Abstract
The apicomplexan parasite Cryptosporidium is a significant cause of diarrheal disease worldwide. Previously, we reported that a Cryptosporidium parvum subtilisin-like serine protease activity with furin-type specificity cleaves gp40/15, a glycoprotein that is proteolytically processed into gp40 and gp15, which are implicated in mediating infection of host cells. Neither the enzyme(s) responsible for the protease activity in C. parvum lysates nor those that process gp40/15 are known. There are no furin or other proprotein convertase genes in the C. parvum genome. However, a gene encoding CpSUB1, a subtilisin-like serine protease, is present. In this study, we cloned the CpSUB1 genomic sequence and expressed and purified the recombinant prodomain. Reverse transcriptase PCR analysis of RNA from C. parvum-infected HCT-8 cells revealed that CpSUB1 is expressed throughout infection in vitro. In immunoblots, antiserum to the recombinant CpSUB1 prodomain revealed two major bands, of ∼64 kDa and ∼48 kDa, for C. parvum lysates and proteins “shed” during excystation. In immunofluorescence assays, the antiserum reacted with the apical region of sporozoites and merozoites. The recombinant prodomain inhibited protease activity and processing of recombinant gp40/15 by C. parvum lysates but not by furin. Since prodomains are often selective inhibitors of their cognate enzymes, these results suggest that CpSUB1 may be a likely candidate for the protease activity in C. parvum and for processing of gp40/15. Importantly, the recombinant prodomain inhibited C. parvum infection of HCT-8 cells. These studies indicate that CpSUB1 plays a significant role in infection of host cells by the parasite and suggest that this enzyme may serve as a target for intervention.
Cryptosporidium is a waterborne apicomplexan parasite that causes diarrhea in humans and animals worldwide (20, 37, 39). Infection in immunocompetent individuals is generally asymptomatic or self-limiting. However, severe, prolonged, and possibly fatal illness can occur in immunocompromised hosts, such as AIDS patients. Two major species, Cryptosporidium hominis and C. parvum, cause most human infections (42). Transmission occurs by animal-to-human or human-to-human contact or by consumption of contaminated water and food. Nitazoxanide is the only FDA-approved treatment for cryptosporidiosis in the United States; however, this drug is not effective in immunocompromised individuals (2). Therefore, continuing efforts to develop new and effective interventions are essential.
The processes of attachment, invasion, parasitophorous vacuole formation, and egress are crucial events in C. parvum infection. However, little is known about the specific parasite and host molecules involved in these processes (39). Progress in identifying these molecules and their functional roles has been hindered by the inability to propagate Cryptosporidium in vitro and to genetically manipulate the parasite (39). However, completion of the genome sequences of Cryptosporidium spp. (1, 43) has facilitated the identification of proteins homologous to those which mediate these processes in the related apicomplexans Toxoplasma and Plasmodium. These include surface proteins as well as those secreted from specialized apical complex organelles, such as micronemes, rhoptries, and dense granules (36).
Proteolytic processing of surface and apical complex proteins by parasite proteases is required for invasion of host cells, for assembly and trafficking of proteins, and for egress from host cells (6, 7, 10, 22). A number of these proteins are processed by serine proteases, which are characterized by the presence of a conserved serine residue in the active site (18). Serine protease inhibitors have been shown to block invasion, intracellular development, or egress of Toxoplasma (33) and Plasmodium (6, 7) as well as Cryptosporidium (14, 15, 40), indicating the importance of these proteases in mediating infection.
Subtilisin-like serine proteases (subtilases) are synthesized as inactive preproproteins, which consist minimally of a signal peptide, a propeptide domain (prodomain), and a catalytic domain. Following signal peptide cleavage, the prodomain is autocatalytically cleaved but remains noncovalently bound and functions as an intramolecular chaperone for correct folding and maturation of the active enzyme (34). Prodomains are frequently potent and selective inhibitors of their cognate enzymes (23).
We and others previously cloned and characterized Cryptosporidium gp40/15, a precursor glycoprotein that is proteolytically processed to yield surface glycopeptides gp40 and gp15, which are involved in mediating infection (reviewed in reference 39). We recently showed that gp40/15 is processed by human furin, as well as by a subtilisin-like protease activity with furin-type specificity, in C. parvum lysates (40). Importantly, a specific furin inhibitor, decanoyl-Arg-Val-Lys-Arg-chloromethylketone (Dec-RVKR-cmk) abrogated C. parvum infection of intestinal epithelial cells in vitro, suggesting that furin-like serine protease activity may be involved in mediating host-parasite interactions. Neither the enzyme(s) responsible for the serine protease activity in C. parvum lysates nor those that process gp40/15 have been identified. Typical furin or other proprotein convertase genes are not present in Cryptosporidium genomes. However, genes encoding the subtilisin-like proteases CpSUB1 and ChSUB1 have been identified in the C. parvum and C. hominis genomes (14, 40). In this study, we characterize CpSUB1 and present data to suggest that this enzyme contributes to the serine protease activity of C. parvum lysates, processes gp40/15, and plays an important role in infection of host cells in vitro.
MATERIALS AND METHODS
Parasites.
C. parvum (Iowa isolate) oocysts were obtained from Bunch Grass Farm, Deary, ID, and stored in phosphate-buffered saline (PBS) at 4°C until use. Prior to use, oocysts were treated with 1.75% (vol/vol) sodium hypochlorite for 10 min on ice and then washed five times with 20 mM phosphate buffer, pH 7.2, containing 150 mM sodium chloride (PBS) by centrifugation at 8,000 × g for 3 min at 4°C. Hypochlorite-treated oocysts were excysted by incubation at 37°C for 1 h in 0.75% taurocholic acid in PBS.
For Western blotting, C. parvum lysate was prepared as follows. Hypochlorite-treated oocysts (108/ml) were excysted for 2 h at 37°C in the presence of 0.75% taurocholic acid in 1 ml Dulbecco's modified Eagle's medium (DMEM). Protease inhibitors (protease inhibitor cocktail set III; Calbiochem, La Jolla, CA) were then added, and the mixture of unexcysted oocysts and sporozoites was concentrated into a pellet by centrifugation at 10,000 × g for 30 min at 4°C. Shed proteins were obtained as described previously (11). C. parvum lysate for protease assays was prepared as described previously (40).
