Abstract
Accumulation of infectious Plasmodium sporozoites in Anopheles spp. salivary glands marks the final step of the complex development of the malaria parasite in the insect vector. Sporozoites are formed inside midgut-associated oocysts and actively egress into the mosquito hemocoel. Traversal of the salivary gland acinar cells correlates with the sporozoite's capacity to perform continuous gliding motility. Here, we characterized the cellular role of the Plasmodium berghei sporozoite invasion-associated protein 1 (SIAP-1). Intriguingly, SIAP-1 orthologs are found exclusively in apicomplexan hemoprotozoa, parasites that are transmitted by arthropod vectors, e.g., Plasmodium, Babesia, and Theileria species. By fluorescent tagging with mCherry, we show that SIAP-1 is expressed in oocyst-derived and salivary gland-associated sporozoites, where it accumulates at the apical tip. Targeted disruption of SIAP-1 does not affect sporozoite formation but causes a partial defect in sporozoite egress from oocysts and abolishes sporozoite colonization of mosquito salivary glands. Parasites with the siap-1(−) mutation are blocked in their capacity to perform continuous gliding motility. We propose that arthropod-transmitted apicomplexan parasites specifically express secretory factors, such as SIAP-1, that mediate efficient oocyst exit and migration to the salivary glands.
Protozoan parasites of the phylum Apicomplexa have adopted an obligate intracellular life-style in a wide range of animal hosts. Apicomplexan parasites share many characteristics, including a set of apical specialized secretory organelles, active substrate-dependent locomotion, and compartmentalization of biosynthetic pathways in apicoplasts. Transmission to the vertebrate hosts occurs via tailor-made motile parasite stages, termed sporozoites, which are formed inside oocysts, the only extracellular replication phase during the complex apicomplexan life cycles. However, apicomplexan parasites differ fundamentally in their transmission modes. Coccidian parasites, such as Toxoplasma gondii, the causative agent of toxoplasmosis, and cryptosporidia, which can cause life-threatening diarrhea, form oocysts that are taken up orally via contaminated food or water, respectively. After accidental ingestion, sporozoites are liberated from the oocysts and breach or directly invade the intestinal endothelium to commence an infection. In marked contrast, sporozoites of apicomplexan hemoprotozoa are inoculated intradermally by arthropod vectors, e.g., Anopheles mosquitoes or ticks. Sporozoites then actively leave the inoculation site and enter the blood circulation (3). In the case of Plasmodium species, the causative agents of malaria, sporozoites are formed in midgut-associated oocysts (16), actively exit the oocyst (1, 23), and penetrate the distal portion of the lateral salivary gland lobes where they eventually accumulate, rendering the infected mosquito infectious to the vertebrate host (4).
Colonization of the salivary glands by Plasmodium parasites is driven by a number of stage-specific surface proteins (9), including (i) the major sporozoite surface protein circumsporozoite protein (CSP) (18), (ii) the sporozoite adhesin apical membrane antigen/erythrocyte binding-like protein (MAEBL) (8), and (iii) the sporozoite invasin thrombospondin-related anonymous protein (TRAP) (7, 10, 17, 24). However, none of these proteins are conserved across apicomplexan hemoprotozoa, indicating a high degree of parasite/invertebrate host coadaptation. Toward a better understanding of the basic molecular mechanisms of sporozoite motility and arthropod-mediated transmission, we searched for sporozoite-specific candidates that are shared between Plasmodium spp., Babesia spp., and Theileria spp.
SIAP-1/ag17/S5 (PFD0425w) was first discovered in a systematic screen for Plasmodium falciparum sporozoite antigens recognized by sera from individuals that were immunized with irradiated sporozoites and termed antigen 17 (ag17) (2). Subsequently, the Plasmodium yoelii ortholog was isolated in a suppression subtractive hybridization screen for genes that are specifically upregulated in sporozoites compared to blood stages (6). More recently, the P. falciparum ortholog was identified as a gene that is transiently upregulated in activated P. falciparum sporozoites (14).
In this study, we fluorescently tagged the Plasmodium berghei SIAP-1 gene (PbSIAP-1) and show enrichment of the protein in the apical pole of the sporozoite. Targeted gene deletion resulted in viable blood stage parasites that displayed a specific and complete block in sporozoite transmission to the vertebrate host.
MATERIALS AND METHODS
Experimental animals.
Animals were from Charles River Laboratories. All animal work was conducted in accordance with European regulations and approved by the state authorities (Regierungspräsidium Karlsruhe).
Reverse transcriptase PCR (RT-PCR).
Total RNA was purified from Plasmodium berghei ANKA (GFP507cl1)-infected mosquito midguts (oocysts), salivary glands (sporozoites), and infected HepG2 cells (early liver stages) or mouse erythrocytes (mixed blood stages) using the RNeasy kit (Qiagen). Reverse transcription was performed using the RETROscript kit (Ambion). Real-time quantitative PCR was performed on cDNA preparations using the ABI 7500 sequence detection system and Power SYBR green PCR master mix (Applied Biosystems), according to the manufacturer's instructions. Quantitative PCR was performed in triplicate, with 1 cycle of 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 55°C for 15 s, and 60°C for 45 s. Standard curves were generated for all primers using wild-type (WT) cDNA serial dilutions and gave amplification efficiencies of 90 to 100%. Data were analyzed with SDS 1.3.1 software (Applied Biosystems). Relative transcript abundance was normalized to the GFP gene, which is constitutively expressed in P. berghei ANKA GFP507cl1 (5). The following primers were used for real-time PCR: GFP forward (5′-GATGGAAGCGTTCAACTAGCAGACC-3′), GFP reverse (5′-AGCTGTTACAAACTCAAGAAGGACC-3′), SIAP-1 forward (5′-CAGCAATTAGGGACAGTGATGG-3′), SIAP-1 reverse (5′-AATAATGGACACCTCCGTGTGG-3′), HSP70 forward (5′-TGCAGCAGATAATCAAACTC-3′), and HSP70 reverse (5′-ACTTCAATTTGTGGAACACC-3′).
Generation of the mCherry-tagged SIAP-1 parasite line.
