Abstract
The replication terminator protein Fob1 of Saccharomyces cerevisiae specifically interacts with two tandem Ter sites (replication fork barriers) located in the nontranscribed spacer of ribosomal DNA (rDNA) to cause polar fork arrest. The Fob1-Ter complex is multifunctional and controls other DNA transactions such as recombination by multiple mechanisms. Here, we report on the regulatory roles of the checkpoint proteins in the initiation and progression of recombination at Fob1-Ter complexes. The checkpoint adapter proteins Tof1 and Csm3 either positively or negatively controlled recombination depending on whether it was provoked by polar fork arrest or by transcription, respectively. The absolute requirements for these proteins for inducing recombination at an active replication terminus most likely masked their negative modulatory role at a later step of the process. Other checkpoint proteins of the checkpoint adapter/mediator class such as Mrc1 and Rad9, which channel signals from the sensor to the effector kinase, tended to suppress recombination at Fob1-Ter complexes regardless of how it was initiated. We have also discovered that the checkpoint sensor kinase Mec1 and the effector Rad53 were positive modulators of recombination initiated by transcription but had little effect on recombination at Ter. The work also showed that the two pathways were Rad52 dependent but Rad51 independent. Since Ter sites occur in the intergenic spacer of rDNA from yeast to humans, the mechanism is likely to be of widespread occurrence.
Recombination is an important part of DNA replication because it promotes uninterrupted fork progression and complete duplication of the genome by facilitating restart of prematurely arrested replication forks caused by extrinsic or intrinsic factors (15). Despite this important beneficial effect, recombination, if not stringently controlled, poses the inherent risk of inducing genome instability by causing loss or gain or transposition of sequences. The ribosomal DNA (rDNA) of budding yeast provides an excellent model system for investigation of the control of replication fork arrest-induced recombination at Ter sites (see reference 45 for a recent review). Although much information is available on the choice between intrachromatid and interchromatid recombination in rDNA (24), much less is known about the initiation of recombination and its control at Ter sites.
Budding yeast rDNA is present as ∼200 tandem repetitions of a ∼9.1-kb unit sequence in chromosome number XII (34). Each rDNA unit contains, from left to right (Fig. 1A), the sequence encoding the 35S precursor rRNA that is transcribed by RNA polymerase I; an intergenic, nontranscribed spacer region I (IGS I) that contains tandem replication termini Ter2 and Ter1 (also called replication fork barriers) (8); and the sequence encoding 5S RNA that is transcribed by RNA polymerase III, followed by a second intergenic spacer (IGS II) that contains an origin of DNA replication (ARS) (7). Replication is initiated from the ARS and initially progresses bidirectionally until it meets the Ter1 and Ter2 sites, at which it is arrested in a polar mode. The Ter1 and Ter2 sequences specifically bind to the multifunctional terminator protein called Fob1, which causes polar fork arrest at the protein-DNA complexes (23, 32). The programmed fork arrest prevents the replication forks from invading the region of the 35S transcript progressing from the opposite direction. The convergence of oppositely moving transcription and replication is known to cause fork stalling and physiologically unscheduled recombination (40).
FIG. 1.
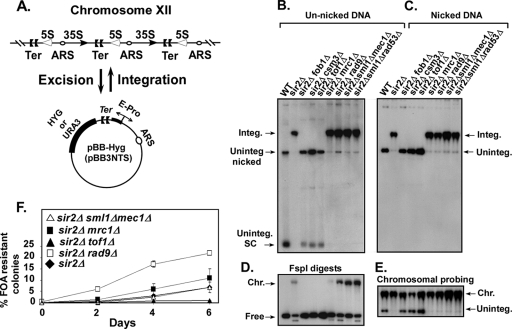
Effect of checkpoint genes on plasmid integration into and excision from the Ter sites of rDNA. (A) Schematic representation of the plasmid integration and excision assays; the plasmid pBB3NTS (pBB-Hyg) contained EXP sequence (which includes the Ter sites and the E-pro promoter). (B) Autoradiogram of a Southern blot showing the intracellular distribution of the reporter plasmid pBB-Hyg DNA resolved without nicking the DNA in an 0.5% agarose gel; a labeled plasmid-specific probe was used for detection of the plasmid DNA. integ., integrated; uninteg., unintegrated; SC, supercoiled DNA; WT, wild type. The lanes are self-explanatory. (C) Autoradiogram of a Southern blot showing the DNA samples shown in panel B but after nicking with NB.BsrD1. (D) Same as in panel B except that the DNA samples were digested with FspI; the lanes from left to right are as labeled in panels B and C. (E) Same as in panel C but probed with labeled chromosomal rDNA to identify the location of the integrated plasmid bands. (F) Kinetics of excision of pBB3NTS (URA3) plasmid rDNA from strains containing the integrated form of the plasmid reporter. Effects of individual deletions of the checkpoint genes tof1, mrc1, and rad9 (all present in a sir2Δ background) on loss of URA3 plasmid as a function of time of growth in nonselective medium. The nine lanes in panels D and E correspond exactly to the nine lanes in panels B and C.
Ter sites are known to be recombinogenic in prokaryotes (16), and in budding and fission yeasts, these act as hot spots for recombination. The process is regulated at multiple levels (45). Interchromatid recombination presumably helps to maintain the homogeneity of the rDNA repeat sequences, whereas maintenance of the rDNA repeat length homeostasis requires controlled illegitimate, intrachromatid exchanges (25, 26). Ribosomal repeat length expansion or contraction, which presumably occurs in response to physiological cues, is caused by Fob1-mediated recombination (25). Recombination at Ter is regulated by Fob1-mediated recruitment of the histone deacetylase called Sir2. Sir2 and Net1 are two of the component proteins of the RENT (regulators of nucleolar silencing and telophase exit) complex (38), which inhibits intrachromatid recombination by recruitment of cohesin in two ways. First, it represses transcription initiated at the bidirectional promoter called E-pro, located in the EXP (expansion) sequence adjacent to Ter in the IGS I (Fig. 1A) (24). Transcription directed toward the Ter region apparently disrupts cohesin assembly and removes this impediment to intrachromatid recombination (24). Second, Fob1 physically interacts with the RENT complex, which in turn interacts with Tof2 and the monopolin complex. The latter attracts cohesins to the Ter region by protein-protein interaction (17, 18). Sir2 also suppresses recombination at rDNA by preventing its accessibility to recombination proteins through alteration of the chromatin structure (14).
Many extrinsic (e.g., hydroxyurea) and intrinsic factors can cause genome-wide unscheduled fork arrest either at DNA lesions or due to depletion of the deoxynucleoside triphosphate pool. The stalled forks are prone to replisomal disassembly and fork collapse that can cause genome instability. Cells respond to stalled forks by invoking a signal transduction pathway called S-phase checkpoint control that delays cell cycle progression, turns on DNA repair genes, and stabilizes the stalled forks against replisomal disassembly and collapse (13, 41). The stalled fork is stabilized by a fork protection complex consisting of Tof1, Csm3, and Mrc1 proteins (21). The S-phase checkpoint pathway of budding yeast consists of a sensor protein kinase called Mec1 and its associated protein Ddc2; the latter binds to a single-stranded DNA binding protein complex, RPA (replication protein A), which accumulates on unraveled single-stranded DNA present near stalled forks. The sensor is followed in the cascade by mediators (Mrc1, Tof1, Csm3, and Rad9) that in turn activate an effector kinase called Rad53 that phosphorylates the target genes to cause cell cycle arrest and turns on genes needed for repair of DNA lesions at which the forks have stalled (13). Checkpoint proteins are not just activated by stress but are also important modulators of DNA transactions under normal conditions (11, 36).