Cloning and sequencing of CpSUB1.
Primers encompassing the open reading frame of C. parvum Iowa II 1MB.729 (GenBank accession no. BX526834) (CpSUB1-F and CpSUB1-R) were designed (Table 1), and the sequence was PCR amplified from genomic DNA isolated from Iowa isolate oocysts, using a DNA isolation kit (Gnome kit; QBIOgene, Irvine, CA). The PCR product was extracted from 2% agarose gels, using a QIAQuick gel extraction kit (Qiagen Inc., Valencia, CA), and cloned into the Topo pCRII vector (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Plasmids were purified using a Qiagen miniprep kit (Qiagen), and the nucleotide sequences were determined by automated DNA dye terminator cycle sequencing at the Tufts University core facility, employing a Perkin-Elmer ABI 377 sequencer. Nucleotide sequences were analyzed using Vector NTI software (Invitrogen) and the ExPASy Molecular Biology Server (http://us.expasy.org).
TABLE 1.
Primers used for PCR cloning and RT-PCR
| Primer | Nucleotide sequence (5′-3′)a |
|---|---|
| CpSUB1-F | ATGAAAAAGGTAAATATTTTCAAGTTGTTG |
| CpSUB1-R | TCATGAATCTAACCTACCAAATTCATT |
| CpSUB1-CDF | AAAGGATCTGGAGTATATTAT |
| CpSUB1-CDR | AATATTTAATGTCCCAGAGG |
| CpSUB1-PDF | GGTATTGAGGGTCGCAGATCTAAAGTTATTCCAGGA |
| CpSUB1-PDR | AGAGGAGAGTTAGAGCCATATTCAACTTCATCACTATG |
Underlined nucleotides represent overhangs required for ligation-independent cloning into the pET 32Xa/LIC vector.
Cell culture.
Human ileocecal adenocarcinoma cells (HCT-8 cells) were cultured in DMEM (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum, 25 mM HEPES, 4 mM l-glutamine, 25 mM sodium pyruvate, 100 U of penicillin, and 100 μg of streptomycin per ml at 37°C in 5% CO2.
RT-PCR.
C. parvum oocysts (4 × 106 per flask) were used to infect confluent HCT-8 cell monolayers grown in T-25 tissue culture flasks. Total RNA was isolated from infected and uninfected HCT-8 cells at 0, 6, 12, 24, 48, and 72 h postinfection, using an RNeasy kit (Qiagen). Contaminating DNA was removed using a DNase-free kit (Ambion, Austin, TX). RNA was reverse transcribed to cDNA by using the Superscript first-strand system (Invitrogen). cDNA was amplified by PCR for 35 cycles (94°C for 1 min, 64°C for 1 min, and 72°C for 1.5 min), using primers (CpSUB1-CDF and CpSUB1-CDR) spanning the predicted catalytic domain (Table 1). cDNA from uninfected HCT-8 cells was used as a negative control, and reactions without reverse transcriptase (RT) were performed in parallel to control for amplification of contaminating DNA. RT-PCR for human β-actin was performed as a loading control, and PCR on genomic DNA was performed as a positive control.
Cloning, expression, and purification of CpSUB1 prodomain.
The prodomain sequence (nucleotides 364 to 672) of CpSUB1 was amplified from C. parvum genomic DNA by using the primers CpSUB1-PDF and CpSUB1-PDR (Table 1) and was cloned into the pET 32Xa/LIC expression vector (Novagen, Madison, WI). The recombinant fusion protein was overexpressed in Escherichia coli BL21(DE3) cells following induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Gold Biotechnology, Inc., St. Louis, MO). To determine whether the fusion protein was soluble, E. coli cells expressing the fusion proteins were harvested from 1-ml cultures by centrifugation at 16,000 × g for 10 min and were lysed with BugBuster protein extraction reagent (Novagen) in the presence of Benzonase nuclease (Novagen) and protease inhibitor cocktail set III. The supernatant and the pellet (containing inclusion bodies) were separated by centrifugation at 16,000 × g for 20 min at 4°C, resuspended in PBS, and resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The fusion protein was detected by Coomassie blue R-250 staining and Western blotting, using horseradish peroxidase (HRP)-conjugated S protein (which binds to the S tag present in the fusion protein).
For large-scale protein purification, 1 liter of LB broth containing 100 μg/ml ampicillin was inoculated with 20 ml of fresh overnight culture and grown to an optical density at 600 nm of 0.6 at 37°C with vigorous shaking prior to induction with 1 mM IPTG for 4 h. The bacteria were harvested by centrifugation at 6,500 × g for 20 min at 4°C, and the pellet was stored at −20°C until use. The cells were resuspended in 50 mM Tris-HCl, pH 8.0, containing 10 mM EDTA and 1% Triton X-100, 1 mg/ml of lysozyme was added, and the mixture was incubated on ice for 30 min. The cells were then lysed in the presence of protease inhibitor cocktail set III by sonication using a Sonic Dismembrator model 100 (Fisher Scientific, Pittsburgh, PA) sonicator for six 10-s bursts at 200 to 300 W, with a 10-s cooling period between each burst. Inclusion bodies were collected by centrifugation at 10,000 × g for 10 min, washed, solubilized, and refolded using a protein refolding kit (Novagen) according to the manufacturer's instructions. The refolded protein was resuspended in binding buffer (50 mM sodium phosphate, pH 8.0, containing 300 mM NaCl and 10 mM imidazole), and the recombinant protein was affinity purified using nickel-nitrilotriacetic superflow (Qiagen) columns according to the manufacturer's instructions. The eluted protein was concentrated by ultrafiltration at 4°C, using an Amicon YM 10 membrane (Millipore, Billerica, MA). The purified protein was detected by SDS-PAGE followed by staining with Coomassie blue R-250 and Western blotting with HRP-conjugated S protein. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce Chemical Co., Rockford, IL) according to the manufacturer's instructions.
