Abstract
Spores are essential particles for the survival of many organisms, both prokaryotic and eukaryotic. Among the eukaryotes, fungi have developed spores with superior resistance and dispersal properties. For the human fungal pathogens, however, relatively little is known about the role that spores play in dispersal and infection. Here we present the purification and characterization of spores from the environmental fungus Cryptococcus neoformans. For the first time, we purified spores to homogeneity and assessed their morphological, stress resistance, and surface properties. We found that spores are morphologically distinct from yeast cells and are covered with a thick spore coat. Spores are also more resistant to environmental stresses than yeast cells and display a spore-specific configuration of polysaccharides on their surfaces. Surprisingly, we found that the surface of the spore reacts with antibodies to the polysaccharide glucuronoxylomannan, the most abundant component of the polysaccharide capsule required for C. neoformans virulence. We explored the role of capsule polysaccharide in spore development by assessing spore formation in a series of acapsular strains and determined that capsule biosynthesis genes are required for proper sexual development and normal spore formation. Our findings suggest that C. neoformans spores may have an adapted cell surface that facilitates persistence in harsh environments and ultimately allows them to infect mammalian hosts.
Sporulation is a common strategy used by bacteria, fungi, and protozoa to survive environmental conditions that cannot support vegetative growth. Spores serve two purposes, namely, rest during harsh conditions to allow germination once the environment is conducive to growth and dispersal of the organism to new territories that may be better suited for growth (25).
The resistance properties of fungal spores under harsh conditions have been well characterized with the model yeast Saccharomyces cerevisiae. S. cerevisiae diploid cells produce spores that are adapted for withstanding stresses such as lytic enzymes, desiccation, and high temperatures (36). The stress resistance properties of S. cerevisiae spores have been linked genetically and biochemically to components of the spore coat, which is compositionally and architecturally distinct from the cell wall of yeast cells (4-6).
Mechanisms of spore dispersal are particularly well developed in fungi. Many fungi produce specialized structures called fruiting bodies that are optimized for dispersal of spores into the environment (7). For example, filamentous fungi produce stalks with spores (conidiophores) which grow vertically to expose spores to wind, splashes, or animal vectors for dispersal (14). Another fungal spore dispersal structure is the ascus, a sac surrounding spores, which can be used to propel spores into stronger wind or water currents (46). In addition to fruiting bodies, fungal spores themselves are also morphologically adapted for dispersal, with an aerodynamic shape and tough spore wall to withstand harsh conditions (14).
Even though dispersed spores are thought to be the infectious agents of many plant fungal pathogens, relatively little is known about spores that cause disease in humans. Many human fungal pathogens, such as Coccidioides immitis, Blastomyces dermatitidis, Histoplasma capsulatum, and Aspergillus fumigatus, can infect humans via the respiratory route as spores, but few studies to determine the properties of these spores have been carried out (37). Another inhaled fungal pathogen is Cryptococcus neoformans, an opportunistic pathogen that causes meningoencephalitis in mammals (10). C. neoformans is unique among the human fungal pathogens because it is amenable to a wide variety of molecular and genetic analyses in the laboratory (22). For this reason, C. neoformans may be an efficient model for understanding the nature of spores from human fungal pathogens.
C. neoformans causes disease primarily in immunocompromised individuals, including AIDS patients, transplant recipients, and patients undergoing cancer chemotherapy (10). Although the infectious particle of C. neoformans in nature is unknown, a model of the natural route of infection has been created based on an abundance of clinical data. In this model, spores or desiccated yeast cells from the environment are inhaled and establish an asymptomatic infection in the lung (22). In healthy individuals, these particles generally do not cause disease; however, in immunocompromised people, the infection disseminates to the central nervous system and progresses to meningoencephalitis, which is uniformly fatal without treatment (15). Spores are predicted to have an advantage in this model because of their small size (∼1 to 2 μm), which would allow them to penetrate the alveoli within the lung more efficiently than yeast cells (∼3 μm in diameter when desiccated and ∼5 to 10 μm in diameter when hydrated) (16). In addition, the fruiting bodies of C. neoformans, like those of many fungi, appear to be adapted for efficient spore dispersal, with aerial hyphae and exposed spore chains (17).
Sporulation in C. neoformans is a result of two distinct pathways. Spores can be produced when yeast cells of opposite mating types (a and α) encounter one another under appropriate environmental conditions. The yeast cells fuse and form filaments, which replicate until an unknown signal leads to the formation of a basidium (fruiting body) and long spore chains (27). An alternative pathway leading to sporulation is α or monokaryotic fruiting. Fruiting occurs when α cells are grown under nutrient-limiting conditions, causing them to form filaments and basidia with short spore chains (49). In both cases, the filaments are aerial, growing away from the substrate, presumably facilitating spore dispersal.
Little is known about the developmental processes that lead to spore production in C. neoformans, and virtually nothing is known about the nature of the spore particle. One challenge to the study of C. neoformans spores has been the absence of a method for isolating large numbers of pure spores. Although microdissection has been an effective method for isolating spores for genetic analyses (26), this method cannot yield sufficient numbers of spores for biochemical or molecular studies. Previous attempts to isolate large numbers of pure spores have not been successful or have yielded mixed cell-spore populations unsuitable for most studies. Filtration methods have been implemented to isolate spores for virulence studies, but these methods are not easily reproduced, and small yeast cells can contaminate samples (45). Large numbers of yeast cells in a sexually developing population make it impossible to isolate spores by filtration methods commonly used for filamentous fungi. To circumvent these difficulties, we developed a method to purify spores to homogeneity using density gradient centrifugation.
From crosses undergoing sexual development, we isolated small elliptical particles by using density gradients and confirmed that they were spores. These purified spores were then characterized in detail, using scanning electron microscopy, transmission electron microscopy, and fluorescence microscopy. We also assessed spore resistance to a variety of environmental conditions. In addition, we probed the surfaces of spores, using a library of lectins to identify spore-specific surface carbohydrates. Finally, we evaluated the spore for the presence of the capsule polysaccharide glucuronoxylomannan (GXM). Using these approaches, we have characterized C. neoformans spores in detail for the first time, and we have discovered that capsule biosynthesis genes are required for normal sexual development and spore biogenesis.
MATERIALS AND METHODS
Strain manipulations and media.
All strains used were of the serotype D background (Cryptococcus neoformans var. neoformans). All were handled using standard techniques and media as described previously (1, 42). Sexual development assays were conducted on V8 medium at room temperature in the dark for 2 to 4 days. Sexual development was evaluated by observing the peripheries of test spots on V8 medium. The wild-type strains used in crosses were JEC20 (a) and JEC21 (α) (28).
Gradient purification of C. neoformans spores.
Cultures of JEC20 crossed with JEC21 were scraped off V8 agar plates after 4 to 5 days. The mass of cells, consisting of yeast cells, filaments, basidia, and spores, was suspended in 60% Percoll (Fisher) containing 0.1% Triton X-100 and made isotonic with phosphate-buffered saline (PBS). The suspension was then centrifuged at 10,000 × g for 30 min in an SW 40Ti ultracentrifuge rotor (Beckman-Coulter) to produce a self-generating gradient. A band of spores could be visualized near the bottom of the gradient, with all other types of cells remaining near the top of the gradient. A 1-ml tuberculin syringe was used to puncture through the side of the tube and harvest the band of spores.
Isolation and analysis of recombinant spores.
