Abstract
Phosphorylation on tyrosine residues is a key signal transduction mechanism known to regulate intercellular and intracellular communication in multicellular organisms. Despite the lack of conventional tyrosine kinases in the genome of the single cell organism Trypanosoma brucei, phosphorylation on trypanosomal protein tyrosine residues has been reported for this parasite. However, the identities of most of the tyrosine-phosphorylated proteins and their precise site(s) of phosphorylation were unknown. Here, we have applied a phosphotyrosine-specific proteomics approach to identify 34 phosphotyrosine-containing proteins from whole-cell extracts of procyclic form T. brucei. A significant proportion of the phosphotyrosine-containing proteins identified in this study were protein kinases of the CMGC kinase group as well as some proteins of unknown function and proteins involved in energy metabolism, protein synthesis, and RNA metabolism. Interestingly, immunofluorescence microscopy using anti-phosphotyrosine antibodies suggests that there is a concentration of tyrosine-phosphorylated proteins associated with cytoskeletal structures (basal body and flagellum) and in the nucleolus of the parasite. This localization of tyrosine-phosphorylated proteins supports the idea that the function of signaling molecules is controlled by their precise location in T. brucei, a principle well known from higher eukaryotes.
Reversible protein phosphorylation by protein kinases and phosphatases is a major regulatory mechanism of most cellular processes in eukaryotic organisms (7). Dysregulation of protein phosphorylation networks is responsible for a myriad of diseases from cancers to immune disorders and neurodegenerative diseases (8, 9). These findings have prompted the characterization of the protein kinase complements (“kinomes”) of a number of organisms (2, 6, 14, 16, 18, 21, 24, 27, 32, 41), and an important turning point came with the realization that parasite kinomes are substantially different from those of the hosts they infect. One major difference is the absence of genes coding for any recognizable tyrosine-specific kinases in the genome of Trypanosoma brucei (29; I. R. E. Nett, D. M. A. Martin, D. Miranda-Saavedra, D. Lamont, J. D. Barber, A. Mehlert, and M. A. J. Ferguson, submitted for publication). T. brucei causes human African trypanosomiasis (also known as African sleeping sickness) and is responsible for ∼30,000 deaths per annum (35). However, despite the lack of conventional tyrosine kinases in this parasite and related trypanosomes, there is evidence that several proteins are phosphorylated on tyrosine residues in these organisms (10, 30). Tyrosine phosphatase activity has also been observed in cell extracts of bloodstream form and procyclic form T. brucei (1). More recently, the identification and biochemical characterization of a T. brucei protein tyrosine phosphatase 1 suggest a role for tyrosine phosphorylation in the regulation of the trypanosome life cycle (36). In addition, the characterization of the T. brucei protein phosphatase complement (“phosphatome”) revealed the presence of 19 dual-specificity protein phosphatases. However, orthologues of the human mitogen-activated protein kinase (MAPK) phosphatases are missing in the T. brucei genome (3), although plant-like MAPK phosphatase homologues are present. This is an interesting observation, since our large-scale phosphoproteomics analysis of bloodstream form T. brucei cells (Nett et al., submitted for publication) revealed phosphorylation on tyrosine residues of 13 protein kinases, of which two belong to the MAPK family (GeneDB accession no. Tb927.6.4220 and Tb10.61.0250).
Phosphorylation of protein tyrosine residues regulates important cell functions in higher eukaryotes, but the role of this posttranslational modification is largely unknown for T. brucei. Here, we used a phosphotyrosine-specific mass spectrometry (MS)-based approach to identify proteins carrying this modification in the procyclic form of the parasite and reveal that phosphorylation of tyrosine residues within canonical sequence motifs is conserved in T. brucei. However, by using anti-phosphotyrosine-specific antibodies, we show a localization pattern for phosphotyrosine-containing proteins in T. brucei that is substantially different from that in mammalian cells.
MATERIALS AND METHODS
Cell preparation and lysis.
The procyclic form T. brucei cell line 29-13-6 (44) was cultured in SDM-79 medium (5), containing G418 (Gibco) at 15 μg/ml and hygromycin B (Roche) at 50 μg/ml. Human HeLa cells were grown in Dulbecco modified Eagle medium (Gibco) supplemented with 10% fetal calf serum (Gibco) and 2 mM l-glutamine (Gibco) at 37°C.
For the isolation of phosphotyrosine-containing peptides from trypanosomes, 1 liter of cells was grown to a density of 1 × 107 cells/ml at 28°C in a water-jacketed incubator. Cells were treated with 800 μM hydrogen peroxide for 30 min before lysis. Cell lysis and phosphopeptide preparation were performed using the PhosphoScan P-Tyr-100 kit (Cell Signaling) according to the manufacturer's instructions.
Cell lysis for Western blotting.
Before lysis, cells were washed three times in ice-cold phosphate-buffered saline (PBS) buffer and then lysed in ice-cold RIPA lysis buffer (10 mM Tris-HCl, pH 7.5; 1 mM sodium-β-glycerophosphate; 1 mM sodium-pyrophosphate; 1 mM sodium fluoride; 5 mM EDTA; 0.5% NP-40; 0.2% sodium-deoxycholate; 0.2% sodium dodecyl sulfate [SDS]; EDTA-free protease inhibitor tablet [Roche]; 100 μM activated sodium-orthovanadate) at a ratio of 1 × 109 cells per 1 ml of lysis buffer. The lysate was sonicated two times for 30 s at 80% power of an ultrasonic processor machine (Jencons) at 4°C and centrifuged at 14,000 rpm for 20 min at 4°C. The supernatant was transferred into a fresh tube, and the protein concentration determined using the Micro BCA protein assay kit (Pierce) according to the manufacturer's instructions. Approximately 10 μg of proteins were mixed with the appropriate volume of 4× Laemmli sample buffer and 10× sample-reducing agent (Invitrogen), heated, and separated on a precast Novex 4 to 12% Bis-Tris SDS-polyacrylamide electrophoresis gel. After electrophoresis, proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, and the membrane was blocked with 4% bovine serum albumin in Tris-buffered saline (TBS) (150 mM NaCl, 50 mM Tris-HCl, pH 7.2) containing Triton X-100 (0.2%) for 1 h at room temperature. The membrane was incubated with the anti-phosphotyrosine monoclonal antibody 4G10 (1 mg/ml; Upstate) at a dilution of 1:10,000 for 1 h at room temperature, then washed, and incubated with secondary antibody (anti-mouse horseradish peroxidase; Roche) at 1:5,000. Western blots were developed using the ECL plus Western blotting detection reagent (GE Healthcare) according to the manufacturer's instructions and were exposed to Hyperfilm (GE Healthcare).
