&D Systems Europe Ltd, Lille, France).21
Sorted B-cell production of IL-6 was measured from aliquots of cell-free culture supernatants (n = 10) with or without stimulation. Measurements of IL-6 production were made using a specific enzyme-linked immunosorbent assay (ELISA) from commercial kits (R&D Systems Europe Ltd) according to the manufacturer’s instructions. Absorbance at 450 nm was measured with an ELISA reader (Multiskan EX; Labsystem, Helsinki, Finland).
Statistical analysis
The data obtained in this study were evaluated using the Mann–Whitney U-test for unpaired observations. P values < 0·05 were considered significant.
Results
TLR9 is expressed on the surface of peripheral blood B cells and non-B cells
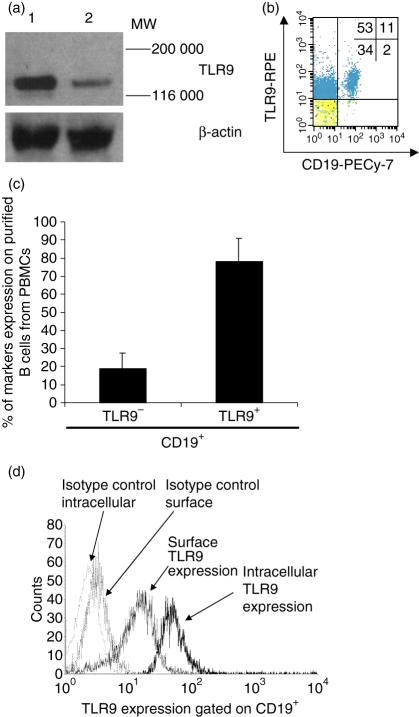
To examine TLR9 expression on PBMC subsets, we first analyzed non-permeabilized PBMCs by flow cytometry. We found that subsets of both CD19+ and CD19− cells were labelled with anti-TLR9 mAb (Fig. 1). Western blot analysis showed that the anti-TLR9 mAb bound specifically to TLR9 (Fig. 1a). The TLR9-expressing cells accounted for roughly 64% of all PBMCs: 53% were CD19− cells (non-B cells) and 11% were CD19+ cells (B cells) (Fig. 1b). Within the B-cell population (i.e. the CD19+ subset), 78% ± 12·9 were TLR9+ and only 19% ± 8·3 were TLR9− (Fig. 1c). Next, we investigated more precisely the localization of TLR9 expression. Purified B cells were either fixed (non-permeabilized) or permeabilized for surface or intracellular labelling, respectively, with phycoerythrin-linked anti-TLR9. Flow cytometry indicated that B-cell expression of TLR9 was 76% ± 2·9 for non-permeabilized and 90% ± 3·3 for permeabilized cells. The mean fluorescence in non-permeabilized cells was also less than that in permeabilized cells (Fig. 1d). We further investigated the possibility that immunocomplexes could influence the TLR9 expression via Fcγ-receptors. When these receptors were blocked, we observed no significant differences in B-cell surface expression of TLR9 compared to the results reported above (data not shown).
Figure 1.
Cell-surface Toll-like receptor 9 (TLR9) expression on B cells sorted from peripheral blood mononuclear cells (PBMCs). (a) Western blot analysis of TLR9 protein expressed in human PBMC lysate (lane 1) and positive control (lane 2). The estimated molecular weight of TLR9 is 120 000; β-actin was used as the loading control. The Western blot was performed three times with similar results. (b) TLR9 was detected by antibody labelling and flow cytometry analysis after gating for CD19+. (c) Summary of flow cytometry analysis of TLR9 expression by CD19+ B cells. The mean percentage of CD19+ B cells that were positive and negative for TLR9 expression is shown (mean ± SD from 10 independent experiments). (d) Detection of cell surface and intracellular TLR9 expression by flow cytometry. Flow cytometry representations of TLR9 expression on B-cell surface and intracellular locations (one representative experiment out of three). Cells stained with the isotype control antibody provided the background level of TLR9 expression.
