Abstract
Calcineurin is a conserved protein phosphatase that plays a critical role in Ca2+ signaling and stress responses. Previously, a new class of conserved calcineurin-binding proteins, the calcipressins, was identified. However, the role of these proteins remains controversial, and both inhibitory and stimulatory effects on calcineurin were observed. In this study, we investigate the role of CbpA, the Aspergillus fumigatus member of the calcipressin family, and report that deletion of the cbpA gene resulted in reduced hyphal growth and limited attenuated virulence. Interestingly, under high-calcium-level conditions, the ΔcbpA strain displayed improved Ca2+ tolerance compared to the wild-type strain and revealed increased expression of vcxA, chsA, and cnaA, which encode the vacuolar Ca2+/H+ exchanger VcxA, chitin synthase A, and the calcineurin catalytic subunit CnaA, respectively. The increased transcript levels of these three genes were reversed in the presence of the calcineurin inhibitor FK506, indicating a calcineurin-dependent mechanism. Overexpression of cbpA resulted in decreased transcription of vcxA, chsA, and cnaA, associated with wild-type sensitivity to Ca2+. Taken together, our study highlights the importance of CbpA in the regulation of hyphal growth and calcium adaptation of A. fumigatus and provides evidence that CbpA may serve as a feedback inhibitor in some aspects of calcineurin functions.
Calcineurin is a conserved Ca2+/calmodulin-dependent, serine-threonine-specific phosphatase which mediates a broad range of cellular functions, including T-cell activation, neurite extension (8), long-term memory (19), and cardiac hypertrophy (21). For many of these physiological processes, calcineurin exerts its effects by dephosphorylating the nuclear factor of activated T cells (NFAT), resulting in translocation of NFAT to the nucleus and induction of calcineurin-dependent genes (4). The protein phosphatase activity of calcineurin is activated upon association with Ca2+-bound calmodulin, which displaces the autoinhibitory domain and allows substrates access to the catalytic site.
Due to the diversity of the pathways regulated by calcineurin, it is not surprising that several endogenous regulators other than Ca2+/calmodulin have recently been identified, including a novel family of calcineurin-binding proteins, the calcipressins, which are conserved from yeasts (Saccharomyces cerevisiae) to humans (13, 26). The founding member of this protein family, DSCR1 (also known as Adapt78 or MCIP1), is located on chromosome 21 in an area termed the Down syndrome candidate region (DSCR), and overexpression of DSCR1 is associated with Down syndrome and Alzheimer's disease (25). In humans, two additional calcipressin genes, DRCR1L1 (MCIP2) and DSCR1L2 (MCIP 3), have been identified (26). Previous studies have shown that DSCR1 and DSCR1L1 inhibit the phosphatase activity of calcineurin by binding to a region between the catalytic and calcineurin B-binding domains of the calcineurin A subunit (11). However, DSCR1 is unique among all members of the calcipressin family, as it is transcriptionally upregulated by calcineurin activity (13) and can therefore function in a negative feedback loop of the calcineurin pathway (37). Most of the described calcineurin regulators show an inhibitory effect either on the phosphatase activity of calcineurin, as in the case of AKAP79 and CHP (14), or on the translocation of its target NFAT, as observed with Cabin1/Cain (18). However, the mechanism of the DSCR protein family remains controversial. While DSCR1 can bind to the calcineurin A subunit and thereby inhibit its phosphatase activity, some cardiac studies revealed that DSCR1 can also facilitate the calcineurin pathway, depending on the nature of the stimulus (13, 24, 35).
The importance of this novel protein family is further emphasized by the existence of orthologs in yeasts. In Cryptococcus neoformans, Cbp1 has been identified as a member of the calcipressin family, which is required for mating but not for growth at 37°C (9). In Saccharomyces cerevisiae, overexpression of the homolog Rcn1 inhibits two independent functions of calcineurin: the induction of the transcription factor Tcn1 and the inhibition of the Ca2+/H+ exchanger Vcx1. However, the rcn1 deletion mutant also exhibits reduced calcineurin activity, indicating that similar to the human ortholog DSCR1, Rcn1 can both inhibit and stimulate the calcineurin pathway (13).
To investigate the physiological role of the A. fumigatus calcipressin CbpA, we deleted cbpA by homologous recombination. The ΔcbpA strain displayed reduced hyphal growth and a modest reduction in virulence in a murine inhalational model of invasive aspergillosis. The cbpA deletion phenotype also includes calcineurin-dependent increased transcription of the vacuolar Ca2+/H+ exchanger gene vcxA, associated with improved calcium tolerance. Overexpression of cbpA resulted in decreased expression of vcxA, consistent with the model of negative feedback on calcineurin function.
MATERIALS AND METHODS
Strains, media, and growth conditions.
A. fumigatus wild-type strain AF293 and its uracil-uridine auxotroph mutant AF293.1 (23) were used in all experiments described below. All A. fumigatus cultures were grown on glucose minimal medium (GMM) (27) at 37°C unless otherwise specified. For inducible-promoter experiments, a modified Aspergillus minimal medium that contained 1% (wt/vol) glucose as the sole carbon source and 20 mM Mg(NO3)2 as the sole nitrogen source for promoter induction was used (12). For routine cloning, Escherichia coli DH5α competent cells (New England Biolabs, Ipswich, MA) were grown in Luria-Bertani broth (Fisher Scientific, Pittsburg, PA) supplemented with 50 μg/ml carbenicillin (Sigma-Aldrich, St. Louis, MO) at 37°C.