To track auxotrophic markers in spore progeny, parental strains with deletions in the ADE2 (α) and URA5 (a) genes were crossed (JEC50 and JEC43, respectively) (35, 48). Strains were resuspended together in PBS and streaked onto V8 juice agar. Sexual development was allowed to take place for 4 to 5 days prior to spore purification. Spores were isolated using a Percoll gradient, plated onto yeast-peptone-dextrose (YPD) agar, and grown at 30°C for 2 days. Resulting colonies were patched on YPD agar and grown at 30°C overnight before they were replica plated onto synthetic dextrose medium without uracil or without adenine. Replica plates were then evaluated for cell growth after 24 h at 30°C.
Fluorescence staining with lectins and anti-GXM antibody.
Cells were suspended in 10 mM HEPES buffer with 0.15 M NaCl, 0.1 mM CaCl2, and 0.1 mM MnCl2. Fluorescein-conjugated Datura stramonium lectin (DSL) or rhodamine-conjugated concanavalin A (ConA; Vector Labs) was then added at a 1:50 dilution. Counterstaining was performed using calcofluor white MR2 (Sigma) at 3.5 mg/ml. The cells were incubated in these stains for 20 min on ice and then washed twice in HEPES buffer. The following lectins did not bind to either spores or yeast cells: Glycine max SBA, Ricinus communis strain 120 RCA 120, Arachis hypogeae PNA, Dolichos biflorus DBA, Phaseolus vulgaris PHA-L, Phaseolus vulgaris PHA-E, Jacalin, Erythrina cristagalli ECL, Griffonia simplicifolia GSL-1, Griffonia simplicifolia GSL-2, Sophora japonica SJA, and Vicia villosa VVA.
Capsule staining was carried out using a panel of anti-GXM antibodies directly conjugated to Alexa Fluor 594 (Invitrogen) (3). The staining was performed by suspending yeast cells or spores in PBS containing 0.1% bovine serum albumin. The cells were incubated with 50 μg/ml of anti-GXM antibody for 30 min and then washed three times using PBS with 0.1% bovine serum albumin. All counterstains, such as calcofluor white MR2 (Sigma), 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen), and DSL (Vector Labs), were added at the same time as the antibody.
Electron microscopy.
Spores were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 100 mM sodium phosphate buffer, pH 7, for 12 h at 4°C. Spores were washed free of fixative and then applied to poly-l-lysine-coated coverslips (Sigma) and allowed to adhere for 1 hour. Coverslips with adhered spores were then soaked in 4% osmium tetroxide for 1 hour. Coverslips were then dehydrated using an ethanol (EtOH) series with 10-minute incubations in 50% (once), 75% (once), and 100% (three times) EtOH, followed by incubations in a hexamethyldisilazane (HMDS) (Sigma) series consisting of 10 min in 1:1 HMDS-EtOH and three 10-min soaks in 100% HMDS. The coverslips were then air dried at room temperature before being sputter coated with palladium. A Hitachi S-570 scanning electron microscope was used to visualize the samples.
Transmission electron microscopy.
C. neoformans spores were immersion fixed for 2 h in 2.5% glutaraldehyde and 2.0% paraformaldehyde buffered in 100 mM sodium phosphate buffer (PB) at room temperature. The primary fixed samples were rinsed five times for 5 min each time in PB, postfixed in 1% osmium tetroxide and 1% potassium ferrocyanide in 0.1 M PB for 1 h at room temperature, and rinsed five times for 5 min each in PB. Postfixed and rinsed spores were dehydrated in a graded series of EtOH washes with increasing percentages of EtOH (35%, 50%, 70%, 80%, 90%, and 95% for 10 min each and 100% three times for 10 min each) at room temperature. Fully dehydrated samples were infiltrated with increasing concentrations of PolyBed 812 (Polysciences Inc.) and propylene oxide. Infiltrated samples were placed in embedding capsules (BEEM). Embedding and polymerization took place in fresh PolyBed 812 for 48 h at 60°C. Embedded spores were sectioned on a Leica EM UC6 ultramicrotome at 90 nm. The sections were collected on bare 300-mesh thin-bar copper grids (EMS) and poststained in uranyl acetate and lead citrate. The sectioned samples were viewed at 80 kV on a Philips CM120 transmission electron microscope equipped with a MegaView III camera (Olympus Soft Imaging System).
Assaying environmental stress resistance.
All spores used for stress resistance assays were isolated from crosses between JEC20 and JEC21 by use of a 60% Percoll gradient equilibrated with PBS and containing 0.1% Triton X-100. The yeast strains JEC20 and JEC21 were harvested at mid-log phase after growth in YPD prior to exposure to stress conditions. To examine heat shock, cells were suspended in PBS at ∼300 CFU/0.1 ml. One-hundred-microliter aliquots were incubated in a water bath at 25°C or 42°C. At each time point, the cells were plated on YPD agar, incubated at 30°C for 3 days, and then scored for number of colonies. The value for each time point was then converted to the percentage of survival compared to that at time zero. Diethyl ether resistance was tested by plating ∼300 colonies onto YPD agar and then inverting the plate over Whatman filter paper treated with 1 ml of diethyl ether (Sigma). Survival was assessed by quantifying colony formation after increasing times of diethyl ether exposure. Desiccation resistance was assayed by making 10-fold serial dilutions of spores and yeast cells, starting with 107 cells/ml, and spotting the dilutions onto rich medium and nitrocellulose membranes. The membranes were then placed in sterile petri dishes and allowed to dry at ambient temperature for 48 h. The membranes were then placed on YPD agar, and growth on the membranes was compared to growth on rich medium without desiccation. Oxidative stress resistance was assessed by making 10-fold serial dilutions of spores and parental yeast cells in PBS. The cell suspensions were then mixed with an equivalent volume of 20 mM H2O2. The cell suspensions were incubated in 10 mM H2O2 for 5 minutes before they were plated to YPD and grown at 30°C for 2 days. UV light resistance was assessed by suspending 200 to 300 yeast cells or spores in 100 μl of PBS and spotting the suspension onto the lid of a sterile petri dish. Droplets were exposed to a series of UV dosages from 0 to 25,000 μJ/cm2, using a Stratalinker UV light source (Stratagene). The suspension of yeast cells or spores was then spread onto YPD and grown at 30°C for 2 days.
Capsule-deficient strain construction.
To produce capsule-deficient spores, we created congenic mating pairs of four well-characterized capsule mutants. The starting acapsular strains, cap10Δ, cap60Δ, cap64Δ, and cap67 strains (ATCC 52817-B3501 background), were all of the α mating type, so each mutant strain was crossed with JEC20 (a) on V8 agar (11-13, 23). Once spore chains were visible, spores were purified using a Percoll gradient. Isolated spores (>99% pure) were outgrown, and capsule-deficient colonies were crossed with JEC20 and JEC21 to determine their mating types. a cap mutants were confirmed to be deficient in capsule production by staining with India ink and Alexa Fluor 594-conjugated anti-GXM antibody. a cap mutants were then backcrossed with JEC21 (α), and spores from this cross yielded both a cap and α cap strains for each capsule gene, as follows: a cap10Δ (CHY1528), α cap10Δ (CHY1353), a cap60Δ (CHY1613), α cap60Δ (CHY1529), a cap64Δ (CH1534), α cap64Δ (CHY1355), a cap67 (CHY1377), and α cap67 (CHY1352) strains.
The α cap67 strain used in these studies was the parental strain (ATCC 52817) instead of the F1 α cap67 strain because F1 α cap67 × F1 a cap67 mutants exhibited a more severe phenotype than other capsule mutants, with little or no filamentation and no spores. The α cap67 (ATCC 52817) × F1 a cap67 strain had a sexual development phenotype identical to that of crosses of F1 strains from the other capsule mutants (cap10Δ, cap60Δ, and cap64Δ strains) and produced enough spores to carry out further analyses.