Differential membrane TLR9 expression by B cells according to their membrane marker patterns
To further characterize B cells that express – or do not express – membrane TLR9 within each main subset, we four-colour-labelled blood B cells with mAbs against CD19, CD27, TLR9 and other antigens associated with phenotype, activation, or maturation states. For example, CD23 is only expressed by activated naïve B cells and CD80 is widely expressed early after B-cell activation.22–24
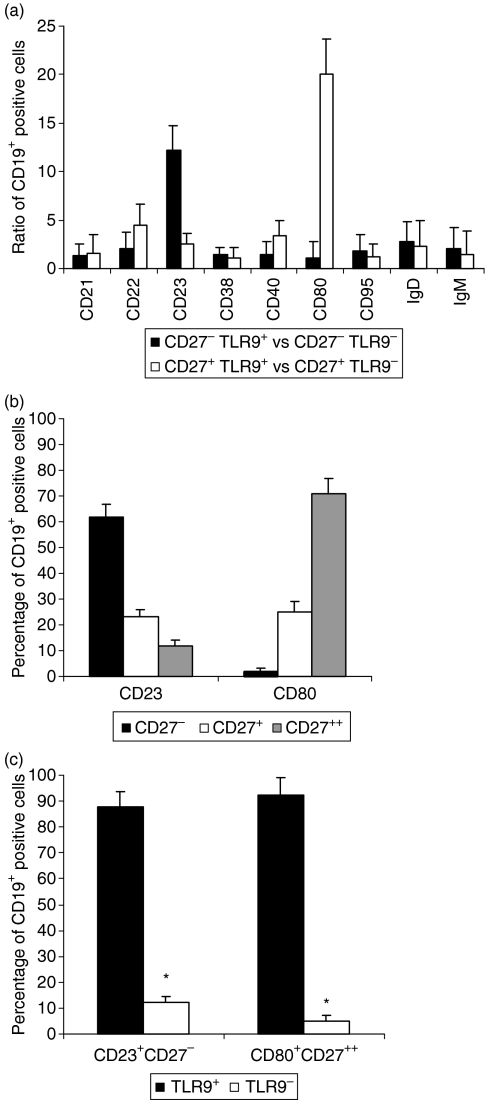
After a large series of analyses, we focused on two B-cell subpopulations, CD27− and CD27+, to determine their differential expression of TLR9. The ratio of TLR9+ to TLR9− expression was 12·20 ± 2·5 in CD27− B cells (CD23+) and 20·06 ± 3·6 in CD27+ B cells (CD80+) (Fig. 2a). To determine whether antibody binding was dependent on the degree of activation, we performed costaining with either CD80 or CD23 in the presence of anti-TLR9 and compared the results to those obtained using the isotype controls. We determined that B-cell surface TLR9 expression was independent of strong activation, because we observed strong TLR9 expression even when B cells were weakly activated (data not shown).
Figure 2.
Flow cytometry analysis of cell surface marker expression by the CD19+ B-cell subpopulation. (a) Cell surface marker associations with Toll-like receptor 9 (TLR9) expression. Ratios of TLR9 expression : non-expression on purified B-cell subsets, including the ratio of CD27− TLR9+ to CD27− TLR9− and the ratio of CD27+ TLR9+ to CD27+ TLR9− (ratios of means ± SD; n = 10), are shown in conjunction with various cell surface markers. (b) Peripheral blood mononuclear cells (PBMCs) were distinguished according to CD23 or CD80 expression. The percentages of low (−), medium (+), and high (++) levels of CD27 expression are shown after gating for CD19+ (mean ± SD; n = 10). (c) PBMCs were distinguished according to CD27 and either CD80 or CD23 expression. The percentage of TLR9-labelled cells is shown after gating for CD19+ (mean ± SD; n = 10).
