Abstract
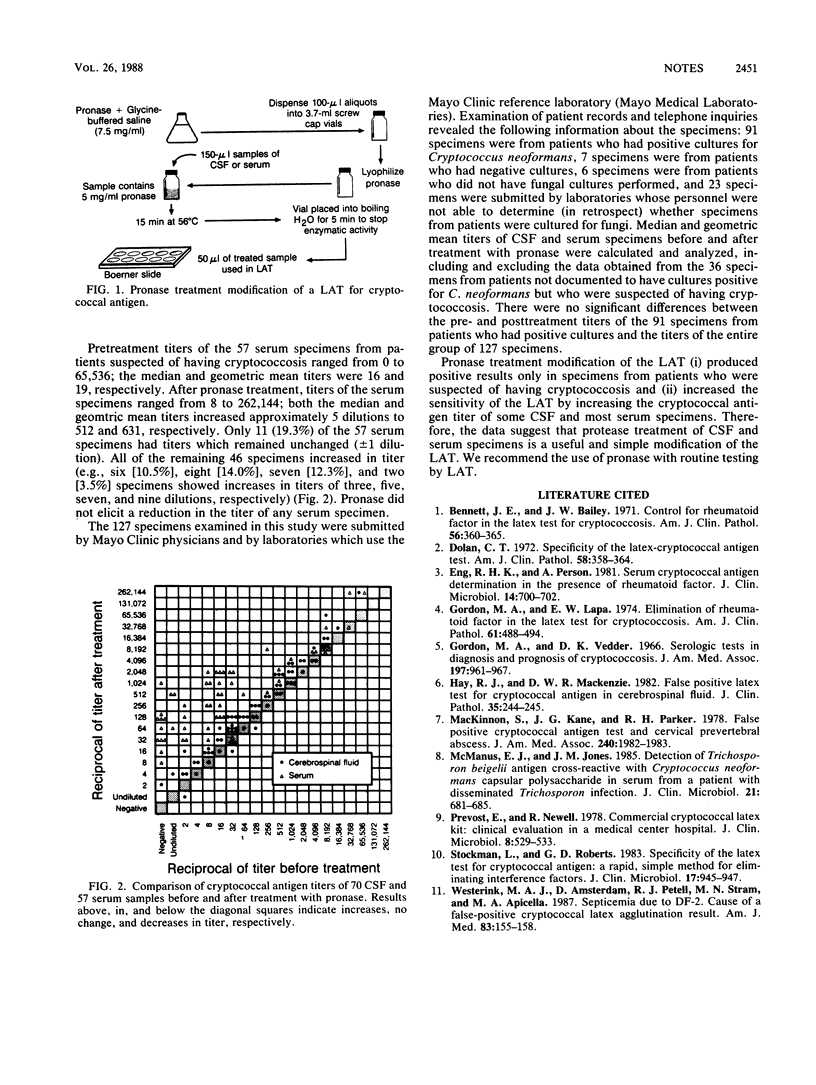
Cryptococcal antigen titers of 70 cerebrospinal fluid and 57 serum specimens from patients suspected of having cryptococcosis were determined both before and after treatment with pronase. Median titers of cerebrospinal fluid specimens before and after treatment were 128 and 128, respectively; mean geometric titers of these specimens before and after treatment were 102 and 204, respectively. Median titers of the serum specimens before and after treatment were 16 and 512, respectively; mean geometric titers of these specimens before and after treatment were 19 and 631, respectively. The modified latex agglutination test did not detect antigen in any of 50 cerebrospinal fluid and 51 serum specimens from patients not suspected of having cryptococcosis. These results suggest that the pronase modification increases the sensitivity of the latex agglutination test and that the modification be routinely incorporated into it.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. E., Bailey J. W. Control for rheumatoid factor in the latex test for cryptococcosis. Am J Clin Pathol. 1971 Sep;56(3):360–365. doi: 10.1093/ajcp/56.3.360. [DOI] [PubMed] [Google Scholar]
- Dolan C. T. Specificity of the latex-cryptococcal antigen test. Am J Clin Pathol. 1972 Oct;58(4):358–364. doi: 10.1093/ajcp/58.5.358. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Person A. Serum cryptococcal antigen determination in the presence of rheumatoid factor. J Clin Microbiol. 1981 Dec;14(6):700–702. doi: 10.1128/jcm.14.6.700-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. A., Lapa E. W. Elimination of rheumatoid factor in the latex test for cryptococcosis. Am J Clin Pathol. 1974 Apr;61(4):488–494. doi: 10.1093/ajcp/61.4.488. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Vedder D. K. Serologic tests in diagnosis and prognosis of cryptococcosis. JAMA. 1966 Sep 19;197(12):961–967. [PubMed] [Google Scholar]
- Hay R. J., Mackenzie D. W. False positive latex tests for cryptococcal antigen in cerebrospinal fluid. J Clin Pathol. 1982 Feb;35(2):244–245. doi: 10.1136/jcp.35.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon S., Kane J. G., Parker R. H. False-positive cryptococcal antigen test and cervical prevertebral abscess. JAMA. 1978 Oct 27;240(18):1982–1983. [PubMed] [Google Scholar]
- McManus E. J., Jones J. M. Detection of a Trichosporon beigelii antigen cross-reactive with Cryptococcus neoformans capsular polysaccharide in serum from a patient with disseminated Trichosporon infection. J Clin Microbiol. 1985 May;21(5):681–685. doi: 10.1128/jcm.21.5.681-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost E., Newell R. Commercial cryptococcal latex kit: clinical evaluation in a medical center hospital. J Clin Microbiol. 1978 Nov;8(5):529–533. doi: 10.1128/jcm.8.5.529-533.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L., Roberts G. D. Corrected version specificity of the latex test for cryptococcal antigen: a rapid, simple method for eliminating interference factors. J Clin Microbiol. 1983 May;17(5):945–947. doi: 10.1128/jcm.17.5.945-947.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink M. A., Amsterdam D., Petell R. J., Stram M. N., Apicella M. A. Septicemia due to DF-2. Cause of a false-positive cryptococcal latex agglutination result. Am J Med. 1987 Jul;83(1):155–158. doi: 10.1016/0002-9343(87)90512-2. [DOI] [PubMed] [Google Scholar]