Antibodies.
Purified recombinant CpSUB1PD protein was resolved by SDS-PAGE and stained with 10% Coomassie blue R-250 in water, and the relevant band (32 kDa) was excised from the gels and emulsified with complete Freund's adjuvant for the initial immunization and with incomplete Freund's adjuvant for subsequent boosts. BALB/c mice were immunized by intraperitoneal injection at 2-week intervals as previously described (11). Rabbit antiserum to recombinant C. parvum gp15 was obtained as described previously (30).
Western blotting.
Western blotting was performed as described previously (11), using mouse anti-CpSUB1PD serum as the primary antibody, with HRP-conjugated goat anti-mouse immunoglobulin G (IgG; Southern Biotech, Birmingham, AL) used for detection. Preimmune mouse sera were used as a control.
IFA.
Immunofluorescence assays (IFAs) of oocysts, sporozoites, and intracellular-stage organisms in C. parvum-infected HCT-8 cells were performed as described previously (29), using mouse anti-CpSUB1PD serum and rabbit anti-gp15 serum as primary antibodies, with detection by Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR), respectively. Slides were examined by differential interference contrast and fluorescence microscopy, using a Zeiss Axioimager Z.1 fluorescence microscope (Carl Zeiss Microscopy, Jena, Germany). z stacks with 0.1-mm spacing were captured with an Orca IEEE1394 digital charge-coupled device camera (Hamamatsu, Hamamatsu, Japan), and the images were deconvolved and merged using Volocity software (Improvision Inc., Lexington, MA) for pseudoconfocal deconvolution microscopy.
Protease inhibition assay.
Protease activity in C. parvum lysates was assayed using the synthetic peptidyl fluorogenic substrate t-butyloxycarbonyl-Arg-Val-Arg-Arg-7-amino-4-methylcoumarin (Boc-RVRR-AMC; Bachem Biosciences, King of Prussia, PA) as described previously (40). To investigate inhibition of protease activity, increasing concentrations of CpSUBPD or control protein (recombinant protein containing the fusion tags only) (11) were preincubated with the lysates for 30 min at 37°C prior to the assay. Inhibition of recombinant gp40/15 (rgp40/15) cleavage by C. parvum lysate was performed as described previously (40).
Infection assay.
Hypochlorite-treated oocysts (2 × 104/well) were preincubated with increasing concentrations of CpSUB1PD or control protein in serum-free DMEM or with serum-free DMEM alone at 37°C for 30 min and then added to HCT-8 cells grown to confluence in 96-well plates. The plates were incubated at 37°C in 5% CO2 for 24 h. After washing of the plates with prewarmed DMEM, the cells were fixed with methanol for 20 min at room temperature and infection was quantified by enzyme-linked immunosorbent assay as described previously (40).
Cytotoxicity and excystation assays.
Possible cytotoxicity of CpSUB1PD for the HCT-8 cells or sporozoites was assayed as described previously (9). The effect of the prodomain on excystation of oocysts was determined as described previously (40).
Statistical analysis.
All assays were performed in triplicate, and the mean and standard error were determined. Experiments were repeated at least three times. Results were analyzed statistically with Graph Pad Prism (Graph Pad Inc., San Diego, CA), using analysis of variance (ANOVA) tests. Differences were considered significant at P values of <0.05.
RESULTS
In silico analysis of CpSUB1.
We previously identified C. parvum Iowa isolate CpSUB1 and C. hominis TU502 ChSUB1 sequences from the Cryptosporidium databases in the CryptoDB genome resource (19) by BLAST searches with the catalytic domains of other apicomplexan subtilases (40). Feng et al. subsequently reported cloning and analyses of the CpSUB1 and ChSUB1 genes from the GCH1 and TU502 isolates, respectively (14). CryptoDB contains two CpSUB1 sequences from two different sequencing efforts, namely, sequence Cgd6_4840 from the C. parvum Iowa U.S. isolate (1) and sequence 1MB.729 from the C. parvum Iowa United Kingdom isolate (3). Both sequences are identical except that 1MB.729 has an additional 456 nucleotides containing a 76-bp intron at the 3′ end. This raised the question as to whether this was a gene duplication. We therefore examined contigs AAEE01000002 (1) and BX526834 (3), where these genes are located. We aligned 12,139 bp of sequence from the two contigs upstream and downstream of the CpSUB1 open reading frames and found that the contig sequences were identical. Therefore, it is likely that the two CpSUB1 sequences are not the result of gene duplication but that when the Cgd6_4840 gene was annotated, the intron and second exon sequences were not included. The complete CpSUB1 sequence from the C. parvum GCH1 isolate sequenced by Feng et al. was very similar to that of the United Kingdom Iowa isolate, with only 10 nucleotide changes (14). To confirm the complete CpSUB1 sequence from the U.S. Iowa isolate, we used primers based on the United Kingdom Iowa sequence to PCR amplify the gene using U.S. Iowa isolate genomic DNA. The sequence of this amplicon was identical to that of the 1MB.729 gene (data not shown).
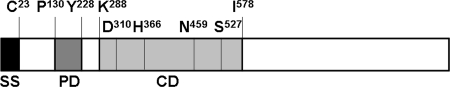
Bioinformatic analysis revealed that the 3,975-bp CpSUB1 coding sequence encodes a 1,324-amino-acid (aa) protein with a predicted molecular mass of 147 kDa and a pI of 5.8269. Figure 1 shows a schematic representation of the predicted domain structure of CpSUB1. The deduced amino acid sequence is predicted to contain a signal peptide with the cleavage site between C23 and N24 (SignalP 3.0 [http://www.cbs.dtu.dk/services/SignalP]). A 99-aa propeptide domain (P130 to Y228) and a 291-aa peptidase S8 catalytic domain (K288 to I578), diagnostic of subtilisin-like serine proteases, were predicted by the InterPro program (http://www.ebi.ac.uk/interpro/). The catalytic domain of CpSUB1 contains the conserved catalytic triad residues D310, H366, and S527 and the oxyanion hole residue N459 with the expected spacing (31). CpSUB1 is not predicted to contain a transmembrane domain or a C-terminal hydrophobic sequence for addition of a glycosylphosphatidylinositol (GPI) anchor. Five predicted N-glycosylation sites and one O-glycosylation site were identified using the NetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc) and NetOGlyc 3.1 (http://www.cbs.dtu.dk/services/NetOGlyc/) programs, respectively.