Capsule transfer assay.
Capsule transfer assays using capsule-deficient spores were conducted as previously described (38). Briefly, capsule-containing medium was made by culturing JEC21 in minimal medium (synthetic dextrose liquid) for 5 days and then clarifying the medium using centrifugation and filtration. Acapsular spores and parental yeast cells were incubated in 2 μl/ml of capsule-containing medium diluted in PBS. After washing away free capsule, the cells were stained with anti-GXM antibody (F12D2) conjugated to Alexa Fluor 594.
RESULTS
Purification of C. neoformans spores.
During C. neoformans sexual development, many distinct cell types are formed. To isolate spores from yeast cells, filaments, and basidia, we carried out density gradient centrifugation. Crosses between a and α cells were carried out on V8 agar plates, and approximately 1 gram of the resulting cell mixture was scraped off the plates and suspended in 10 ml of 60% Percoll equilibrated with PBS containing 0.1% Triton X-100. The resulting suspension was subjected to centrifugation at 10,000 × g for 30 min. After centrifugation, several bands were visible to the naked eye. Microscopic analysis of fractions from the gradient revealed a band at the bottom of the gradient containing small particles (1 to 3 μm) that appeared consistent with the size and shape of spores (17).
We validated that the particles from the gradient were spores by evaluating the distribution of parental markers. Differentially marked parental strains, a ura5 and α ade2 strains, were crossed on V8 agar and subjected to density gradient centrifugation. Particles of 1 to 3 μm that were predicted to be spores were isolated, counted on a hemacytometer, and grown on nutrient-rich medium. Individual colonies were picked and evaluated for auxotrophies. Colonies were replica plated onto minimal medium without uracil, minimal medium without adenine, and rich medium. Three replicates of 100 to 200 colonies were scored for their growth phenotypes on these media, and the fraction of colonies with each phenotype was calculated. The distribution of markers was found to be Mendelian in nature, with each possible phenotype accounting for approximately 25% of the colonies (the percentages of Ura+ Ade−, Ura− Ade+, Ura− Ade−, and Ura+ Ade+ colonies were 25.9, 24.3, 27.9, and 22%, respectively). If these cells were a mixture of only the parental strains or diploids, we would not have recovered the recombinant genotypes consistent with spores, because no cells with both auxotrophies could have been produced. We therefore concluded that the particles isolated from the bottom of the gradients were the spore progeny of the two parental strains. We also concluded that the spores were pure because contamination by parental yeast cells would have altered the marker distribution, resulting in a non-Mendelian distribution of phenotypes. Overall, spores recovered by this gradient purification method have a germination frequency of >90%, suggesting that the vast majority of spores from crosses are viable. In addition, gradient-purified spores are very stable; if they are not exposed to rich growth medium, spores do not germinate into yeast cells and remain viable in PBS for at least several months (data not shown).
Spores have a large surface coat.
To compare the general structures of spores and yeast cells, both cell types were visualized by light and fluorescence microscopy. Figure 1A shows both yeast cells and spores in a bright-field image. As observed previously, yeast cells are large and spherical relative to spores, which are much smaller and elongated (17). When stained with calcofluor white (which binds chitin) to reveal the cell wall and with SYTOX green to reveal nuclei, both cell types are visible under fluorescence microscopy, with cell wall material highlighted in blue and nuclei highlighted in green. Yeast cells are ∼5 μm in diameter and contain a single nucleus (Fig. 1B). Spores are much smaller than yeast cells overall. Quantitative measurements of several hundred spores similar to those shown in Fig. 1C provided an average spore size of 1.5 μm ± 0.1 μm by 3 μm ± 0.2 μm.
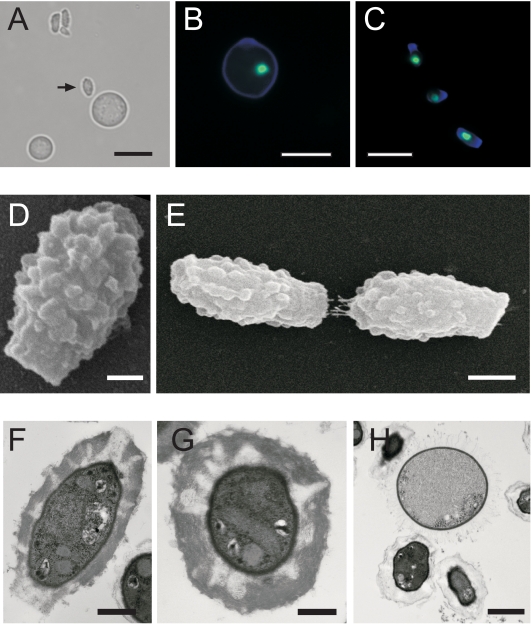
FIG. 1.
C. neoformans spores have a morphology distinct from that of yeast cells. (A) Bright-field micrograph revealing the relative differences in size and shape between a spore (arrow) and a nearby yeast cell. Bar, 5 μm. (B and C) Yeast cell (B) and spores (C) stained with calcofluor white (blue cell walls) and SYTOX green (green nuclei). Bars, 5 μm. (D) SEM micrograph showing the ultrastructure of the spore. Bar, 500 nm.(E) SEM micrograph showing two spores end to end. Bar, 1 μm. (F) TEM micrograph of a longitudinal section of a spore. Bar, 500 nm. (G) TEM micrograph of a latitudinal section of a spore. Bar, 250 nm. (H) TEM micrograph of two spores adjacent to a yeast cell. Bar, 1 μm.
To further investigate the morphology of spores, we carried out scanning electron microscopy (SEM). SEM micrographs revealed that spores are highly polar, with a flat, “stalk-like” structure at one end and a narrower “tip” at the other end (Fig. 1D). Nearly all spores in the population shared this morphology. It is possible that these structures are important for spore chain formation, as seen in Fig. 1E, where two spores are oriented in a head-to-tail manner, suggesting that spore chains may consist of similarly oriented spores. The head-to-tail arrangement was always observed when spores were in a chain-like formation. SEM also revealed that the spore ultrastructure is composed of a highly textured, heterogeneous surface.
To illuminate the internal architecture of spores, we carried out transmission electron microscopy (TEM). TEM micrographs (Fig. 1F to H) confirmed a polar cell with a stalk-like structure at one end and a thick spore coat (∼250 nm), making up a significant proportion of the volume of the spore (Fig. 1F). Cross sections also indicated that the spore coat is not completely homogenous (Fig. 1G) but contains striated zones of low electron density that may correspond to the textured surface of the spore seen using SEM.
Strikingly, this thick coat contrasts sharply with the encapsulated cell wall of vegetatively growing yeast cells (Fig. 1H) and with previously reported TEM micrographs of yeast cells under capsule-inducing conditions in which yeast cells with capsule have a mesh-like capsule layer (18). To rule out the possibility that our purification protocol, which used detergent to prevent spore aggregates, might alter the structure of the spore coat, we carried out density gradients using bovine serum albumin (0.1% final concentration) in place of the detergent (0.1% Triton X-100). Both TEM and lectin staining gave identical images with Triton X-100 and bovine serum albumin, indicating that the spore coat structure is not fundamentally altered by exposure to low concentrations of detergent (data not shown).