Although unscheduled fork arrest caused by genotoxic stress can induce a checkpoint response, physiologically programmed fork arrest at Ter sites of rDNA does not seem to elicit such a response. The intra-S checkpoint proteins Tof1 and Csm3, but not Mrc1, promote stable programmed fork arrest at Ter (9, 31, 44) by counteracting Rrm3 helicase/“sweepase” (31). Rrm3 is known to facilitate fork passage through many nonhistone protein-DNA complexes that are tightly bound to DNA throughout the genome (43).
Given that fork arrest at Ter triggers recombination and in the light of the information presented above, one would hypothesize that Tof1/Csm3 should promote recombination at replication termini of rDNA by counteracting the negative effect of Rrm3 on fork arrest. One would further predict that Mrc1 and Rad9, the mediators of replication and DNA damage response checkpoint pathways, respectively (48), which do not play a role in promoting fork arrest (9, 31, 44), should not promote recombination at Ter. Although some information on the roles of checkpoint proteins in recombination caused by nonphysiological fork arrest is available (29), the possible regulatory role of checkpoint adapter proteins on recombination at natural Ter sites has not been completely elucidated in the nucleolar milieu.
Investigations of recombination at ectopically placed Ter sites outside the rDNA array have yielded different results in different systems. For example, placement of inverted Ter sites at an ectopic location in budding yeast showed no evidence of enhanced recombination at this site and fork arrest at this location was not dependent on Mec1 and Rad53, the sensor and the effector kinases of the checkpoint pathway, respectively (9). On the other hand, an ectopically placed sequence consisting of the promoter (I element) and enhancer (E element) that includes the Ter sites, when located in chromosome III, promoted illegitimate recombination (HOT1 activity). HOT1-activated recombination requires both transcription by RNA polymerase I and Fob1 binding to Ter located in the E element (22, 35, 46). Fob1 protein induces HOT1 recombination not by arresting replication forks (47) but by acting as a transcription factor that promotes RNA polymerase I transcription (34). In Schizosaccharomyces pombe, placement of Ter sites (RTS) at ectopic locations caused enhanced recombination and genome instability when the cognate terminator protein Rtf1p and the intra-S-phase checkpoint protein SWI1 (and presumably SWI3) were provided (2, 27).
The proteins encoded by the RAD52 epistasis group of genes that promote homologous recombination (HR) have been reviewed previously (33). Rad52 and Rad59 are single-stranded DNA-annealing proteins that act together but somewhat differently from each other; Rad54 is a motor protein; Rad55 and Rad57 are facilitators of the homology search protein Rad51, which is the equivalent of prokaryotic RecA protein. Rad50 and yKu80 are proteins involved in the nonhomologous end-joining (NHEJ) pathway.
In this work, we endeavored to test some of the hypotheses stated above by measuring the frequencies of integration of a plasmid containing the EXP sequence (which includes Ter and E-pro) into chromosomal rDNA in the absence of the SIR2 gene (5, 30) and of the excision of the integrated sequences from rDNA. We investigated the roles played by the various checkpoint proteins in recombination at Fob1-Ter complexes with or without associated fork arrest. We show that the intra-S-phase checkpoint genes TOF1 and CSM3 promoted recombination at Ter within the rDNA array but suppressed recombination at the ectopically placed HOT1 locus that included the enhancer (including Ter)-promoter sequences of the precursor 35S rRNA. In contrast, MRC1 and RAD9 mediators/adapters suppressed recombination at both Ter and HOT1 to various degrees. The Mec1 sensor and Rad53 effector were positive modulators of HOT1 recombination triggered by RNA polymerase I transcription. The results revealed that the intra-S-phase checkpoint proteins Tof1 and Csm3 have dual and contrasting roles in regulating recombination at Fob1 binding sites depending on whether the recombination was triggered by fork arrest at Ter or by an alternative mechanism involving transcription by RNA polymerase I. Recombination was also modulated either positively or negatively by other members of the replication checkpoint signal transduction pathway.
MATERIALS AND METHODS
Construction of strains.
All strains constructed as a part of this work and those received from other sources are listed in Table 1. The strain LPY11 (W303 Δsir2:HIS3; courtesy of Loraine Pillus) was used to delete all checkpoint and recombination genes using appropriate markers such as G418 or TRP1 (28).
TABLE 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| W303 | MATa (leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) | R. Rothstein |
| LPY11 | W303a sir2Δ:HIS3 | L. Pillus |
| Lfob1 | LPY11 fob1Δ:G418 | This study |
| Lcsm3 | LPY11 csm3Δ:G418 | This study |
| Ltof1 | LPY11 tof1Δ:G418 | This study |
| Lmrc1 | LPY11 mrc1Δ:G418 | This study |
| Lrad9 | LPY11 rad9Δ:G418 | This study |
| Lrad50 | LPY11 rad50Δ:G418 | This study |
| Lrad51 | LPY11 rad51Δ:G418 | This study |
| Lrad52 | LPY11 rad52Δ:G418 | This study |
| Lrad54 | LPY11 rad54Δ:G418 | This study |
| Lrad55 | LPY11 rad55Δ:G418 | This study |
| Lrad59 | LPY11 rad59Δ:G418 | This study |
| Lrrm3 | LPY11 rrm3Δ:G418 | This study |
| Ltr13 | LPY11 tof1Δ:G418 (crelox G418 lost) rrm3Δ:G418 | This study |
| Lmus81 | LPY11 mus81Δ:G418 | This study |
| Lslx4 | LPY11 slx4Δ:G418 | This study |
| Lms9 | LPY11 mus81Δ:G418 (crelox G418 lost) slxΔ:G418 | This study |
| WDHY1638 | W303 RAD5 sml1Δ mec1Δ | W. D. Heyer |
| Lmec1 | WDHY1638 sir2Δ:G418 | This study |
| W2105-17b | W303 sml1Δ:URA3 rad53Δ:HIS3 rad5 | R. Rothstein |
| Lrad53 | W2105-17b sir2Δ:G418 | This study |
| K5665 | RLK1-3C MATα his4-260 ade2-1 ura3-52 canR | R. Keil |
| Kfob1 | K5665 fob1Δ:G418 | This study |
| Kcsm3 | K5665 csm3Δ:G418 | This study |
| Ktof1 | K5665 tof1Δ:G418 | This study |
| Kmrc1 | K5665 mrc1Δ:G418 | This study |
| Krad9 | K5665 rad9Δ:G418 | This study |
| Krad51 | K5665 rad51Δ:G418 | This study |
| Krad52 | K5665 rad52Δ:G418 | This study |
| Krad54 | K5665 rad54Δ:G418 | This study |
| Krad55 | K5665 rad55Δ:G418 | This study |
| Krad59 | K5665 rad59Δ:G418 | This study |
| Krrm3 | K5665 rrm3Δ:G418 | This study |
| Ktr1 | K5665 rrm3Δ:G418 (crelox G418 lost) tof1Δ:G418 | This study |
| Ksml1 | K5665 sml1Δ:G418 | This study |
| Kmec1 | K5665 sml1Δ:G418 (crelox G418 lost) mec1Δ | This study |
| Krad53 | K5665 sml1Δ:G418 (crelox G418 lost) rad53Δ | This study |
Plasmid integration assay.