For targeted fluorescent tagging of PbSIAP-1, a fragment corresponding to the 3′ end of the PbSIAP-1 open reading frame (ORF) without the stop codon was amplified by PCR using P. berghei genomic DNA as the template and primers mCherry-SIAP-1for (5′-ATAAGAATGCGGCCGCGCATCTGAAAGTAAAGGAAAATGGGTC GC-3′; NotI site is underlined) and mCherry-SIAP-1rev (5′-GGGCTAGCTGAATTGTCCACGAAAACACTGTAACTATAGG-3′; NheI site is underlined). The PCR product was cloned into the B3D+mCherry vector (15) immediately upstream of the mCherry sequence and the PbDHFR/TS 3′ untranslated region (UTR), resulting in the SIAP-1/mCherry targeting plasmid. The SIAP-1/mCherry plasmid was linearized with SpeI, and parasite transfection, positive selection, and parasite cloning were performed as described previously (5). Integration-specific PCR amplification of the mCherry-tagged SIAP-1 was generated using specific primer combinations. We obtained one parasite population that was used for a systematic expression and localization analysis. Expression of the mCherry fusion proteins was analyzed through direct detection of the red fluorescence of mCherry by confocal microscopy. Images were acquired on a Zeiss LSM510 confocal system (Zeiss, Germany) and processed with Adobe Photoshop software (Adobe Systems, Inc.).
Generation of the siap-1(−) parasites.
For targeted disruption of the SIAP-1 gene, two PCR fragments flanking the SIAP-1 ORF were amplified from genomic Plasmodium berghei DNA. The 5′ UTR amplification with the primer combination 5′SIAP-1-Rep_for (5′-GGGGTACCGTGTTTTCATGCTATATGTACATTTGC-3′; KpnI site is underlined) and 5′SIAP-1-Rep_rev (5′-CCCAAGCTTGATGATATTGACCGTAAAATCC-3′; HindIII site is underlined) resulted in a 634-bp 5′ UTR fragment. Cloning into the standard P. berghei transfection vector b3D.DT^H.^D resulted in pSE-41. Next, a 788-bp 3′ UTR fragment was amplified with primers 5′SIAP-1-Rep_ for (5′-CGGGATCCCCTATAGTTACAGTGTTTTCG-3′; BamHI site is underlined) and 5′SIAP-1-Rep_ rev (5′-GGACTAGTGTTCGATATACCTTGCAGATTCC-3′; SpeI site is underlined) and cloned into pSE-41. This resulted in the SIAP-1 targeting vector pSE-65, which contained both fragments flanking the positive selection marker T. gondii dihydrofolate reductase/thymidylate synthase (dhfr/ts), allowing replacement of the endogenous SIAP-1 locus upon a double crossover event and subsequent selection with the antifolate pyrimethamine (19). P. berghei transfection was done with 10 μg KpnI/SpeI-digested pSE-65 and gradient-purified P. berghei NK65 and P. berghei ANKA (clone GFP507cl1) schizonts (5). Positive selection for successful integration of the targeting plasmid was carried out by providing 70 μg/ml pyrimethamine with the drinking water for a period of 8 days. Transfer of the emerging P. berghei population into naive animals confirmed pyrimethamine resistance. Genomic DNAs for selected parasite populations were genotyped by an integration-specific PCR using primers Tgfor (5′-CCCGCACGGACGAATCCAGATGG-3′) and SIAP-1_int_check2_rev (5′-CTGTGAATGTGTATATTGTGCATATGCC-3′). Clonal siap1(−) parasite populations were obtained by limiting dilution into 15 naive NMRI mice and confirmed by integration-specific PCR.
As a complementary approach, we generated SIAP-1 knockout parasites specifically expressing green fluorescent protein (GFP) in sporozoites. For this purpose, we first amplified by PCR a fragment comprising the CSP promoter region and the GFP coding sequence, using primers GFPfor (5′-ATAAGAATGCGGCCGCGACATGCATATGTGTTGGTTGTAATTGAGG-3′; NotI site is underlined) and GFPrev (5′-CGCGGATCCTTATTTGTATAGTTCATCCATGCC-3′; BamHI site is underlined) and genomic DNA from the PbFluspo parasite line (11) as a template. Cloning of this fragment into the B3D+ vector (15) using NotI/BamHI resulted in the vector B3D+CSPGFP. 5′ and 3′ fragments of the PbSIAP-1 gene were then amplified using primers SIAP-1_REP1 (5′-TCCCCGCGGAGTTAACTCGAAAAAAATAAGGACG-3′; SacII site is underlined) and SIAP-1_REP2 (5′-ATAAGAATGCGGCCGCTTTTAAGATATACCCCTTAATGAGAGC-3′; NotI site is underlined) and primers SIAP-1_REP3 (5′-CCCAAGCTTGATGTATGTATAATACCGAAAATTGG-3′; HindIII site is underlined) and SIAP-1_REP4 (5′-CGGGGTACCCGCCTTATTTAAGGGATAAAGAATGGG-3′; KpnI site is underlined), respectively, and genomic DNA from WT P. berghei. These two fragments were then cloned into the B3D+CSPGFP vector, using the respective restriction endonucleases to generate the targeting construct SIAP-1-GFPrep. Transfection, selection, and cloning were then performed as described above. Genotyping PCR using specific primer combinations confirmed the expected recombination event.
Phenotypical analysis during the Plasmodium life cycle in vivo.
Blood stage development was analyzed in vivo in asynchronous infections using NMRI mice. Gametocyte differentiation and exflagellation of microgametes were detected in mice before mosquito feedings. Anopheles stephensi mosquito rearing and maintenance was carried out under a 14 h light/10 h dark cycle with 75% humidity at 28°C. Once infected, Anopheles mosquitoes were kept at 80% humidity and 20°C. Sporozoite populations were separated and analyzed as described previously (21). Briefly, for determination of sporozoite infectivity and numbers of midgut- and salivary gland-associated sporozoites, infected mosquitoes were dissected at days 10, 14, and 17 after feeding, respectively. Hemocoel sporozoites were recovered at days 14, 16, and 18 after feeding. For determination of infectivity, sporozoites isolated from mosquito midguts, hemocoel, or salivary glands were injected intravenously at the numbers indicated into young Sprague-Dawley rats. Patency was checked daily by microscopic examination of Giemsa-stained blood smears.