In 10 experiments with blood from healthy donors, two-thirds (62% ± 5) of the CD19+ CD27− B cells were CD23+ (Fig. 2b) and 71% ± 6 of the CD19+ CD27high B cells were CD80+ (Fig. 2c). TLR9 expression was principally observed (> 85%) within the CD19+ CD27− CD23+ and the CD19+ CD27high CD80+ subsets (Fig. 2c).
Functional TLR9 expression on B-cell subsets CD19+ CD27− CD23+ and CD19+ CD27high CD80+ is associated with sustained IL-6 secretion
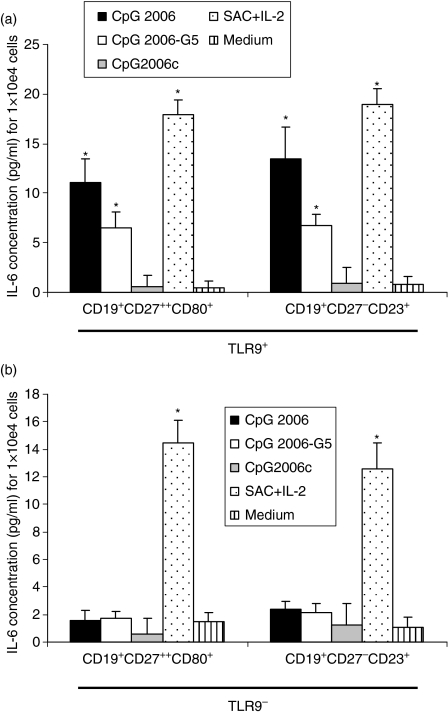
Interleukin-6 is a growth factor that stimulates activated B cells upon binding to the IL-6 receptor (CD126); IL-6 binding is required for plasma cell differentiation and immunoglobulin production.25,26 To determine whether the TLR9+ B-cell subset could produce IL-6 upon stimulation, sorted CD19+ CD27− CD23+ and CD19+ CD27high CD80+ B-lymphocyte subsets were stimulated with IL-2, IL-10 and soluble CD40L in the absence or presence of ODN 2006. The aim was to detect B-lymphocyte IL-6 production after TLR9 stimulation with CpG-ODN. We found that costimulation with ODN 2006 significantly upregulated IL-6 production, as expected from other studies.4,27 CD19+ CD27high CD80+ cells produced 11·1 pg/ml ± 2·3 IL-6 compared to 0·6 pg/ml ± 1·1 in the control, P<0·01, and CD19+ CD27− CD23+ cells produced 13·5 pg/ml ± 3·2 IL-6 compared to 0·9 pg/ml ± 1·6 in the control, P<0·01 (Fig. 3). In contrast, we found that, compared to ODN 2006, co-stimulation with ODN 2006-G5 produced significantly less IL-6; CD19+ CD27high CD80+ cells produced 6·5 pg/ml ± 1·6 IL-6 (P<0·05 versus control) and CD19+ CD27− CD23+ cells produced 6·7 pg/ml ± 1·2 IL-6 (P<0·05 versus control) (Fig. 3a).
Figure 3.
Detection of interleukin-6 (IL-6) in the culture supernatants of the indicated B-cell subsets stimulated with oligodeoxydinucleotide (ODN; CpG 2006, CpG 2006-G5) or control ODN (CpG 2006c) for 48 hr. (a) CD19+ CD27− CD23+ TLR9+ and CD19+ CD27high CD80+ TLR9+, (b) CD19+ CD27− CD23+ TLR9− and CD19+ CD27high CD80+ TLR9− B cells (8000 cells/well) were exposed to IL-2 (10 ng/ml), IL-10 (50 ng/ml), and to a recombinant CD40L–FLAG-tag fusion protein (sCD40L) along with 1 μg/ml CD40L enhancer. Cells were stimulated with 2 μm final concentrations of human stimulatory CpG-ODN type B (CpG 2006 and CpG 2006-G5), control ODNs (CpG 2006c), or Staphylococcus aureus Cowan strain (0·01%) with 50 ng/ml human recombinant IL-2 (SACIL-2). IL-6 was quantified by enzyme-linked immunosorbent assay after 48 hr; background levels were subtracted. Data are representative of three experiments (mean ± SD). Asterisks (*) indicate a statistically significant effect compared to control ODN stimulation (P<0·05).