The plasmid integration assay was carried out essentially as described in references 5 and 30) with slight modifications. Plasmid pBB3NTS (45) containing a URA3 marker was transformed into appropriate strains by the lithium acetate method and plated on SD-Ura− plates. A few colonies of ∼1 mm in diameter were mixed by the inoculation loop and then streaked on a new SD-Ura− plate. After three or six cycles of streaking, a few colonies were pooled and then inoculated into 10 ml of SD-Ura− medium. DNA was prepared from overnight cultures (32). Strains containing plasmid pBB-Hyg were grown in the same way except that the culture medium was yeast extract-peptone-dextrose (YPD) plus hygromycin (200 μg/ml). DNA samples, unnicked or nicked by NB.BsrD1 (New England Biolabs), were fractionated in an 0.8% agarose gel in the presence of ethidium bromide. Southern transfer and probing by pUC18 were carried out as described in reference 31. Plasmid integration was carried out using both pBB3NTS and pBB-Hyg plasmids, and they are shown in the appropriate figure legends. Excision assays were carried out with strains in which plasmid pBB3NTS was completely integrated into the chromosome.
Excision assay.
Strains containing the integrated pBB3NTS (URA3) plasmid were first grown overnight in liquid SD-Ura− medium. Overnight cultures were then inoculated into fresh SC complete medium to allow cells to lose the integrated plasmid. At appropriate times (days), cultures were diluted and plated on YPD and SD medium containing 1 g/liter of 5-fluoroorotic acid (5-FOA). Plates were incubated at 30°C for 3 days before colonies were counted. Each day, the liquid cultures were diluted in SC medium, grown for 24 h, and plated on the appropriate day.
HOT1 assay.
The wild-type strain or its derivatives were grown overnight in SD-Ura− medium. Cultures were inoculated into fresh YPD medium and grown at 30°C. Cultures were diluted at different time points and spread on YPD and SD-FOA+ plates in triplicate. Plates were incubated at 30°C. The percent HOT1 activity was calculated from the ratio of FOA-resistant colonies and the total number of colonies growing on YPD plates multiplied by 100.
2D gel electrophoretic assay.
Two-dimensional (2D) gel analysis of fork arrest was carried out as described in reference 6.
RESULTS
Effect of checkpoint proteins on recombination at Ter.
First, we wished to investigate the possible impact of replication checkpoint proteins of the adapter class on recombination provoked by fork arrest at Ter. Two of the adapter proteins, namely, Tof1 and Csm3, but not Mrc1 and Rad9, are required for promotion of stable fork arrest at Ter sites (9, 31, 44). We investigated the impact of the loss of the various individual checkpoint proteins on recombination at Ter by utilizing the observation that a plasmid containing the recombinogenic EXP region that includes the tandem Ter sites (24) is readily integrated into the chromosomal rDNA array in the absence of Sir2 in a Fob1-dependent fashion (5). It was essential to eliminate Sir2 activity because it is known to suppress intrachromatid but not interchromatid recombination in rDNA (26). While intrachromatid recombination was critical for the plasmid integration and excision assay (Fig. 1A), interchromatid recombination was phenotypically “silent.” We transformed the reporter plasmid pBB3-Hyg (32) into the sir2Δ strain (LPY11) and its isogenic derivatives, which contained deletions of various checkpoint genes of the adapter/mediator class such as TOF1, CSM3, MRC1, and RAD9. In addition, we also transformed the plasmid into the strains from which SIR2 was deleted along with the checkpoint sensor MEC1 gene and the effector RAD53 gene (Table 1 shows all strains). We measured the percentage of the total intracellular plasmid DNA that was integrated into the chromosome of each of these strains. The transformants were grown for 40 to 60 generations on YPD-plus-hygromycin plates. We determined plasmid integration by extracting and resolving the intracellular DNA in agarose gels followed by Southern blotting and hybridization of the blots to a labeled plasmid-specific probe. The free and integrated forms of plasmid DNA were quantified with a phosphorimager (Fig. 1B and C). The intracellular pBB-Hyg plasmid DNA in the wild-type cells remained almost exclusively in the unintegrated form in either the supercoiled or the relaxed state (Fig. 1B and C). In the sir2Δ strain, almost the entire population of plasmid DNA was integrated and it migrated in a band that corresponded to the chromosomal DNA (Fig. 1B and C). By reprobing the blots with a labeled rDNA probe, we confirmed that this band corresponded to sheared chromosomal DNA (Fig. 1E). We confirmed that the plasmid integration was FOB1 dependent by examining the intracellular plasmid DNA in the sir2Δ fob1Δ strain and found that it existed almost completely in the unintegrated state (Fig. 1B and C). It should be noted that flipping the orientation of the Ter site with respect to the origin by 180° abolished plasmid integration, thereby showing that the integration was dependent on polar fork arrest at the terminus (data not shown).
We then examined the distributions of intracellular plasmid DNA in each of the sir2Δ derivatives that contained single deletions of the checkpoint genes. Southern blots of the DNA samples resolved in agarose gels and probed with a labeled pUC18 DNA probe showed that in the sir2Δ csm3Δ and sir2Δ tof1Δ strains, the plasmids remained in the free form (Fig. 1B and C). In contrast, ∼98% of the intracellular plasmid DNA was integrated into the chromosome in the mrc1Δ, rad9Δ, mec1Δ, and rad53Δ derivatives (Fig. 1B and C). In order to simplify the gel electrophoresis patterns and thereby make it easier to quantify the results, we nicked the plasmid DNA samples before electrophoresis with the restriction enzyme NB.BsmI or NB.BsrD1, which nicks but does not cut both strands of the DNA at its recognition site(s). Phosphorimager analysis of the resulting blots was performed, and the data confirmed that, whereas in the tof1Δ and csm3Δ strains plasmid integration was almost completely blocked, ∼98% of the plasmid DNA was integrated into chromosome in the sir2Δ mrc1Δ, sir2Δ rad9Δ, mec1Δ, and rad53Δ strains (Fig. 1C). In order to ascertain that the band migrating above the nicked circular plasmid DNA was chromosomally integrated, we stripped the blot shown in Fig. 1C, reprobed it with a labeled chromosomal rDNA, and found that the upper band in lanes 1 to 7 hybridized to the labeled probe (Fig. 1E). Did the plasmid DNA integrate in a single cluster, or were these integrated at diverse locations in rDNA? We addressed this question by digesting the DNA with FspI. It should be noted that, whereas the plasmid DNA contains a single FspI site, none are present in the rDNA repeats. Digestion of the DNA with FspI showed (assuming complete digestion) that ∼90% of the plasmid DNA was integrated in a single cluster (Fig. 1D). In order to confirm that the plasmids were integrated into chromosomal rDNA, we double digested the DNA samples with FspI and NheI (this site is present in the rDNA repeats but not in the plasmid sequence). The DNA blots were also probed with an rDNA probe, and the results were consistent with the conclusion that the bulk of the integrations occurred within the rDNA (not shown).