In vitro experiments.
For analysis of gliding motility, sporozoites isolated from infected mosquito salivary glands were deposited on glass slides coated with bovine serum albumin and incubated at 37°C for 30 min. Trails left behind gliding parasites were then visualized using anti-CSP antibodies (12), followed by anti-mouse Alexa Fluor 488 antibodies (Molecular Probes). For analysis of exoerythrocytic form development, we used HuH7 cells cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics. P. berghei sporozoites were added in triplicate wells, incubated for 2 h at 37°C, and washed off. After 48 h, exoerythrocytic forms were revealed using primary antibodies against Plasmodium heat shock protein 70 (HSP70) (20), followed by the anti-mouse Alexa Fluor 488 secondary antibody. In vitro analysis of the PbSIAP-1/mCherry parasites was performed in HepG2 cells, as described previously (15).
RESULTS
SIAP-1 is conserved in arthropod-transmitted Apicomplexa spp. and is specifically expressed in P. berghei mosquito stages.
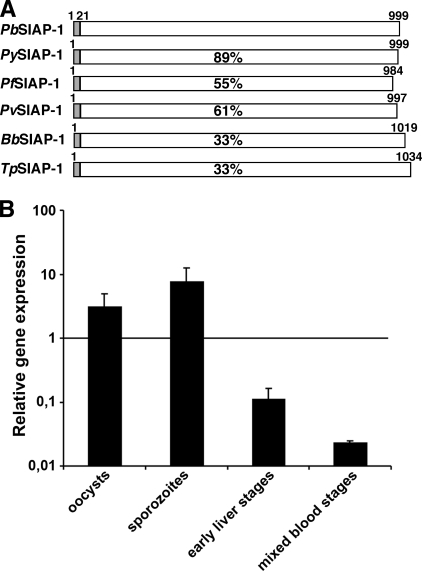
We initiated our characterization of SIAP-1/ag17/S5 by an in silico analysis of orthologous genes in Plasmodium species and related apicomplexan parasites (Fig. 1A). The SIAP-1 genes from the model rodent-infecting Plasmodium species P. yoelii and P. berghei encode proteins of 999 amino acid residues each. SIAP-1 members appear to be potentially secreted proteins and are readily retrieved from annotated Plasmodium genomes. Remarkably, SIAP-1 orthologs are present in the Babesia and Theileria genomes (see Fig. S1 in the supplemental material) but absent in Toxoplasma and Cryptosporidium, indicating that its presence may be restricted to arthropod-transmitted apicomplexan parasites (Fig. 1A). Analysis of SIAP-1 protein sequence indicates the presence of a signal peptide but no identified conserved domain (see Fig. S1 in the supplemental material).
FIG. 1.
Plasmodium SIAP-1. (A) Schematic diagram of the primary structures of the apicomplexan SIAP-1 proteins. The primary structure of P. berghei SIAP-1 (PB000251.01.0; reannotated) and the orthologs of P. yoelii (PY00455), P. falciparum (PFD0425w), Plasmodium vivax (Pv000815), Babesia bovis (XP_001609197), and Theileria parva (XP_765499) are indicated with white boxes. The predicted cleavable signal peptide is represented in gray. Amino acid sequence identities are indicated as percentages of identical residues compared to the P. berghei sequence. (B) Quantitative RT-PCR analysis of P. berghei SIAP-1 gene expression in midgut oocysts (day 12 postinfection of mosquitoes), salivary gland sporozoites (day 18 postinfection of mosquitoes), early liver stages (6 h postinfection of HepG2 cells) and mixed blood stages. Relative gene expression was normalized to GFP expression level and is shown as the mean (± standard deviation) of the results from two independent experiments.
We next analyzed SIAP-1 transcript abundance during sporozoite maturation in the rodent malaria model parasite P. berghei. cDNAs generated from asexual blood stages, liver stages, oocysts, and salivary gland-associated sporozoites were used as templates for quantitative RT-PCR (Fig. 1B). PbSIAP-1 expression was highest in young oocysts and sporozoites and rapidly decreased after sporozoite invasion of hepatocytes. In good agreement with data obtained using P. yoelii (6), expression of PbSIAP-1 was very low in blood stages. Collectively, SIAP-1 expression correlates with a presumed role in the arthropod vector.
PbSIAP-1 is localized at the apical tip and the surface of sporozoites.
In order to investigate the cellular localization and the expression timing of PbSIAP-1, we generated parasite lines expressing the endogenous SIAP-1 protein fused to the mCherry red fluorescent protein (13). This was achieved by transfection of a targeting vector that contains an amino-terminally truncated PbSIAP-1 copy and in-frame fusion of the mCherry coding region, followed by the DHFR/TS 3′ UTR (see Fig. S2A in the supplemental material). Upon a single crossover event, integration of this construct is predicted to result in an allelic duplication (19), resulting in an mCherry-tagged full-length copy and a nontranscribed 5′ truncated version of the PbSIAP-1. Transfection was performed in P. berghei ANKA parasites expressing GFP (5), leading to green fluorescent parasites that express a red fluorescent SIAP-1 protein. Genotyping by PCR using specific primer combinations confirmed the desired integration event (see Fig. S2B in the supplemental material). Importantly, PbSIAP-1/mCherry parasites completed the parasite life cycle normally. They were indistinguishable from WT parasites in development and growth of asexual stages (data not shown). PbSIAP-1/mCherry parasites produced gametocytes, and exflagellation of male gametocytes was similar to that in WT parasites (data not shown). Transmission to Anopheles stephensi mosquitoes and oocyst development were also normal compared to WT parasites. PbSIAP-1/mCherry oocysts produced sporozoites, which invaded mosquito salivary glands. In two independent infection experiments, we found 20,000 and 16,700 salivary gland-associated sporozoites per mosquito, respectively, numbers which are similar to those obtained with WT parasites (see Fig. 4D). More importantly, PbSIAP-1/mCherry sporozoites were infective as demonstrated by complete liver stage development in vitro (Fig. 2E) and in vivo. C57/BL6 mice inoculated intravenously with 5,000 PbSIAP-1/mCherry sporozoites (n = 5) or exposed to the bites of five infected mosquitoes (n = 5) all developed a patent parasitemia at day 3 or day 4 after inoculation, respectively, similar to those infected with WT parasites. Collectively, these data demonstrate that the C-terminal tagging of SIAP-1 had no detrimental effect on P. berghei infectivity.