As a negative control, we used the same conditions to stimulate the TLR9− B cells isolated from the same two populations (CD19+ CD27high CD80+ and CD19+ CD27− CD23+) with ODN 2006, ODN 2006-G5 and control ODN; under these conditions, we noted no significant modulation of IL-6 production (Fig. 3b). As a positive control, the functional capacity of sorted B cells was tested by stimulation with S. aureus Cowan strain and human recombinant IL-2 (Fig. 3a,b). The results showed that the functional capacity of the B-cell subpopulations was readily detectable.
Discussion
TLRs play important roles in innate immunity and in bridging innate and adaptive immune responses.28 In this study, we noted that 64% of PBMCs expressed TLR9, and 53% were non-B cells. Although TLR9 is predominantly expressed on plasmacytoid dendritic cells and B cells, various studies show that monocytes, natural killer cells and T cells can express weak but detectable levels of TLR9.8 In addition, at the mRNA level, human T cells have been shown to express several TLR isoforms, suggesting that TLR ligands can directly influence T-cell function. For example, several studies have shown that T cells express TLR4 and TLR9, and that binding to TLR4 and TLR9 ligands induced an activation/proliferation response.29,30 It has also been suggested that the detection of baseline levels of TLR9 in human monocytes indicates that monocytes are able to modulate TLR9 expression along distinct differentiation pathways, depending on the cytokine milieu and the microbial molecules present.8 Although some studies indicated that purified monocytes did not react to CpG-ODNs, others have shown that monocyte-derived DCs (cultured in the presence of granulocyte–macrophage colony-stimulating factor and IL-4) expressed TLR9 and reacted directly to CpG-ODN.31,32
B lymphocytes and other immune cells, including dendritic cells, bridge both types of immunity because they are able to bind pathogens through several types of receptors (FcγRs, complement receptors, and PRRs), present antigens to selected T-cell types, and coactivate those T cells. Human B cells express several TLRs. Specifically, peripheral B lymphocytes and tonsillar B cells express TLRs 1, 2, 6, 7, 9 and 10; tonsillar B cells also express TLR4.33 It has been particularly challenging to clarify the location of TLR9 on B lymphocytes. Initially it was thought that TLR9 was only expressed intracellularly, and not on the B-cell surface. However, recently several groups, including ours, have demonstrated the presence of TLR9 on the surface of B cells. Other groups have reported very low surface TLR9 expression levels on only a small percentage of B cells (∼ 5%).34 Although all these studies used the same reagents (i.e. the same mAb clones), we cannot rule out the influence of different experimental conditions.34 Still other studies have demonstrated that B cells must be permeabilized to detect intracellular TLR9; in those studies, virtually all B cells were TLR9+.4,34,35
The precise function of TLR9 is not yet known; it is expressed by a variety of immune cells and it appears that discrete subpopulations of different cell types display TLR9. The natural ligand for TLR9 is methylated CpG DNA, which is primarily found in bacteria and some parasites. B-type CpG-ODNs are also found in some vertebrate species, and are powerful B-lymphocyte activators.6 B-type CpG-ODNs induce B-lymphocyte entry into G1 phase; rapid secretion of cytokines including IL-6, IL-10 and IL-12;36–38 and overexpression of FcγRs and molecules characteristic of activated stages, including CHM II, CD80, CD867,39 and CD40L (CD154).40
Priming with interferons or B-cell antigen receptor (BCR) -mediated delivery of CpG–antigen complexes may induce antigen-specific follicular B cells to become responsive to complex TLR9 ligands; this may lead to an increase in B-cell proliferation and promote immunoglobulin secretion and isotype switching.