Although the plasmid integration assay showed that TOF1 and CSM3 genes were essential for recombination at Ter, it did not have sufficient sensitivity to reveal more subtle effects caused by the deletions of MRC1, RAD9, MEC1, and RAD53 checkpoint genes. We wished to investigate this possibility by performing the reverse assay, i.e., measurements of the rate of excision of pBB3NTS plasmid present in the integrated state in the rDNA. We allowed plasmid excision to occur in the various genetic backgrounds mentioned above and counted the percentage of 5-FOA-resistant colonies generated as a function of prior duration of growth in nonselective (complete) medium. The generation of ura3 colonies was not due to silencing of the marker in rDNA because the SIR2 silencer, which encodes the NAD-dependent histone deacetylase, had been deleted from all the strains. The data not only confirmed the absolute necessity of TOF1/CSM3 for FOB1-dependent recombination at Ter but also revealed that the rate of excision was increased in the sir2Δ rad9Δ and the sir2Δ mrc1Δ strains in comparison with the sir2Δ control. While the inhibitory impact of RAD9 on plasmid excision was unambiguous, the effect of mrc1Δ was modest in comparison with the sir2Δ control (Fig. 1F). The deletion of MEC1 had no significant effect on plasmid excision. The data are consistent with the conclusion that RAD9 and probably MRC1 were negative modulators of plasmid excision (Fig. 1F). While performing the excision experiments, we have considered the possibilities that the growth rates of the 5-FOA-resistant colonies of different genotypes, the location of integration, etc., might affect excision rates. However, experiments were performed with independent isolates, and the results were generally consistent with those conducted on plasmid integration whenever it was possible to do this comparison (as in Fig. 1).
Effect of the tof1Δ rrm3Δ double deletion on recombination.
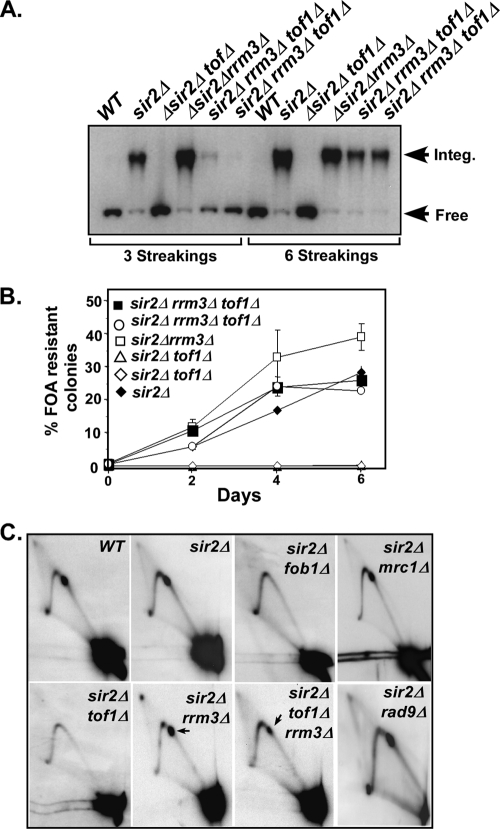
We have previously reported that Tof1 and Csm3 promote stable fork arrest by counteracting the effect of the helicase Rrm3, which allows fork passage through the Fob1-Ter complex (30). Rrm3 is a 5′-3′ helicase that is known to promote genome-wide fork passage through nonhistone protein-DNA barriers (19, 42). Tof1 and Csm3 partially restored fork arrest at Ter by counteracting the tendency of Rrm3 helicase to facilitate fork passage past Fob1-Ter complexes. We wished to determine if the negative modulation of Rrm3 by the Tof1-Csm3 complex was also manifested in a similar manner in plasmid integration and excision in a sir2Δ background. We constructed a sir2Δ rrm3Δ tof1Δ strain and compared integration of pBB-Hyg in this strain with its integration in sir2Δ, sir2Δ tof1Δ, and sir2Δ rrm3Δ strains (Fig. 2). The autoradiograms of Southern blots of agarose gels showed that, after three cycles of serial streaking and growth on selective medium, >98% of the input plasmid DNA had integrated into chromosomal rDNA in the sir2Δ and sir2Δ rrm3Δ cells. In contrast, as expected, no plasmid integration was detectable in the sir2Δ tof1Δ strain. Furthermore, in the sir2Δ tof1Δ rrm3Δ cells, less than 30% of the input plasmid DNA was found in the integrated state after three rounds of streaking and growth on selective medium. Complete plasmid integration could be achieved only after a total of six cycles of streaking and serial growth of the latter strain (Fig. 2A). The data are consistent with the interpretation that Tof1 activity, although essential for plasmid integration in the presence of the Rrm3 sweepase, was not strictly necessary when there was no Rrm3 present in the cell milieu. The lower rate of plasmid integration appeared to be commensurate with the partial restoration of fork arrest in the sir2Δ fob1Δ rrm3Δ strain in comparison with the sir2Δ control. In order to confirm the data by another approach, we measured plasmid excision kinetics in the same genotypes used for plasmid integration. We transformed the plasmid pBB3NTS into sir2Δ, sir2Δ rrm3Δ, and sir2Δ fob1Δ rrm3Δ strains. We deleted the TOF1 gene from the sir2Δ strain in which integration had occurred. As expected, there was a 1.5-fold increase in the rate of excision in the sir2Δ rrm3Δ strain in comparison with the sir2Δ strain, but plasmid excision was completely abolished in the sir2Δ tof1Δ strain (data from two independent isolates are shown). The rate of plasmid excision, in contrast, was restored to nearly the level of that of the sir2Δ control in the sir2Δ tof1Δ rrm3Δ genotype (Fig. 2B).
FIG. 2.
Contribution of the interplay between TOF1 and RRM3 to plasmid integration and excision. (A) Autoradiogram of a representative Southern blot showing the effect of tof1Δ rrm3Δ double deletions on plasmid integration (pBB-Hyg) in comparison with the single deletions, all in a common sir2Δ background after three and six serial streakings and growth in the selective medium. (B) Effects of single and double deletions (tof1Δ, rrm3Δ, and tof1Δ rrm3Δ) on pBB3NTS (URA3) plasmid excision. (C) Autoradiograms of 2D gels showing replication fork arrest at Ter in different strains; the arrows show the termination spots generated by fork arrest at the two closely spaced Ter1 and Ter2 sites. WT, wild type.
We wished to make sure that, in the genetic background used here, sir2Δ did not alter the requirements for regulation of fork arrest that have been investigated before in SIR2 genotypes by performing 2D gel electrophoresis (31). The autoradiograms of 2D gels of replication intermediates confirmed that fork arrest was abolished in the sir2Δ tof1Δ strain in comparison with the sir2Δ control. It was slightly elevated over that of the control in the sir2Δ rrm3Δ strain. Also consistent with our previous work, in the sir2Δ tof1Δ rrm3Δ strain the fork arrest at Ter was partially restored in comparison with the sir2Δ control (Fig. 2C). The data support the conclusion that in the sir2Δ genotype, Tof1 promoted stable fork arrest by counteracting the Rrm3 sweepase, thereby restoring fork arrest-mediated recombination at Ter. Deletions of the MRC1 and RAD9 mediators did not abolish fork arrest (Fig. 2C). The absence of these genes did not abolish plasmid integration or excision triggered by replication termination (Fig. 2A and B). Rather, these checkpoint mediator proteins seemed to suppress recombination, probably at a later step following fork arrest (Fig. 1F). A possible mechanistic explanation of these observations is presented in the Discussion. Deletion of MEC1 and RAD53 did not have any visible impact on fork arrest at Ter (data not shown).