C. neoformans spores are resistant to environmental stress.
To evaluate the resistance of spores and yeast cells to extreme environmental conditions, spores and parental yeast cells were subjected to simulated environmental stresses and evaluated for survival. Spore survival was assayed in response to high temperature, desiccation, oxidative stress, chemical insult, and UV exposure. To assess temperature resistance, spores and parental yeast cells (mid-log phase) were suspended in PBS and incubated for various times at a series of temperatures from 25°C to 42°C before being plated onto rich medium (YPD). At 25°C, both spores and yeast cells survived until the final time point, i.e., 2 h (Fig. 2A, top panel). However, at higher temperatures, spores exhibited greater resistance than yeast cells did. At 42°C, >50% of the spores survived after 15 min, whereas both parental yeast strains were inviable after 3 min at 42°C (Fig. 2A, bottom panel). Stationary-phase yeast cells were also evaluated in this assay and were inviable after 3 min at 42°C, showing sensitivity to high temperature identical to that of mid-log-phase yeast cells (data not shown).
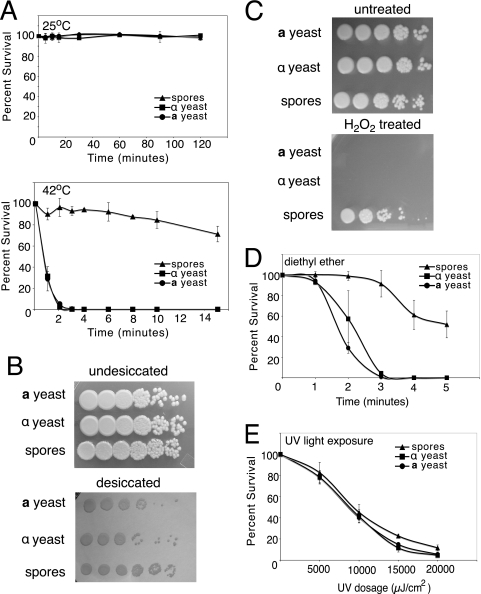
FIG. 2.
Spores are more resistant to environmental stress than yeast cells. All environmental stress resistance experiments were carried out using wild-type a and α yeast cells (JEC20 and JEC21, respectively) and spores purified from a cross between them. (A) Resistance to heat shock. Both a and α yeast cells (circles and squares, respectively) and spores (triangles) were incubated in PBS at 25°C or 42°C before they were plated on rich growth medium. On each graph, the x axis shows time of incubation and the y axis shows percent survival. (B) Resistance to desiccation. The top panel shows 10-fold serial dilutions of spores and parental yeast cells grown on rich medium. The bottom panel shows the same dilutions spotted onto a nitrocellulose membrane and incubated at room temperature for 2 days before the membrane was transferred to rich medium. Rows are labeled according to cell type (top, a yeast; middle, α yeast; and bottom, spores). (C) Resistance to oxidative stress. Both a and α yeast cells and spores were diluted in 10-fold serial dilutions and incubated in 10 mM H2O2 before they were plated to rich medium. Rows are labeled according to cell type (top, a yeast; middle, α yeast; and bottom, spores). (D) Resistance to diethyl ether. Both a and α yeast cells and spores were plated on rich growth medium and then exposed to diethyl ether vapors. The x axis shows time of exposure, and the y axis shows percent survival of spores and yeast cells after vapor exposure. (E) Exposure to UV light. Both a and α yeast cells and spores were diluted to 300 cells in 10 μl water and spotted on a solid surface. The spots were exposed to increasing dosages of UV light before they were plated to rich medium. The x axis shows the dose of UV in μJ/cm2, and the y axis shows percent survival compared to untreated cells.
To assess desiccation resistance, spores and yeast cells were spotted onto nitrocellulose membranes and placed in a desiccation chamber for 48 h. The membranes were then transferred to nutrient-rich agar and grown at 30°C to assess survival. Undesiccated yeast cells and spores grew to the same extent on rich medium (Fig. 2B, top panel). However, spores were more resistant to desiccation than parental yeast cells (Fig. 2B, bottom panel). In fact, over the course of four independent experiments, spores were consistently at least 10-fold more resistant to desiccation than the parental yeast cells.
Oxidative stress resistance was assessed by exposing spores and mid-log-phase yeast cells in solution to H2O2. Tenfold serial dilutions of 107 cells/ml were suspended in PBS containing 10 mM H2O2 for 5 min, plated on rich medium, and grown at 30°C. Untreated yeast cells and spores grew equally well (Fig. 2C, top panel). However, yeast cells were all killed by 10 mM H2O2 after 5 min of exposure. In contrast, spores survived incubation in 10 mM H2O2 (Fig. 2C, bottom panel). Stationary-phase yeast cells had a susceptibility to oxidative stress identical to that of mid-log-phase yeast cells, with no stationary-phase yeast cells surviving after 5 min of exposure to 10 mM H2O2 (data not shown).
General chemical resistance was evaluated by exposure to diethyl ether. Spores and yeast cells were plated on rich medium and exposed to diethyl ether vapors emanating from Whatman paper saturated with diethyl ether. Spores were more resistant to exposure to diethyl ether vapors, with >50% survival after 5 min, whereas the parental yeast strains showed no survival at this time point (Fig. 2D).
Although spores were more resistant than yeast cells to these diverse forms of stress, spores were not significantly more resistant to another form of stress, UV light. To assess UV resistance, PBS containing 200 to 300 yeast cells or spores was exposed to increasing doses of UV light, ranging from 5,000 μJ/cm2 to 20,000 μJ/cm2. Survival was assessed by spreading the treated cells onto rich medium and scoring the CFU. Spores and yeast cells showed no significant differences in survival after exposure to UV (Fig. 2E). Overall, these resistance data indicate that spores are more resistant to environmental stress than yeast cells, but this differential resistance does not apply to all types of stress.
Spores and yeast cells display different polysaccharide profiles on their surfaces.
Many fungal spores have a protective coat made of glycoproteins and polysaccharides, which protects them from the environment (4-6). The coat of C. neoformans spores could be important for survival in its ecological niche and in its interaction with a mammalian host. To begin to understand the nature of the C. neoformans spore coat, we probed both yeast cells and spores for surface-exposed sugar moieties by using lectins. Lectins are proteins with carbohydrate binding abilities that can be fluorescently labeled and used to stain different sugars. Twenty-one fluorescently labeled lectins were initially screened against purified spores, and those lectins that bound spores were used to stain a mixture of spores and yeast cells to assess the specificity of each lectin for spores.
Two lectins had spore-specific binding characteristics. Datura stramonium lectin (DSL), which binds oligomers of N-acetylglucosamine, bound to the spore surface but not to the surfaces of yeast cells (Fig. 3A, left panel) or filaments (Fig. 3A, right panel). Concanavalin A (ConA), which binds internal α-linked mannose in glycoprotein core structures, also showed spore-specific binding. This lectin had been discovered previously to bind the bud scars of C. neoformans yeast cells (19, 21). However, like DSL, ConA binds the entire surface of the spore but does not bind filaments or basidia (Fig. 3B). These data provide evidence that not only is the surface of the spore morphologically distinct from that of yeast cells (as seen in the SEM and TEM images of Fig. 1) but that the exposed spore surface is also compositionally distinct.
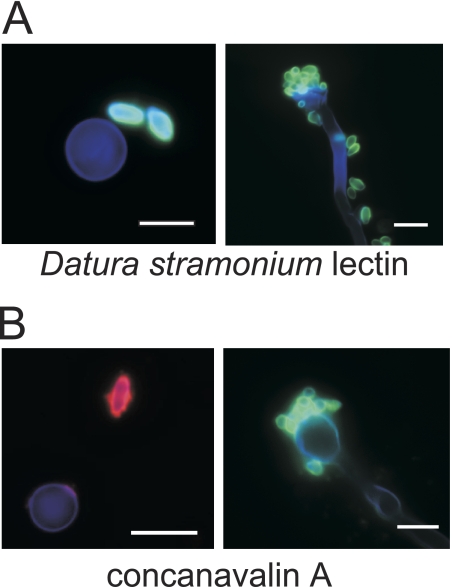
FIG. 3.