Moreover, both IFN priming and CD40-mediated activation, together with autocrine secretion of IL-6 and IL-109 and signalling initiated by B-cell activating factor from the tumour necrosis factor family, are requisite for CpG-DNA-mediated isotype switching toward complement-fixing IgG isotypes.41 Recently, Cunningham-Rundles et al. investigated B cell activation induced by TLR9.35 They showed that CpG-DNA did not upregulate expression of CD86 on common variable immune deficiency (CVID) B cells, despite costimulation by the BCR, and did not induce production of IL-6 or IL-10 (as would healthy B cells). Although TLR9 was found in the cytoplasm and on the surface of ODN-activated healthy B cells, both TLR9 and its mRNA were deficient in CVID B cells. Moreover, these TLR9 deficiencies were not related to the proportion of CD27+ memory B cells. The data therefore suggest that there are TLR9 activation defects in CVID that would prevent CpG-DNA-initiated innate immune responses; these defects may lead to loss of B-cell function.
The aim of the present investigation was to further characterize TLR9+ B-cell subset(s). We found TLR9 expression on circulating peripheral B cells from healthy blood donors, which was generally consistent with other reports.33,34 We further identified two distinct subpopulations of B cells, CD19+ CD27− CD23+ and CD19+ CD27high CD80+ cells, that expressed higher levels of membrane TLR9 than other subpopulations. Our results showed that both naïve and memory B cells exhibit TLR9 expression; this agrees with data from other groups.
Upon stimulating B-lymphocyte subsets with IL-2, IL-10 and sCD40L in the absence or presence of ODN 2006 or ODN 2006-G5, the CD19+ CD27− CD23+ and CD19+ CD27high CD80+ B-cell subsets produced elevated amounts of IL-6 in comparison to other subsets. This suggested that TLR9 engagement is followed by sustained IL-6 production. Moreover, we found that surface TLR9 exhibited better responsiveness to ODN 2006 than ODN 2006-G5.
The physiological significance of B-cell expression of TLR9 has not yet been determined. Our results are consistent with others showing that human B cells are stimulated by a TLR9/CpG interaction, and that both naïve and memory B cells respond, to different extents, by proliferation and differentiation into immunoglobulin-secreting cells.10,42,43 However, our study did not confirm the data reported by Bernasconi et al.10 and Jung et al.43 For instance, we found that CD23+ CD27− TLR9+ B cells (naïve B cells) produced as much IL-6 as or more IL-6 than CD80+ CD27high TLR9+ B cells (memory B cells). Jung et al. used an in vitro human purified tonsillar B-cell culture system that mimics the primary or secondary immune response in vivo. In that system, CpG DNA clearly augmented the proliferation and generation of plasma cells from naïve and memory B cells.43 However, the Bernasconi et al. reported that in human naïve B cells, TLR9 expression was rapidly induced following BCR triggering; they also reported that human memory B cells proliferated and differentiated into immunoglobulin-secreting cells in response to CpG, but naïve B cells only did so when simultaneously stimulated through the BCR.10
In spite of these recent efforts, little is known about the precise function of TLR9+ B cells. They very likely play a role in protection mechanisms, similar to other TLR-positive cells.7,34,44 Both naïve and non-naïve B cells may play a protective role by sensing certain types of infectious pathogenic danger (e.g. CpG DNA)1 or by sensing autoimmune clones.45,46 However, the significance of TLR9 expression by distinct subsets of peripheral B cells is still largely unexplained. One could speculate that naïve B cells are designed to sense bacterial and viral (or even parasitic) danger, via pathogen-derived CpG DNA. Alternatively, it has been shown that engagement of the BCR on naïve B cells by their cognate antigen can lead to upregulation of TLR9 expression.10
The potential role of TLRs in B-cell activation and antibody production in vivo is intriguing because T-cell-dependent antigen-specific antibody response appears to require engagement of TLR on B cells.47 TLR9 stimulation on memory B cells may drive cell activation and subsequent immunoglobulin production,10,42 but its effect on naïve B cells is less clear and more controversial.22,48
Our findings add to the current understanding of differentiation of circulating blood (memory) B cells through TLR9 stimulation. We showed that one ligand that signals infectious danger can stimulate naïve and memory B-cell subsets to produce large amounts of IL-6. In turn, the cytokine IL-6 exerts pleiotropic effects on a variety of cells, including B cells themselves, and influences B-cell maturation into antibody-producing cells. These data support a role for TLR9 signalling in B-cell production of antibodies in response to danger.