To examine phosphotyrosine-containing proteins of cytosol and cytoskeletal fractions, aliquots of 3 × 107 procyclic form T. brucei cells were washed in PBS buffer and lysed in either 100 μl ice-cold MME buffer (10 mM MOPS, pH 6.9; 1 mM EGTA; 1 mM MgSO4) containing EDTA-free protease inhibitor tablet (Roche), 0.2% Triton X-100, and a phosphatase inhibitor cocktail of 1 mM sodium-β-glycerophosphate, 1 mM sodium-pyrophosphate, 1 mM sodium fluoride, and 100 μM activated sodium-orthovanadate or in 100 μl RIPA lysis buffer (see above). The MME lysate was incubated on ice for 30 min and centrifuged at 13,000 × g for 2 min at 4°C. The supernatant was transferred into a fresh tube, and the pellet was washed three times with 1 ml MME buffer containing 0.2% Triton X-100 with protease and phosphatase inhibitors, as described previously. The insoluble cytoskeletons were resolubilized in 100 μl RIPA lysis buffer, and 20-μl aliquots (6 × 106 cell equivalents) of the MME soluble fraction and the RIPA-solubilized cytoskeletal fraction were resolved on a 4 to 12% NuPAGE gel (Invitrogen), alongside 20 μl of the whole-cell RIPA-lysate, and transferred to PVDF. Replicate blots were incubated with either alkaline phosphatase buffer (50 mM Tris-HCl, pH 8.5; 5 mM MgCl2) containing 15 units of shrimp alkaline phosphatase at 30°C overnight or with 10 μg of recombinant glutathione S-transferase-SHP-1 in 50 mM Tris-HCl (pH 7.5), 0.1 mM EGTA, 0.03% Brij 35, 0.1% β-mercaptoethanol. The control blot was incubated in glutathione S-transferase-SHP-1 buffer. Blots were rinsed three times with TBS containing 0.2% Triton X-100 (TBS-T), and Western blotting for phosphotyrosine was performed as described previously. Western blots were stripped in 50 mM Tris-HCl (pH 7), 2% SDS, 0.1% β-mercaptoethanol for 30 min at 65°C and then rinsed three times with TBS containing 0.2% Triton X-100. For a loading control, blots were incubated with the anti-tubulin clone DM1A (1 mg/ml; Abcam) at a dilution of 1:10,000 and then with goat anti-mouse horseradish peroxidase (1:10,000) and developed by enhanced chemiluminescence.
Immunolocalization.
Mid-log procyclic trypanosomes (∼1 × 107 cells/ml) were settled onto coverslips for 15 min, and in the case of cytoskeleton preparations, settled cells were incubated with MME buffer (see above) on ice for 10 min with three changes of buffer. Isolated flagellar complexes were obtained by incubating the cells with MME buffer containing 1 M NaCl, 0.2% Triton X-100, and protease and phosphatase inhibitors, as described previously, on ice for 15 min with three changes of buffer. Cells were washed in PBS and fixed in either 4% paraformaldehyde (PFA) in PBS or 100% ice-cold methanol. Coverslips were blocked with 2% fish gelatin in TBS/0.2% Triton X-100 for 1 h at room temperature before antibody labeling with approximately 0.25 μg per coverslip of 4G10 antibody (Upstate), fluorescein isothiocyanate-conjugated 4G10 antibody (Upstate), PY-20 antibody (Transduction Laboratories), or PY-100 antibody (Cell Signaling Technologies) or with a 1:30 dilution in 2% fish gelatin in TBS/0.2% Triton X-100 of BBA4 or Rib72 monoclonal antibody tissue culture supernatant (kind gifts of Keith Gull). Rib72 labeling was visualized with anti-mouse immunoglobulin G (IgG) Alexa Fluor 633 (2 μg/ml; Invitrogen) secondary antibody and 4G10 labeling with either anti-mouse IgG Alexa Fluor 633 (2 μg/ml; Invitrogen) or anti-mouse IgG Alexa Fluor 488 (2 μg/ml; Invitrogen). BBA4 labeling was visualized with anti-mouse IgM Alexa Fluor 488 (2 μg/ml; Invitrogen). Cells were counterstained with anti-mouse fluorescein isothiocyanate-conjugated anti-α tubulin (1.5 μg/ml; Sigma) and/or 4′-6-diamidino-2-phenylindole (2 μg/ml; Sigma) before mounting with Vectashield (Vector Laboratories). For staining of the nucleolus, procyclic cells were incubated with a carboxy-terminal tetramethyl rhodamine isothiocyanate (TRITC)-labeled deca-arginine peptide, TRITC-D(R10) (Peptide Specialty Laboratories GmbH), at a 7 μM final concentration for 1 h at 28°C. Cells were washed once in PBS, resuspended in SDM-79 medium and incubated for another 2 h at 28°C. Cells were then harvested, fixed in 4% PFA/PBS, and mounted as described above.
For the dephosphorylation assay, methanol-fixed trypanosomes were incubated with 2 ml alkaline phosphatase buffer (see above) containing 10 units of alkaline shrimp phosphatase in a six-well plate overnight at 37°C. Positive control cells were incubated in 2 ml alkaline phosphatase buffer only, without the addition of alkaline shrimp phosphatase.
For immunofluorescence studies of human HeLa cells, 2 × 105 cells were seeded onto a square coverslip (22 mm by 22 mm) and incubated at 37°C for 24 h. The cells were washed twice with 1 ml of PBS and fixed in 4% PFA/PBS. Antibody incubations and mounting of cells were performed as described for trypanosomes.
Images were collected using a DeltaVision Spectris restoration wide-field deconvolution microscope (Applied Precision LLC) equipped with a CoolSnap HQ cooled charge-coupled-device camera. Optical sections were processed using SoftWoRx software (Applied Precision LLC) and Adobe Photoshop (Adobe).
LC-MS.
Liquid chromatography (LC) was performed on a fully automated UltiMate 3000 Nano LC system (Dionex) fitted with a 1- by 5-mm precolumn PepMap C18 (LC Packings, Dionex) and a 75-μm by 15-cm reverse-phase C18 nano-column (LC Packings, Dionex). Samples were loaded in 0.1% formic acid containing 2% acetonitrile (buffer A) and separated using a binary gradient consisting of buffer A and buffer B (90% acetonitrile, 0.08% formic acid). Peptides were eluted with a linear gradient from 5 to 40% buffer B over 130 min. The high-pressure liquid chromatography system was coupled to an LTQ-Orbitrap mass spectrometer (Thermo Electron) equipped with a proxeon nanospray ionization source.
With the LTQ-Orbitrap mass spectrometer, a survey scan was performed over a mass range of m/z 335 to 1,800 in the Orbitrap analyzer (R = 60,000), each triggering five tandem MS (MS/MS) LTQ acquisitions of the five most intense ions. The Orbitrap mass analyzer was internally calibrated on the fly using the lock mass of polydimethylcyclosiloxane at m/z 445.120025.
Raw peak list files obtained from the LTQ-Orbitrap were converted to Mascot generic files using Raw2msm software (gift from Matthias Mann) and were searched against concatenated forward and reverse sequence databases consisting of amino acid sequences from T. brucei strain 927 and translated open reading frames from T. brucei strain 427 using the Mascot search engine (Mascot V2.1; Matrix Science). The search criteria were as follows: up to two missed cleavages were allowed; carbamidomethylation, oxidation, and phosphorylation were set as variable modifications. The precursor ion mass tolerance was set to 10 ppm and 0.8 Da for all MS/MS spectra acquired in the LTQ mass analyzer.