FIG. 1.
Schematic representation of deduced primary structure of CpSUB1. The CpSUB1 protein consists of 1,324 amino acid residues, with a predicted signal sequence (SS) from residues 1 to 23, a predicted prodomain (PD) from residues 130 to 228, and a predicted subtilisin catalytic domain (CD) from residues 288 to 578. The relative positions of the catalytic triad residues D (aspartate), H (histidine), and S (serine) and the oxyanion hole residue N (asparagine) are shown.
CpSUB1 is expressed throughout intracellular development.
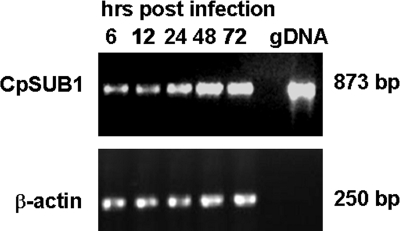
RT-PCR of RNA from HCT-8 cells infected with C. parvum oocysts for increasing periods of time revealed that the CpSUB1 gene is expressed throughout infection in vitro (Fig. 2). Control reactions including RT-PCR on RNA from uninfected cells and reactions performed in the absence of RT were negative (data not shown). These results confirm the findings of Feng et al. (14). However, precise quantification of CpSUB1 expression at different times after infection will need to be performed by quantitative real-time RT-PCR.
FIG. 2.
Expression of CpSUB1 in C. parvum-infected HCT-8 cells. RT-PCR was performed on total RNA extracted from C. parvum-infected HCT-8 cells at various times postinfection, using CpSUB1 catalytic domain-specific primers. Genomic DNA (gDNA) was used as a positive control, and β-actin was used as a loading control.
Cloning, overexpression, and purification of the CpSUB1 prodomain.
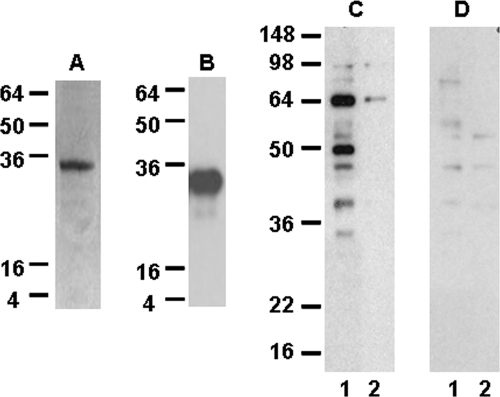
In order to initiate studies on the functional activity of CpSUB1, we cloned the sequence encoding the prodomain into the pET-32 Xa/LIC vector and overexpressed the recombinant fusion protein (designated CpSUB1PD) in E. coli. The fusion protein was present in inclusion bodies but could be solubilized, refolded, and purified in soluble form (Fig. 3). The molecular mass of CpSUB1PD is 32 kDa, as predicted.
FIG. 3.
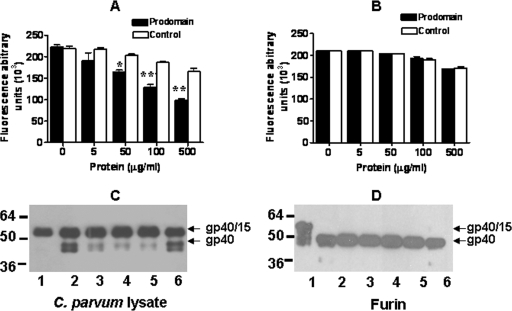
Purification of recombinant CpSUB1PD and Western blot analysis of parasite proteins recognized by antisera to recombinant CpSUB1PD. The recombinant CpSUB1PD protein was purified by metal affinity chromatography, and the purified protein was analyzed by 12% SDS-PAGE and Coomassie blue R250 staining (A) or by Western blotting with S protein (B) as described in Materials and Methods. (C and D) C. parvum lysate (lane 1) and shed proteins (lane 2) were subjected to 12% SDS-PAGE under reducing conditions, transferred to polyvinylidene difluoride, and probed with antisera to CpSUB1PD (C) or preimmune sera (D). Molecular size markers in kDa are indicated on the left of the gel and blots.
Antisera to CpSUB1PD recognize C. parvum proteins.
Western blotting of C. parvum lysates with antisera to CpSUB1PD revealed two major bands, of approximately 64 and 48 kDa, and minor bands, at approximately 55 and 30 kDa (Fig. 3). Upon prolonged exposure of the blots, faint bands of approximately 98 and 147 kDa were also sometimes detected. The 147-kDa band may represent the precursor preprotein, while the lower-molecular-mass bands may represent proteolytic processing products, as is the case for other subtilases. The ∼64-kDa band was the only one identified in proteins shed during excystation. There was no reactivity of the major bands with preimmune sera.
Immunolocalization of CpSUB1 in C. parvum sporozoites and intracellular-stage organisms.
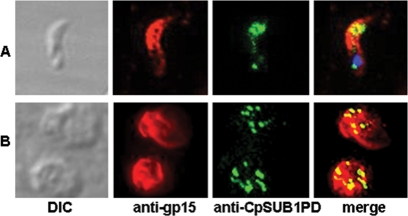
To investigate the localization of CpSUB1 in the parasite, we performed IFAs with anti-CpSUB1PD sera, using pseudoconfocal deconvolution microscopy. CpSUB1 was colocalized with gp15, an antigen that is present on the surfaces of sporozoites and merozoites (11). The results revealed reactivity of the antisera with internal organelles in the apical region of C. parvum sporozoites and intracellular merozoites in infected C. parvum HCT-8 cells (Fig. 4). The precise identification of Cryptosporidium organelles cannot be determined by light microscopy, and thus organellar localization of CpSUB1 will require immunoelectron microscopy.