Generation of ΔcbpA and complemented (ΔcbpA+cbpA) strains of A. fumigatus.
A gene replacement construct was designed to replace the entire cbpA coding region with the Aspergillus parasiticus pyrG gene by homologous recombination. Approximately 1.2 kb of the 5′ flanking region and 1.1 kb of the 3′ flanking region of cbpA were amplified and cloned into the plasmid pJW24 (a gift from Nancy Keller, University of Wisconsin, Madison, WI), containing the 3.1-kb A. parasiticus pyrG gene. The replacement construct was used as a PCR template to create a 5.4-kb fragment for transformation into A. fumigatus strain AF293.1. Protoplast transformations were performed as previously described (3, 5, 6, 29), with slight modifications. Briefly, conidia were inoculated into 250 ml GMM broth and incubated for 16 h at 28°C. The germinated spores were filtered and mixed with 40 ml osmotic medium (1.2 M MgSO4, 10 mM sodium phosphate buffer) and 200 mg lysing enzymes (Sigma-Aldrich, St. Louis, MO). After 4 h of incubation at 28°C at 50 rpm, the protoplasts were captured with 20 ml of trapping buffer (0.6 M sorbitol, 0.1 M Tris-HCl [pH 7]) and centrifuged at 5,000 rpm for 15 min. The protoplasts were removed from the interface and resuspended in STC (1.2 M sorbitol, 10 mM CaCl2, 10 mM Tris-HCl [pH 7.5]). For transformation, 110 μl of protoplasts was mixed with 2.5 μg of the PCR product and 50 μl of 60% polyethylene glycol 3350 (Sigma-Aldrich, St. Louis, MO). After incubation on ice for 30 min, an additional 950 μl of polyethylene glycol and 50 μl of 1 M CaCl2 were added, mixed gently, and incubated at room temperature for 20 min. The protoplasts were collected by centrifugation at 13,000 rpm for 5 min, resuspended in 2 ml STC, and spread onto GMM plates supplemented with 1.2 M sorbitol and 1% yeast extract. Transformants were selected for growth in the absence of uracil-uridine supplementation. Replacement of cbpA was confirmed by Southern analysis using the digoxigenin PCR labeling system (Roche Applied Science, Indianapolis, IN) and a probe for the 5′ cbpA flanking sequence.
To complement cbpA at its native locus, a construct was designed according to the method of Bhabhra et al. (2) to undergo a single crossover insertion adjacent to the disruption site. The complement allele, which used pTOPO 2.1 (Invitrogen, Carlsbad, CA) as the vector, contained the entire cbpA gene plus a flanking sequence 2.5 kb upstream and 500 bp downstream and the hygromycin B resistance gene cassette (hph) (33). The single crossover event was expected to occur between the PacI-linearized vector and the PacI site in the promoter region of the cbpA mutant allele (see Fig. 2B). Transformants were screened for growth in the presence of hygromycin B, and complementation of cbpA was verified by Southern analysis as described above.
FIG. 2.
Replacement and complementation of the cbpA gene. (A). Schematic representation of the genomic locus of the wild-type and ΔcbpA strains. The cbpA gene was replaced with the pyrG gene by homologous recombination. (B) Complementation of cbpA resulting from a single crossover between the PacI site in the promoter region of the cbpA mutant allele and the PacI-linearized vector, containing the entire cbpA gene plus a 2-kb 5′ and 500-bp 3′ flanking sequence. (C) Southern analysis with EcoRV-digested genomic DNA and the cbpA left flank probe (P). The appearance of a 2.9-kb fragment in the ΔcbpA strain indicated successful gene replacement of cbpA by pyrG. The regeneration of the 3.5-kb wild-type (WT) EcoRV fragment and the appearance of a 7-kb EcoRV fragment comprising the pCB1636 plasmid sequence confirmed a successful reintroduction of cbpA into the ΔcbpA background at the native locus.
Radial growth and conidiation analyses.
For both radial growth and conidiation analyses, starting cultures of 1 × 104 conidia of wild-type, ΔcbpA, and ΔcbpA+cbpA strains were grown on GMM agar at 37°C. For radial growth analysis, the colony diameters were determined every 24 h over a period of 5 days. To analyze conidiation, conidia were harvested after 96 h of incubation and quantified as the total numbers per ml and per mm2 of growth. Radial growth analysis under calcium stress was performed in the presence of 200 mM CaCl2 with or without the calcineurin inhibitor FK506 at a subinhibitory concentration (5 ng/ml). All experiments were performed in triplicate.
Gene expression analysis by real-time reverse transcription-PCR (RT-PCR).