RESULTS
Detection of tyrosine-phosphorylated proteins by immunoblotting using 4G10 anti-phosphotyrosine antibody.
To initiate an analysis of tyrosine-phosphorylated proteins in T. brucei, we investigated the usefulness of commercially available anti-phosphotyrosine antibodies to bind to trypanosomal phosphotyrosine-containing proteins by Western blotting. The 4G10 anti-phosphotyrosine antibody (Upstate) revealed several protein bands, especially high-molecular-weight proteins, in total cell lysates of both procyclic (Fig. 1, lane 1) and bloodstream form T. brucei (see Fig. S1 in the supplemental material). The association of phosphotyrosine-containing proteins with microtubular structures, indicated by immunofluorescence microscopy (see below), was also analyzed by Western blotting using whole-cell, TX-100-soluble and cytoskeleton extracts of procyclic form T. brucei cells (Fig. 1). Anti-phosphotyrosine reactive proteins were detected in both fractions to different degrees (Fig. 1, compare lanes 2 and 3). A higher proportion of tyrosine-phosphorylated proteins was found in the cytoskeleton extract (Fig. 1, lane 3) than for TX-100-soluble proteins obtained from an equal amount of cells (Fig. 1, lane 2). To assess the possibility of nonspecific antibody binding, parallel incubations of PVDF membranes containing the same amount of procyclic cell lysate were performed in the absence (Fig. 1, control) and presence of alkaline phosphatase (Fig. 1) or the phosphotyrosine-specific phosphatase SHP-1 (Fig. 1). A reduction of signal intensity was clearly visible for the majority of the bands after treating the PVDF membrane with alkaline phosphatase; however, the signal was completely abolished after treating PVDF membranes with the protein tyrosine phosphatase SHP-1. These results confirm the specificity of the 4G10 antibody for phosphotyrosine-containing proteins.
FIG. 1.
Detection of T. brucei tyrosine-phosphorylated proteins by immunoblotting. Aliquots of total cell lysates (T) and TX-100-soluble (S) and insoluble (P) fractions from 6 × 106 procyclic form trypanosome equivalents were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting with 4G10 antibody before (Control, lanes 1 to 3) and after alkaline phosphatase (alk. phosphatase, lanes 4 to 6) or SHP-1 phosphotyrosine-specific phosphatase (SHP-1, lanes 7 to 9) treatment of the blot. Following the 4G10 Western blotting, the membranes were stripped and probed with anti-tubulin antibodies as a loading control (lower panels). α-pTyr, anti-phosphotyrosine.
Tyrosine phosphorylation is hydrogen peroxide sensitive.
We next examined the level of tyrosine phosphorylation after treating the cells with different concentrations of hydrogen peroxide (Fig. 2). Hydrogen peroxide has been shown to be involved in the regulation of redox signaling pathways by inactivating protein tyrosine phosphatases and, as a result, increasing tyrosine phosphorylation of target proteins (33). In procyclic trypanosomes, first responses to the hydrogen peroxide treatment were detected at concentrations of 200 μM, and detection levels reached their maximum in cell lysates obtained from cells that had been incubated with 800 μM hydrogen peroxide for 20 min or 30 min (Fig. 2). In bloodstream form cells (see Fig. S2 in the supplemental material), clear response to the hydrogen peroxide treatment was detected at 400 μM of the oxidizing agent at all time points measured (17, 23, and 30 min), which could be increased with higher hydrogen peroxide concentrations up to 800 μM where the signal reached saturation. These results suggest the possible expression of hydrogen peroxide-sensitive phosphatases in the trypanosome and/or the presence of redox-regulated protein tyrosine kinases. The latter, if they exist, would have to be dual-specificity kinases, since conventional tyrosine kinase genes are absent in T. brucei. It is worth noting that peroxovanadium [bpV(phen)] had no effect on phosphotyrosine levels under conditions in which it had clear effects on mammalian cell control experiments run alongside (data not shown).
FIG. 2.
T. brucei cells are sensitive to treatment with hydrogen peroxide. Procyclic trypanosomes were treated with 200 μM, 400 μM, and 800 μM hydrogen peroxide for 10 min, 20 min, and 30 min before cell lysis. An aliquot of 15 μg protein was loaded in each lane. Lane 1, protein lysate of untreated cells. Bands that show a clear response to the hydrogen peroxide treatment are indicated with an asterisk. α-pTyr, anti-phosphotyrosine.
Immuno-affinity purification of phosphotyrosine-containing peptides from procyclic form T. brucei.
In order to identify T. brucei tyrosine-phosphorylated proteins, we immunoprecipitated tyrosine-phosphorylated peptides from tryptic digests of whole-cell lysates of the procyclic form and analyzed the immunoprecipitate by LC-MS/MS. Procyclic trypanosomes were treated with 800 μM hydrogen peroxide for 30 min before cell lysis to increase the levels of phosphorylation on tyrosine residues and thus improve the detection of phosphorylated proteins by MS. In total, 45 phosphotyrosine-containing peptide isoforms from 34 proteins were identified in two separate experiments using approximately 40 mg starting material (see Table S1 in the supplemental material). An example of a fragmentation mass spectrum of a tyrosine-phosphorylated peptide is shown in Fig. 3. A mass increment of 243 Da, which corresponds to a phosphorylated tyrosine residue (pY), was observed for residue Y188 in the phosphopeptide sequence GVGVNVTSpYVVTR of GeneDB accession no. Tb927.6.1780. The loss of a phosphate group in the form of phosphoric acid (H3PO4) was observed for T186, suggesting this residue as a second site of phosphorylation in the same peptide.
FIG. 3.
Mass spectrometric analysis of T. brucei phosphotyrosine-containing peptides. Shown is the fragmentation spectrum of the diphosphorylated peptide GVGVNVpTSpYVVTR (p indicates phosphorylated residue) of a putative TbMAPK (GeneDB accession no. Tb927.6.1780) measured on an LTQ-Orbitrap mass spectrometer. Phosphorylation at the threonine and tyrosine residues of the TSY motif could be deduced due to the neutral loss of phosphoric acid starting from the y7 and b7 ions (−P) and the observed mass increment of 243 Da (+P) between the y4 and y5 ions, respectively.
The majority of proteins found to be phosphorylated on tyrosine residues were protein kinases belonging to the CMGC kinase group, and one kinase was designated a NEK group member (Table 1). T. brucei protein kinases were annotated according to the classification described in the work of Nett et al. (submitted for publication). Of the CMGC kinases, we identified 10 putative T. brucei mitogen-activated protein kinases (TbMAPK) homologous to the 15 putative MAPKs encoded in the Leishmania genome (42), including the experimentally characterized TbMAPK2 (28) (Table 1). We also detected the T. brucei homologue of LmjMPK15, which falls into the T. brucei CDK kinase family (Tb10.329.0030).