FIG. 4.
CpSUB1 localizes to the apical region of sporozoites and merozoites. IFA was performed on sporozoites (A) and intracellular-stage organisms (B), using anti-CpSUB1PD and anti-gp15 sera. CpSUB1 (green) was colocalized with gp15 (red). Images were deconvolved to improve clarity. The sporozoite nucleus was stained with DAPI (4′,6-diamidino-2-phenylindole) (blue). There was no specific reactivity with preimmune sera (not shown).
The CpSUB1 prodomain inhibits serine protease activity of C. parvum but not the activity of furin.
Prodomains of other subtilases (23), including PfSUB1 (21), have been shown to be potent and specific inhibitors of their cognate enzymes. To determine whether CpSUB1 contributed to the furin-like serine protease activity we previously reported for C. parvum lysates (40), we preincubated the lysates with the recombinant CpSUB1 prodomain or a control recombinant protein containing only the fusion tags (11) before measuring protease activity. Figure 5A shows that CpSUB1PD significantly inhibited the protease activity in a dose-dependent manner compared to the control protein (P = 0.038). To assess the selectivity of the inhibition by CpSUB1PD, we examined its effect on the activity of recombinant human furin. The results indicated that there was no inhibition of furin activity even at the highest concentration tested (Fig. 5B). These results suggest that CpSUB1 contributes to the serine protease activity of C. parvum lysates.
FIG. 5.
The CpSUB1 prodomain inhibits protease activity and processing of rgp40/15 by the C. parvum lysate but not by furin. C. parvum lysate (A) or furin (B) was preincubated with increasing amounts of CpSUB1 prodomain or the control protein, and activity was measured by Boc-RVRR-AMC cleavage. Each graph represents data pooled from three separate experiments, each performed in triplicate and analyzed by two-way ANOVA (*, P < 0.05; **, P < 0.001). The error bars represent the standard deviations. C. parvum lysate (C) or furin (D) was preincubated with CpSUB1 prodomain or control protein before the addition of rgp40/15. Cleavage of rgp40/15 was analyzed by Western blotting with S protein. Lanes 1, untreated rgp40/15; lanes 2, rgp40/15 treated with C. parvum lysate or furin; lanes 3 to 5, rgp40/15 treated with C. parvum lysate or furin plus 150 (lanes 3), 750 (lanes 4), or 1,500 (lanes 5) ng CpSUB1 prodomain; lanes 6, rgp40/15 treated with C. parvum lysate or furin plus 1,500 ng control protein.
The CpSUB1 prodomain inhibits the processing of gp40/15 by the C. parvum serine protease activity but not by furin.
We previously showed that rgp40/15 was proteolytically processed by the serine protease activity in C. parvum lysates as well as by human furin (40). In order to determine whether CpSUB1 is responsible for this processing, we preincubated the C. parvum lysate or human furin with increasing concentrations of CpSUB1PD or the control fusion protein and assayed for cleavage of rgp40/15. Figure 5C shows that CpSUB1PD (lanes 3, 4, and 5) but not the control protein (lane 6) almost completely inhibited processing of rgp40/15 by the C. parvum lysates. In contrast, neither CpSUB1PD nor the control protein had any effect on cleavage of rgp40/15 by furin (Fig. 5D). These results suggest that gp40/15 may be a substrate for CpSUB1.
The CpSUB1 prodomain inhibits C. parvum infection of HCT-8 cells.
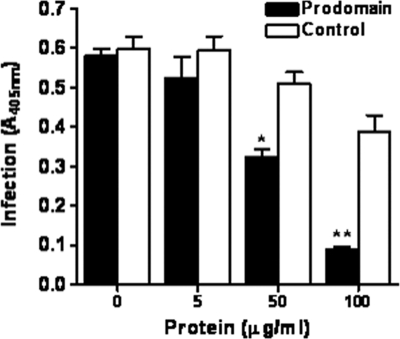
Previous studies have shown that serine protease inhibitors decrease C. parvum infection in vitro (14, 15, 40). To investigate the role of CpSUB1 in C. parvum infection, we determined the effect of CpSUB1PD on infection of host cells by the parasite in vitro. Figure 6 shows that CpSUB1PD significantly inhibited C. parvum infection of HCT-8 cells in a dose-dependent manner compared to the control recombinant protein. Inhibition of C. parvum infection of HCT-8 cells by CpSUB1PD was not due to cytotoxicity of CpSUB1PD for either the host cells or the parasite (data not shown). In addition, CpSUB1PD had no effect on excystation of C. parvum oocysts (data not shown), indicating that inhibition of infection was not due to an inhibitory effect on excystation. These findings suggest that CpSUB1 plays a key role in infection of host cells.
FIG. 6.
Recombinant CpSUB1 prodomain inhibits C. parvum infection of HCT-8 cells. HCT-8 cells were infected for 24 h with oocysts that had been preincubated with increasing concentrations of CpSUB1PD or control protein for 30 min. Infection was quantified by enzyme-linked immunosorbent assay. The graph represents data pooled from three separate experiments, each performed in triplicate and analyzed by two-way ANOVA (*, P < 0.05; **, P < 0.01). The error bars represent the standard deviations.
DISCUSSION
Studies employing specific protease inhibitors have suggested that a serine protease(s) plays a key role in C. parvum infection in vitro (14, 15, 40) and raised the possibility that it may serve as a target for development of drugs against cryptosporidiosis. Recently, genes encoding serine proteases, including subtilisin-like and rhomboid proteases, have been identified from the Cryptosporidium genomes (12, 14, 39, 40). In this study, we focused on the subtilisin-like serine protease or subtilase CpSUB1.