A final concentration of 1 × 106 conidia/ml of wild-type and ΔcbpA strains was inoculated into 10 ml GMM broth and incubated at 37°C for 18 h. After 20 min of pretreatment with FK506 (20 ng/ml), CaCl2 was added to give a final concentration of 200 mM. The liquid cultures were incubated for an additional 20 min prior to RNA extraction. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) and Qiagen RNeasy columns (Qiagen, Inc., Valencia, CA) and was treated with DNase I (Ambion, Austin, TX). Approximately 500 ng of the RNA was subjected to first-strand cDNA synthesis (Invitrogen, Carlsbad, CA). Real-time PCR assays were performed with 20-μl reaction volumes that contained 1× iQ SYBR green master mix, 0.2 μM of each primer (see the supplemental material), and 2 μl of a 1:5 dilution of the cDNA. Real-time PCRs from one representative experiment were performed in duplicate, and the expression levels of all genes of interest were normalized to β-tubulin levels. For each sample, the 2−ΔΔCt method of analysis was used to determine the changes in gene expression.
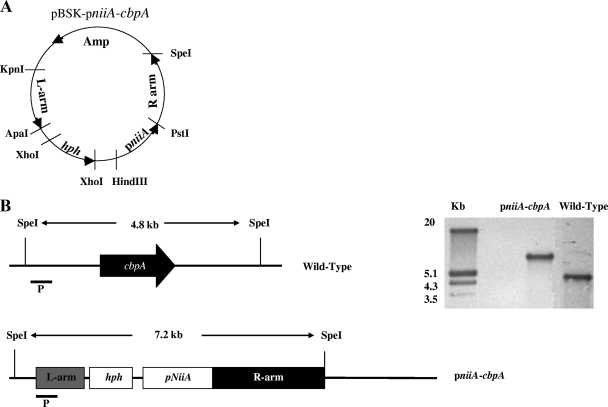
Construction of the pniiA-cbpA strain.
The cbpA endogenous promoter was replaced by the A. fumigatus nitrate-inducible promoter according to the method of Hu et al. (12) (see Fig. 5A). Briefly, 1.2 kb of the 5′ flanking sequence of cbpA was amplified by PCR from AF293 genomic DNA. The PCR product (L arm) was digested with ApaI and KpnI and cloned into pBluescript II SK(−) at the ApaI/KpnI sites. The hygromycin B resistance cassette was amplified from plasmid pCB1636 (34), digested with XhoI, and subcloned into pBluescript II SK(−). The pniiA sequence was amplified from AF293 genomic DNA, digested with HindIII and PstI, and cloned into pBluescript at the HindIII/PstI sites. Approximately 1.5 kb from the ATG start codon of the cbpA gene was amplified (R arm), digested with PstI and SpeI, and cloned into the pniiA side of the plasmid. The resulting pniiA-cbpA cassette was used as a PCR template, and 2.5 μg of the obtained PCR product was transformed into the wild-type strain. Replacement of the cbpA endogenous promoter was confirmed by Southern analysis using a probe for the 5′ cbpA flanking sequence.
FIG. 5.
Generation of the pniiA-cbpA strain. (A) The promoter replacement construct comprises the hygromycin B resistance cassette, 1.2 kb upstream (L arm), 1.5 kb downstream from the predicted ATG start codon of the cbpA gene (R arm), and 1 kb of the nitrate-inducible promoter sequence (pniiA). (B) Southern analysis of SpeI-digested genomic DNA using the left flank probe (P) displayed an expected 4.8-kb fragment in the wild-type strain and a 7.2-kb fragment in the pniiA-cbpA mutant, confirming successful replacement of the cbpA endogenous promoter by the nitrate-inducible promoter pniiA.
Murine inhalational model of invasive aspergillosis.
Briefly, 6-week-old CD1 male mice (Charles River Laboratories) were immunosuppressed with cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ) at 150 mg/kg of body weight intraperitoneally on days −2 and +3 of infection and with triamcinolone acetonide (Kenalog-40; Bristol-Myers Squibb, Princeton, NJ) at 40 mg/kg subcutaneously on days −1 and +6 of infection as previously described (6, 29). Mice were housed under sterile conditions and supplied with sterile drinking water supplemented with vancomycin (1 mg/ml), gentamicin (0.2 mg/ml), and clindamycin (1 mg/ml). In a Hinners inhalational chamber, four groups of 20 unanesthetized mice were each exposed by inhalation to 40 ml of an aerosolized suspension (1 × 109 conidia/ml) containing the AF293 wild-type, ΔcbpA mutant, and ΔcbpA+cbpA strains or a diluent control (0.05% Tween 80) for 30 min. The animals were evaluated for morbidity and mortality in a blinded fashion twice daily. Survival was plotted on a Kaplan-Meier curve for each Aspergillus strain, and the log rank test was used for pairwise comparison of survival (GraphPad Prism 5.0; San Diego, CA). Statistical significance was determined by a two-tailed test and set at P values of <0.05.
Histopathologic evaluation of fungal burden.
To evaluate in vivo histopathologic progression of disease, four groups of four additional mice were similarly infected in the murine inhalational model with each strain and euthanized at defined time points (days 4 and 7 after infection). Lungs were harvested and embedded in 10% neutral buffered formalin prior to being sectioned and stained with Gomori's methenamine silver and hematoxylin-eosin to characterize inflammation and fungal invasion. A veterinary pathologist evaluated each lung section in a blinded fashion to quantify infection according to a five-point pulmonary infarct score that incorporated necrosis, hemorrhage, edema, and hyphal presence.