TABLE 1.
Tyrosine-phosphorylated protein kinases of whole-cell procyclic trypanosomesa
| T. brucei GeneDB accession no.c | GeneDB annotation | Kinase group | Kinase family | MW (in thousands)d | Phosphopeptideb | Reference |
|---|---|---|---|---|---|---|
| Tb10.70.7040 | CRK1 | CMGC | CDK | 34.3 | 11 IGEGSpYGVVFR 21 | 37, 38, 39 |
| Tb10.389.1730* | MAPK11, putative | CMGC | MAPK | 47.3 | 173 EESDQGEHMTDpYVTMR 188 | |
| Tb10.6k15.2790 | MAPK1, putative | CMGC | MAPK | 39.3 | 160 CFNTQGGDNDLTEpYIATR 177 | |
| Tb927.7.2420 | GSK3α, putative | CMGC | GSK3 | 55.3 | 274 NVPpYIFSR 281 | |
| Tb927.3.690* | PK, putative | CMGC | MAPK | 63.8 | 170 ARPPYTDpYVSTR 181 | |
| Tb927.7.3580 | PK, putative | NEK | 66.5 | 429 FNGGpYNSSSVSGNGVVK 445 | ||
| Tb10.70.2070* | MAPK2, putative | CMGC | MAPK | 49.7 | 166 EQVARPVLTDpYIMTR 180 | |
| Tb11.01.4230 | PK, putative | CMGC | CLK | 53.1 | 99 KVTpYALPNQSR 109 | |
| Tb10.329.0030 | PK, putative | CMGC | CDK | 64.4 | 234 DAQASDTFpYVCTR 246 | |
| Tb927.8.3770* | PK, putative | CMGC | MAPK | 46.9 | 182 EDTQDPNKTHpYVTHR 196 | |
| Tb09.211.0960* | PK, putative | CMGC | MAPK | 41.7 | 152 GLHVSQPLTEpYVSTR 166 | |
| Tb10.70.2210 | CRK3 | CMGC | CDK | 35.0 | 26 MDILGEGTpYGVVYR 39 | 39 |
| Tb11.02.0640 | PK, putative | CMGC | DYRK/class II | 51.5 | 266 LFTpYIQSR 273 | |
| Tb11.01.8550* | ECK1 | CMGC | MAPK | 72.1 | 155 GNYTEpYVATR 164 | 15 |
| Tb10.61.1850* | MAPK9, putative | CMGC | MAPK | 42.7 | 158 SRPPFTEpYVSTR 169 | |
| Tb10.61.0250 | MAPK2 | CMGC | MAPK | 41.8 | 180 DDQCTQTSALTEpYVVTR 196 | 28 |
| Tb927.6.1780* | PK, putative | CMGC | MAPK | 46.1 | 180 GVGVNVTSpYVVTR 192 | |
| Tb927.7.7360 | CRK2 | CMGC | CDK | 39.2 | 52 VGEGSpYGIVYK 62 | 38, 39 |
| Tb10.61.3140 | PK, putative | CMGC | GSK3 | 40.3 | 178 LAADEPNVApYICSR 191 |
Shown are proteins identified in our phosphotyrosine-specific proteomics study with at least one tyrosine-phosphorylated residue.
pY indicates a tyrosine-phosphorylated residue. Numbers preceding and following the sequences specify the start and the end of the phosphopeptide sequence.
TbMAPKs with a fully phosphorylated TXY motif are indicated with an asterisk.
MW, molecular weight.
The signature TXY motif in the activation loop of MAPKs (25) was found fully phosphorylated in 8 of the 10 TbMAPKs (Table 1), and the remaining 3 kinases (including the identified TbCDK) were phosphorylated only on the tyrosine residue. The TXY motif present in the putative TbMAPKs showed a rather variable central amino acid; five TbMAPKs were found to contain a TEY motif, three TbMAPKs displayed a TDY motif, and the remaining two TbMAPKs had either THY or TSY. Another variation of the TXY motif was found in the T. brucei homologue of LmjMPK15, which showed a TFY signature motif in the activation segment.
Other tyrosine-phosphorylated proteins identified in our study are involved in protein synthesis (GeneDB accession no. Tb927.5.3120 translation initiation factor, putative; ribosomal protein S27, putative), energy metabolism (glycosomal phosphoenolpyruvate carboxykinase; ATP-dependent phosphofructokinase), and RNA metabolism (ATP-dependent DEAD/H RNA helicase, putative). Tyrosine-containing peptides were also found for nonclassified proteins (10 “hypothetical proteins”) (see Table 2).
TABLE 2.
Non-protein kinase tyrosine-phosphorylated proteins of whole-cell procyclic trypanosomesa
| T. brucei GeneDB accession no. | Protein description | MW (in thousands)c | Phosphopeptideb | Reference |
|---|---|---|---|---|
| Tb927.5.3120 | Translation initiation factor, putative | 34.9 | 287 SVQAVGSATpYSAQVGKR 303 | |
| Tb11.01.1475 | Ribosomal protein S27, putative | 9.6 | 25 LVQGPNSpYFMDVK 37 | |
| Tb927.2.4210 | Glycosomal phosphoenolpyruvate carboxykinase; glycosomal protein P60 | 58.5 | 172 EQVILGTEpYAGEMK 185 | |
| Tb927.3.3270 | TbPFK ATP-dependent phosphofructokinase; 6-phospho-1-fructokinase | 53.5 | 54 DKTDYIMpYNPRPR 66 | 23 |
| Tb09.211.3510 | ATP-dependent DEAD/H RNA helicase, putative | 82.7 | 138 FDVDFpYDRPR 147 | |
| Tb09.211.0170 | Hypothetical protein, conserved | 28.8 | 96 FTGGpYSSTPYVTSDAR 111 | |
| Tb927.4.2040 | Hypothetical protein, conserved | 20.8 | 2 PSpYPPRDEYR 11 | |
| Tb10.6k15.2400 | Hypothetical protein, conserved | 97.7 | 536 GAEEAQAALpYAVGK 549 | |
| Tb11.02.0420 | Hypothetical protein, conserved | 21.4 | 7 CIICAGNSAHpYR 18 | |
| Tb10.70.3380 | Hypothetical protein, conserved | 52.9 | 34 SILGKpYPIR 42 | |
| Tb09.160.1160 | Hypothetical protein, conserved | 85.9 | 19 IGGDpYEWSNTLAR 31 | |
| Tb11.01.2800 | Hypothetical protein, conserved | 41.6 | 287 THDpYDEPQELIR 298 | |
| Tb927.4.3130 | Hypothetical protein, conserved | 30.6 | 178 VGVTSGpYANTQGR 190 | |
| Tb927.3.1400 | Hypothetical protein, conserved | 37.2 | 279 YLQSQNCRPTGNAGpYGGGNGPASSAEVR 306 | |
| Tb10.6k15.3460 | Hypothetical protein, conserved | 284.7 | 385 HVMEQHpYGTIAK 396 |
Shown are proteins identified in our phosphotyrosine-specific proteomics study with at least one tyrosine-phosphorylated residue.
pY indicates a tyrosine-phosphorylated residue. Numbers preceding and following the sequences specify the start and the end of the phosphopeptide sequence.