Spores have specific lectin binding properties. Spores and yeast cells were stained with calcofluor white (to stain all cell types) and fluorescently labeled lectins (to show spore-specific binding). Stained cells were visualized by fluorescence microscopy. Bars, 5 μm. (A) Spore-specific staining by DSL (green), which binds N-acetylglucosamine oligomers. The left panel shows a yeast cell (∼5 μm in diameter; blue) and two spores (∼3 μm long; green). The right panel shows a long filament cell with a terminal basidium (club-like structure of ∼5 μm in diameter; blue), with spores from collapsed chains on the end and scattered along the filament (∼3 μm long; green). (B) Spore-specific staining by ConA (visualized in red in the left panel in and green in the right panel), which binds α-mannose residues. The left panel shows a yeast cell (∼5 μm in diameter; blue) and a spore (∼3 μm long; red). The right panel shows a basidium (club-like structure of ∼5 μm in diameter; blue) with collapsed spore chains attached (∼3 μm long; green).
Anti-GXM antibodies bind the spore surface.
A polysaccharide found on the surfaces of yeast cells is GXM, the major component of the C. neoformans capsule. A large polysaccharide capsule is produced in response to the host environment, and it has been shown to be essential for virulence of C. neoformans (8). To assess what role GXM might play in spore architecture, we used a fluorophore-labeled anti-GXM antibody (F12D2) to stain both spores and yeast cells for the presence of GXM (Fig. 4A). Because the surfaces of yeast cells and spores were morphologically dissimilar and exhibited distinct exposed surface polysaccharides, we predicted that spores would likely not harbor anti-GXM epitopes on the spore surface. Surprisingly, however, spores showed robust staining with the anti-GXM antibody. As expected, yeast cells stained with anti-GXM antibody but not with spore-specific DSL (Fig. 4A, top panels). In contrast, spores stained with both anti-GXM antibody and DSL (Fig. 4A, bottom panels), with anti-GXM staining appearing external to staining with DSL. Spores were also probed using a panel of anti-GXM antibodies raised against purified GXM from serotype A, B, C, and D yeast cells (Table 1) (3). In each case, the anti-GXM antibody signal appeared to occur in patches over the entire spore surface, with apparent “hot spots” in some locations and other areas showing little or no binding (Fig. 4B). These findings suggest that an anti-GXM reactive molecule is part of the spore coat. Alternatively, however, binding of the anti-GXM antibodies to the spore surface could have been the result of GXM transfer from yeast cells to the spores during the purification process. Capsule transfer has been shown previously to occur when acapsular strains of yeast are exposed to capsular material in solution (38). To clarify the origin of spore-associated anti-GXM antibody binding, we carried out capsule transfer assays with capsule-producing yeast cells and acapsular spores.
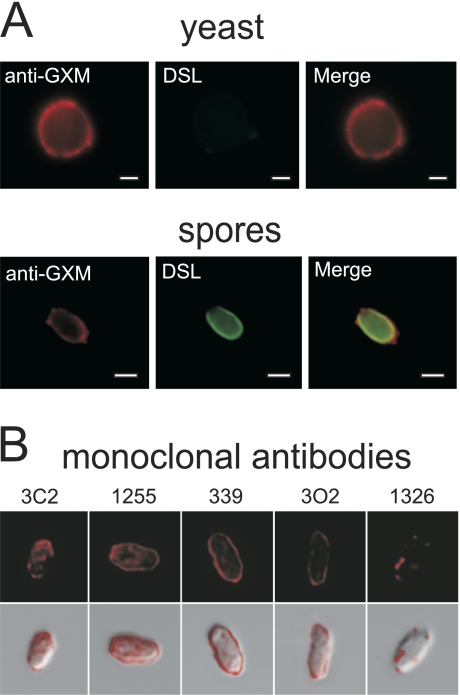
FIG. 4.
The spore surface is bound by antibodies to the capsular polysaccharide GXM. (A) The top panels show a yeast cell stained with the anti-GXM antibody F12D2 (red) and with DSL (no signal). The bottom panels show an identically stained spore brightly labeled with both anti-GXM antibody (red) and DSL (green), indicating the presence of anti-GXM epitopes on the surfaces of spores. Bars, 1 μm. (B) The top panels show the binding of five independent monoclonal anti-GXM antibodies (red). The bottom panels display a merge of anti-GXM antibody staining with differential interference contrast bright-field images of spores.
TABLE 1.
Monoclonal antibodies used for spore staininga
| Monoclonal antibody | GXM used for immunization | Immunoglobulin G Isotype | Serotype reactivity |
|---|---|---|---|
| 3C2 | Serotype C | 1 | A, B, C, D |
| 471 | Serotype A | 1 | A, B, C, D |
| 339 | Serotype B | 1 | A, B, D |
| 1255 | Serotype A | 1 | A, B, D |
| 302 | Serotype D | 1 | A, D |
| 1326 | Serotype A | 1 | A, D |
| F12D2 | De-O-Ab | 3 | A, B, C, D |
Monoclonal antibodies were raised against GXM purified from the culture medium of yeast cells. GXM from serotypes A, B, C, and D were used as antigens, producing some antibodies that bind all four serotypes and some that are specific for a subset of serotypes. The different reactivities of the antibodies indicate that this panel binds to several different epitopes within the GXM structure.
De-O-acetylated serotype A GXM.
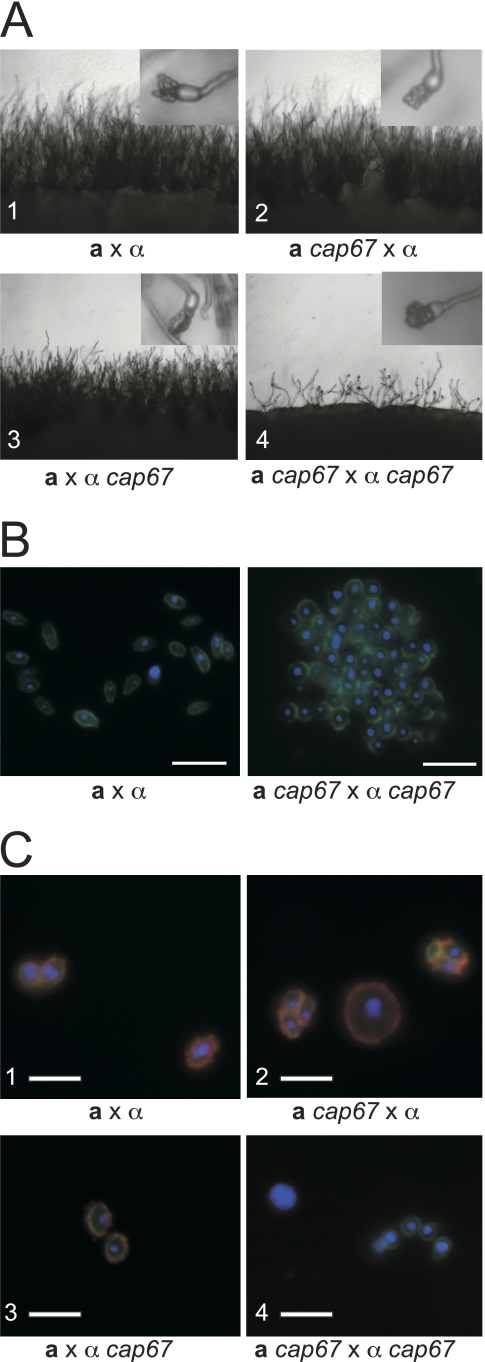
To generate acapsular spores, we first needed to generate acapsular yeast strains in congenic backgrounds. We chose four independent capsule mutants that had been characterized previously (cap10Δ, cap60Δ, cap64Δ, and cap67 strains) and backcrossed each of them into the congenic serotype D (JEC20 or JEC21) background to generate parent starting strains for crosses between acapsular strains. Acapsular spores were generated by crossing the resulting congenic, capsule-deficient a and α strains. All four capsule mutants led to the same outcome: crossing of capsule-deficient strains led to diminished sexual development (Fig. 5A), producing fewer filaments and basidia than either wild-type crosses (a × α) or unilateral crosses (capΔ mutant × wild type). Spore production by the capsule-deficient strains was limited, and these spores formed large clumps instead of typical spore chains (Fig. 5A, insets). Microscopy of spores from capsule-deficient parents isolated using Percoll gradients revealed that these spores formed large aggregates that could not be disrupted with Triton X-100 detergent at concentrations of up to 1.0% or by physical disruption (Fig. 5B).