RESULTS
Identification of A. fumigatus CbpA.
The putative A. fumigatus homolog of S. cerevisiae Rcn1 was identified by a BLASTP search using the full-length Rcn1 protein sequence (YKL159C). The result revealed four matches, with Afu2g13060 showing the conserved FxISPPxSPP motif, present in all members of the calcipressin family of calcineurin-binding proteins (13). The reciprocal BLASTP search against the S. cerevisiae genome database revealed the yeast protein YKL159C as the best match, suggesting that Afu2g13060 is the putative A. fumigatus homolog of Rcn1. The potential homolog Afu2g13060, designated CbpA, is a small protein of 288 amino acids that shares a 31% identity with S. cerevisiae Rcn1. The ClustalW alignment (http://www.ebi.ac.uk/clustalw/) of CbpA and related proteins from Saccharomyces cerevisiae, Cryptococcus neoformans, and Homo sapiens displayed the highest sequence conservation in the central span of about 20 amino acids, containing two conserved serine-proline repeats in the motif FxISPPxSPP (Fig. 1). Unlike the mammalian calcipressins, the RNA recognition motif (32), comprising approximately 80 amino acids located near the N terminus, is absent in fungi. Analysis of the promoter region of the cbpA gene has revealed a single calcineurin dependent-response element, AGCCTC (15), and a stress-responsive element, AGGGGA (10) (data not shown).
FIG. 1.
Multiple alignment of Aspergillus fumigatus CbpA with predicted homologs from Homo sapiens, Cryptococcus neoformans, and Saccharomyces cerevisiae. Identical residues are in black, and similar amino acids are in gray. The most conserved central motif is underlined.
Characterization of the ΔcbpA strain.
To assess the physiological function of CbpA, a ΔcbpA strain was generated by replacing the entire cbpA coding region with the A. parasiticus pyrG gene (Fig. 2A). Replacement of cbpA by pyrG was verified by Southern analysis with a probe for the 5′ flanking region outside the disruption allele (Fig. 2C). To complement cbpA at its native locus, a construct was designed to undergo a single crossover insertion adjacent to the disruption site (Fig. 2B). Southern analysis revealed the regeneration of the wild-type 3.5-kb EcoRV fragment and the appearance of a 7-kb EcoRV fragment in the complemented allele, indicating that cbpA was reintroduced at its native locus (Fig. 2C).
Radial growth analysis on minimal medium showed that the hyphal growth of the ΔcbpA strain was decreased compared to that of the wild-type and the ΔcbpA+ΔcbpA strains (Fig. 3A and B), but not as severely as previously observed in the calcineurin A (ΔcnaA) mutant and the zinc finger transcription factor (ΔcrzA) mutant (6, 29). Although analysis of conidiation revealed similar numbers of harvested conidia per ml, the ΔcbpA strain showed an approximately double density when analyzed for numbers of conidia per mm2 of growth (data not shown). Increased densities of conidia per mm2 were also seen in the ΔcnaA and ΔcrzA strains (data not shown), conceivably a stress response to the lack of these genes. Despite the reduced hyphal growth, the ΔcbpA strain did not reveal any aberration in the morphology of hyphae or conidia (data not shown).
FIG. 3.
Hyphal growth analysis of the wild-type, ΔcbpA, and ΔcbpA+cbpA strains. (A) Culture morphology of the wild-type, ΔcbpA, and ΔcbpA+cbpA strains on GMM after 4 days of growth at 37°C. (B) The ΔcbpA strain displayed an approximately 30% decrease in radial growth over a 5-day time course compared with the wild-type and complemented strains.
To examine whether the reduced radial growth of the ΔcbpA strain was attributable to defects in the biosynthesis of cell wall components, we quantified the amounts of β-1,3-glucan and chitin in the ΔcbpA strain compared to those in the wild-type strain as described in references 16 and 30. The results showed no significant difference in β-1,3-glucan and chitin content between the two strains (data not shown), indicating that CbpA is involved in hyphal growth but plays only limited roles in asexual development and cell wall synthesis.
The ΔcbpA strain displayed improved calcium tolerance.
To investigate the role of CbpA in the calcineurin pathway, we compared the radial growths of the wild-type and the ΔcbpA strains under high-calcium-level conditions (200 mM CaCl2) in the presence or absence of the calcineurin inhibitor FK506. Surprisingly, despite the reduced hyphal growth on minimal medium, when transferred to medium containing CaCl2, the ΔcbpA strain exhibited a greater radial hyphal growth than the wild-type strain (Fig. 4). The increased calcium tolerance of the ΔcbpA strain was completely reversed in the presence of FK506, suggesting that deletion of cbpA contributed to calcium tolerance in a calcineurin-dependent manner.
FIG. 4.
Hyphal growth in the presence of CaCl2. A total of 1 × 104 conidia were inoculated onto GMM plates containing CaCl2 (200 mM) with or without the calcineurin inhibitor FK506 (5 ng/ml). The radial growth was documented after 72 h at 37°C. The experiment was performed in triplicate and repeated three times with similar results. WT, wild type.