Impact of deletions of the genes of the RAD52 epistasis group on recombination at Ter.
Are plasmid integration and excision at Ter caused by HR or by NHEJ? To address this question, we investigated the dependence of plasmid integration on the gene products of the RAD52 epistasis group needed for HR and on yKU80, which encodes a DNA end binding protein needed for NHEJ. We measured the frequency of plasmid integration in the different genotypes described below and discovered that plasmid integration was absolutely dependent on RAD52. We made sure that loss of plasmid integration in rad52Δ cells was not due to loss of fork arrest (data not shown). Within the limits of the resolution of the plasmid integration assay, we could not detect any effect of the deletion of RAD50 or yKU80 on plasmid integration, leading to the conclusion that NHEJ did not play a significant role in plasmid integration at Ter (Fig. 3A). Individual deletions of RAD51, RAD54, RAD55, and RAD59 in the common sir2Δ background did not reveal any significant impact on the frequency of plasmid integration after three cycles of serial streaking on selective medium (Fig. 3A). We also performed plasmid excision assays in the same genetic backgrounds mentioned above and discovered that plasmid excision did not require RAD51, RAD50, RAD54, and RAD55 (Fig. 3B).
FIG. 3.
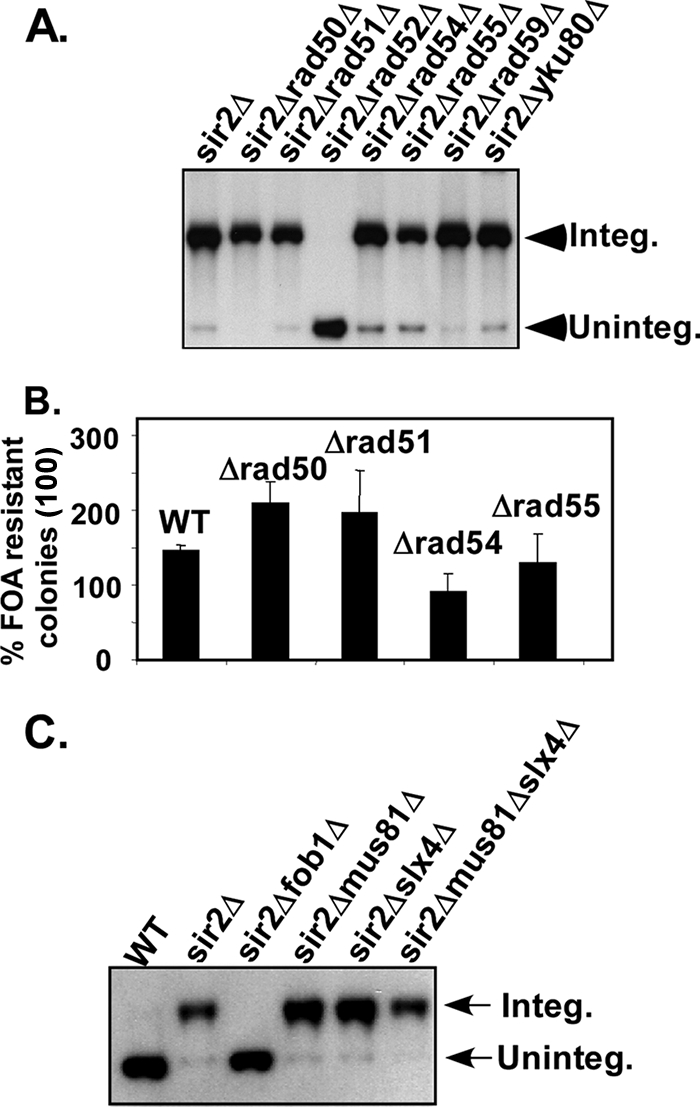
Impact of deletions of genes involved in HR and NHEJ on plasmid integration and excision. (A) Autoradiogram of a representative Southern blot of nicked DNA showing the status of intracellular plasmid DNA pBB3NTS (URA3) in isogenic strains containing various deletions. The lanes are self-explanatory. (B) Plasmid excision kinetics showing the impact of various deletions of recombination genes on the excision of pBB3NTS (URA3). (C) Effect of deletions of two structure-specific endonucleases (slx4Δ, mus81Δ, and slx4Δ mus81Δ) on plasmid pBB3NTS (URA3) integration; the lanes are self-explanatory. WT, wild type.
We performed 2D gel analyses of replication intermediates from each of the strains containing the deletions of the recombination genes and observed no difference in the extent of fork arrest at Ter in any of the deletion derivatives, in comparison with the strain containing the single sir2Δ deletion (data not shown).
Recombination initiation at Ter does not require SLX4.
Which gene product(s) catalyzed the double-strand (DS) break at Ter to initiate the recombination process? Previous work has revealed that in the absence of Sgs1 helicase, which resolves Holliday junctions and removes “chicken foot” structures caused by fork reversal, two classes of structure-specific nucleases (and a third group of genes encoding ubiquitin ligase) are essential for resolution and processing of replication intermediates in the rDNA and for survival of budding yeast (20). The first class of structure-specific endonucleases encoded by the SLX1/SLX4 genes preferentially cuts at DNA forks. The second class consists of the endonuclease gene MUS81 and its partner MMS4 (20). In fission yeast the homolog of the SLX1/SLX4 complex is the principal nuclease that acts at natural replication termini of rDNA to promote recombination (12). Are SLX4 and/or MUS81 also involved in initiation of recombination at Ter of budding yeast when SGS1 is present? In order to address this question, we performed a quantitative plasmid integration assay as described above in the appropriate genotypic backgrounds (Fig. 3C). The results showed that after three cycles of serial streaking on selective plates, plasmid integration was not detectably reduced in the mus81Δ, slx4Δ, or mus81Δ slx4Δ strains (in the common sir2Δ background). There was little if any free plasmid present in the autoradiograms, suggesting that deletions of slx4 and mus81 did not reduce the rate of plasmid integration. The data are consistent with the interpretation that neither SLX1/SLX4 nor MUS81/MMS4 appeared to be essential for initiation of recombination at the Ter sites of Saccharomyces cerevisiae.
Several checkpoint genes including TOF1 and CSM3 inhibited recombination at the HOT1 site.
The HOT1 site consists of two cis-acting elements, namely, E (the enhancer) and I (initiator). HOT1 recombination is stimulated by RNA polymerase I that binds to the I element, and it also depends on Fob1 binding to the Ter site present in the E element, where the Fob1-Ter complex serves to activate RNA polymerase I. However, HOT1 activity does not depend on fork arrest (45). These observations provided us with an opportunity to investigate the impact of checkpoint proteins and the Rad52 epistasis group proteins on recombination that was dependent on the Fob1-Ter complex but was not triggered by replication termination (47). We investigated whether TOF1 and CSM3, along with other checkpoint genes belonging to mediator, sensor, and effector classes, also modulated HOT1 activity. We constructed strains of the appropriate genotypes that also included the HOT1 locus placed in chromosome III (Table 1). Recombination was measured by the excision of a URA3 reporter by induction of illegitimate recombination by HOT1 between two flanking his4 sequences (Fig. 4A). We scored the appearance of ura3 colonies on 5-FOA plates as a function of increasing cumulative periods of prior growth in nonselective medium. The data are consistent with the conclusion that, in contrast to the absolute requirement of TOF1 and CSM3 genes for integrative recombination and plasmid excision at an active Ter site, these genes suppressed HOT1 activity. The strains containing rad9Δ and mrc1Δ also showed various degrees of derepression of HOT1 activity (Fig. 4B). The data suggested that Tof1 and Csm3 proteins might be protecting a DNA-protein complex from being processed and channeled into the HOT1-mediated intrachromatid recombination pathway. Deletion of the FOB1 gene served as a negative control and, as expected, completely eliminated HOT1 activity.