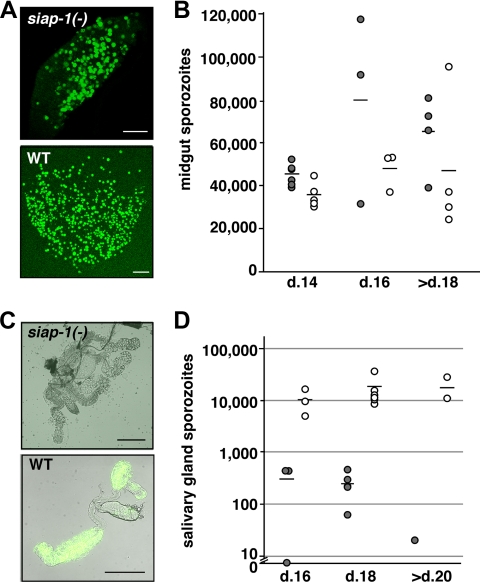
FIG. 4.
siap-1(−) sporozoites are impaired in egress from oocysts. (A) Representative immunofluorescence pictures of infected A. stephensi midguts at day 14 after infection. (B) Quantification of oocyst-associated sporozoites per infected mosquito for siap-1(−) (gray circles) and WT (white circles) parasites. The bars represent the mean values. (C) Representative merged bright field and immunofluorescence pictures of infected A. stephensi salivary glands at day 17 after infection. (D) Quantification of salivary gland-associated sporozoites per infected mosquito for siap-1(−) (gray circles) and WT (white circles) parasites. Note the logarithmic scale. Bars, 200 μm.
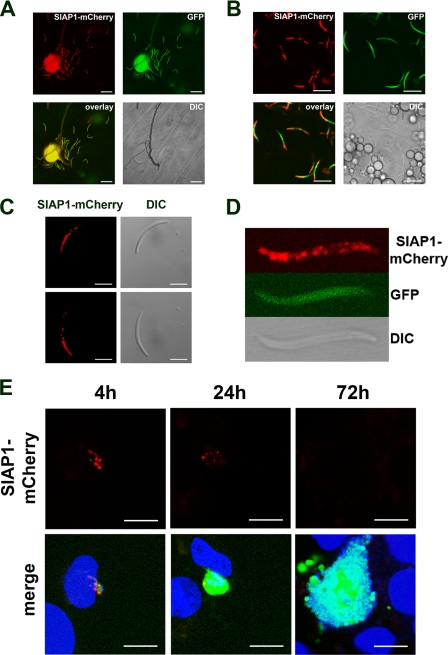
FIG. 2.
Expression and localization of PbSIAP-1/mCherry in sporozoites. Expression of the mCherry-tagged SIAP-1 (red) was analyzed by confocal fluorescence microscopy of SIAP-1/mCherry P. berghei parasites constitutively expressing GFP (green). (A) Midgut sporozoites. Bar, 20 μm. (B) Salivary gland-associated sporozoites. Bar, 10 μm. (C) Fluorescent imaging of a motile sporozoite. Sequential acquisition of the red fluorescence and differential interference contrast (DIC) confirms SIAP-1 accumulation mainly at the apical tip. Note that the distribution of SIAP-1/mCherry is not modified as the sporozoite glides. Bar, 5 μm. (D) Higher magnification of a fixed salivary gland-associated sporozoite. (E) Confocal microscopy analysis of HepG2 cell cultures 4 h, 24 h, and 72 h after infection with P. berghei parasites expressing GFP (green) and SIAP-1/mCherry (red). Nuclei were stained with Hoechst 33342 (blue). Bars, 10 μm.
We first performed live cell imaging of blood stages of the transgenic PbSIAP-1/mCherry. In good agreement with our transcription analysis and published data (6), we could not detect a red signal throughout the blood stage development of the malaria parasite (data not shown). In contrast, the fusion protein was abundantly expressed in oocyst-derived and salivary gland sporozoites (Fig. 2A and B). Remarkably, in both immature and mature sporozoites, PbSIAP-1/mCherry showed a polarized distribution predominant at the apical tip of the parasites, as confirmed by examination of motile sporozoites (Fig. 2C). Interestingly, in addition to this apical concentration, PbSIAP-1/mCherry was also detected as distinct patches distributed along the parasite (Fig. 2C). These patches were sometimes clearly distributed at the periphery of the parasite, suggesting a possible association of SIAP-1 with the sporozoite pellicle (Fig. 2D). This pattern is consistent with immunofluorescence data generated with antibodies against P. falciparum SIAP-1 (14). Interestingly, we observed no change in the distribution of PbSIAP-1/mCherry during the gliding of motile sporozoites (Fig. 2C).
We further examined the fate of PbSIAP-1/mCherry after sporozoite invasion of HepG2 hepatocytic cells. In transforming sporozoites 4 h postinfection, PbSIAP-1/mCherry was detected in distinct patches, without clear apical polarization (Fig. 2E). These dotted structures were still detected 24 h postinfection, although at a lower intensity, but had completely disappeared in mature liver stages 72 h postinfection (Fig. 2E), consistent with the absence of PbSIAP-1/mCherry expression observed in blood stages.
Generation of PbSIAP-1 knockout parasites.