Although subtilases have been identified in a number of apicomplexan parasites, the best-characterized subtilases are those of Plasmodium falciparum and Toxoplasma gondii (7, 10, 22, 41). For P. falciparum, three subtilases are currently annotated in PlasmoDB, but only PfSUB1 and PfSUB2 have been characterized (41). PfSUB1 is a secreted protein that may be essential for survival, as the PfSUB1 gene cannot be disrupted (8, 21, 32, 41). This enzyme is discharged from newly described organelles called “exonemes” into the parasitophorous vacuolar space, where it mediates proteolytic processing of at least two members of the serine-rich antigen enzyme family and facilitates egress of merozoites from the host erythrocyte (44). PfSUB2, a transmembrane microneme protein which functions as a “sheddase” for the surface adhesins MSP-1 and AMA-1, is also encoded by an essential gene (4, 16, 17). In T. gondii, two of at least eight putative subtilases have been characterized. TgSUB1 is a GPI-anchored microneme protein whose substrate(s) is not known (5, 22, 26). The gene encoding TgSUB1 was recently shown to be nonessential (V. Lagal and K. Kim, submitted for publication). TgSUB2, a transmembrane protein localized to rhoptries, functions as a rhoptry maturase and is encoded by an essential gene (22, 27). Other described subtilases include NcSUB1, a secreted subtilase in Neospora caninum present in micronemes (24, 25), and BdSUB1, a subtilase of Babesia divergens, also a secreted protein which localizes to dense granules (28). The functions and substrates of these enzymes are unknown.
Analysis of the deduced amino acid sequence of CpSUB1 revealed the characteristic domain structure of subtilases, with a signal peptide, a propeptide domain, and a catalytic domain. Like PfSUB1 and BdSUB1, CpSUB1 is not predicted to have a transmembrane domain or a GPI anchor attachment site, suggesting that it is a soluble protein. This is consistent with the finding that at least one of the protein bands revealed by antisera to the recombinant propeptide domain is “shed” during excystation. The catalytic domain displays the distinctive catalytic triad and oxyanion hole residues and is 38 to 45% homologous to those of other apicomplexan subtilases (14, 40). However, consistent with the finding that prodomains of subtilisin-like proteases generally have no sequence conservation, there is no significant homology with other apicomplexan subtilases in the region of the prodomain sequence.
Antisera to the CpSUB1 prodomain showed several bands (two major and three minor) for C. parvum lysates that were of lower molecular masses than the predicted 147 kDa of the complete protein. This observation is consistent with the finding that other apicomplexan subtilases are processed (6, 15, 25, 26) and suggests that like these enzymes, CpSUB1 is synthesized as an inactive preprotein, which then undergoes posttranslational processing into intermediately processed species and the mature enzyme. Ongoing studies are directed at confirming this by biosynthetic labeling and pulse-chase experiments. Autocatalytic processing is very typical for subtilases and represents a mechanism of controlled protease activation in which the inactive precursor is converted to an active enzyme only when it reaches the appropriate subcellular compartment (35). All other apicomplexan subtilases characterized thus far are localized to apical complex organelles. CpSUB1 is also present in the apical region of invasive-stage organisms, although the organellar localization has not yet been determined.
Previously, we reported a serine protease activity with furin-type specificity in C. parvum lysates that cleaves the synthetic substrate Boc-RVRR-AMC as well as the precursor glycoprotein gp40/15. Since typical furin or other proprotein convertases are not present in the C. parvum genome, we hypothesized that CpSUB1 may contribute to the serine protease activity of the lysates as well as to processing of gp40/15. To investigate this possibility, we exploited the finding that subtilase prodomains are often potent and selective inhibitors of their cognate enzymes. For example, the recombinant propeptides of PfSUB1 and PfSUB2 have been shown to inhibit the proteolytic activity of these enzymes (17, 21). The recombinant PfSUB1 propeptide is a rapid-equilibrium, high-affinity competitive inhibitor, with an experimentally determined Ki(app) for inhibition of recombinant PfSUB1 of 5.3 ± 0.4 nM (21). We found that the recombinant CpSUB1 prodomain inhibited cleavage of Boc-RVRR-AMC, as well as that of gp40/15, in a dose-dependent manner compared to a control protein containing only the fusion tags. The inhibition was specific for the protease activity in the C. parvum lysate, since the prodomain had no effect on furin-mediated cleavage of Boc-RVRR-AMC and gp40/15. These results suggest that CpSUB1 is likely to be responsible for both the protease activity of C. parvum lysates and cleavage of gp40/15. However, confirmation of this possibility and determination of the inhibitory kinetics of the propeptide domain will require the expression of enzymatically active CpSUB1.
To date, CpSUB1 is the only subtilase that has been shown to be expressed in C. parvum. Another sequence encoding a putative subtilase-like protein is present in the Cryptosporidium genomes, but this sequence is incomplete at both the 5′ and 3′ ends and the serine residue of the catalytic triad is replaced by an asparagine. It remains to be determined whether this gene expresses an active enzyme. The only other serine proteases identified in the Cryptosporidium genomes are rhomboids (13). These are conserved, intramembrane proteases that cleave their substrates within their transmembrane domains (38). Since rhomboid proteases do not display subtilisin-like protease activity or furin-type specificity, they are not likely to be responsible for the Boc-RVRR-AMC cleavage by C. parvum lysates. In addition, since gp40/15 is not a transmembrane protein and does not contain a Spitz-like substrate motif, it is not a putative substrate for rhomboid proteases.
Serine protease inhibitors, including those which inhibit subtilisin-like proteases, have been shown to inhibit C. parvum infection in vitro (14, 15). Since the CpSUB1 prodomain inhibited the processing of gp40/15 into gp40 and gp15, antigens that are important for infection in vitro, it was of interest to determine the effect of CpSUB1PD on infection. We found that CpSUB1PD significantly inhibited C. parvum infection of HCT-8 cells in a dose-dependent manner compared to the control protein. This finding is consistent with our previous study showing that the furin inhibitor Dec-RVKR-cmk, which inhibits the C. parvum lysate protease activity and blocks processing of gp40/15, also abrogates infection in vitro. These findings suggest that processing of gp40/15 by CpSUB1 may play an important role in mediating C. parvum infection. However, it is also possible that inhibition of infection may be related to CpSUB1 processing of other proteins involved in infection.