MW, molecular weight.
Localization studies of tyrosine-phosphorylated proteins in T. brucei.
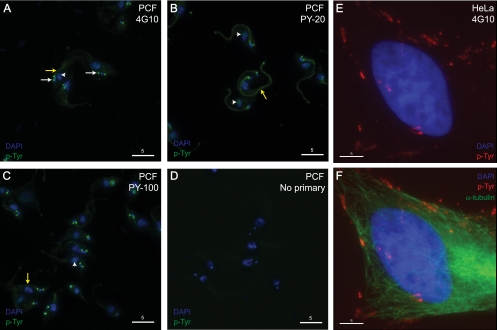
We next investigated the localization pattern of tyrosine-phosphorylated proteins using the anti-phosphotyrosine monoclonal antibodies 4G10, PY-20, and PY-100 on PFA-fixed procyclic trypanosomes (Fig. 4). Phosphotyrosine-containing proteins appeared to localize specifically to punctate structures in the posterior of the cell (Fig. 4A) and to the nucleus (Fig. 4A to C). A phosphotyrosine signal was also observed along the flagellum (Fig. 4A to C), which faded out toward its distal tip, particularly in the case of 4G10 and PY-100 staining. It is worth noting that the fixation method (4% PFA versus 100% methanol) had no effect on the overall staining pattern; however, the recognition of phosphotyrosine-containing structures using methanol-fixed procyclic form cells seemed to be weaker compared to that using PFA fixation (data not shown). A no-primary antibody control was also included to demonstrate that the staining was a consequence of the primary antibody and not a nonspecific background (Fig. 4D). The specificity of the anti-phosphotyrosine antibody 4G10 to recognize phosphotyrosine-containing residues on fixed trypanosomes was tested by treating coverslips with alkaline phosphatase overnight and then with 4G10 staining and immunofluorescence microscopy (see Fig. S3 in the supplemental material). The treatment with alkaline phosphatase abolished the immunofluorescence signal, demonstrating the selectivity of the 4G10 antibody after cell fixation and ruling out the possibility of cross-reactivity.
FIG. 4.
Indirect immunofluorescence using anti-phosphotyrosine antibodies. Whole-cell procyclic trypanosomes were fixed in 4% PFA and stained with 4G10 (A), PY-20 (B), and PY-100 (C). (D) A no-primary-antibody control was included and demonstrated that the staining was a consequence of the primary anti-phosphotyrosine antibodies. Tyrosine-phosphorylated proteins (in green) localized to punctate structures in the posterior of the cells (white arrows) and were also found along the length of the flagellum (yellow arrows) and in the nucleus (white arrowheads). (E and F) Control HeLa cells labeled with 4G10 showed concentrated anti-phosphotyrosine staining (in red) at focal adhesion regions. DNA (blue) is visualized by 4′-6-diamidino-2-phenylindole (DAPI) staining. Anti-α-tubulin is shown in green. p-Tyr, tyrosine-phosphorylated proteins. White bar corresponds to a length of 5 μm.
In order to investigate whether the proteins visualized by the anti-phosphotyrosine antibodies were associated with microtubular structures, procyclic form T. brucei total cytoskeletons were extracted with detergent, fixed in 4% PFA, and immunostained with the anti-phosphotyrosine antibodies 4G10, PY-20, and PY-100 (Fig. 5). Immunofluorescence images of the cytoskeleton preparations gave the same staining pattern that was previously observed with whole cells, except for the nucleus (compare with Fig. 4). Identical results were obtained with bloodstream form T. brucei cytoskeletons using the anti-phosphotyrosine antibody 4G10 (see Fig. S4 in the supplemental material). The strong signal for the punctate structures in the posterior of the cell was present in both life cycle stages, and the position and shape of this organelle indicated the labeling of the basal body (mature and immature probasal body). The lack of any gap between the signal for the basal body and the labeling of the flagellum (Fig. 5A), as well as the fact that the signal for the flagellum connects to only one (the mature basal body), strongly pointed to the flagellum signal being axonemal rather than paraflagellar rod or flagellum attachment zone filament (Keith Gull, personal communication). Moreover, the signal did not seem to vary much through the cell cycle, as it could be detected on the old and new flagella (Fig. 5B).
FIG. 5.
Indirect immunofluorescence analysis of cytoskeletal preparations using anti-phosphotyrosine antibodies. Procyclic form cytoskeletons were fixed in 4% PFA and stained with 4G10 (A), PY-20 (B), and PY-100 (C). (D) A no-primary-antibody control was included and demonstrated that the staining was a consequence of the primary anti-phosphotyrosine antibodies. The staining pattern suggested labeling of the basal bodies in the posterior of the cell and was observed along the length of the flagellum (in green). DNA (blue) is visualized by 4′-6-diamidino-2-phenylindole (DAPI) staining. White bar corresponds to a length of 5 μm.
In order to define the individual components within the cytoskeleton that the phosphotyrosine signal was associated with, we performed immunolocalization studies using 4G10 in association with the monoclonal antibodies BBA4 and Rib72. BBA4 is a specific marker for the proximal poles of both the basal and probasal bodies (45) and labels the basal body structure as a two-dot pattern in the posterior of a trypanosome cell (12). Colabeling experiments indicate that phosphotyrosine-containing proteins are localized toward the distal end of both the basal and probasal bodies, as the staining patterns for 4G10 and BBA4 show very close proximity but do not overlap (Fig. 6C). This specific labeling has previously been observed in procyclic trypanosomes when using the anti-phosphopeptide monoclonal antibody MPM2 (12). In addition, the phosphotyrosine signal seems to be on the distal end of the basal body rather than around this organelle, as determined by taking differential interference contrast images of anti-phosphotyrosine 4G10-labeled flagellum extractions (Fig. 6E).
FIG. 6.
Colocalization studies of phosphotyrosine staining using the anti-basal body marker BBA4. Indirect immunofluorescence of procyclic form T. brucei cytoskeletons colabeled with the anti-phosphotyrosine antibody 4G10 (A) and BBA4 (B), a specific marker for the proximal pole of both basal and probasal structures. Images shown in panels A and B are shown merged in panel C. Differential interference contrast (DIC) images of T. brucei flagella were taken (D) and merged with immunofluorescence images labeled with the anti-phosphotyrosine 4G10 antibody (E). White arrows, labeling of the basal body. Colabeling structures that were observed are enlarged and surrounded by a white box. p-Tyr, tyrosine-phosphorylated proteins. White bar corresponds to a length of 5 μm.