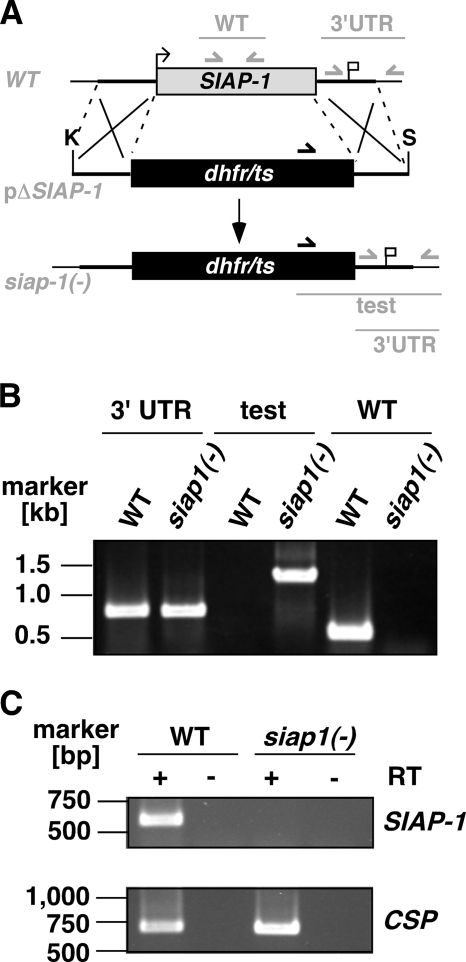
To address the function of SIAP-1 during the Plasmodium life cycle, we generated loss-of-function P. berghei parasites. We used a replacement strategy (Fig. 3A) to disrupt the endogenous SIAP-1 gene copy by double-crossover homologous recombination (19). A targeting construct comprising 5′ and 3′ fragments of SIAP-1 flanking the pyrimethamine resistance cassette was used for positive selection after parasite transfection. The parental blood stage population from a successful transfection was used to isolate one disruptant clone, which was subsequently used for phenotypical analysis. Occurrence of the double crossover was confirmed by PCR in pyrimethamine-resistant parasites, using primers specific for the recombination event (Fig. 3B). The WT locus was not detected in siap-1(−) parasites, confirming the homogeneity of the expected recombination. While CSP control transcripts were detected in WT as well as siap-1(−) sporozoites by RT-PCR, SIAP-1 transcripts were detected only in WT, but not in siap-1(−) sporozoites, confirming the successful depletion of SIAP-1 in the mutants (Fig. 3C).
FIG. 3.
Targeted deletion of the P. berghei SIAP-1 gene. (A) Replacement strategy for targeted gene disruption of PbSIAP-1. The WT SIAP-1 locus (WT) is targeted with a KpnI (K)/SpeI (S)-linearized replacement plasmid (pΔSIAP-1) containing the 5′ and 3′ UTR of PbSIAP-1 and the positive selection marker TgDHFR-TS. After double-crossover homologous recombination, the SIAP-1 ORF is substituted by the selection marker, resulting in the mutant siap-1(−) allele. Replacement- and WT-specific test primer combinations and expected fragments are shown as lines. (B) Replacement-specific PCR analysis. Confirmation of the predicted gene targeting is done with primer combinations that amplify only a signal in the recombinant locus (test). The absence of a WT-specific signal in the clonal siap-1(−) parasite population confirms the purity of the mutant parasite line. (C) Depletion of SIAP-1 transcripts in siap-1(−) parasites. cDNA from WT and siap-1(−) sporozoites were used as a template for SIAP-1-specific PCRs (top). Amplification of the circumsporozoite protein (CSP) transcripts was used as a positive control (bottom).
PbSIAP-1 is required for infection of mosquito salivary glands and transmission to the mammalian host.
We next examined the phenotype of siap-1(−) parasites during the P. berghei life cycle. As expected from successful disruption of the SIAP-1 gene in blood stages, siap-1(−) parasites were indistinguishable from WT parasites in development and growth of asexual stages (data not shown). SIAP-1-deficient parasites produced gametocytes, and exflagellation of male gametocytes was similar to that in WT parasites (data not shown). These data demonstrate that SIAP-1 is not required during asexual blood stage development and sexual differentiation.
We next investigated transmission of SIAP-1 knockout parasites to Anopheles stephensi mosquitoes. We let mosquitoes feed on mice infected with siap-1(−) or WT parasites, and at different time points after the blood meal, mosquitoes were dissected and examined for parasite development. Examination at days 10 and 14 demonstrated that siap-1(−) parasites formed high numbers of oocysts and oocyst-derived sporozoites, respectively, as did WT parasites (Fig. 4A and B). We noticed a persistence of high numbers of midgut-associated sporozoites per infected mosquito beyond day 18 after infection (Fig. 4B). Strikingly, at day 16 after mosquito feeding, the number of siap-1(−) sporozoites associated with salivary glands was dramatically reduced compared to that of WT parasites (Fig. 4C and D). This defect was also observed when salivary glands were examined later, beyond day 20. These results indicate that siap-1(−) sporozoites have a defect in the egress from oocysts and/or the invasion of salivary glands.
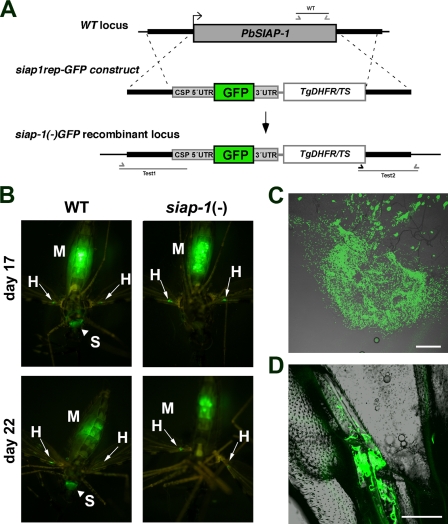
To discriminate between these two possibilities, we performed in vivo imaging of whole infected mosquitoes. For this purpose, we generated an additional SIAP-1-deficient line using a replacement cassette containing the GFP coding sequence under the control of the CSP promoter, cloned in tandem with the positive selection marker Tgdhfr/ts (Fig. 5A). After integration of this construct in recombinant P. berghei parasites, we obtained a siap-1(−)/GFP parasite line, characterized by deletion of the SIAP-1 gene (see Fig. S3 in the supplemental material) and expression of high levels of GFP in sporozoites, similar to that in the Pbfluspo line (11), which was used as a WT control.