To better understand the basis of increased calcium tolerance of the ΔcbpA strain, we examined the transcriptional response to calcium stress, focusing on nine genes involved in cell wall biosynthesis, calcium homeostasis, and the calcineurin pathway. Real-time RT-PCR data revealed that five genes showed >2-fold changes in expression level in the ΔcbpA strain in response to Ca2+ (Table 1). One of these genes is vcxA, which encodes the vacuolar Ca2+/H+ exchanger VcxA. Although an increased transcription level of vcxA was also observed in the wild-type strain under calcium stress, this occurred to a much lesser extent and was apparently not sufficient to improve phenotypic calcium tolerance in the wild-type strain. In both strains, the increased transcription of vcxA was diminished in the presence of the calcineurin inhibitor FK506, indicating that the transcriptional regulation of vcxA occurs, at least in part, via a calcineurin-dependent mechanism. Interestingly, an increased expression level in the calcineurin A (cnaA) gene was observed in the ΔcbpA strain, suggesting that CbpA may be involved in feedback regulation of calcineurin. Of all examined cell wall-related genes, only the chitin synthase A (chsA) gene had an increased transcription level in the ΔcbpA strain in a calcineurin-dependent manner. However, despite the increased transcription of chsA in the ΔcbpA strain, the chitin content in the ΔcbpA strain under calcium stress remained similar to that in the wild-type strain (data not shown). The ΔcbpA strain also showed increased transcript levels of fksA and chsC, which encode the β-1,3-glucan synthase and chitin synthase C, respectively. However, this occurred more likely via a calcineurin-independent mechanism, as the observed increased transcription was not reversed by FK506. Despite the increased transcription of fksA, the ΔcbpA strain displayed a hyphal morphology similar to that of the wild-type strain in the presence of the β-1,3-glucan synthase inhibitor caspofungin (data not shown).
TABLE 1.
Profiles of gene expression in response to Ca2+
| Gene | Function | Fold expression changea
|
|||
|---|---|---|---|---|---|
| Ca2+
|
Ca2+-FK506
|
||||
| Wild type | ΔcbpA | Wild type | ΔcbpA | ||
| cnaA | Calcineurin catalytic subunit | 1.52 | 4 | 1.74 | 0.59 |
| vcxA | Vacuolar H+/Ca2+ exchanger | 4.76 | 111.43 | 0.24 | 38.05 |
| chsA | Chitin synthase A | 0.73 | 25.99 | 0.01 | 4 |
| chsC | Chitin synthase C | 0.57 | 3.03 | 0.17 | 4 |
| fksA | β-1,3-Glucan synthase catalytic subunit | 1.52 | 3.73 | 0.2 | 21.86 |
Data shown are relative to the levels for the corresponding nontreated strains and are averages for duplicates from one representative experiment.
Overexpression of cbpA decreased the expression of vcxA, chsA, and cnaA in response to CaCl2.
To provide further support for the involvement of CbpA in the transcriptional regulation of vcxA, chsA, and cnaA, we then monitored the expression of these three genes under overexpression of cbpA. We placed cbpA under the control of the nitrogen-regulated promoter of the A. fumigatus nitrite reductase gene, niiA, which is induced by nitrate in the absence of the repressor ammonium (1, 22). The cbpA endogenous promoter was replaced by the nitrate-inducible promoter by homologous recombination (Fig. 5A), and the replacement was confirmed by Southern analysis (Fig. 5B). Real-time RT-PCR revealed a fivefold increase in transcript levels of cbpA under inducing conditions, confirming that conditional overexpression of cbpA was achieved. The increased expression of cbpA resulted in decreased transcription of vcxA, cnaA, and chsA in response to Ca2+ [0.3-, 0.29-, and 0.59-fold, respectively, relative to the levels for the wild-type strain in GMM-Mg(NO3)2-CaCl2; these results are averages for duplicates from one representative experiment].
CbpA plays a limited role in pathogenicity in a murine model of invasive pulmonary aspergillosis.
The virulence of the ΔcbpA strain in an inhalational murine model of pulmonary invasive aspergillosis was investigated as previously described (6, 29). Infection with the wild-type strain showed 90% mortality by 14 days postinfection, whereas infection with the ΔcbpA strain resulted in a moderate reduction in mortality (Fig. 6A). Although the reduced mortality reached statistical significance (P = 0.02), histopathological analysis of infected lung tissue revealed a massive hyphal invasion and extensive inflammatory responses in mice infected with the ΔcbpA strain, similar to what was found for the wild-type and ΔcbpA+cbpA strains (Fig. 6B).
FIG. 6.
CbpA plays a limited role in pathogenesis of A. fumigatus. (A) Kaplan-Meier survival curve. Infection with the wild-type strain resulted in 90% mortality by 14 days postinfection, while infection with the ΔcbpA mutant displayed a moderate reduction in mortality (P = 0.02). (B) Lung histopathology at day 7 postinfection. (Top row) Gomori's methenamine silver staining demonstrated extensive hyphal proliferation in the lung tissues of mice infected with the wild-type, ΔcbpA, and cbpA complemented strains. (Bottom row) Hematoxylin and eosin staining shows similar necrosis and inflammation levels in the three strains. Magnification, ×400.