Acknowledgments
We gratefully acknowledge the technical help of Béatrice Arnaud, Dorothée Patti and Céline Blanchard. We would like to thank Sophie Acquart and Françoise Boussoulade (EFS Auvergne-Loire, France) for their help in preparing the human blood cells. We thank Odile Sabido (Centre Commun de Cytométrie en Flux, Faculté de Médecine, Université Jean Monnet de Saint-Etienne, France) for cell sorting flow cytometry. We thank Philip Lawrence for kindly revising the manuscript and Prof. M. Cogné (Limoges – UMR CNRS 6101) for helpful discussions. Financial support was received through grants from the ‘Convention interrégionale Massif Central – Réseau Switch – MENRT 01Y0242b’ and from the Regional Blood Bank – EFS Auvergne-Loire, France. The study was partly funded by EFS Auvergne-Loire.
References
- 1.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–50. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 3.McCluskie MJ, Weeratna RD, Davis HL. The role of CpG in DNA vaccines. Springer Semin Immunopathol. 2000;22:125–32. doi: 10.1007/s002810000014. [DOI] [PubMed] [Google Scholar]
- 4.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–48. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 7.Cognasse F, Acquart S, Beniguel L, Sabido O, Chavarin P, Genin C, Garraud O. Differential production of immunoglobulin classes and subclasses by mucosal-type human B-lymphocytes exposed in vitro to CpG oligodeoxynucleotides. Clin Chem Lab Med. 2005;43:22–31. doi: 10.1515/CCLM.2005.003. [DOI] [PubMed] [Google Scholar]
- 8.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 9.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi NL, Onai N, Lanzavecchia A. A role for toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 11.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–8. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 12.Cognasse F, Beniguel L, El HR, Sabido O, Chavarin P, Genin C, Garraud O. HIV-gp160 modulates differentially the production in vitro of IgG, IgA and cytokines by blood and tonsil B lymphocytes from HIV-negative individuals. Clin Exp Immunol. 2003;132:304–8. doi: 10.1046/j.1365-2249.2003.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cognasse F, Chavarin P, Acquart S, Sabido O, Beniguel L, Genin C, Richard Y, Garraud O. Differential downstream effects of CD40 ligation mediated by membrane or soluble CD40L and agonistic Ab: a study on purified human B cells. Int J Immunopathol Pharmacol. 2005;18:65–74. doi: 10.1177/039463200501800108. [DOI] [PubMed] [Google Scholar]
- 14.Cognasse F, Sabido O, Beniguel L, Genin C, Garraud O. A flow cytometry technique to study nuclear factor-kappa (NFκB) translocation during human B cell activation. Immunol Lett. 2003;90:49–52. doi: 10.1016/s0165-2478(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 15.Callard RE, Rigley KP, Smith SH, Thurstan S, Shields JG. CD19 regulation of human B cell responses. B cell proliferation and antibody secretion are inhibited or enhanced by ligation of the CD19 surface glycoprotein depending on the stimulating signal used. J Immunol. 1992;148:2983–7. [PubMed] [Google Scholar]
- 16.Del Nagro CJ, Otero DC, Anzelon AN, Omori SA, Kolla RV, Rickert RC. CD19 function in central and peripheral B-cell development. Immunol Res. 2005;31:119–32. doi: 10.1385/IR:31:2:119. [DOI] [PubMed] [Google Scholar]
- 17.Ellyard JI, Avery DT, Phan TG, Hare NJ, Hodgkin PD, Tangye SG. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 2004;103:3805–12. doi: 10.1182/blood-2003-09-3109. [DOI] [PubMed] [Google Scholar]
- 18.Farstad IN, Carlsen H, Morton HC, Brandtzaeg P. Immunoglobulin A cell distribution in the human small intestine: phenotypic and functional characteristics. Immunology. 2000;101:354–63. doi: 10.1046/j.1365-2567.2000.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartz H, Mendoza Y, Gebker M, Fischborn T, Heeg K, Dalpke A. Poly-guanosine strings improve cellular uptake and stimulatory activity of phosphodiester CpG oligonucleotides in human leukocytes. Vaccine. 2004;23:148–55. doi: 10.1016/j.vaccine.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Agematsu K, Ochs HD, Sugane K. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol. 2003;108:128–37. doi: 10.1016/s1521-6616(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–13. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 23.Campana D, Janossy G, Bofill M, et al. Human B cell development. I. Phenotypic differences of B lymphocytes in the bone marrow and peripheral lymphoid tissue. J Immunol. 1985;134:1524–30. [PubMed] [Google Scholar]