FIG. 5.
siap-1(−) sporozoites fail to colonize the mosquito salivary glands. (A) Replacement strategy for targeted gene disruption of PbSIAP-1 and insertion of a GFP expression cassette. The WT SIAP-1 locus is targeted with a SacII/KpnI-linearized replacement plasmid (siap1rep-GFP construct) containing the 5′ and 3′ UTR of PbSIAP-1, the positive selection marker TgDHFR-TS, and the GFP coding sequence under the control of the CSP promoter region. After double crossover homologous recombination, the SIAP-1 ORF is substituted by the selection marker and the GFP cassette, resulting in the mutant siap-1(−)/GFP allele. (B) Fluorescence imaging of whole mosquitoes infected with WT or siap-1(−)/GFP parasites, day 17 and day 22 after the infectious blood meal. Note the presence of green fluorescent WT parasites in the mosquito midgut (M), hemocoel (H, best visualized in the wings with arrows), and salivary glands (S, arrowheads). siap-1(−)/GFP parasites were only detected in the midgut (M) and the hemocoel (H, arrows), but not in salivary glands of infected mosquitoes. (C) Fluorescence microscopy of a dissected mosquito midgut 21 days after infection with siap1(−)/GFP parasites. Note the presence of oocysts and the release of high numbers of sporozoites. Bar, 200 μm. (D) Fluorescence microscopy of a dissected mosquito wing 21 days after infection with siap-1(−)/GFP parasites, demonstrating the presence of sporozoites in the hemocoel. Bar, 50 μm.
We first examined the WT (Pbfluspo) parasites at day 17 and day 22 after mosquito infection (Fig. 5B). Parasites could be detected in three different compartments, the midgut, the salivary glands, and the wings, the latter apparently reflecting the presence of parasites in the hemocoel (Fig. 5B). In sharp contrast, siap-1(−)/GFP parasites were observed only in the midguts and wings of infected mosquitoes but not in the salivary glands (Fig. 5B). Closer examination of the midguts of siap-1(−)/GFP parasite-infected mosquitoes on day 21 postinfection revealed the presence of numerous oocysts, most of which quickly ruptured after dissection of the midgut, releasing high numbers of sporozoites (Fig. 5C). This is consistent with a defect in egress from oocysts in SIAP-1-deficient parasites. However, this defect is not complete, since siap-1(−)/GFP sporozoites could be found in the hemocoel, as demonstrated by closer examination of the wings of infected mosquitoes (Fig. 5D). These sporozoites were either nonmotile or displayed a limited nonproductive motility (data not shown), as typically observed with oocyst-derived sporozoites.
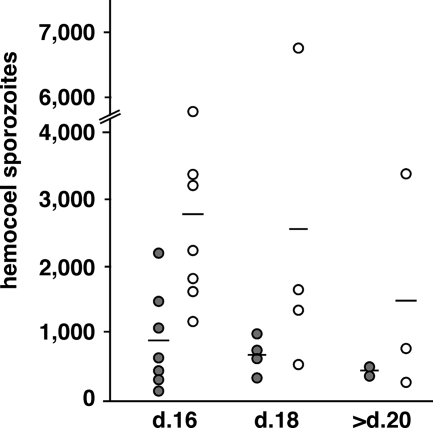
To provide a quantitative assessment of siap-1(−) sporozoite egress, we isolated hemolymph from infected mosquitoes and determined the numbers of hemocoel sporozoites (Fig. 6). We consistently recovered siap-1(−) sporozoites from hemocoel, but the numbers were typically lower compared to those of WT parasite-infected mosquitoes. This result differs markedly from TRAP knockout sporozoites, which egress normally but are impaired in their capacity to invade salivary glands, resulting in a significant accumulation of hemocoel sporozoites (10). Lower hemocoel sporozoite numbers of the siap-1(−) parasites indicate a defect in exiting oocysts. However, the siap-1(−) phenotype is an intermediary between the WT and the ones observed for egress mutants, e.g., the CSP region II mutant and the Pbsera5/ecp1(−) parasites (1, 23). Collectively, these data confirm that deletion of SIAP-1 causes a partial defect in the egress from oocysts and indicate an additional effect on invasion of salivary glands.
FIG. 6.
Quantitative assessment of siap-1(−) sporozoite release into the mosquito hemocoel. Shown are quantifications of hemocoel sporozoites per infected mosquito for siap-1(−) (gray circles) and WT (white circles) parasites. The bars represent the mean values.
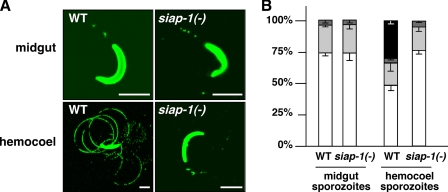
We next examined the gliding locomotion of the siap-1(−) sporozoites (Fig. 7). Midgut-associated sporozoites isolated from siap-1(−) parasite-infected mosquitoes are indistinguishable from sporozoites isolated from WT parasite-infected mosquitoes and display infrequent, nonproductive motility. Continuous gliding locomotion, as seen in a proportion of WT hemocoel sporozoites, was completely absent from siap-1(−) sporozoites, indicating an important role for SIAP-1 in this process.
FIG. 7.
Defective gliding locomotion in siap-1(−) sporozoites. (A) Representative immunofluorescence pictures of sporozoites isolated from Anopheles midguts or hemocoels. Sporozoites were deposited onto coated coverslips, and trails were visualized with the anti-circumsporozoite protein (CSP) antibody. Note that siap-1(−) hemocoel sporozoites lack productive motility and are indistinguishable from midgut-associated sporozoites. (B) Quantification of the WT and siap1(−) motility patterns. Shown is the percentage of nonmotile, i.e., detached (white) and attached (light gray), sporozoites and sporozoites that display nonproductive (dark gray) and productive (black) motility. In contrast to WT sporozoites that mature upon egress to the mosquito hemocoel, siap-1(−) parasites remain nongliding.