DISCUSSION
Recent studies of the molecular mechanisms of fungal morphogenesis and virulence have revealed an essential role for calcineurin in pathogenic fungi. In the human pathogens C. neoformans, Candida albicans, and A. fumigatus, calcineurin regulates morphogenesis and is required for virulence in animal models, making it an attractive antifungal target (31). The calcineurin inhibitors FK506 and cyclosporine A have been highly successful in modern transplantation medicine; however, systemic toxicity and pathobiology of calcineurin-dependent signaling (36) may preclude the administration of these calcineurin inhibitors for treatment of patients with invasive fungal infections. Thus, an increased understanding of the molecular mechanisms of the calcineurin pathway is essential and may open new opportunities for preventive and therapeutic intervention by a more appropriate drug-targeting design. Within the last decade, several endogenous inhibitors of calcineurin have been identified, including a novel conserved family of calcineurin-binding proteins, the calcipressins (13, 26). In this study, we identified the A. fumigatus ortholog CbpA and characterized its role in calcineurin signaling. Deletion of the corresponding gene cbpA resulted in reduced hyphal growth, but not as severely as observed in the previously reported ΔcnaA and ΔcrzA strains (6, 29). Surprisingly, unlike the ΔcnaA and ΔcrzA strains, the ΔcbpA strain displayed a higher tolerance to Ca2+ than the wild type.
The increased calcium tolerance could be the result of alteration in either the transport or the storage of these ions. Expression analysis of genes involved in calcium transport revealed a greater increase in transcript levels of the vacuolar Ca2+/H+ exchanger gene vcxA in the ΔcbpA strain than in the wild type. The increased transcription of vcxA was diminished in the presence of FK506, indicating that transcriptional regulation of vcxA is, at least in part, calcineurin dependent. In contrast, the expression levels of pmrA and pmcA, which encode the P-type Ca2+ ATPases PmrA and PmcA, remained virtually unchanged in the wild-type and ΔcbpA strains (data not shown). Our data are in concordance with a study of S. cerevisiae which revealed that Vcx1 can catalyze Ca2+ sequestration into the vacuole more efficiently than Pmc1 when exposed to a sudden pulse of increased cytosolic free Ca2+ (20). However, although the functions of the vacuole Ca2+/H+ exchanger appear to be similar in filamentous fungi and yeast, a significant difference is observed between A. fumigatus and S. cerevisiae regarding the regulation of this Ca2+ pump. While the S. cerevisiae vacuole Ca2+/H+ exchanger Vcx1 is inhibited by calcineurin, probably via a posttranslational mechanism (13), we observed a stimulatory effect of calcineurin on VcxA at the transcriptional level. This finding is correlated with the data obtained from Aspergillus nidulans (28). In that study, the expression of vcxA was increased in the presence of CaCl2 in a calcineurin/crzA-dependent manner. It was also shown that the ΔcrzA strain displayed reduced expression of vcxA, associated with increased sensitivity to Ca2+. Thus, it is most likely that the observed enhanced calcium tolerance of the ΔcbpA strain is the result of the increased activity of VcxA. The increased transcript levels of vcxA in the ΔcbpA strain indicate an inhibitory effect of CbpA on this calcineurin-dependent gene. Additional support for a negative role for CbpA in calcineurin regulation of vcxA is provided by the finding that the expression of this gene was decreased when CbpA was overexpressed. The question remains, why should the cells inhibit the activity of VcxA when this Ca2+ pump can provide improved tolerance during calcium stress? One possible explanation is that downregulation of VcxA function would propagate Ca2+ signals, allowing optimal induction of crucial Ca2+-mediated signaling pathways. Thus, the hyphal growth rate during calcium stress may be compromised in order to maintain other essential Ca2+-dependent functions, such as protein processing and folding.
Unlike the C. neoformans calcipressin Cbp1, which did not significantly affect the expression of calcineurin (9), A. fumigatus CbpA may be involved in the transcriptional regulation of calcineurin, as judged from the increased transcription of the calcineurin catalytic subunit gene, cnaA, in the ΔcbpA strain in response to Ca2+. Further support for this hypothesis is provided by the observation that the transcription of cnaA was reduced when cbpA was overexpressed. However, it is also possible that the effect of cbpA deletion or overexpression may lead to indirect effects in the cells that altered the cnaA transcriptional response. Additional studies are needed to elucidate the physiological function of CbpA in the regulation of the calcineurin signaling pathway in A. fumigatus.