Since CpSUB1PD is not expected to penetrate host cells, inhibition of infection implies that the proteolytic events involved occur at the parasite surface. Many proteolytic processing events, such as that of the Plasmodium merozoite proteins MSP-1 and AMA-1 by PfSUB2, take place at the surface (17). This is facilitated by secretion of PfSUB2 onto the surfaces of merozoites (16). Similarly, CpSUB1, which is present in the apical region of invasive-stage organisms, may be secreted from apical complex organelles onto the surface.
In summary, the results of this study indicate that CpSUB1 is expressed during infection in vitro, localizes to the apical region of invasive-stage organisms, is likely to contribute to the protease activity of C. parvum lysates and to processing of gp40/15, and may be important in mediating infection in vitro. Additional studies are required to conclusively demonstrate the role of this enzyme in C. parvum-host cell interactions and to determine whether it may serve as a target for intervention.
Acknowledgments
This work was supported by NIH grants RO1 AI05786 and R21 AI077476 (to H.D.W.) and by grant RO1 AI46985 (to K.K.). J.W. was supported by NIH training grant T32 AI007329. We also acknowledge support from National Institute of Allergy and Infectious Diseases conference grant R13AI078718. P.T. was supported by a scholarship from Siriraj Hospital, Mahidol University, Bangkok, Thailand.
We thank Anne Kane, Tufts Medical Center, for preparation of media, plasmids, and recombinant proteins and the Tufts University Core Facility for nucleotide sequencing. We also thank Anne Kane, Jiazhou Qiu, and Andrew G. Plaut, Tufts Medical Center, for help and advice with the protease assays and for stimulating discussions.
Footnotes
Published ahead of print on 23 January 2009.
REFERENCES
- 1.Abrahamsen, M. S., T. J. Templeton, S. Enomoto, J. E. Abrahante, G. Zhu, C. A. Lancto, M. Deng, C. Liu, G. Widmer, S. Tzipori, G. A. Buck, P. Xu, A. T. Bankier, P. H. Dear, B. A. Konfortov, H. F. Spriggs, L. Iyer, V. Anantharaman, L. Aravind, and V. Kapur. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304441-445. [DOI] [PubMed] [Google Scholar]
- 2.Abubakar, I., S. H. Aliyu, C. Arumugam, P. R. Hunter, and N. K. Usman. 2007. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007CD004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankier, A. T., H. F. Spriggs, B. Fartmann, B. A. Konfortov, M. Madera, C. Vogel, S. A. Teichmann, A. Ivens, and P. H. Dear. 2003. Integrated mapping, chromosomal sequencing and sequence analysis of Cryptosporidium parvum. Genome Res. 131787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barale, J. C., T. Blisnick, H. Fujioka, P. M. Alzari, M. Aikawa, C. Braun-Breton, and G. Langsley. 1999. Plasmodium falciparum subtilisin-like protease 2, a merozoite candidate for the merozoite surface protein 1-42 maturase. Proc. Natl. Acad. Sci. USA 966445-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder, E. M., V. Lagal, and K. Kim. 2008. The prodomain of Toxoplasma gondii GPI-anchored subtilase TgSUB1 mediates its targeting to micronemes. Traffic 91485-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman, M. J. 2008. Malarial proteases and host cell egress: an ‘emerging’ cascade. Cell. Microbiol. 101925-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman, M. J. 2004. Proteases in host cell invasion by the malaria parasite. Cell. Microbiol. 6893-903. [DOI] [PubMed] [Google Scholar]
- 8.Blackman, M. J., H. Fujioka, W. H. Stafford, M. Sajid, B. Clough, S. L. Fleck, M. Aikawa, M. Grainger, and F. Hackett. 1998. A subtilisin-like protein in secretory organelles of Plasmodium falciparum merozoites. J. Biol. Chem. 27323398-23409. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 583488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carruthers, V. B., and M. J. Blackman. 2005. A new release on life: emerging concepts in proteolysis and parasite invasion. Mol. Microbiol. 551617-1630. [DOI] [PubMed] [Google Scholar]
- 11.Cevallos, A. M., X. Zhang, M. K. Waldor, S. Jaison, X. Zhou, S. Tzipori, M. R. Neutra, and H. D. Ward. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 684108-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowse, T., and D. Soldati. 2004. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr. Opin. Microbiol. 7388-396. [DOI] [PubMed] [Google Scholar]
- 13.Dowse, T. J., and D. Soldati. 2005. Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parasitol. 21254-258. [DOI] [PubMed] [Google Scholar]
- 14.Feng, X., D. E. Akiyoshi, G. Widmer, and S. Tzipori. 2007. Characterization of subtilase protease in Cryptosporidium parvum and C. hominis. J. Parasitol. 93619-626. [DOI] [PubMed] [Google Scholar]
- 15.Forney, J. R., S. Yang, C. Du, and M. C. Healey. 1996. Efficacy of serine protease inhibitors against Cryptosporidium parvum infection in a bovine fallopian tube epithelial cell culture system. J. Parasitol. 82638-640. [PubMed] [Google Scholar]
- 16.Hackett, F., M. Sajid, C. Withers-Martinez, M. Grainger, and M. J. Blackman. 1999. PfSUB-2: a second subtilisin-like protein in Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 103183-195. [DOI] [PubMed] [Google Scholar]
- 17.Harris, P. K., S. Yeoh, A. R. Dluzewski, R. A. O'Donnell, C. Withers-Martinez, F. Hackett, L. H. Bannister, G. H. Mitchell, and M. J. Blackman. 2005. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 1241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedstrom, L. 2002. Serine protease mechanism and specificity. Chem. Rev. 1024501-4524. [DOI] [PubMed] [Google Scholar]