Finally, we investigated the role of SIAP-1 in transmission of sporozoites to the mammalian host (Table 1). Rats were exposed to the bites of mosquitoes infected with WT or siap-1(−) parasites. Subsequently, animals were checked daily by blood smear examination to detect the appearance of a blood stage infection. In contrast to WT parasites, which induced parasitemia in all exposed animals, none of the rats exposed to siap-1(−) parasites developed a patent blood stage infection (Table 1). We obtained the same result in C57/BL6 mice. All mice exposed to the bites of 20 mosquitoes infected with siap-1(−) parasites (n = 2) or siap-1(−)/GFP parasites (n = 5) remained blood smear negative. Furthermore, we bypassed natural transmission and injected mice with siap-1(−) sporozoites isolated from salivary glands, hemocoel, or midguts of infected mosquitoes. Infected animals typically remained blood smear negative (Table 1). As observed previously with the trap(−) mutant (17), only very high inocula, i.e., 50,000 salivary gland sporozoites or 1 million midgut-associated sporozoites, induced patently infected animals.
TABLE 1.
In vivo infectivity of siap-1(−) and WT parasites
| Location of sporozoite population | Parasite population | Method of inoculation or no. of sporozoitesa | No. of infected animals/ inoculated animals | Prepatent period (days)b |
|---|---|---|---|---|
| Salivary gland | siap-1(−) | Mosquito bite | 0/4 | NA |
| WT | Mosquito bite | 8/8 | 3.5 | |
| siap-1(−) | 50,000 | 1/1 | 6 | |
| siap-1(−) | 10,000 | 0/5 | NA | |
| WT | 10,000 | 2/2 | 3.5 | |
| Hemocoel | siap-1(−) | 10,000 | 1/4 | 6 |
| siap-1(−) | 5,000 | 0/3 | NA | |
| WT | 1,000 | 10/10 | 4.7 | |
| Midgut | siap-1(−) | 1,000,000 | 1/1 | 5 |
| siap-1(−) | 400,000 | 1/2 | 7 | |
| siap-1(−) | 100,000 | 1/7 | 7 | |
| WT | 100,000 | 10/10 | 5.4 |
Young Sprague-Dawley rats were injected intravenously with indicated numbers of sporozoites or exposed to the bites of 5 to 10 infected mosquitoes at days 17 to 20 after mosquitoes were infected.
NA, not applicable.
Collectively, our results demonstrate that SIAP-1 plays a dual role in parasites’ egress from midgut-associated oocysts and sporozoite colonization of salivary glands. Both cellular functions are directly linked to sporozoite locomotion, a vital parasite activity that is abolished in SIAP-1 loss-of-function lines. These defects result in a complete block of sporozoite transmission from highly infected mosquitoes.
DISCUSSION
In this study, we identified the first conserved gene among apicomplexan hemoprotozoa that plays an essential role in sporozoite transmission to the mammalian host. SIAP-1 loss-of-function sporozoites display a specific defect in sporozoite maturation. Anopheles mosquitoes infected with siap-1(−) parasites cannot transmit malaria to susceptible rodents, despite the normal formation of sporozoites in their midguts. Mutant parasites fail to mature, as seen by the lack of continuous gliding locomotion in hemocoel sporozoites and colonization of the final target organ, the salivary glands. This phenotype correlates with a transient accumulation in midgut oocysts and is reminiscent of the previously described egress mutants (1, 23). However, a direct role in oocyst rupture is unlikely because siap-1(−) sporozoites were consistently recovered outside the midgut.
One possible function of SIAP-1 is a role in parasite locomotion. This notion is supported by the complete absence of trail formation and entry into salivary glands. In addition, tagging of the endogenous SIAP-1 copy revealed a striking distribution with apical polarization and accumulation to additional specific areas. Intriguingly, these SIAP-1-positive patches remain constant upon sporozoite motility. Therefore, an immediate role, for instance in association with TRAP family invasins, in rearward distribution of tight junctions between sporozoite receptors and the substratum is unlikely. Because of the paucity of typical microfilaments in extracellular stages of apicomplexan parasites we could not address whether the composition of the motor machinery in vivo is altered in siap-1(−) sporozoites. The distribution pattern of PbSIAP-1/mCherry combined with the absence of trail formation suggest that SIAP-1 may localize to structures located under the sporozoite surface and may be involved in gliding motility, possibly by interacting with the inner membrane complex. Alternatively, we cannot exclude an extracellular function, perhaps in preparation for active locomotion. In P. falciparum, SIAP-1 is processed and released into the supernatant upon incubation at 37°C (14), a feature shared with the apical membrane antigen 1 and TRAP. Presently, it is unknown where SIAP-1 is clipped. Due to the presence of a predicted cleavable signal sequence, we fluorescently tagged SIAP-1 at the carboxy terminus. Hence, we may only be able to visualize the unprocessed full-length protein, and localization of the mature form may differ, including dissociation into the extracellular milieu and deposition onto trails.
At this stage, we cannot formally exclude a protective role of SIAP-1 against clearance in mosquitos. Sporozoites that fail to reach and penetrate the salivary glands are quickly cleared by the mosquito immune system (22). Previous studies with trap(−) and maebl(−) parasites showed that a block in life cycle progression translates into accumulation of hemocoel sporozoites. Absence of SIAP-1 on the sporozoite surface may render sporozoites more accessible to the mosquito innate immune system, resulting in enhanced clearance from the mosquito hemocoel.
Our results with siap-1(−) parasites clearly demonstrate that SIAP-1 is required for efficient egress from oocysts and also for invasion of the mosquito salivary glands. In particular, using a novel approach that allows sensitive detection of fluorescent parasites in whole infected mosquitoes, we could show that a proportion of siap-1(−) sporozoites could exit from oocysts and access the hemocoel. Nevertheless, these parasites were not able to efficiently invade salivary glands. We hypothesize that the defect in motility in siap-1(−) sporozoites is responsible for the dual defect in egress from oocysts and invasion of salivary glands, resulting in a complete block in sporozoite transmission to the vertebrate host.