The in vivo virulence study using the inhalational murine model revealed a modest reduction in mortality in animals infected with the ΔcbpA strain. The attenuated virulence could be the result of enhanced activity of calcineurin caused by the loss of its potential endogenous inhibitor CbpA. One possible consequence of the unrestrained calcineurin activity is an increased activity level of the vacuolar Ca2+/H+ exchanger VcxA, which would lead to prolonged sequestration of Ca2+ into the vacuole and decrease the availability of free cytosolic Ca2+, required for induction of essential Ca2+-dependent signaling pathways. A connection between Ca2+ signaling, stress tolerance, and virulence was observed in the C. neoformans mutant lacking the EcaI gene, which encodes the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (7). The ecaI deletion mutant displayed attenuated virulence at 37°C in four different host models, probably attributable to the impaired Ca2+ transport into the endoplasmic reticulum. On the other hand, our histopathological data showed that the lungs of the mice infected with the ΔcbpA strain reveal extensive hyphal invasion and inflammation similar to those in the animals infected with the wild-type and cbpA complemented strains. Furthermore, the ΔcbpA and wild-type strains displayed equal sensitivities to the oxidants menadione and H2O2 and comparable hyphal growths at 40°C (data not shown). Thus, it is more likely that the contribution of CbpA to virulence and disease establishment is only limited.
Since the homologs of CbpA from other species were shown to bind to calcineurin and regulate its function, we additionally sought to verify if CbpA could also bind to calcineurin (data not shown). We analyzed the ability of CbpA to bind calcineurin A by expressing CnaA as a glutathione S-transferase fusion protein, using the pGEX6P-1 bacterial expression vector and performing the in vitro binding assay using purified recombinant CbpA. Yeast two-hybrid assays were also performed by using the respective genes cloned into the pGAD424 and pGBT9 vectors and screening for the growth of the transformants in the absence of histidine or adenine. In both experiments, we could not detect binding of CbpA to calcineurin A. Since interactions between proteins depend on specific conditions (such as Ca2+ in vivo) which cannot be totally recapitulated in vitro, we cannot completely rule out the possibility of their interaction, especially considering the fact that its orthologs interacted with calcineurin. However, it is interesting to note that A. fumigatus CbpA shows conservation only in the region of the SPPXSPP repeat, which is known to be phosphorylated by GSK3 and mitogen-activated protein kinase and could probably be the site of dephosphorylation by calcineurin. Since certain Ca2+-dependent interactions are often not recapitulated using the yeast two-hybrid system (17) and some interactions between proteins depend on specific in vitro conditions, further studies are needed to establish the exact mechanism of regulation of calcineurin by CbpA.
In conclusion, we demonstrate that CbpA is involved in hyphal growth and calcium homeostasis. Our data also provide evidence that CbpA is a potential endogenous inhibitor of calcineurin. However, because the phenotypes of the ΔcbpA strain were not as severe as those observed in the ΔcnaA and ΔcrzA strains, CbpA is apparently not required for all aspects of calcineurin functions. Therefore, continued studies for identification of other regulators and downstream partners of calcineurin may bring deeper insights in this complex pathway and make it more accessible for antifungal targeting.
Supplementary Material
Acknowledgments
We thank Andrew Alspaugh, Aimee Zaas, Michael Price, and Wenqi Hu for comments and suggestions and Leslie Eibest for assistance with the scanning electron microscopy.
N.P. was supported by a Faculty of Medicine Siriraj Hospital fellowship grant, and W.J.S. was supported by a K08 award (A1061149), a Basic Science Faculty Development grant from the American Society for Transplantation, and a Children's Miracle Network grant.
Footnotes
Published ahead of print on 27 February 2009.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Amaar, Y. G., and M. M. Moore. 1998. Mapping of the nitrate-assimilation gene cluster (crnA-niiA-niaD) and characterization of the nitrite reductase gene (niiA) in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Genet. 33206-215. [DOI] [PubMed] [Google Scholar]
- 2.Bhabhra, R., M. D. Miley, E. Mylonakis, D. Boettner, J. Fortwendel, J. C. Panepinto, M. Postow, J. C. Rhodes, and D. S. Askew. 2004. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect. Immun. 724731-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bok, J. W., and N. P. Keller. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree, G. R. 2001. Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 2762313-2316. [DOI] [PubMed] [Google Scholar]