FIG. 4.
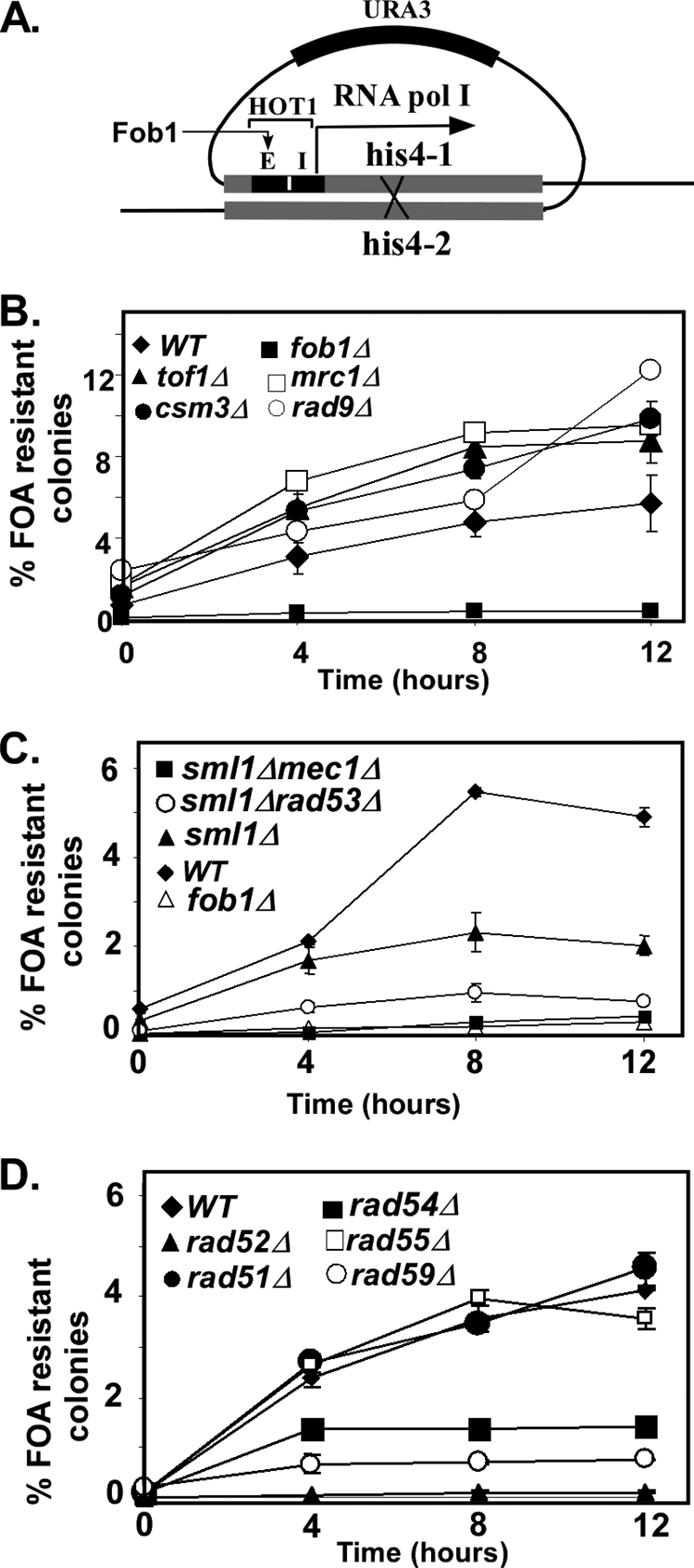
Impact of deletions of genes encoding checkpoint proteins and recombination proteins on HOT1 activity. (A) Schematic diagram showing the HOT1 assay; the E elements (containing the Fob1 binding site) and the I elements are shown; recombination between the flanking his4 genes excises URA3, which has no ARS and therefore is eliminated from the cells. (B) HOT1 activity in the absence of checkpoint adapter proteins. (C) HOT1 activity in the absence of MEC1 and RAD53. (D) HOT1 activity in different strains with individual deletions of the various members of the RAD52 epistasis group. WT, wild type.
We further investigated the possible modulatory effects of the sensor kinase Mec1 and the effector kinase Rad53 on HOT1 by constructing the appropriate strains and measuring HOT1 activity as described above (Fig. 4A). Both the mec1Δ and the rad53Δ strains showed a significant decrease in HOT1 activity, suggesting that the sensor and the effector kinases stimulated transcription-dependent recombination triggered by the binding of Fob1 to the Ter site embedded in the enhancer (E) element (Fig. 4A and C). It should be noted that it was necessary to construct double sml1Δ mec1Δ and sml1Δ rad53Δ deletions because, without the removal of the SML1 gene, which is a repressor of ribonucleotide nucleotide reductase, deletions of either MEC1 or RAD53 cause cell lethality (51). The data also showed a lesser reduction of HOT1 activity upon individual deletion of the SML1 gene (Fig. 4C).
HOT1 activity and the RAD52 epistasis group.
It was already known that HOT1 activity was partially dependent on RAD50 and completely on RAD52 (50). In order to investigate the impacts of the other members of the RAD52 epistasis group, we constructed appropriate strains containing individual deletions of the genes (Table 1) and measured HOT1 activity by quantifying the emergence of 5-FOA-resistant colonies as a function of prior growth in nonselective medium. The data confirmed the conclusion that HOT1 activity was completely dependent on the RAD52 gene and further revealed that it was partially dependent on RAD54 and RAD59 but not on RAD51 and RAD55 (Fig. 4D). Therefore, the HOT1 recombination pathway appears to be different in this regard from the fork arrest-dependent recombination pathway that results in plasmid integration/excision at Ter. A summary of the results is shown schematically in Fig. 5.
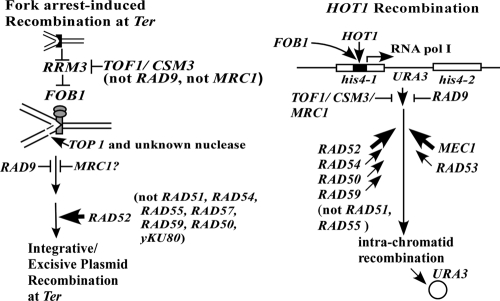
FIG. 5.
Schematic diagram that summarizes the data on the impact of various checkpoint proteins and members of the RAD52 epistasis group on plasmid integration and excision at Ter and on HOT1 activity. The heavy arrows at RAD52 and lighter ones at RAD54 and RAD59 indicate that RAD52 was absolutely essential for both plasmid integration and HOT1 activity; the latter genes played a stimulatory role in HOT1 recombination. The question mark next to MRC1 in the plasmid integration/excision pathway indicates that the inhibitory effect was rather modest compared with that of the wild type. Previous work has shown that RAD50 was partially required for HOT1 activity. The heavy arrow next to MEC1 indicates that this gene makes a significant positive contribution to HOT1 activity.