- 24.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–7. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarte K, De Vos J, Thykjaer T, et al. Generation of polyclonal plasmablasts from peripheral blood B cells: a normal counterpart of malignant plasmablasts. Blood. 2002;100:1113–22. [PubMed] [Google Scholar]
- 26.Jego G, Bataille R, Pellat-Deceunynck C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 2001;97:1817–22. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Poeck H, Jahrsdoerfer B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–63. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 28.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5:62–9. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 29.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176:3885–9. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- 30.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–73. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoene V, Peiser M, Wanner R. Human monocyte-derived dendritic cells express TLR9 and react directly to the CpG-A oligonucleotide D19. J Leukoc Biol. 2006;80:1328–36. doi: 10.1189/jlb.0106011. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Alvarez R, Roderiquez G, Guan E, Caldwell Q, Wang J, Phelan M, Norcross MA. CpG-independent synergistic induction of beta-chemokines and a dendritic cell phenotype by orthophosphorothioate oligodeoxynucleotides and granulocyte–macrophage colony-stimulating factor in elutriated human primary monocytes. J Immunol. 2005;174:6113–21. doi: 10.4049/jimmunol.174.10.6113. [DOI] [PubMed] [Google Scholar]
- 33.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;236:140–5. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Eaton-Bassiri A, Dillon SB, Cunningham M, Rycyzyn MA, Mills J, Sarisky RT, Mbow ML. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun. 2004;72:7202–11. doi: 10.1128/IAI.72.12.7202-7211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–87. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 36.Anitescu M, Chace JH, Tuetken R, Yi AK, Berg DJ, Krieg AM, Cowdery JS. Interleukin-10 functions in vitro and in vivo to inhibit bacterial DNA-induced secretion of interleukin-12. J Interferon Cytokine Res. 1997;17:781–8. doi: 10.1089/jir.1997.17.781. [DOI] [PubMed] [Google Scholar]
- 37.Yi AK, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996;157:5394–402. [PubMed] [Google Scholar]
- 38.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol. 2002;168:4711–20. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]
- 39.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 41.Lenert PS. Targeting toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther. 2006;8:203. doi: 10.1186/ar1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 43.Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol. 2002;169:2368–73. doi: 10.4049/jimmunol.169.5.2368. [DOI] [PubMed] [Google Scholar]
- 44.Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol Scand. 2001;59:124–30. doi: 10.1080/000163501750266701. [DOI] [PubMed] [Google Scholar]
- 45.Goodnow CC. Immunology. Discriminating microbe from self suffers a double toll. Science. 2006;312:1606–8. doi: 10.1126/science.1129797. [DOI] [PubMed] [Google Scholar]
- 46.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J Immunol. 2005;174:1775–81. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 47.Pasare C, Medzhitov R. Control of B-cell responses by toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 48.Huggins J, Pellegrin T, Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27-naive human B cells. Blood. 2007;109:1611–9. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]