- 19.Heiges, M., H. Wang, E. Robinson, C. Aurrecoechea, X. Gao, N. Kaluskar, P. Rhodes, S. Wang, C. Z. He, Y. Su, J. Miller, E. Kraemer, and J. C. Kissinger. 2006. CryptoDB: a Cryptosporidium bioinformatics resource update. Nucleic Acids Res. 34D419-D422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, D. B., and A. C. White. 2006. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. N. Am. 35291-314. [DOI] [PubMed] [Google Scholar]
- 21.Jean, L., F. Hackett, S. R. Martin, and M. J. Blackman. 2003. Functional characterization of the propeptide of Plasmodium falciparum subtilisin-like protease-1. J. Biol. Chem. 27828572-28579. [DOI] [PubMed] [Google Scholar]
- 22.Kim, K. 2004. Role of proteases in host cell invasion by Toxoplasma gondii and other Apicomplexa. Acta Trop. 9169-81. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y., Z. Hu, F. Jordan, and M. Inouye. 1995. Functional analysis of the propeptide of subtilisin E as an intramolecular chaperone for protein folding. Refolding and inhibitory abilities of propeptide mutants. J. Biol. Chem. 27025127-25132. [DOI] [PubMed] [Google Scholar]
- 24.Louie, K., and P. A. Conrad. 1999. Characterization of a cDNA encoding a subtilisin-like serine protease (NC-p65) of Neospora caninum. Mol. Biochem. Parasitol. 103211-223. [DOI] [PubMed] [Google Scholar]
- 25.Louie, K., R. Nordhausen, T. W. Robinson, B. C. Barr, and P. A. Conrad. 2002. Characterization of Neospora caninum protease, NcSUB1 (NC-P65), with rabbit anti-N54. J. Parasitol. 881113-1119. [DOI] [PubMed] [Google Scholar]
- 26.Miller, S. A., E. M. Binder, M. J. Blackman, V. B. Carruthers, and K. Kim. 2001. A conserved subtilisin-like protein TgSUB1 in microneme organelles of Toxoplasma gondii. J. Biol. Chem. 27645341-45348. [DOI] [PubMed] [Google Scholar]
- 27.Miller, S. A., V. Thathy, J. W. Ajioka, M. J. Blackman, and K. Kim. 2003. TgSUB2 is a Toxoplasma gondii rhoptry organelle processing proteinase. Mol. Microbiol. 49883-894. [DOI] [PubMed] [Google Scholar]
- 28.Montero, E., L. M. Gonzalez, M. Rodriguez, Y. Oksov, M. J. Blackman, and C. A. Lobo. 2006. A conserved subtilisin protease identified in Babesia divergens merozoites. J. Biol. Chem. 28135717-35726. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor, R. M., K. Kim, F. Khan, and H. D. Ward. 2003. Expression of Cpgp40/15 in Toxoplasma gondii: a surrogate system for the study of Cryptosporidium glycoprotein antigens. Infect. Immun. 716027-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor, R. M., J. W. Wanyiri, A. M. Cevallos, J. W. Priest, and H. D. Ward. 2007. Cryptosporidium parvum glycoprotein gp40 localizes to the sporozoite surface by association with gp15. Mol. Biochem. Parasitol. 15680-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawlings, N. D., E. O'Brien, and A. J. Barrett. 2002. MEROPS: the protease database. Nucleic Acids Res. 30343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajid, M., C. Withers-Martinez, and M. J. Blackman. 2000. Maturation and specificity of Plasmodium falciparum subtilisin-like protease-1, a malaria merozoite subtilisin-like serine protease. J. Biol. Chem. 275631-641. [DOI] [PubMed] [Google Scholar]
- 33.Shaw, M. K., D. S. Roos, and L. G. Tilney. 2002. Cysteine and serine protease inhibitors block intracellular development and disrupt the secretory pathway of Toxoplasma gondii. Microbes Infect. 4119-132. [DOI] [PubMed] [Google Scholar]
- 34.Shinde, U., and M. Inouye. 1996. Propeptide-mediated folding in subtilisin: the intramolecular chaperone concept. Adv. Exp. Med. Biol. 379147-154. [DOI] [PubMed] [Google Scholar]
- 35.Siezen, R. J., and J. A. Leunissen. 1997. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6501-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soldati, D., B. J. Foth, and A. F. Cowman. 2004. Molecular and functional aspects of parasite invasion. Trends Parasitol. 20567-574. [DOI] [PubMed] [Google Scholar]
- 37.Tzipori, S., and H. Ward. 2002. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 41047-1058. [DOI] [PubMed] [Google Scholar]
- 38.Urban, S. 2006. Rhomboid proteins: conserved membrane proteases with divergent biological functions. Genes Dev. 203054-3068. [DOI] [PubMed] [Google Scholar]
- 39.Wanyiri, J., and H. Ward. 2006. Molecular basis of Cryptosporidium-host cell interactions: recent advances and future prospects. Future Microbiol. 1201-208. [DOI] [PubMed] [Google Scholar]
- 40.Wanyiri, J. W., R. O'Connor, G. Allison, K. Kim, A. Kane, J. Qiu, A. G. Plaut, and H. D. Ward. 2007. Proteolytic processing of the Cryptosporidium glycoprotein gp40/15 by human furin and by a parasite-derived furin-like protease activity. Infect. Immun. 75184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Withers-Martinez, C., L. Jean, and M. J. Blackman. 2004. Subtilisin-like proteases of the malaria parasite. Mol. Microbiol. 5355-63. [DOI] [PubMed] [Google Scholar]
- 42.Xiao, L., and U. M. Ryan. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17483-490. [DOI] [PubMed] [Google Scholar]
- 43.Xu, P., G. Widmer, Y. Wang, L. S. Ozaki, J. M. Alves, M. G. Serrano, D. Puiu, P. Manque, D. Akiyoshi, A. J. Mackey, W. R. Pearson, P. H. Dear, A. T. Bankier, D. L. Peterson, M. S. Abrahamsen, V. Kapur, S. Tzipori, and G. A. Buck. 2004. The genome of Cryptosporidium hominis. Nature 4311107-1112. [DOI] [PubMed] [Google Scholar]
- 44.Yeoh, S., R. A. O'Donnell, K. Koussis, A. R. Dluzewski, K. H. Ansell, S. A. Osborne, F. Hackett, C. Withers-Martinez, G. H. Mitchell, L. H. Bannister, J. S. Bryans, C. A. Kettleborough, and M. J. Blackman. 2007. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 1311072-1083. [DOI] [PubMed] [Google Scholar]