DISCUSSION
Efficient synthesis of macromolecular components of high abundance such as rRNA in eukaryotes is facilitated by the existence of multiple copies of the template DNA that are present in tandemly repeated copies in the rDNA cluster(s). In some organisms, as many as several thousand copies of rDNA repeats are maintained, sometimes on several different chromosomes (4). However, the maintenance of numerous tandem copies of the same sequence poses special challenges to the organism, not the least of which is prevention of excessive recombination mediated by the replication terminator proteins (e.g., Fob1) that might cause disassembly of the array into extrachromosomal rDNA circles, genome instability, and perhaps even premature cellular aging (37).
This work provides new insights into the control of initiation and progression of recombination at Fob1-Ter complexes and shows that the checkpoint mediator protein Tof1 and its binding partner Csm3, which are the homologs of the mammalian timeless (TIM) and timeless-interacting protein partner (TIPIN) and the fission yeast SWI1 and SWI3, respectively (4), promote recombination at Ter by preserving fork arrest by counteracting the activity of Rrm3 helicase/sweepase. The sweepase tends to promote fork progression past the Fob1-Ter complex in the absence of Tof1 or Csm3 (31). It should be noted that Mrc1, which together with Tof1 and Csm3 forms a fork protection complex (21), is not involved in termination of replication and consequently was not required for initiating recombination at Ter. Rather, Mrc1 showed a modest inhibitory effect on this recombination as indicated by the plasmid excision assay. The protein is known to be involved in sister chromatid cohesion (49). Since Sir2 inhibits plasmid integration (5), our experiments were carried out in a sir2Δ background in which derepression of the E-pro bidirectional promoter causes transcription-mediated removal of some of the cohesin from the Ter region, thereby at least partially removing a major barrier to intrachromatid recombination (24). Perhaps in the mrc1Δ strain the removal of residual cohesin from the Ter region caused a correspondingly modest stimulation of plasmid excision.
The present work also showed that RAD9, a mediator of the DNA damage checkpoint pathway (48), is also a negative regulator of recombination as revealed by the plasmid excision experiments. RAD9 is also known to be an inhibitor of recombination at a hot spot located at or near a tRNA gene in budding yeast (1). Although the mechanistic details of RAD9-mediated inhibition of recombination are hitherto unknown, like TOF1 and MRC1, RAD9 could be promoting retention of residual cohesion at Ter or by another mechanism that prevents the termination complex from being processed as a recombination intermediate.
Most recombination events are believed to be initiated by a DS break on DNA (39), although there is some evidence that recombination and restart of some stalled forks could be effected without a DS break by template switching and generation of a recombination intermediate (T. Carr, personal communication). What might be catalyzing a DS break at stalled forks at Ter? Our work shows that neither the structure-specific endonucleases Slx1 and Slx4 nor the Mus81/Mms4 complex was involved in the process. However, there is a report that the homologous Slx1 and Slx4 of S. pombe seem to be needed for this function in fission yeast (12). We have recently discovered that topoisomerase I is one of the enzymes that enhances recombination, probably by generating the DS breaks at Ter (B. K. Mohanty and D. Bastia, unpublished data).
It is interesting that the transcription-catalyzed, Fob1-dependent but fork arrest-independent HOT1 recombination was inhibited by Tof1 and Csm3. It is possible that in addition to their absolute requirement for initiating recombination at Ter, Tof1 and Csm3 could also be serving as inhibitors of recombination by promoting cohesin assembly around Ter at a step(s) following fork arrest. If so, such an effect would be masked from detection due to the absolute requirement for these two proteins at the recombination initiation step. In summary, observations presented here support the conclusion that, depending on whether recombination was initiated by fork arrest at Ter or by RNA polymerase I-catalyzed transcription at the HOT1 locus, the Tof1-Csm3 complex acted as a positive or negative regulator of recombination, respectively.
Neither plasmid integration/excision at Ter nor HOT1 activity required the homology search protein Rad51, but both were absolutely dependent on the DNA strand-annealing protein Rad52 (33). However, HOT1 activity but not recombination at Ter seemed to be stimulated by Rad59 (strand-annealing protein) and Rad54 (motor protein), indicating additional differences between these two modes of HR provoked by Fob1. Our results showing the dispensability of RAD51 for recombination at Ter are not inconsistent with the observation that recombination intermediates accumulate in a DNA polymerase α mutant of budding yeast in a Rad51-independent mode (52) and that recombination at the Ter sites of fission yeast rDNA is also Rad51 independent (12). The impact of the various checkpoint proteins and the Rad52 group of proteins on recombination at Ter and at HOT1 is summarized in Fig. 5.
It is interesting that Mec1 significantly enhanced HOT1 activity. This checkpoint sensor kinase is known to activate HR, as measured by a gap-filling assay, by phosphorylating Rad55, which is a facilitator of the homology search protein Rad51 (3). During meiosis, the sensor promotes recombination by phosphorylating the Hop1 protein (10). Neither the recombination at an active Ter site nor HOT1 activity, as reported in this work, required Rad55 or Rad51. Therefore, it is reasonable to predict that the sensor kinase Mec1 is likely to have a different target(s) in the Fob1-dependent HOT1 recombination pathways.
In conclusion, despite the paucity of detailed mechanistic biochemistry of checkpoint pathways, the molecular biological and genetic analyses carried out in this work have provided new insights into how recombination at replication termini is modulated by checkpoint proteins. Since Ter sites are conserved in the intergenic spacer regions of rDNA from yeast to humans (4), some of the conclusions reached here should have general significance.
Acknowledgments
We thank Steve Brill, Bernardo Schvartzman, Andres Aguilera, Ralph Keil, Lorrain Pillus, Wolf Heyer, and Rodney Rothstein for gifts of yeast strains and plasmids. We also thank Tony Carr, Tom Petes, and Steve Brill for useful comments on the manuscript.
This work was supported by grants from NIAID and NIGMS to D.B.
Footnotes
Published ahead of print on 20 February 2009.