FIG. 5.
Capsule mutants have defects in sexual development and spore formation. (A) All panels show the periphery of a cross on V8 medium under a light microscope at low magnification (×200). Insets each show a single basidium under higher magnification (×400). (1) Wild-type a × α cross. (2 and 3) Wild-type a or α strain crossed with corresponding a cap67 or α cap67 strain. (4) a cap67 × α cap67 cross. The few basidia that were produced did not develop proper spore chains and yielded clumps of spores (inset in panel 4). Identical results were obtained for all additional capsule deletion strains tested (cap10Δ, cap60Δ, and cap64Δ strains). (B) Spores were stained with DAPI (blue) and DSL (green) and visualized by fluorescence micros- copy. (Left) Normal morphology of spores derived from a wild-type cross. (Right) Spores derived from a cap67 × cap67 cross forming an aggregated mass of spores. (C) Spores from wild-type and cap67 strains stained with DAPI to reveal nuclei (blue), with anti-GXM antibody (F12D2) to detect capsule (red), and with DSL to identify spores (green) were visualized by immunofluorescence microscopy. All three color channels were merged into a single image for each sample. (1) Spores derived from a wild-type a by α cross (JEC20 × JEC21). (2 and 3) Spores and yeast cells from crosses between wild-type and cap67 strains (CHY1377 × JEC21 and JEC20 × ATCC 52817, respectively). (4) Spores and yeast cells isolated from a cross between two cap67 strains (CHY1377 × ATCC 52817). Bars, 5 μm.
When spores from crosses between wild-type strains (a × α), cap67 mutant × wild-type crosses, and cap67 mutant × cap67 mutant crosses were stained with DSL and anti-GXM antibody, we found that spores from wild-type and unilateral crosses (cap67 mutant × wild type) were bound by both the lectin and the anti-GXM antibody (Fig. 5C, panels 1 to 3). In contrast, spores from cap67 × cap67 strains were bound only by DSL and showed no binding by the anti-GXM antibody (Fig. 5C, panel 4). As expected, capsule-deficient parental yeast strains did not stain with anti-GXM antibody (data not shown). Spores and yeast cells from crosses using cap10Δ, cap60Δ, and cap64Δ strains yielded results identical to those for cap67 strains (data not shown).
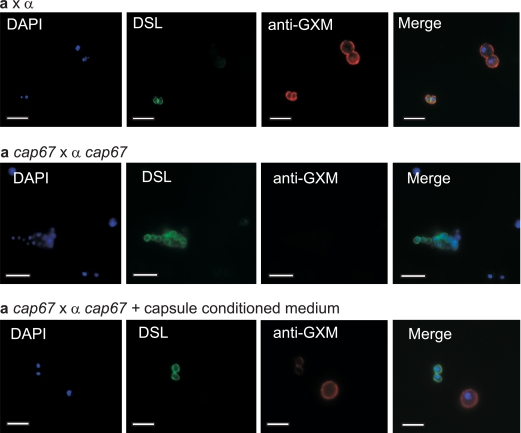
Spores resulting from crosses between acapsular parental yeast strains could not have been exposed to GXM during purification. To evaluate whether the GXM epitope present on wild-type spores resulted from exposure to GXM during purification, we carried out capsule transfer assays (38). Capsule-containing medium was created by growing wild-type yeast cells in minimal broth for 5 days. The yeast cells were then removed via centrifugation and filtration. Yeast cells and spores from acapsular strain crosses were then incubated in this capsule-containing medium for 1 hour at 25°C and then washed. Staining was then carried out with DAPI, DSL, and anti-GXM antibody on (i) wild-type spores and yeast cells, (ii) acapsular spores and yeast cells, and (iii) capsule-treated spores and yeast cells. As expected, the nuclei of all cells stained with DAPI (blue), and spores alone stained with DSL (green) (Fig. 6). Also expected were the findings that wild-type spores and yeast cells had approximately equal levels of anti-GXM antibody staining (red) (Fig. 6, top panels) and that spores from capsule-deficient parents and acapsular yeast did not fluoresce when stained with anti-GXM antibody (Fig. 6, middle panels). In contrast, spores from capsule-deficient parents and acapsular yeast that were incubated in capsule-conditioned medium and then stained with anti-GXM antibody (red) showed a distinct staining pattern: yeast cells stained brightly (similar to their wild-type counterparts), and spores showed only very faint staining (Fig. 6, bottom panels). The merged image of all three fluorescence channels shows that capsule transfer does not recapitulate the wild-type levels of anti-GXM antibody staining on spores. Compared to the staining of wild-type spores and yeast cells, which had approximately equal staining intensities, it appears that GXM was not transferred to spores efficiently. These results indicate that capsule transfer cannot account for wild-type spore staining with anti-GXM antibody, showing that in fact the GXM epitope is a substantial component of the spore coat produced during sporulation.
FIG. 6.
Capsule is not efficiently transferred to the surfaces of capsule-deficient spores. Yeast cells and spores were labeled with DAPI (blue) to reveal nuclei, with DSL (green) to identify spores, and with anti-GXM antibody F12D2 (red) to highlight polysaccharide epitopes. The top row of panels shows yeast cells and spores from a wild-type cross. Note that wild-type spores and yeast cells have similar intensities of staining with anti-GXM antibody. The middle row shows yeast cells and spores from an a cap67 × α cap67 cross, in which no anti-GXM antibody staining is detectable. The bottom row of panels shows yeast cells and spores stained after incubation in capsule-conditioned medium. Anti-GXM antibody staining shows brightly stained yeast cells, with only faint staining of the spores. The final panel in each row is a merged image of all three fluorescence channels used.
DISCUSSION
Fungal spores function as excellent dispersal agents because of their generally ovoid shapes and environmental resistance properties. For these reasons, spores are the suspected infectious particles in many human fungal infections, but little is known about the properties of these spores. Using the pathogenic fungus C. neoformans, we have succeeded in isolating upwards of 108 spores per preparation, a number sufficient for comprehensive studies of their basic properties. Our results show that spores differ from yeast cells in many ways, including the presence of a distinct surface coat, the display of a unique array of exposed polysaccharides, and increased resistance to environmental stresses. We also discovered that the spore surface is bound by antibodies to GXM, a capsular polysaccharide previously characterized as a virulence trait of yeast cells. We discovered that capsule biosynthesis genes are required for both efficient sexual development and spore formation, playing a critical role in preventing spore aggregation that suggests a role for capsular polysaccharides in facilitating spore dispersal.
Resistance properties of spores.