We next investigated the localization of phosphotyrosine-containing proteins observed along the length of the flagellum by using the monoclonal anti-axonemal Rib72 antibody. Rib72 extends from the basal body to the tip of the flagellum but is not present in the basal body itself (Keith Gull, personal communication). Colabeling experiments with 4G10 (Fig. 7B) and Rib72 (Fig. 7C) revealed that the staining pattern of both monoclonal antibodies overlapped in most of the areas (Fig. 7D). Thus, the phosphotyrosine signal occurs as a continuous pattern between the basal body and the tip of the flagellum, indicating that T. brucei tyrosine-phosphorylated proteins are associated with, or are immediately adjacent to, the flagellum axoneme. However, part of the phosphotyrosine signal does not colocalize with the Rib72 stain but separates from the axoneme and occurs along the cell body (Fig. 7D). This observation suggested that phosphotyrosine-containing proteins could also be associated with membranous structures close to the flagellum in T. brucei. An axonemal location of phosphotyrosine proteins was also supported by flagellum extraction of procyclic form cells and staining with the anti-phosphotyrosine 4G10 antibody (Fig. 6E). The phosphotyrosine signal on the basal body and up the flagellum survived the high-salt treatment applied to obtain these isolated flagellum preparations, indicating that phosphotyrosine-containing proteins are tightly associated with structures forming the flagellum.
FIG. 7.
Phosphotyrosine-containing proteins are associated with the flagellum axoneme. Cytoskeleton preparations of procyclic form T. brucei (A) were colabeled with the anti-phosphotyrosine 4G10 antibody (B) and the anti-axonemal Rib72 antibody (C). The overlap of both staining patterns in most of the areas (D) strongly suggested that the anti-phosphotyrosine signal is axonemal. The area of the phosphotyrosine signal that did not colocalize with the Rib72 staining is indicated with arrows (D). White bar corresponds to a length of 5 μm.
To determine the subnuclear localization of phosphotyrosine-containing proteins observed by immunofluorescence microscopy (Fig. 4B), we used a fluorescent marker synthetic peptide [TRITC-D(R10)] that targets the nucleolus (22). This subcompartment of the nucleus was chosen for the colocalization studies because the nucleolar T. brucei protein NOPP44/46 (GeneDB accession no. Tb927.8.760) has been identified as having developmentally regulated tyrosine phosphorylation (11, 31). The nucleolus-specific TRITC-D(R10) label colocalized with the anti-phosphotyrosine signal in a distinct region of the nucleus (see Fig. S5 in the supplemental material), thus confirming previous evidence by the Parsons group of tyrosine-phosphorylated proteins in the nucleolus.
DISCUSSION
In this study, we used advanced MS-based phosphotyrosine-specific proteomics to shed light on the extent of protein tyrosine phosphorylation in T. brucei. We show that protein tyrosine phosphorylation is a relatively abundant posttranslational modification in an organism that lacks conventional tyrosine kinases (Nett et al., submitted for publication). Of the 34 phosphotyrosine-containing proteins we detected in this study, 19 protein kinases and 2 metabolic kinases (phosphoenolpyruvate carboxykinase and 6-phospho-1-fructokinase) could be identified, which suggests that in T. brucei tyrosine phosphorylation preferentially occurs on proteins that possess putative kinase activity. Of the 19 protein kinases, phosphorylation sites on 11 T. brucei homologues of Leishmania MAPKs could be identified. MAPK family members require phosphorylation of both a threonine residue and a tyrosine residue in the activation loop for full enzymatic activity of the kinase. We found the highly conserved TXY motif in the activation segment of MAPKs fully phosphorylated on eight of the identified putative TbMAPKs. Phosphorylation of the tyrosine residue allows the activation loop to adopt the active conformation, and subsequent phosphorylation of the threonine residue activates the kinase fully by promoting the correct alignment of the catalytic residues (17). Thus, the remaining putative TbMAPKs found to be phosphorylated on only the tyrosine residue, including the previously characterized TbMAPK2 (28), are likely to represent the kinase in an inactive state. These results suggest that the activation of MAPKs in this highly divergent parasite follows the same mechanistic principles as those in higher eukaryotes. The expression of phosphorylated STE kinases, which are thought to represent TbMAPK upstream activator proteins (29) in bloodstream form trypanosomes (Nett et al., submitted for publication), could be evidence for the existence of canonical signal transduction via MAPK in T. brucei.
Other protein kinases identified in this study include the cell division cycle 2 (Cdc2)-related protein kinases CRK1 (GeneDB accession no. Tb10.70.7040), CRK2 (GeneDB accession no. Tb927.7.7360), and CRK3 (GeneDB accession no. Tb10.70.2210), all of which were found to be tyrosine phosphorylated in their N terminus (Y16, Y57, and Y34, respectively). All three CRK isoforms have previously been detected phosphorylated on the same residues in our global phosphoproteome study of bloodstream form T. brucei (Nett et al., submitted for publication), suggesting that life cycle stage-specific tyrosine phosphorylation of these residues can be excluded. Interestingly, the phosphorylated tyrosine sites are flanked by an S15 in CRK1, S56 in CRK2, and T33 in CRK3, which could correspond to the conserved human CDK1 T14 and Y15 residues. Phosphorylation of the conserved Y15 of CDK1 by the Wee1 dual-specificity tyrosine kinase inactivates the CDK complex (20), and as a Wee1 kinase is encoded by the T. brucei genome, it is possible that CRKs in T. brucei are regulated by a similar mechanism.
Our detection of previously identified trypanosomal phosphorylation sites within the activation segments of both isoforms of the glycogen synthase kinase-3 (GeneDB accession no. Tb927.7.2420 and Tb10.61.3140) and a DYRK homologue (GeneDB accession no. Tb11.02.0640) (Nett et al., submitted for publication) strongly supports the view that known kinase-activating sites are conserved in T. brucei and indicates that several segments of known signal transduction pathways are conserved in this organism.
This study also provided an insight into the localization of tyrosine-phosphorylated proteins in both life cycle stages of T. brucei. Proteins carrying this posttranslational modification were found to be concentrated at the cytoskeleton, specifically with the axoneme and the basal bodies of the flagellum, as well as the nucleolar compartment of the parasite, as determined by immunofluorescence microscopy. This is in stark contrast to the localization pattern of tyrosine-phosphorylated proteins in mammalian cells, in which the signal for this posttranslational modification is concentrated at the plasma membrane in focal adhesion points that are known as locations for high tyrosine kinase activity (40). In these studies, it is difficult to correlate phosphotyrosine proteome data with localization data, since the former are based on the purification of phosphotyrosine-containing tryptic peptides, whereas the latter are based on antibody recognition in the context of folded and fixed proteins. Therefore, we cannot say what proportion of the phosphoproteome is associated with the cyctoskeletal and nucleolar compartments nor what subset of phosphoproteins might be specific for those locations. Nevertheless, the lack of conventional receptor tyrosine kinases and nonreceptor tyrosine kinases in T. brucei might be one aspect of the different staining patterns observed between trypanosomes and mammalian cells. The different locations of phosphotyrosine-containing proteins in the human system and the parasite might also directly reflect different functions of phosphotyrosine-based signaling in the organisms due to their substantially different requirements for survival.