REFERENCES
- 1.Admire, A., L. Shanks, N. Danzl, M. Wang, U. Weier, W. Stevens, E. Hunt, and T. Weinert. 2006. Cycles of chromosome instability are associated with a fragile site and are increased by defects in DNA replication and checkpoint controls in yeast. Genes Dev. 20159-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. S., F. Osman, and M. C. Whitby. 2005. Replication fork blockage by RTS1 at an ectopic site promotes recombination in fission yeast. EMBO J. 242011-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashkirov, V. I., J. S. King, E. V. Bashkirova, J. Schmuckli-Maurer, and W. D. Heyer. 2000. DNA repair protein Rad55 is a terminal substrate of the DNA damage checkpoints. Mol. Cell. Biol. 204393-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastia, D., and B. K. Mohanty. 2006. Termination of DNA replication, p. 155-174. In M. DePamphilis (ed.), DNA replication and human disease. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 5.Benguria, A., P. Hernandez, D. B. Krimer, and J. B. Schvartzman. 2003. Sir2p suppresses recombination of replication forks stalled at the replication fork barrier of ribosomal DNA in Saccharomyces cerevisiae. Nucleic Acids Res. 31893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer, B. J., and W. L. Fangman. 1987. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51463-471. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, B. J., and W. L. Fangman. 1988. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell 55637-643. [DOI] [PubMed] [Google Scholar]
- 8.Brewer, B. J., D. Lockshon, and W. Fangman. 1992. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell 71267-271. [DOI] [PubMed] [Google Scholar]
- 9.Calzada, A., B. Hodgson, M. Kanemaki, A. Bueno, and K. Labib. 2005. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 191905-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carballo, J. A., A. L. Johnson, S. G. Sedgwick, and R. S. Cha. 2008. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell 132758-770. [DOI] [PubMed] [Google Scholar]
- 11.Cha, R. S., and N. Kleckner. 2002. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297602-606. [DOI] [PubMed] [Google Scholar]
- 12.Coulon, S., P. H. Gaillard, C. Chahwan, W. H. McDonald, J. R. Yates III, and P. Russell. 2004. Slx1-Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol. Biol. Cell 1571-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 2741664-1672. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb, S., and R. E. Esposito. 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56771-776. [DOI] [PubMed] [Google Scholar]
- 15.Haber, J. 1999. DNA recombination: the replication connection. Trends Biochem. Sci. 24271-275. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi, T., H. Nishitani, and T. Kobayashi. 1995. A new type of E. coli recombinational hotspot which requires for activity both DNA replication termination events and the Chi sequence. Adv. Biophys. 31133-147. [DOI] [PubMed] [Google Scholar]
- 17.Huang, J., I. L. Brito, J. Villen, S. P. Gygi, A. Amon, and D. Moazed. 2006. Inhibition of homologous recombination by a cohesin-associated clamp complex recruited to the rDNA recombination enhancer. Genes Dev. 202887-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, J., and D. Moazed. 2003. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 172162-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivessa, A. S., J. Q. Zhou, V. P. Schulz, E. K. Monson, and V. A. Zakian. 2002. Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 161383-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaliraman, V., and S. J. Brill. 2002. Role of SGS1 and SLX4 in maintaining rDNA structure in Saccharomyces cerevisiae. Curr. Genet. 41389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katou, Y., Y. Kanoh, M. Bando, H. Noguchi, H. Tanaka, T. Ashikari, K. Sugimoto, and K. Shirahige. 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 4241078-1083. [DOI] [PubMed] [Google Scholar]
- 22.Keil, R. L., and G. S. Roeder. 1984. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell 39377-386. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, T. 2003. The replication fork barrier site forms a unique structure with Fob1p and inhibits the replication fork. Mol. Cell. Biol. 239178-9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi, T., and A. R. Ganley. 2005. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 3091581-1584. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi, T., D. J. Heck, M. Nomura, and T. Horiuchi. 1998. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev. 123821-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi, T., T. Horiuchi, P. Tongaonkar, L. Vu, and M. Nomura. 2004. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117441-453. [DOI] [PubMed] [Google Scholar]
- 27.Lambert, S., A. Watson, D. M. Sheedy, B. Martin, and A. M. Carr. 2005. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell 121689-702. [DOI] [PubMed] [Google Scholar]
- 28.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 29.Lucca, C., F. Vanoli, C. Cotta-Ramusino, A. Pellicioli, G. Liberi, J. Haber, and M. Foiani. 2004. Checkpoint-mediated control of replisome-fork association and signalling in response to replication pausing. Oncogene 231206-1213. [DOI] [PubMed] [Google Scholar]
- 30.Mayan-Santos, M. D., M. L. Martinez-Robles, P. Hernandez, J. B. Schvartzman, and D. B. Krimer. 2008. A redundancy of processes that cause replication fork stalling enhances recombination at two distinct sites in yeast rDNA. Mol. Microbiol. 69361-375. [DOI] [PubMed] [Google Scholar]
- 31.Mohanty, B. K., N. K. Bairwa, and D. Bastia. 2006. The Tof1p-Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 103897-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanty, B. K., and D. Bastia. 2004. Binding of the replication terminator protein Fob1p to the Ter sites of yeast causes polar fork arrest. J. Biol. Chem. 2791932-1941. [DOI] [PubMed] [Google Scholar]
- 33.Paques, P., and J. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petes, T. D. 1979. Yeast ribosomal DNA genes are located on chromosome XII. Proc. Natl. Acad. Sci. USA 76410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serizawa, N., T. Horiuchi, and T. Kobayashi. 2004. Transcription-mediated hyper-recombination in HOT1. Genes Cells 9305-315. [DOI] [PubMed] [Google Scholar]
- 36.Shechter, D., V. Costanzo, and J. Gautier. 2004. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell Biol. 6648-655. [DOI] [PubMed] [Google Scholar]
- 37.Sinclair, D. A., K. Mills, and L. Guarente. 1998. Molecular mechanisms of yeast aging. Trends Biochem. Sci. 23131-134. [DOI] [PubMed] [Google Scholar]
- 38.Straight, A. F., W. Shou, G. J. Dowd, C. W. Turck, R. J. Deshaies, A. D. Johnson, and D. Moazed. 1999. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97245-256. [DOI] [PubMed] [Google Scholar]
- 39.Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein, and F. W. Stahl. 1983. The double-strand-break repair model for recombination. Cell 3325-35. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, Y., T. Horiuchi, and T. Kobayashi. 2003. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev. 171497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tercero, J. A., and J. F. Diffley. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412553-557. [DOI] [PubMed] [Google Scholar]
- 42.Torres, J. Z., J. B. Bessler, and V. A. Zakian. 2004. Local chromatin structure at the ribosomal DNA causes replication fork pausing and genome instability in the absence of the S. cerevisiae DNA helicase Rrm3p. Genes Dev. 18498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres, J. Z., S. L. Schnakenberg, and V. A. Zakian. 2004. Saccharomyces cerevisiae Rrm3p DNA helicase promotes genome integrity by preventing replication fork stalling: viability of rrm3 cells requires the intra-S-phase checkpoint and fork restart activities. Mol. Cell. Biol. 243198-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tourriere, H., G. Versini, V. Cordon-Preciado, C. Alabert, and P. Pasero. 2005. Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol. Cell 19699-706. [DOI] [PubMed] [Google Scholar]
- 45.Tsang, E., and A. Carr. 2008. Replication fork arrest, recombination and the maintenance of ribosomal DNA stability. DNA Repair (Amsterdam) 101613-1623. [DOI] [PubMed] [Google Scholar]
- 46.Voelkel-Meiman, K., R. L. Keil, and G. S. Roeder. 1987. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell 481071-1079. [DOI] [PubMed] [Google Scholar]
- 47.Ward, T. R., M. L. Hoang, R. Prusty, C. K. Lau, R. L. Keil, W. L. Fangman, and B. J. Brewer. 2000. Ribosomal DNA replication fork barrier and HOT1 recombination hot spot: shared sequences but independent activities. Mol. Cell. Biol. 204948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinert, T. A., and L. H. Hartwell. 1993. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics 13463-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, H., C. Boone, and H. L. Klein. 2004. Mrc1 is required for sister chromatid cohesion to aid in recombination repair of spontaneous damage. Mol. Cell. Biol. 247082-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zehfus, B. R., A. D. McWilliams, Y. H. Lin, M. F. Hoekstra, and R. L. Keil. 1990. Genetic control of RNA polymerase I-stimulated recombination in yeast. Genetics 12641-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao, X., A. Chabes, V. Domkin, L. Thelander, and R. Rothstein. 2001. The ribonucleotide reductase inhibitor Sml1 is a new target of the Mec1/Rad53 kinase cascade during growth and in response to DNA damage. EMBO J. 203544-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou, H., and R. Rothstein. 1997. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell 9087-96. [DOI] [PubMed] [Google Scholar]