By characterizing spores and subjecting them to specific environmental conditions, we have begun to build a framework for understanding the properties of spores that might make them effective dispersed particles. We anticipate that dispersed particles would minimally require resistance to dry conditions, temperature fluctuations, and UV radiation as well as the ability to remain in the spore form. In these studies, spores were generally more resistant than yeast cells to many stresses, including high temperature and desiccation. The spore resistance to desiccation was particularly interesting given that one common hypothesis in the field is that desiccated yeast cells could be infectious particles (16). Although this may still be the case, we found in our experiments that yeast cells are quite sensitive to desiccation and lose viability rapidly. On the other hand, spores were at least an order of magnitude more resistant to dry conditions than yeast cells, indicating that in the environment spores would perhaps have a better chance of persisting under dry conditions. These findings are also important because there have been questions as to whether C. neoformans spores can withstand harsh environmental conditions in nature because of relatively low germination frequencies of spores after microdissection in the laboratory. Our data indicate that these low germination frequencies are not due to the inherent fragility of spores.
In fact, spores are also resistant to high levels of UV radiation. Although spores showed little increased resistance relative to yeast cells, both are quite UV resistant relative to other microbes (2). Our UV light exposure tests were designed to mimic exposure to direct sunlight at high altitude for several minutes, a condition which may be encountered in nature (34). Our tests, however, do not account for longer wavelengths of UV light that occur in nature, where spores might show more dramatic increased resistance to UV irradiation than yeast cells. Furthermore, our tests do not account for the fact that many environmental fungi, including C. neoformans, can produce melanin when provided with phenolic precursor molecules (41, 47). Our tests were conducted under nonmelanizing conditions, and spores derived from sexual development on V8 agar do not appear to be melanized. It is certainly possible that in nature melanin may play a more prominent role in UV resistance, but this possibility remains to be addressed.
Surface properties of spores.
A key in understanding spore resistance and dispersal lies in determining the nature of the spore coat. This thick coat is likely to play key roles in protecting the spore from harsh environmental conditions by allowing it to survive dispersal through wind or water and in interfacing with a host immune system.
We initially focused on identifying sugar moieties on the spore surface because spores from all fungi for which information is known and from many bacteria incorporate polysaccharides into their surfaces. Given the visual properties of the spore coat, it was surprising that the spore surface was efficiently bound by monoclonal antibodies to the polysaccharide GXM. GXM is a well-studied virulence determinant for C. neoformans yeast cells. In response to the host environment, yeast cells produce surface capsule polysaccharide in large amounts, and cells that cannot produce GXM (acapsular strains) cannot cause disease in most animal models of infection (50).
Sexual development conditions are not capsule inducing. Yeast cells from populations undergoing sexual development on V8 agar form only a small amount of capsule polysaccharide that can be seen by TEM (Fig. 1H). Spores from the same conditions produce a thick coat with no resemblance to the mesh-like structures associated with yeast GXM. However, it is very clear that spores are bound efficiently by anti-GXM antibodies. Given that four strains containing independent mutations in the capsule biosynthesis pathway do not produce spores that react with anti-GXM antibodies and that many independently derived anti-GXM antibodies give the same staining pattern on spores, we concluded that the most likely scenario is that GXM is exposed on the surfaces of spores. Formally, however, we cannot rule out that the capsule biosynthesis pathway could contribute to the biosynthesis of capsule-like polysaccharides or glycoconjugates with epitopes similar to those of GXM. Future studies of C. neoformans sexual development and spores may provide interesting opportunities to further characterize the capsule biosynthesis pathway.
Interestingly, our data also show that genes in the capsule biosynthesis pathway are required for the processes of sexual development and sporulation, a phenotype that has not been reported previously for acapsular strains. All of the congenic capΔ strains that were crossed with one another exhibited defects in sexual development, including limited filamentation and low rates of sporulation. The limited number of spores that did form were structurally abnormal, presumably because GXM is required for the formation of a normal spore coat. Unfortunately, electron microscopic analysis of these abnormal spores proved impossible because their extreme aggregation made them refractory to standard fixation and embedding techniques. Other approaches will need to be implemented to further explore the coats of spores from acapsular strains and to determine the role that GXM plays in this structure. No matter what the mechanism, however, the presence of an intact capsule biosynthesis pathway appears to be very important in providing dispersal properties to spores, either directly by functioning akin to the hydrophobin molecules of other fungi or indirectly by masking other surface factors or structures that lead to aggregation (44). These findings indicate that the traditional view of GXM as a virulence trait may need to be expanded to include its likely role in spore dispersal in the environment.
Spore production in nature.
Surprisingly, no filamentous, spore-containing structures have been observed directly in the environment for any of the Cryptococcus species. However, environmental sampling has consistently identified small particles consistent with the presence of spores from samples containing Cryptococcus (39, 40). Furthermore, population genetic studies in Africa provide evidence for freely recombining populations of a and α strains of C. neoformans in nature, suggesting that crosses between a and α strains produce recombinant progeny (most likely in the form of spores) (33). Population studies also reveal that the majority of Cryptococcus populations outside sub-Saharan Africa show very little evidence of recombination, suggesting primarily clonal population structures (20, 32). In these populations, it is suspected that spore production takes place via monokaryotic (or α) fruiting. In this developmental pathway, α cells (in the absence of a cells) form filaments and spores when exposed to extreme desiccation and starvation conditions (30, 49). Based on the findings that most environmental isolates (>95%) are of a single mating type (α) and that α-α fusion events have been detected in nature, it has been proposed that α fruiting spores are the likely dispersed form of C. neoformans in the environment in most parts of the world (9, 29, 31).
Because of the technical limitations posed by the small number of spores that are formed during fruiting in the laboratory, we have not yet succeeded in garnering a sufficient number of spores for a comprehensive analysis; however, because of the many similarities between sexual development and α fruiting, we predict that α spores from fruiting will have many of the properties of spores produced in crosses between a and α cells.
Independent of the mechanism of sporulation, all spores will encounter challenging conditions in nature. While resistance to temperature and desiccation are proposed to be key to surviving in the environment, it has been proposed that survival will also require resistance to other microorganisms, including predation by soil amoebae such as Acanthamoeba castellanii (43). Our tests of resistance to oxidative stress suggest that spores may have an advantage in response to predation, as amoebae phagocytose prey and kill via the actions of reactive oxygen species. This property may also confer resistance to the activity of host immune cells after inhalation into the mammalian lung. Alveolar macrophages are responsible for engulfing invading particles and destroying them, largely through the production of reactive oxygen and nitrogen species (24). The resistance of spores to oxidative stress suggests that spores could potentially evade oxygen-dependent host defense mechanisms, thus facilitating infection of a host.
For nearly 30 years, studies of C. neoformans spores have been hampered by the inability to reliably isolate large numbers of pure spores. Now, using pure populations of spores, we have discovered many of the basic properties of this particle and established a solid foundation for future studies. Understanding this basic biology of spores is essential for future studies of spores as dispersal agents and as infectious particles. The ability to study the spores of C. neoformans makes this human fungal pathogen a facile model with which the tools developed by the field can be applied to investigating spore production. Continuing studies of spores promise to reveal insights not only into Cryptococcus biology but also into the biology of other human fungal pathogens.
Acknowledgments
We thank the University of Wisconsin Medical School Electron Microscope Facility for TEM sample processing and imaging and R. Albrecht and J. Heintz for SEM and light microscopy technical assistance. We thank T. Doering for providing acapsular strains and helpful insights. We thank C. Kowalski and C. Kent for technical assistance and R. Borchardt and K. Klein for laboratory support. We are also grateful to R. Brazas, J. Ekena, C. Fox, E. Kruzel, B. Stanton, M. Staudt, and J. Woods for critical readings of and comments on the manuscript.