Apicomplexan sporogony in ticks differs largely from Plasmodium sporozoite formation in Anopheles mosquitoes. For instance, Babesia and Theileria sporozoite formation is initiated after kinete invasion of salivary glands and occurs inside a multinucleated sporoblast/sporont residing in a hypertrophied salivary gland cell. After exiting the sporoblast, sporozoites can be directly injected with the saliva into the vertebrate host, largely omitting the need for sporozoite-encoded proteins that function in the tick vector. Therefore, functional orthologs of Plasmodium TRAP and/or MAEBL may instead be expressed by the motile kinete. SIAP-1 stands out as it appears to be structurally conserved across apicomplexan hemoprotozoa and may play similar roles in the final maturation steps of Babesia and Theileria sporozoites. Potential roles in sporozoite exit from the sporoblast or sporont and gliding locomotion will be difficult to address by genetic approaches in these parasites. Expression profiling during the parasite life cycles and complementation of the siap-1(−) phenotypes described in this study will reveal the degree of similarity between the orthologous genes.
Ultimately, identifying the conserved cellular roles of SIAP-1 will provide important insights into basic principles of sporozoite biology and of arthropod-borne parasitic diseases.
Supplementary Material
Acknowledgments
We thank Marion Steinbuechel for assistance with WT parasite-infected mosquito experiments, Diana Scheppan for assistance with whole mosquito pictures, and Kristin Götz for technical assistance.
This work was supported in part by grants from the research focus Tropical Medicine Heidelberg of the Medical Faculty of Heidelberg University, the Deutsche Forschungsgemeinschaft (SFB 544; no. B10), the European Commission (BioMalPar; no. 23), the Joachim Siebeneicher Foundation and the Chica and Heinz Schaller Foundation. O.S. is a recipient of a Marie Curie Intra-European Fellowship and an EMBO Long-Term Fellowship.
Footnotes
Published ahead of print on 30 January 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aly, A. S., and K. Matuschewski. 2005. A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J. Exp. Med. 202225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doolan, D. L., S. Southwood, D. A. Freilich, J. Sidney, N. L. Graber, L. Shatney, L. Bebris, L. Florens, C. Dobano, A. A. Witney, E. Appella, S. L. Hoffman, J. R. Yates III, D. J. Carucci, and A. Sette. 2003. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. USA 1009952-9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frischknecht, F. 2007. The skin as interface in the transmission of arthropod-borne pathogens. Cell. Microbiol. 91630-1640. [DOI] [PubMed] [Google Scholar]
- 4.Frischknecht, F., P. Baldacci, B. Martin, C. Zimmer, S. Thiberge, J. C. Olivo-Marin, S. L. Shorte, and R. Ménard. 2004. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell. Microbiol. 6687-694. [DOI] [PubMed] [Google Scholar]
- 5.Janse, C. J., B. Franke-Fayard, G. R. Mair, J. Ramesar, C. Thiel, S. Engelmann, K. Matuschewski, G. J. van Gemert, R. W. Sauerwein, and A. P. Waters. 2006. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol. 14560-70. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser, K., K. Matuschewski, N. Camargo, J. Ross, and S. H. Kappe. 2004. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol. Microbiol. 511221-1232. [DOI] [PubMed] [Google Scholar]
- 7.Kappe, S., T. Bruderer, S. Gantt, H. Fujioka, V. Nussenzweig, and R. Ménard. 1999. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J. Cell Biol. 147937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariu, T., M. Yuda, K. Yano, and Y. Chinzei. 2002. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J. Exp. Med. 1951317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matuschewski, K. 2006. Getting infectious: formation and maturation of Plasmodium sporozoites in the Anopheles vector. Cell. Microbiol. 81547-1556. [DOI] [PubMed] [Google Scholar]
- 10.Matuschewski, K., A. C. Nunes, V. Nussenzweig, and R. Ménard. 2002. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 211597-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan, R., V. Thathy, M. M. Mota, J. C. Hafalla, R. Ménard, and K. Vernick. 2001. Fluorescent Plasmodium berghei sporozoites and pre-erythrocytic stages: a new tool to study mosquito and mammalian host interactions with malaria parasites. Cell. Microbiol. 3371-379. [DOI] [PubMed] [Google Scholar]
- 12.Potocnjak, P., N. Yoshida, R. S. Nussenzweig, and V. Nussenzweig. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 1511504-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 221567-1572. [DOI] [PubMed] [Google Scholar]
- 14.Siau, A., O. Silvie, J.-F. Franetich, S. Yalaoui, C. Marinach, L. Hannoun, G.-J. van Gemert, A. J. F. Luty, E. Bischoff, P. H. David, G. Snounou, C. Vaquero, P. Froissard, and D. Mazier. 2008. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte invasion. PLoS Pathog. 4e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvie, O., K. Goetz, and K. Matuschewski. 2008. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 4e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinden, R. E., and K. Matuschewski. 2005. The sporozoite, p. 169-190. In I. Sherman (ed.), Molecular approaches to malaria. American Society for Microbiology Press, Washington, DC.
- 17.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Ménard. 1997. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90511-522. [DOI] [PubMed] [Google Scholar]
- 18.Tewari, R., D. Rathore, and A. Crisanti. 2005. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell. Microbiol. 7699-707. [DOI] [PubMed] [Google Scholar]
- 19.Thathy, V., and R. Ménard. 2002. Gene targeting in Plasmodium berghei. Methods Mol. Med. 72317-331. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji, M., D. Mattei, R. S. Nussenzweig, D. Eichinger, and F. Zavala. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 8016-21. [DOI] [PubMed] [Google Scholar]
- 21.Vanderberg, J. P. 1975. Development of infectivity by the Plasmodium berghei sporozoite. J. Parasitol. 6143-50. [PubMed] [Google Scholar]
- 22.Vaughan, J. A., L. Hensley, and J. C. Beier. 1994. Sporogonic development of Plasmodium yoelii in five anopheline species. J. Parasitol. 80674-681. [PubMed] [Google Scholar]
- 23.Wang, Q., H. Fujioka, and V. Nussenzweig. 2005. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 1e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wengelnik, K., R. Spaccapelo, S. Naitza, K. J. H. Robson, C. J. Janse, F. Bistoni, A. P. Waters, and A. Crisanti. 1999. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 185195-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.