Work by Das et al. (11) has identified a tyrosine-phosphorylated NOPP44/46 protein with a nucleolar localization in procyclic trypanosomes. This observation is consistent with our detection of a phosphotyrosine signal in the nucleolus of procyclic form cells by immunofluorescence. It remains unclear whether our microscopy studies also detected the NOPP44/46 protein, as it was not detected in our focused tyrosine phosphoproteomics screen of whole procyclic form cells. However, analysis of the NOPP44/46 amino acid sequence (GeneDB accession no. Tb927.8.760) reveals that, of the five tyrosine residues it contains, at least four of them are undetectable by MS. One is the C-terminal residue in the sequence RY (the tryptic product would be too small to detect), and three reside in tryptic peptides with masses of 6,947 Da (two sites) and 7,440 Da (one site) that are too large to provide meaningful MS-MS spectra. Only the N-terminal tyrosine residue (Y) in the sequence MEGFYGVEVSAGQKVK would be potentially detectable by our method. However, there is no guarantee that this is a phosphorylation site in NOPP44/46, and in any phosphoproteome analysis there is no guarantee that every occupied site will be detected. NOPP44/46 has been shown to bind nucleic acid (11), and RNA interference of the T. brucei-specific protein in the procyclic form affects large-subunit rRNA processing (19). This raises the interesting question of whether growth control and development of T. brucei are mediated by nucleolus-localized tyrosine-phosphorylated proteins, with ribosome synthesis as the obvious target of regulation. A more targeted phosphoprotomics study of T. brucei nucleoli could provide answers to the questions of how these nucleolus-localized tyrosine-phosphorylated proteins operate and if there is a connection to known signaling pathways.
The observation of tyrosine-phosphorylated proteins located on the basal body and along the axoneme of the flagellum extending to its distal tip is intriguing, as it suggests that signaling molecules could be associated with microtubules, which has been shown with mammalian cells (26). In fact, in mammalian cells, many kinases, such as MAPK, MEK1, MEK2, Raf1 (MEKK), CDK5, and CDC2, as well as many phosphatases, such as protein phosphatase 2A (PP2A), protein phosphatase 2B (PP2B), and protein phosphatase 1 (PP1), were detected in microtubule pellets, indicating that microtubule assembly and stability might be regulated by an interplay of these signaling molecules. The same study also revealed that the pool of microtubule-associated MAPK was constitutively active, which would be consistent with our observation that the phosphotyrosine signal along the flagellum and on the basal body was cell cycle independent.
Interestingly, a recent study of the green alga Chlamydomonas reinhardtii has shown that the tyrosine-phosphorylated active form of GSK3 was enriched in the flagellum (43). RNA interference of Chlamydomonas reinhardtii GSK3 resulted in cells that had no flagella, suggesting a role for GSK3 in assembly and maintenance of flagella, presumably through the regulation of intraflagellar transport. Our study showed that tyrosine-phosphorylated proteins are associated with the flagellum and the basal body, which is an organelle that provides a platform for recruiting proteins involved in intraflagellar transport (34). In addition, we identified TbGSK3 phosphorylated in its activation loop, suggesting that the kinase is active in the parasite. Both flagellum formation and basal body segregation are vital for T. brucei (4, 13), and our findings indicate that tyrosine-phosphorylated proteins might play a role in these essential processes.
Supplementary Material
Acknowledgments
We thank Keith Gull at the University of Oxford for providing the BBA4 and Rib72 monoclonal antibodies, Silvana van Koningsbruggen at the University of Dundee for the TRITC-D(R10) synthetic peptide, and Lucia Güther for procyclic cells. We also thank Sam Swift of the Centre for High Resolution Imaging and Processing at the University of Dundee for help with light microscopy.
I.R.E.N. was supported by a Wellcome Trust Prize Ph.D. studentship. M.A.J.F. is supported by a Wellcome Trust program grant (085622). L.D. is supported by an MRC program grant and the Dundee DSTT consortium (Astra Zeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Co., Merck KGaA, and Pfizer).
Footnotes
Published ahead of print on 30 January 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Bakalara, N., A. Seyfang, T. Baltz, and C. Davis. 1995. Trypanosoma brucei and Trypanosoma cruzi: life cycle-regulated protein tyrosine phosphatase activity. Exp. Parasitol. 81302-312. [DOI] [PubMed] [Google Scholar]
- 2.Bimbó, A., Y. Jia, S. L. Poh, R. K. Karuturi, N. den Elzen, X. Peng, L. Zheng, M. O'Connell, E. T. Liu, M. K. Balasubramanian, and J. Liu. 2005. Systematic deletion analysis of fission yeast protein kinases. Eukaryot. Cell 4799-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley, R., H. Tariq, H. McElhinney, B. Szöor, J. Huxley-Jones, R. Stevens, K. Matthews, and L. Tabernero. 2007. The TriTryp phosphatome: analysis of the protein phosphatase catalytic domains. BMC Genomics 8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broadhead, R., H. R. Dawe, H. Farr, S. Griffiths, S. R. Hart, N. Portman, M. K. Shaw, M. L. Ginger, S. J. Gaskell, P. G. McKean, and K. Gull. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440224-227. [DOI] [PubMed] [Google Scholar]
- 5.Brun, R., and Schönenberger. 1979. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36289-292. [PubMed] [Google Scholar]
- 6.Caenepeel, S., G. Charydczak, S. Sudarsanam, T. Hunter, and G. Manning. 2004. The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc. Natl. Acad. Sci. USA 10111707-11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, P. 2000. The regulation of protein function by multisite phosphorylation-a 25 year update. Trends Biochem. Sci. 25596-601. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, P. 2001. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 2685001-5010. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, P. 2002. Protein kinases-the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 1309-315. [DOI] [PubMed] [Google Scholar]
- 10.Cool, D. E., and J. J. Blum. 1993. Protein tyrosine phosphatase activity in Leishmania donovani. Mol. Cell. Biochem. 127143-149. [DOI] [PubMed] [Google Scholar]
- 11.Das, A., G. C. Peterson, S. B. Kanner, U. Frevert, and M. Parsons. 1996. A major tyrosine-phosphorylated protein of Trypanosoma brucei is a nucleolar RNA-binding protein. J. Biol. Chem. 27115675-15681. [DOI] [PubMed] [Google Scholar]
- 12.Davidge, J. A., E. Chambers, H. A. Dickinson, K. Towers, M. L. Ginger, P. G. McKean, and K. Gull. 2006. Trypanosome IFT mutants provide insight into the motor location for mobility of the flagella connector and flagellar membrane formation. J. Cell Sci. 1193935-3943. [DOI] [PubMed] [Google Scholar]