This work was supported by funds from a UW Medical Education Research Committee award to C.M.H., a Burroughs Wellcome Fund Career Award in the Biomedical Sciences to C.M.H., and grant R01 AI064287 to C.M.H. from the NIH. M.R.B. was supported by a UW Molecular Biosciences training grant from the NIH (T32GM0721532), and S.S.G. was supported by a UW Genomic Sciences Training Program grant from the NIH (T32HG002760).
Footnotes
Published ahead of print on 30 January 2009.
REFERENCES
- 1.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1998. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet. Biol. 251-14. [DOI] [PubMed] [Google Scholar]
- 2.Arrage, A. A., T. J. Phelps, R. E. Benoit, and D. C. White. 1993. Survival of subsurface microorganisms exposed to UV radiation and hydrogen peroxide. Appl. Environ. Microbiol. 593545-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, S., P. Thorkildson, and T. R. Kozel. 2003. Monoclonal antibodies reactive with immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans. Clin. Diagn. Lab. Immunol. 10903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briza, P., M. Breitenbach, A. Ellinger, and J. Segall. 1990. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 41775-1789. [DOI] [PubMed] [Google Scholar]
- 5.Briza, P., A. Ellinger, G. Winkler, and M. Breitenbach. 1988. Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. J. Biol. Chem. 26311569-11574. [PubMed] [Google Scholar]
- 6.Briza, P., A. Ellinger, G. Winkler, and M. Breitenbach. 1990. Characterization of a dl-dityrosine-containing macromolecule from yeast ascospore walls. J. Biol. Chem. 26515118-15123. [PubMed] [Google Scholar]
- 7.Brown, J. K., and M. S. Hovmoller. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297537-541. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan, K. L., and J. W. Murphy. 1998. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 471-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bui, T., X. Lin, R. Malik, J. Heitman, and D. Carter. 2008. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, α mating type populations. Eukaryot. Cell 71771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
- 11.Chang, Y. C., and K. J. Kwon-Chung. 1998. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 662230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y. C., and K. J. Kwon-Chung. 1999. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 1815636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 1996. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 641977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke, B. M., and D. G. Jones. 2006. The epidemiology of plant diseases. Springer, Dordrecht, The Netherlands.
- 15.Driver, J. A., C. A. Saunders, B. Heinze-Lacey, and A. M. Sugar. 1995. Cryptococcal pneumonia in AIDS: is cryptococcal meningitis preceded by clinically recognizable pneumonia? J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9168-171. [PubMed] [Google Scholar]
- 16.Ellis, D. H., and T. J. Pfeiffer. 1990. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 336923-925. [DOI] [PubMed] [Google Scholar]
- 17.Erke, K. H. 1976. Light microscopy of basidia, basidiospores, and nuclei in spores and hyphae of Filobasidiella neoformans (Cryptococcus neoformans). J. Bacteriol. 128445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldmesser, M., J. Rivera, Y. Kress, T. R. Kozel, and A. Casadevall. 2000. Antibody interactions with the capsule of Cryptococcus neoformans. Infect. Immun. 683642-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, A. J., R. A. Bird, S. L. Kelly, K. Nishimura, D. Poyner, S. Taylor, and S. N. Smith. 2004. FITC-lectin avidity of Cryptococcus neoformans cell wall and capsular components. Mycologia 961-8. [PubMed] [Google Scholar]
- 20.Franzot, S. P., J. S. Hamdan, B. P. Currie, and A. Casadevall. 1997. Molecular epidemiology of Cryptococcus neoformans in Brazil and the United States: evidence for both local genetic differences and a global clonal population structure. J. Clin. Microbiol. 352243-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray, R. D., and R. H. Glew. 1973. The kinetics of carbohydrate binding to concanavalin A. J. Biol. Chem. 2487547-7551. [PubMed] [Google Scholar]
- 22.Hull, C. M., and J. Heitman. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36557-615. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson, E. S., and W. R. Payne. 1982. UDP glucuronate decarboxylase and synthesis of capsular polysaccharide in Cryptococcus neoformans. J. Bacteriol. 152932-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janeway, C. 2005. Immunobiology: the immune system in health and disease. Garland Science, New York, NY.
- 25.Judelson, H. S., and F. A. Blanco. 2005. The spores of Phytophthora: weapons of the plant destroyer. Nat. Rev. Microbiol. 347-58. [DOI] [PubMed] [Google Scholar]
- 26.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 671197-1200. [PubMed] [Google Scholar]
- 27.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68821-833. [PubMed] [Google Scholar]
- 28.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, X., and J. Heitman. 2006. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 6069-105. [DOI] [PubMed] [Google Scholar]
- 30.Lin, X., C. M. Hull, and J. Heitman. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 4341017-1021. [DOI] [PubMed] [Google Scholar]
- 31.Lin, X., A. P. Litvintseva, K. Nielsen, S. Patel, A. Floyd, T. G. Mitchell, and J. Heitman. 2007. αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 31975-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litvintseva, A. P., L. Kestenbaum, R. Vilgalys, and T. G. Mitchell. 2005. Comparative analysis of environmental and clinical populations of Cryptococcus neoformans. J. Clin. Microbiol. 43556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litvintseva, A. P., R. E. Marra, K. Nielsen, J. Heitman, R. Vilgalys, and T. G. Mitchell. 2003. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot. Cell 21162-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meloni, D., G. R. Casale, A. M. Siani, S. Palmieri, and F. Cappellani. 2000. Solar UV dose patterns in Italy. Photochem. Photobiol. 71681-690. [DOI] [PubMed] [Google Scholar]
- 35.Moore, T. D., and J. C. Edman. 1993. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 131962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neiman, A. M. 2005. Ascospore formation in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69565-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemecek, J. C., M. Wuthrich, and B. S. Klein. 2006. Global control of dimorphism and virulence in fungi. Science 312583-588. [DOI] [PubMed] [Google Scholar]
- 38.Reese, A. J., and T. L. Doering. 2003. Cell wall α-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 501401-1409. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz, A., and G. S. Bulmer. 1981. Particle size of airborne Cryptococcus neoformans in a tower. Appl. Environ. Microbiol. 411225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz, A., R. A. Fromtling, and G. S. Bulmer. 1981. Distribution of Cryptococcus neoformans in a natural site. Infect. Immun. 31560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw, C. E., and L. Kapica. 1972. Production of diagnostic pigment by phenoloxidase activity of Cryptococcus neoformans. Appl. Microbiol. 24824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 9815245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stringer, M. A., R. A. Dean, T. C. Sewall, and W. E. Timberlake. 1991. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 51161-1171. [DOI] [PubMed] [Google Scholar]
- 45.Sukroongreung, S., K. Kitiniyom, C. Nilakul, and S. Tantimavanich. 1998. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med. Mycol. 36419-424. [PubMed] [Google Scholar]
- 46.Trail, F. 2007. Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiol. Lett. 27612-18. [DOI] [PubMed] [Google Scholar]
- 47.Wang, Y., and A. Casadevall. 1994. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 603864-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickes, B. L., U. Edman, and J. C. Edman. 1997. The Cryptococcus neoformans STE12α gene: a putative Saccharomyces cerevisiae STE12 homologue that is mating type specific. Mol. Microbiol. 26951-960. [DOI] [PubMed] [Google Scholar]
- 49.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl. Acad. Sci. USA 937327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaragoza, O., and A. Casadevall. 2004. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proc. Online 610-15. [DOI] [PMC free article] [PubMed] [Google Scholar]