- 5.Cramer, R. A., Jr., M. P. Gamcsik, R. M. Brooking, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, C. J. Balibar, J. R. Graybill, J. R. Perfect, S. N. Abraham, and W. J. Steinbach. 2006. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer, R. A., Jr., B. Z. Perfect, N. Pinchai, S. Park, D. S. Perlin, Y. G. Asfaw, J. Heitman, J. R. Perfect, and W. J. Steinbach. 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 71085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan, W., A. Idnurm, J. Breger, E. Mylonakis, and J. Heitman. 2007. EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 753394-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira, A., R. Kincaid, and K. S. Kosik. 1993. Calcineurin is associated with the cytoskeleton of cultured neurons and has a role in the acquisition of polarity. Mol. Biol. Cell 41225-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox, D. S., and J. Heitman. 2005. Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans. Eukaryot. Cell 41526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitas, F. Z., A. Chapeaurouge, J. Perales, and M. C. Bertolini. 2008. A systematic approach to identify STRE-binding proteins of the gsn glycogen synthase gene promoter in Neurospora crassa. Proteomics 82052-2061. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes, J. J., L. Genesca, T. J. Kingsbury, K. W. Cunningham, M. Perez-Riba, X. Estivill, and S. de la Luna. 2000. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 91681-1690. [DOI] [PubMed] [Google Scholar]
- 12.Hu, W., S. Sillaots, S. Lemieux, J. Davison, S. Kauffman, A. Breton, A. Linteau, C. Xin, J. Bowman, J. Becker, B. Jiang, and T. Roemer. 2007. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsbury, T. J., and K. W. Cunningham. 2000. A conserved family of calcineurin regulators. Genes Dev. 141595-1604. [PMC free article] [PubMed] [Google Scholar]
- 14.Klauck, T. M., M. C. Faux, K. Labudda, L. K. Langeberg, S. Jaken, and J. D. Scott. 1996. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 2711589-1592. [DOI] [PubMed] [Google Scholar]
- 15.Lagorce, A., N. C. Hauser, D. Labourdette, C. Rodriguez, H. Martin-Yken, J. Arroyo, J. D. Hoheisel, and J. Francois. 2003. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 27820345-20357. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidwell, K., J. Dillon, A. Sihota, V. O'Connor, and B. Pilkington. 2004. Determining calmodulin binding to metabotropic glutamate receptors with distinct protein-interaction methods. Biochem. Soc. Trans. 32868-870. [DOI] [PubMed] [Google Scholar]
- 18.Lin, X., R. A. Sikkink, F. Rusnak, and D. L. Barber. 1999. Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J. Biol. Chem. 27436125-36131. [DOI] [PubMed] [Google Scholar]
- 19.Mansuy, I. M., M. Mayford, B. Jacob, E. R. Kandel, and M. E. Bach. 1998. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 9239-49. [DOI] [PubMed] [Google Scholar]
- 20.Miseta, A., R. Kellermayer, D. P. Aiello, L. Fu, and D. M. Bedwell. 1999. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 451132-136. [DOI] [PubMed] [Google Scholar]
- 21.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muro-Pastor, M. I., R. Gonzalez, J. Strauss, F. Narendja, and C. Scazzocchio. 1999. The GATA factor AreA is essential for chromatin remodelling in a eukaryotic bidirectional promoter. EMBO J. 181584-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov, N., D. P. Kontoyiannis, A. Romans, and G. S. May. 2001. Resistance to itraconazole in Aspergillus nidulans and Aspergillus fumigatus is conferred by extra copies of the A. nidulans P-450 14alpha-demethylase gene, pdmA. J. Antimicrob. Chemother. 4875-81. [DOI] [PubMed] [Google Scholar]
- 24.Porta, S., S. A. Serra, M. Huch, M. A. Valverde, F. Llorens, X. Estivill, M. L. Arbones, and E. Marti. 2007. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum. Mol. Genet. 161039-1050. [DOI] [PubMed] [Google Scholar]
- 25.Rothermel, B., R. B. Vega, J. Yang, H. Wu, R. Bassel-Duby, and R. S. Williams. 2000. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 2758719-8725. [DOI] [PubMed] [Google Scholar]
- 26.Rothermel, B. A., R. B. Vega, and R. S. Williams. 2003. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc. Med. 1315-21. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spielvogel, A., H. Findon, H. N. Arst, L. Araujo-Bazan, P. Hernandez-Ortiz, U. Stahl, V. Meyer, and E. A. Espeso. 2008. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem. J. 414419-429. [DOI] [PubMed] [Google Scholar]
- 29.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 51091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, C. Henn, K. Nielsen, J. Heitman, and J. R. Perfect. 2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 512979-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbach, W. J., J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, and J. Heitman. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5418-430. [DOI] [PubMed] [Google Scholar]
- 32.Strippoli, P., L. Lenzi, M. Petrini, P. Carinci, and M. Zannotti. 2000. A new gene family including DSCR1 (Down syndrome candidate region 1) and ZAKI-4: characterization from yeast to human and identification of DSCR1-like 2, a novel human member (DSCR1L2). Genomics 64252-263. [DOI] [PubMed] [Google Scholar]
- 33.Sweigard, J. A., F. Chumley, A. Carroll, L Farrall, and B. Valent. 1997. A series of vectors for fungal transformation. Fungal Genet. Newsl. 4452-53. [Google Scholar]
- 34.Thon, M. R., E. M. Nuckles, and L. J. Vaillancourt. 2000. Restriction enzyme-mediated integration used to produce pathogenicity mutants of Colletotrichum graminicola. Mol. Plant-Microbe Interact. 131356-1365. [DOI] [PubMed] [Google Scholar]
- 35.Vega, R. B., B. A. Rothermel, C. J. Weinheimer, A. Kovacs, R. H. Naseem, R. Bassel-Duby, R. S. Williams, and E. N. Olson. 2003. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 100669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega, R. B., J. Yang, B. A. Rothermel, R. Bassel-Duby, and R. S. Williams. 2002. Multiple domains of MCIP1 contribute to inhibition of calcineurin activity. J. Biol. Chem. 27730401-30407. [DOI] [PubMed] [Google Scholar]
- 37.Yang, J., B. Rothermel, R. B. Vega, N. Frey, T. A. McKinsey, E. N. Olson, R. Bassel-Duby, and R. S. Williams. 2000. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 87E61-E68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.