- 13.de Graffenried, C. L., H. H. Ho, and G. Warren. 2008. Polo-like kinase is required for Golgi and bilobe biogenesis in Trypanosoma brucei. J. Cell Biol. 181431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisen, J. A., R. S. Coyne, M. Wu, D. Wu, M. Thiagarajan, J. R. Wortman, J. H. Badger, Q. Ren, P. Amedeo, K. M. Jones, L. J. Tallon, A. L. Delcher, S. L. Salzberg, J. C. Silva, B. J. Haas, W. H. Majoros, M. Farzad, J. M. Carlton, R. K. Smith, Jr., J. Garg, R. E. Pearlman, K. M. Karrer, L. Sun, G. Manning, N. C. Elde, A. P. Turkewitz, D. J. Asai, D. E. Wilkes, Y. Wang, H. Cai, K. Collins, B. A. Stewart, S. R. Lee, K. Wilamowska, Z. Weinberg, W. L. Ruzzo, D. Wloga, J. Gaertig, J. Frankel, C. C. Tsao, M. A. Gorovsky, P. J. Keeling, R. F. Waller, N. J. Patron, J. M. Cherry, N. A. Stover, C. J. Krieger, C. del Toro, H. F. Ryder, S. C. Williamson, R. A. Barbeau, E. P. Hamilton, and E. Orias. 2006. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 4e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis, J., M. Sarkar, E. Hendriks, and K. Matthews. 2004. A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal extension. Mol. Microbiol. 531487-1499. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg, J. M., G. Manning, A. Liu, P. Fey, K. E. Pilcher, Y. Xu, and J. L. Smith. 2006. The dictyostelium kinome-analysis of the protein kinases from a simple model organism. PLoS Genet. 2e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9576-596. [PubMed] [Google Scholar]
- 18.Hunter, T., and G. D. Plowman. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem. Sci. 2218-22. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, B. C., D. L. Brekken, A. C. Randall, C. T. Kifer, and M. Parsons. 2005. Species specificity in ribosome biogenesis: a nonconserved phosphoprotein is required for formation of the large ribosomal subunit in Trypanosoma brucei. Eukaryot. Cell 430-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krupa, A., G. Preethi, and N. Srinivasan. 2004. Structural modes of stabilization of permissive phosphorylation sites in protein kinases: distinct strategies in Ser/Thr and Tyr kinases. J. Mol. Biol. 181025-1039. [DOI] [PubMed] [Google Scholar]
- 21.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 2981912-1934. [DOI] [PubMed] [Google Scholar]
- 22.Martin, R. M., G. Tünnemann, H. Leonhardt, and M. C. Cardoso. 2007. Nucleolar marker for living cells. Histochem. Cell Biol. 127243-251. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Oyanedel, J., I. W. McNae, M. W. Nowicki, J. W. Keillor, P. A. Michels, L. A. Fothergill-Gilmore, and M. D. Walkinshaw. 2007. The first crystal structure of phosphofructokinase from a eukaryote: Trypanosoma brucei. J. Mol. Biol. 3661185-1198. [DOI] [PubMed] [Google Scholar]
- 24.Miranda-Saavedra, D., M. J. Stark, J. C. Packer, C. P. Vivares, C. Doerig, and G. J. Barton. 2007. The complement of protein kinases of the microsporidium Encephalitozoon cuniculi in relation to those of Saccharomyces cerevisiae and Schizosaccharomyces pombe. BMC Genomics 8309-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyata, Y., and E. Nishida. 1999. Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem. Biophys. Res. Commun. 266291-295. [DOI] [PubMed] [Google Scholar]
- 26.Morishima-Kawashima, M., and K. S. Kosik. 1996. The pool of map kinase associated with microtubules is small but constitutively active. Mol. Biol. Cell 7893-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison, D. K., M. S. Murakami, and V. Cleghon. 2000. Protein kinases and phosphatases in the Drosophila genome. J. Cell Biol. 150F57-F62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller, I. B., D. Domenicali-Pfister, I. Roditi, and E. Vassella. 2002. Stage-specific requirement of a mitogen-activated protein kinase by Trypanosoma brucei. Mol. Biol. Cell 133787-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons, M., E. A. Worthey, P. N. Ward, and J. C. Mottram. 2005. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons, M., M. Valentine, J. Deans, G. L. Schieven, and J. A. Ledbetter. 1991. Distinct patterns of tyrosine phosphorylation during the life cycle of Trypanosoma brucei. Mol. Biochem. Parasitol. 45241-248. [DOI] [PubMed] [Google Scholar]
- 31.Parsons, M., J. A. Ledbetter, G. L. Schieven, A. E. Nel, and S. B. Kanner. 1994. Developmental regulation of pp44/46, tyrosine-phosphorylated proteins associated with tyrosine/serine kinase activity in Trypanosoma brucei. Mol. Biochem. Parasitol. 6369-78. [DOI] [PubMed] [Google Scholar]
- 32.Plowman, G. D., S. Sudarsanam, J. Bingham, D. Whyte, and T. Hunter. 1999. The protein kinases of Caenorhabditis elegans: a model for signal transduction in multicellular organisms. Proc. Natl. Acad. Sci. USA 9613603-13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee, S. G. 2006. H2O2, a necessary evil for cell signaling. Science 3121882-1883. [DOI] [PubMed] [Google Scholar]
- 34.Scholey, J. M. 2003. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19423-443. [DOI] [PubMed] [Google Scholar]
- 35.Stuart, K., R. Brun, S. Croft, A. Fairlamb, Gürtler, R. E. J. McKerrow, S. Reed, and R. Tarleton. 2008. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Investig. 1181301-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szöor, B., J. Wilson, H. McElhinney, L. Tabernero, and K. R. Matthews. 2006. Protein tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in trypanosomes. J. Cell Biol. 175293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu, X., J. Mancuso, W. Z. Cande, and C. C. Wang. 2005. Distinct cytoskeletal modulation and regulation of G1-S transition in the two life stages of Trypanosoma brucei. J. Cell Sci. 1184353-4364. [DOI] [PubMed] [Google Scholar]
- 38.Tu, X., and C. C. Wang. 2005. Coupling of posterior cytoskeletal morphogenesis to the G1/S transition in the Trypanosoma brucei cell cycle. Mol. Biol. Cell 1697-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu, X., and C. C. Wang. 2005. Pairwise knockdowns of cdc2-related kinases (CRKs) in Trypanosoma brucei identified the CRKs for G1/S and G2/M transitions and demonstrated distinctive cytokinetic regulations between two developmental stages of this organism. Eukaryot. Cell 4755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuori, K. 1998. Integrin signaling: tyrosine phosphorylation events in focal adhesions. J. Membr. Biol. 165191-199. [DOI] [PubMed] [Google Scholar]
- 41.Ward, P., L. Equinet, J. Packer, and C. Doerig. 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiese, M. 2007. Leishmania MAP kinases-familiar proteins in an unusual context. Int. J. Parasitol. 371053-1062. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, N. F., and P. A. Lefebvre. 2004. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot. Cell 31307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirtz, E., S. Leal, C. Ochatt, and G. A. Cross. 1999. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 9989-101. [DOI] [PubMed] [Google Scholar]
- 45.Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines, and K. Gull. 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93491-500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.